Abstract
Although CD90 has been identified as a marker for various kinds of stem cells including liver cancer stem cells (CSCs) that are responsible for tumorigenesis, the potential role of CD90 as a marker for CSCs in gliomas has not been characterized. To address the issue, we investigated the expression of CD90 in tissue microarrays containing 15 glioblastoma multiformes (GBMs), 19 WHO grade III astrocytomas, 13 WHO grade II astrocytomas, 3 WHO grade I astrocytomas and 8 normal brain tissues. Immunohistochemical analysis showed that CD90 was expressed at a medium to high level in all tested high-grade gliomas (grade III and GBM) whereas it was barely detectable in low-grade gliomas (grade I and grade II) and normal brains. Double immunofluorescence staining for CD90 and CD133 in GBM tissues revealed that CD133+ CSCs are a subpopulation of CD90+ cells in GBMs in vivo. Flow cytometry analysis of the expression of CD90 and CD133 in GBM-derived stem-like neurospheres further confirmed the conclusion in vitro. The expression levels of both CD90 and CD133 were reduced along with the loss of stem cells after differentiation. Furthermore, the limiting dilution assay demonstrated that the sphere formation ability was comparable between the CD90+/CD133+ and the CD90+/CD133− populations of GBM neurospheres, which is much higher than that of the CD90−/CD133− population. We also performed double staining for CD90 and a vascular endothelial cell marker CD31 in tissue microarrays which revealed that the CD90+ cells were clustered around the tumor vasculatures in high-grade glioma tissues. These findings suggest that CD90 is not only a potential prognostic marker for high-grade gliomas but also a marker for CSCs within gliomas, and it resides within endothelial niche and may also play a critical role in the generation of tumor vasculatures via differentiation into endothelial cells.
Gliomas constitute the majority of primary brain tumors. The most common kind of glioma are astrocytomas which are further classified into four grades by the WHO-grading scheme. WHO grade I and II are well differentiated and considered as low grade, while WHO grade III and IV are poorly differentiated and considered as high grade, with high grade being more malignant and aggressive. Malignant gliomas are among the most devastating and lethal cancers. Even with state-of-the-art surgery, imaging, radiation, and chemotherapy, the prognosis of high-grade gliomas is still poor, with a two-year survival rate of less than 5% for WHO grade IV astrocytoma (also known as glioblastoma multiforme (GBM))1 (1). The poor survival is primarily because of the recurrence of tumor, which is resistant to standard therapies (2). Therefore, new strategies to treat malignant gliomas are urgently needed.
There is increasing evidence that a small population of cancer stem cells (CSCs) within solid tumors is responsible for tumor formation and ongoing growth (3–5). The existence of CSCs has been demonstrated in GBMs (6–8). These CSCs share similar properties with normal stem cells and have the ability to self-renew, differentiate and drive tumorigenesis. The CSCs are resistant to radiation therapy (9) and chemotherapy (10), and are considered to be the most likely cause of cancer recurrence. It should be noted that recent findings have shown the possibility of targeting CSCs for the treatment of brain tumors (9, 11–21). Discovery of potential therapeutic markers for GBM CSCs is thus of intense interest.
Tissue microarrays are a high-throughput proteomic technique for the identification of biomarkers by analyzing tens to hundreds of tissue samples on a single slide (22, 23). Numerous tissue samples from tissue microarrays are immunohistochemically analyzed at one time under the same histological conditions, permitting high reproducibility between different replicates of each sample and reliable comparison between different samples. As the technique allows in situ visualization of protein expression patterns, it is able to provide information regarding subcellular location of target proteins.
Although CD133 has been identified as a marker for CSCs in GBMs, the CD133− cells also have the capability to generate GBM-like tumors in immunodeficient nude mice or rats (24–27). These studies suggest that CD133 is not necessarily expressed in the CSCs in gliomas and there should be more general and sensitive makers for CSCs in gliomas. Our previous study (28) demonstrated that the expression level of CD90 in a GBM-derived stem-like neurosphere line was dramatically higher than that in three traditional adherent GBM cell lines, indicating a role of CD90 as a potential marker for CSCs in GBMs. CD90, also known as Thy-1, is a heavily glycosylated, glycophosphatidylinositol (GPI)-anchored cell surface protein that has previously been identified as a marker for several kinds of stem cells such as hematopoietic stem cells (29) and bone marrow-derived mesenchymal stem cells (30). Recently, CD90 has also been identified as a marker for liver CSCs (31). Based on these observations, the potential of CD90 as a maker for glioma CSCs was characterized in this study using glioma tissue microarrays.
EXPERIMENTAL PROCEDURES
Tissue Samples
Tissue samples were provided in the form of tissue microarrays (US Biomax, Inc. Rockville, MD USA Catalog No. GL722 and GL807). Fifteen of the 58 samples had a GBM (age: 36 ± 17 years; 6 females and 9 males); 19 samples had a WHO grade III astrocytoma (age: 46 ± 11 years; 7 females and 12 males); 13 samples had a WHO grade II astrocytoma (age: 42 ± 12 years; 5 females and 8 males); three samples had a WHO grade I astrocytoma (age: 42 ± 10 years; 1 females and 2 males); eight samples were from normal subjects (age: 41 ± 10 years; 4 females and 4 males). The tissue samples were originated from different donors and each sample had at least two replicates. The glioma tissue sections are from the tumor areas and do not include the adjacent normal tissues.
Immunohistochemical Analysis of Tissue Microarrays
Immunohistochemical staining was performed using tissue microarrays. The paraffin-embedded 5 μm arrays were dewaxed in xylene for 10 min and rehydrated through a series of alcohol solutions (100% ethanol twice, 90% ethanol, 70% ethanol, 5 min each) to water. Antigen retrieval was achieved by boiling the arrays in a citrate buffer at pH 6.0. Endogenous peroxidase activity was blocked using 6% H2O2. The tissue microarrays were blocked with 2% normal goat serum and incubated with rabbit antihuman CD90 monoclonal antibody (1:100 dilution, Abcam, Cambridge, MA) overnight at 4 °C. Immunodetection was performed using the VECTASTAIN Elite ABC system (Vector laboratories, Burlingame, CA) according to the manufacturer's instructions. Hematoxylin counterstain was used to visualize nuclei. The CD90 expression level in each tissue section was assessed in non-necrotic areas of three separate microscopic fields of view under a magnification of 20× and was represented by the mean of the percentage of CD90+ cells.
Double Immunofluorescence Analysis of Tissue Microarrays
According to the different properties of each individual antibody, a sequential procedure was used for the staining of CD90 and CD133, whereas a simultaneous procedure was used for the staining of CD90 and CD31. Briefly, following deparaffinization, rehydration, antigen retrieval, and endogenous peroxidase blocking of tissue microarrays, double immunofluorescence staining for CD90 and CD133 was performed with incubation of mouse anti-human CD133 monoclonal antibody (1:5 dilution, Miltenyi Biotec, Auburn, CA) overnight at room temperature, followed by anti-human CD90 (1:100 dilution) for 2 h at room temperature. For double immunofluorescence staining of CD90 and CD31, a mixture of mouse anti-human CD31 (1:50 dilution, Novocastra, Newcastle Upon Tyne, UK) monoclonal antibody and anti-human CD90 (1:100 dilution) was used to incubate with the arrays overnight at 4 °C. DyLight 488 anti-rabbit IgG (H+L) and DyLight 549 anti-mouse IgG (H+L) (Vector laboratories, Burlingame, CA) were used for immunofluorescence detection, and 4,6-diamidino-2-phenylindole (DAPI) counterstain was used to visualize nuclear detail.
Cell Culture
The HSR-GBM1 neurosphere cells were maintained in the NeuroCult proliferation medium (Stem Cell Technologies, Vancouver, Canada) supplemented with 10 ng/ml EGF (PeproTech, Rocky Hill, NJ), 10 ng/ml FGFb (PeproTech), and 2 μg/ml heparin (Sigma) as previously described (8, 12). Differentiation of the neurospheres was achieved by plating 0.9–1 × 105 cells/cm2 on a poly-ornithine (15 μg/ml) coated culture plate and maintaining in the NeuroCult differentiation medium (Stem Cell Technologies, Vancouver, BC, Canada) as described previously (8).
Cell Sorting and Limiting Dilution Assay of Sphere Formation
Fluorescence-activated cell sorting experiments were carried out at the Flow Cytometry Core at the University of Michigan. HSR-GBM1 neurospheres were dissociated into single cells and stained with CD90-FITC (BD Biosciences, Lexington, KY; Cat# 555595) and CD133-PE (Miltenyi Biotec, Auburn, CA; Cat# 130–080-801) antibodies for FACS according to the manufactures' instructions. Cells were gated against isotype controls (IgG1-FITC and IgG1-PE for CD90 and CD133, respectively) and analyzed for CD90+, CD133+, CD90+/CD133+, CD90+/CD133−, and CD90−/CD133− populations. Each population of unsorted, CD90+/CD133+, CD90+/CD133−, and CD90−/CD133− cells was sorted into a 96-well plate. A twofold dilution of single cells ranging from 125 to 0 was sorted directly into each well of 96-well plates (repeated eight times) using a BDAria II fluorescence-activated cell sorting machine for the limiting dilution assay. Cells were incubated in the 96-wells for 6 days before examination of sphere formation. Percent of wells that did not form spheres (y-axis) was plotted against the number of cells plated per well (x-axis). The minimum number of cells required to form spheres was calculated from the x-intercept of the graph.
Western Blotting
Western blotting was performed essentially as described before (32). Briefly, 16 μg of proteins from the undifferentiated and differentiated HSR-GBM1 cells were separated by 4–20% SDS-PAGE and transferred to polyvinylidene difluoride membranes (Bio-Rad, CA). The membranes were blocked with 1% bovine serum albumin in PBST (0.05% Tween-20 in PBS) for 2 h, and then incubated with the following antibodies overnight at 4 °C: anti-CD90 (Abnova, Taipei, China), anti-CD133 (Abcam, Cambridge, MA), and anti-β-actin (Abcam, Cambridge, MA). The membranes were washed with PBST three times, incubated with peroxidase-conjugated IgG (H+L) for 1 h and washed another three times with PBST, followed by detection with Supersignal West Pico Chemiluminescent horseradish peroxidase Substrate (Thermo Scientific, IL).
Statistical Analysis
t test was used to compare the differences in CD90 expression levels between different sample groups (i.e. astrocytoma WHO grade I, grade II, grade III, grade IV, and normal brains). Statistical significance was defined as p < 0.05.
RESULTS
CD90 is a Marker for High-grade Gliomas
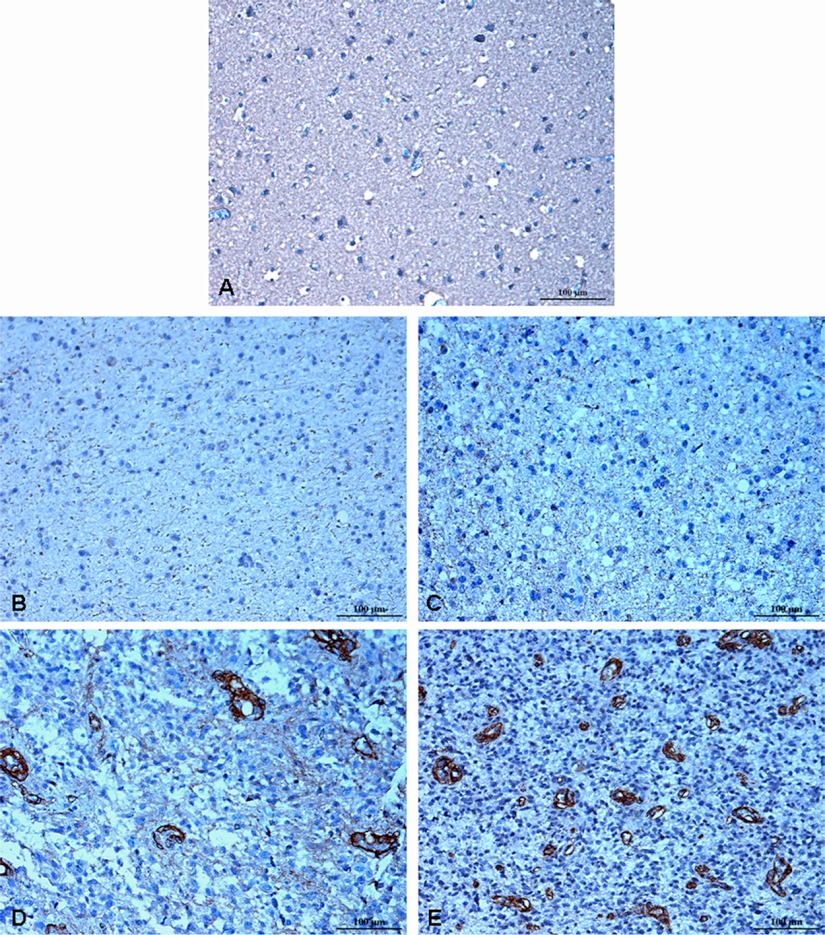
Expression levels of CD90 were assessed immunohistochemically using tissue microarrays containing 50 gliomas of different WHO grades and eight normal subjects. Substantial differences in CD90 staining were observed between high-grade and low-grade gliomas. CD90 was not detectable in normal brains (Fig. 1A), WHO grade I astrocytomas (Fig. 1B), and most of WHO grade II astrocytomas (Fig. 1C). In contrast, the expression of CD90 was dramatically higher in WHO grade III astrocytomas (Fig. 1D) and GBMs (Fig. 1E).
Fig. 1.
Immunohistochemical staining for human CD90 in different grades of astrocytomas and normal brains. Paraffin-embedded tissue microarrays were stained using an anti-human CD90 antibody. CD90 was not detected in normal brain (A), astrocytoma WHO grade I (B) and diffuse astrocytoma WHO grade II (C). In contrast, moderate or strong staining for CD90 was observed in anaplastic astrocytoma WHO grade III (D) and GBM WHO grade IV (E). Hematoxylin counterstain was used to visualize nuclei. Scale bar, 100 μm.
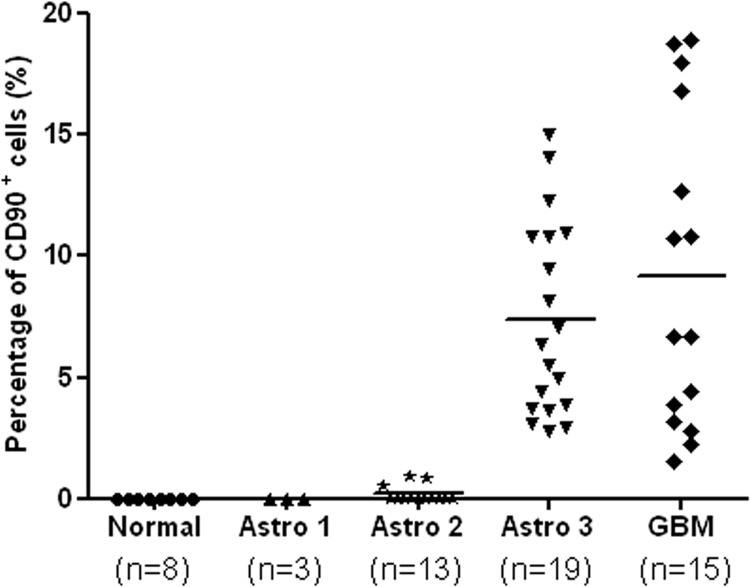
The CD90 expression level in each tissue section was semiquantitatively represented by the percentage of CD90+ cells. The relative CD90 expression levels in normal brains, WHO grade I astrocytomas, WHO grade II astrocytomas, WHO grade III astrocytomas and GBMs are shown in Fig. 2. All normal brains and WHO grade I astrocytomas showed zero CD90 levels. In patients with WHO grade II astrocytomas, CD90 was either not detectable (10 of 13, 77%) or expressed in less than 1% of cells (3 of 13, 23%). As to high-grade gliomas, CD90 was detectable in all tested samples, with an average CD90 level of 7% for WHO grade III astrocytomas (range: 2.8–12.3%; n = 19) and 9% for GBMs (range: 2.2–18.9%; n = 15). No significant difference in CD90 expression was observed between grade I and grade II astrocytomas (p > 0.05), neither was there between grade III astrocytomas and GBM (p > 0.05). However, the expression levels of CD90 in high-grade gliomas were significantly higher than in low-grade gliomas (p < 0.0001). As CD90 was highly expressed in high-grade gliomas but it was almost not expressed in low-grade gliomas and normal brains, CD90 may serve as a marker for high-grade gliomas.
Fig. 2.
Scatter plot of CD90 expression levels in n = 50 patients with different grades of astrocytomas and n = 8 normal subjects. The CD90 expression level of each tissue sample was represented by the percentage of CD90+ cells. Each point corresponds to the CD90 expression level of a single sample. CD90 expression levels were significantly elevated in patients with high-grade astrocytomas (WHO grade III and IV). Astro 1 represents astrocytoma WHO grade I; Astro 2 represents diffuse astrocytoma (WHO grade II); Astro 3 represents anaplastic astrocytoma (WHO grade III); GBM represents glioblastoma multiforme (WHO grade IV).
CD90+ Cells Contain a Subpopulation of CD133+ Cells in GBM
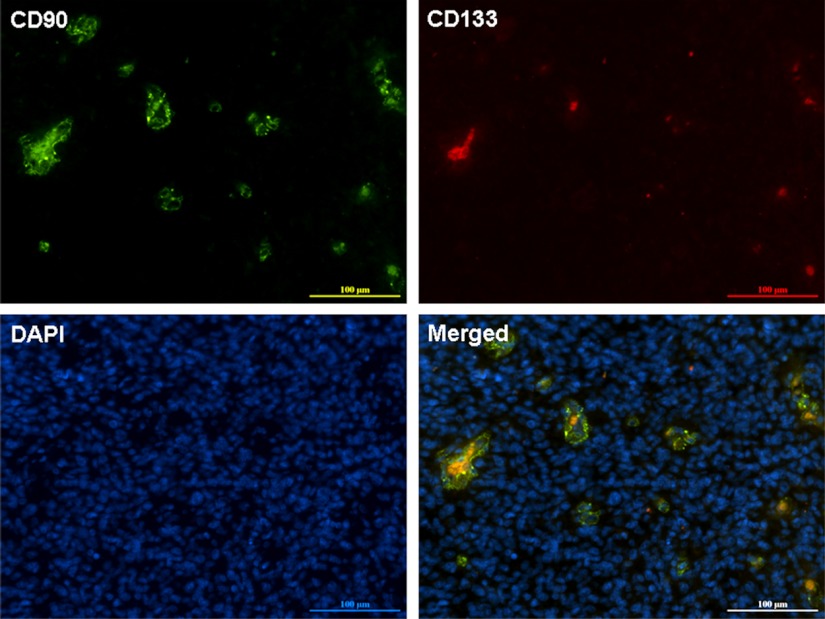
To determine whether the CD90+ cells were also expressing the GBM stem cell marker CD133, we performed double immunofluorescence staining for CD90 and CD133 in primary GBM tissue sections. CD90 and CD133 were co-expressed in GBM, where 100% of the CD133+ cells were also positive for CD90, but only a small part of CD90+ cells were positive for CD133 (Fig. 3). This indicates that the CD133+ stem-like cells are a subpopulation of CD90+ cells in GBMs in vivo.
Fig. 3.
Double immunofluorescence staining for CD90 and CD133 in a GBM section. CD90+ cells contain a subpopulation of CD133+ cells, where the CD90+CD133+ cells are shown in yellow in the merged image. DAPI counterstain was used to visualize nuclei. As a negative control, a WHO grade II astrocytoma was double stained for CD90 and CD133 (data shown in supplemental Fig. S2). Scale bar, 100 μm.
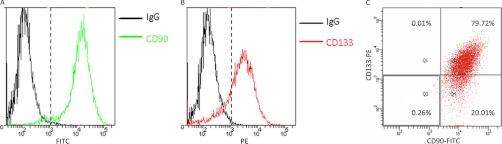
We also tested the expression of CD90 and CD133 in a GBM-derived stem-like neurosphere line HSR-GBM1 which has been extensively used in CSC studies before (8, 12, 15, 28, 33). Flow cytometry analysis showed that CD90 was expressed in more than 99% of HSR-GBM1 cells (Fig. 4A), whereas CD133 was expressed in ∼80% of HSR-GBM1 cells (Fig. 4B). A flow cytometry dot plot showed that ∼99.99% (79.72%/(79.72% + 0.01%)) of CD133+ cells were also expressing CD90, while 79.94% (79.72%/(79.72% + 20.01%)) of CD90+ cells were expressing CD133 (Fig. 4C). The flow cytometry analysis demonstrated that CD133+ cells were a subpopulation of CD90+ cells in GBM-derived stem-like neurospheres.
Fig. 4.
Flow cytometry analysis of the expression of CD90 and CD133 in the stem-like neurosphere HSR-GBM1. Flow cytometry histograms showed more than 99% of HSR-GBM1 cells were CD90-positive (A) and about 80% were CD133 positive (B). C, In the flow cytometry dot plot, the HSR-GBM1 cells were classified into four cell populations based on the expression of CD90 and CD133. The percentage of each subpopulation was shown. FITC, fluorescein isothiocyanate; PE, phycoerythrin.
CD90 is a New Marker for GBM Stem Cells
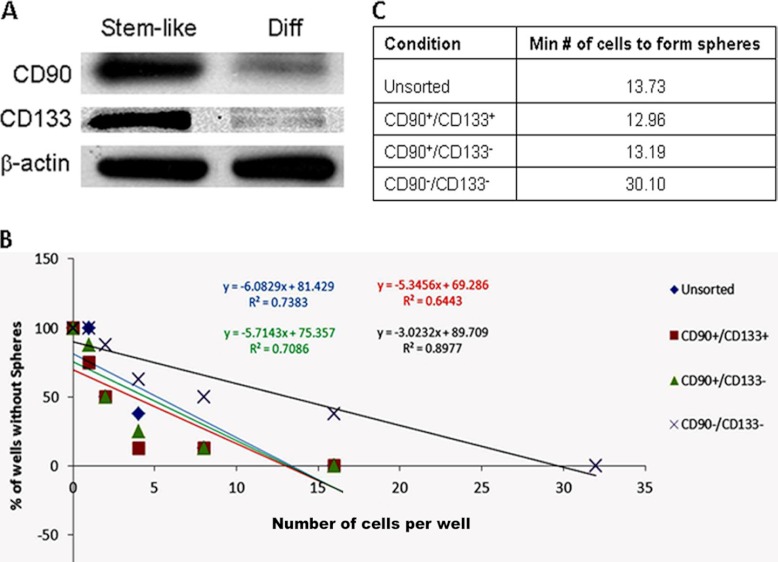
To determine whether CD90 is a GBM CSC marker, we induced the undifferentiated HSR-GBM1 stem-like neurospheres to differentiate them and then analyzed the alteration of CD90 level during the differentiation process. We and others have found that most of stem-like cells were lost after differentiation of HSR-GBM1 cells, as indicated by the reduced expression of the known GBM stem cell marker CD133 (15, 33, 34). Western blotting analysis showed that CD90 was dramatically down-regulated in the differentiated cells compared with the undifferentiated stem-like cells, with a similar differential expression pattern to CD133 (Fig. 5A). These results suggest that both CD90 and CD133 levels were decreased along with the loss of stem-like cells, and CD90 may represent a new marker for CSCs in GBM.
Fig. 5.
CD90 is a marker for GBM stem cells. A, Differential expression of CD90 and CD133 along with the differentiation of the stem-like HSR-GBM1 neurospheres. When the stem-like neurospheres were forced to differentiate, both CD90 and CD133 expression levels were dramatically reduced. Stem-like represents the stem-like HSR-GBM1 neurospheres, which are undifferentiated cells; Diff represents the differentiated HSR-GBM1 cells. B, Populations of unsorted, CD90+/CD133+, CD90+/CD133−, and CD90−/CD133− HSR-GBM1 neurospheres were analyzed for their ability of sphere formation by the limiting dilution assay. y-axis: percent of wells that did not form spheres; x-axis: the number of cells plated per well. C, The minimum number of cells required to form spheres for each population of HSR-GBM1 neurospheres. The number was similar between the unsorted, CD90+/CD133+ and CD90+/CD133− populations. Compared with these populations, the CD90−/CD133− population required the most number of cells (more than twofold) to form spheres.
We further performed a limiting dilution assay to evaluate the self-renewal ability of the CD90+ cells. As a result, the CD90+/CD133+, CD90+/CD133−, and unsorted populations of GBM neurospheres required a similar number of cells to form spheres (Figs. 5B and 5C). Compared with them, the CD90−/CD133− population required more than 2-fold of cells to form spheres. This experiment demonstrated that the CD90+ cells had much higher potential of stemness than the CD90− cells, which confirmed that CD90 was a marker for GBM CSCs.
CD90+ Cells are Clustered Around Tumor Vasculatures in Gliomas
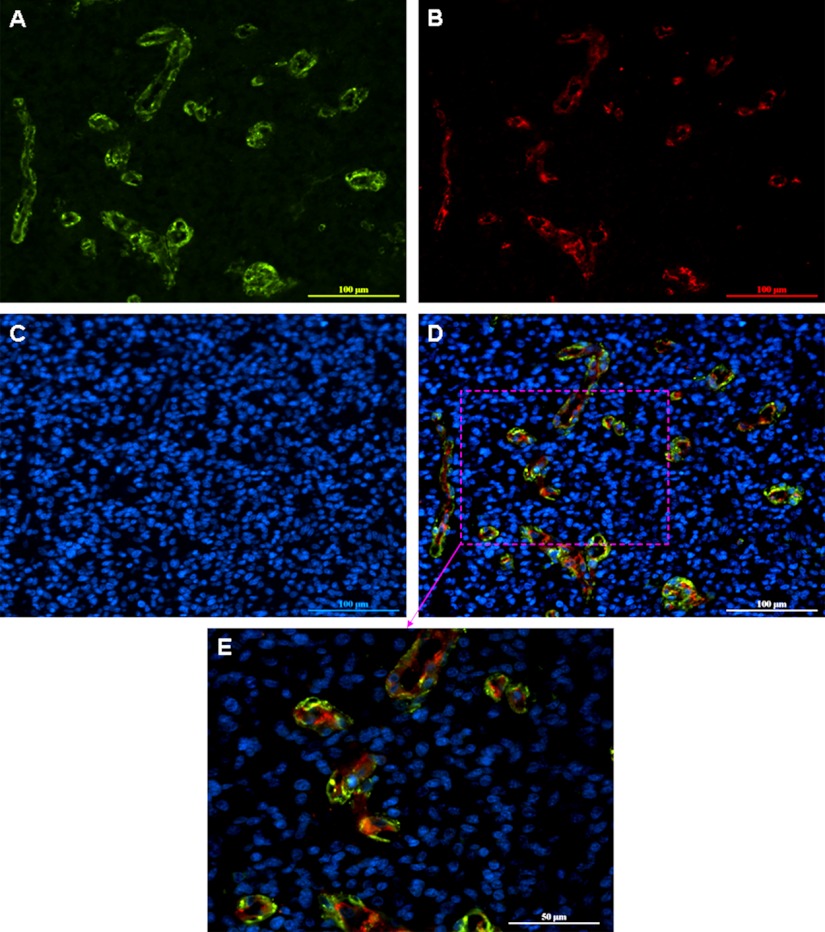
As shown in Figs. 1D and 1E, the CD90+ cells formed vascular-like structures in high-grade gliomas. To test the possible localization of CD90+ cells in tumor vasculature in gliomas, we performed double immunofluorescence staining for CD90 (Fig. 6A) and a vascular endothelial cell marker CD31 (Fig. 6B) in GBMs. The merged images showed that CD90 was either colocalized with CD31 (shown in yellow) or near CD31 (shown in green) in the tumor vasculature structures in GBM (Figs. 6D and 6E). We evaluated the double staining of CD90 and CD133 using 7 GBM tissue samples on a tissue microarray slide (US Biomax Catalog No. GL722). The staining results are reproducible in all the 7 GBM tissue microarrays on the slide (supplemental Fig. S1). These results suggest that CD90+ GBM CSCs may reside within the endothelial niche for their self-renewal, consistent with our recent finding that endothelial cells create a stem cell niche in glioblastoma by providing Notch ligands that nurture self-renewal of cancer stem-like cells (15).
Fig. 6.
Double immunofluorescence staining for CD90 and CD31 in GBM. A GBM section was stained with anti-CD90 (A) and anti-CD31 (B). DAPI counterstain (C) was used to visualize nuclei. Colocalization of CD90 and CD31 was observed in the merged image (D). An area of this merged image was selected and a higher magnification image of the area was also shown (E). Scale bar in A–D, 100 μm; Scale bar in E, 50 μm.
DISCUSSION
In this study, we applied tissue microarrays to analyze the expression of CD90 in different grades of glioma tissues and found that the CD90 levels in high-grade gliomas (WHO grade III and IV) were significantly higher than in low-grade gliomas (WHO grade I and II) and normal brains (Fig. 1 and Fig. 2). These observations suggest that the CD90 expression level may be an indicator of the aggressiveness of gliomas. CD90 showed high sensitivity and specificity as a marker, with high or medium expression levels in all the tested high-grade glioma tissues but barely detectable in low-grade gliomas and normal brains. Therefore, CD90 appears to be a promising new prognostic marker for gliomas.
It should be noted that in contrast to the highly differentiated low-grade gliomas, GBMs are undifferentiated and therefore are more likely to contain undifferentiated CSCs. Because the expression level of CD90 is dramatically increased in GBMs compared with low-grade gliomas, CD90 can be a new marker of these CSCs within GBM tissues. CD90 has been identified as a marker for hematopoietic stem cells (29), bone marrow-derived mesenchymal stem cells (30), hepatic stem cells (35), as well as liver CSCs (31). Although we and others have also observed the expression of CD90 in GBM-derived stem cells (10, 28), the potential of CD90 as a marker for these in vitro cultured stem cells and CSCs in primary glioma tissues remains uncharacterized. To the best of our knowledge, this work is the first to address this issue. In this study, the double immunofluorescence staining of GBM tissues (Fig. 3) and flow cytometry analysis of GBM-derived stem-like neurospheres (Fig. 4) revealed that CD133+ stem cells are a subpopulation of CD90+ cells in GBMs in vivo and in vitro. The expression levels of both CD90 and CD133 were reduced along with the loss of stem cells after differentiation (Fig. 5A). Further a limiting dilution assay demonstrated that the CD90+/CD133+ and CD90+/CD133− populations of GBM neurospheres had comparable ability to form spheres, whereas the CD90−/CD133− population showed much lower potential of stemness (Figs. 5B and 5C). These findings suggest that CD90 is a novel marker for CSCs in high-grade gliomas and it is a more general marker than CD133.
Interestingly, we observed the formation of vascular-like structures by CD90+ cells in high-grade gliomas (Fig. 1). This indicates that CD90+ cells may reside within the endothelial niche to self-renew (15). There has been increasing evidence that stem-like cells within GBMs are able to generate tumor vasculature by endothelial cell differentiation (36, 37), which represents a new mechanism of tumor angiogenesis. In this study, dual staining with CD90 and a vascular endothelial cell marker CD31 confirmed that CD90+ cells were clustered around the tumor vasculatures in GBMs (Fig. 6). These data suggest that the GBM-derived CD90+ stem cells may have the potential to differentiate into vascular endothelial cells.
Our findings may have the following therapeutic implications. First, CD90 is a CSC marker that is more sensitive than CD133, which makes it an ideal drug target for cancer stem cell-based therapies of malignant gliomas. Second, the finding that CD90+ cancer stem cells may play a critical role in the formation of tumor vasculature implies that CD90 may be a good target for anti-angiogenesis treatment of gliomas.
In conclusion, we have shown that CD90 can be used to distinguish high-grade gliomas from low-grade gliomas and normal brains. Further study reveals that CD90 is a new marker for CSCs in high-grade gliomas and it is more general than the known stem cell marker CD133. The CD90+ cells form vascular-like structures and partially co-localize with an endothelial marker CD31 in high-grade glioma tissues, suggesting that CD90+ cells may also reside within the endothelial niche to self-renew. It is also possible that CD90+ cells generate tumor vasculature via differentiation into endothelial cells. These findings provide new insights into the cancer stem cell biology of gliomas.
Acknowledgments
We would like to acknowledge grant support to Dr. Fan from Accelerate Brain Cancer Cure Project Award, American Brain Tumor Association Translational Grant, and Voices Against Brain Cancer Research Grant.
Footnotes
* This work was supported by the National Institute of Health Grant No. R01 49500 (D. M. L.) and the National Cancer Institute Grant No. R21 CA134623 (D. M. L.), R21 CA124441 (D. M. L.) and the National Cancer Institute Grant No. R01CA148621 (Xing Fan).
 This article contains supplemental Figs. S1 and S2.
This article contains supplemental Figs. S1 and S2.
1 The abbreviations used are:
- GBM
- glioblastoma multiforme
- CSC
- cancer stem cell.
REFERENCES
- 1. Ohgaki H., Dessen P., Jourde B., Horstmann S., Nishikawa T., Di Patre P. L., Burkhard C., Schüler D., Probst-Hensch N. M., Maiorka P. C., Baeza N., Pisani P., Yonekawa Y., Yasargil M. G., Lütolf U. M., Kleihues P. (2004) Genetic pathways to glioblastoma: a population-based study. Cancer Res. 64, 6892–6899 [DOI] [PubMed] [Google Scholar]
- 2. Reardon D. A., Rich J. N., Friedman H. S., Bigner D. D. (2006) Recent advances in the treatment of malignant astrocytoma. J. Clin. Oncol. 24, 1253–1265 [DOI] [PubMed] [Google Scholar]
- 3. Reya T., Morrison S. J., Clarke M. F., Weissman I. L. (2001) Stem cells, cancer, and cancer stem cells. Nature 414, 105–111 [DOI] [PubMed] [Google Scholar]
- 4. Visvader J. E., Lindeman G. J. (2008) Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat. Rev. Cancer 8, 755–768 [DOI] [PubMed] [Google Scholar]
- 5. Al-Hajj M., Wicha M. S., Benito-Hernandez A., Morrison S. J., Clarke M. F. (2003) Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. U.S.A. 100, 3983–3988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Singh S. K., Clarke I. D., Terasaki M., Bonn V. E., Hawkins C., Squire J., Dirks P. B. (2003) Identification of a cancer stem cell in human brain tumors. Cancer Res. 63, 5821–5828 [PubMed] [Google Scholar]
- 7. Singh S. K., Hawkins C., Clarke I. D., Squire J. A., Bayani J., Hide T., Henkelman R. M., Cusimano M. D., Dirks P. B. (2004) Identification of human brain tumour initiating cells. Nature 432, 396–401 [DOI] [PubMed] [Google Scholar]
- 8. Galli R., Binda E., Orfanelli U., Cipelletti B., Gritti A., De Vitis S., Fiocco R., Foroni C., Dimeco F., Vescovi A. (2004) Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 64, 7011–7021 [DOI] [PubMed] [Google Scholar]
- 9. Bao S., Wu Q., McLendon R. E., Hao Y., Shi Q., Hjelmeland A. B., Dewhirst M. W., Bigner D. D., Rich J. N. (2006) Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444, 756–760 [DOI] [PubMed] [Google Scholar]
- 10. Liu G., Yuan X., Zeng Z., Tunici P., Ng H., Abdulkadir I. R., Lu L., Irvin D., Black K. L., Yu J. S. (2006) Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol. Cancer 5, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Piccirillo S. G., Reynolds B. A., Zanetti N., Lamorte G., Binda E., Broggi G., Brem H., Olivi A., Dimeco F., Vescovi A. L. (2006) Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature 444, 761–765 [DOI] [PubMed] [Google Scholar]
- 12. Fan X., Khaki L., Zhu T. S., Soules M. E., Talsma C. E., Gul N., Koh C., Zhang J., Li Y. M., Maciaczyk J., Nikkhah G., Dimeco F., Piccirillo S., Vescovi A. L., Eberhart C. G. (2010) NOTCH pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts. Stem Cells 28, 5–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fan X., Matsui W., Khaki L., Stearns D., Chun J., Li Y. M., Eberhart C. G. (2006) Notch pathway inhibition depletes stem-like cells and blocks engraftment in embryonal brain tumors. Cancer Res. 66, 7445–7452 [DOI] [PubMed] [Google Scholar]
- 14. Fan X., Eberhart C. G. (2008) Medulloblastoma stem cells. J. Clin. Oncol. 26, 2821–2827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhu T. S., Costello M. A., Talsma C. E., Flack C. G., Crowley J. G., Hamm L. L., He X., Hervey-Jumper S. L., Heth J. A., Muraszko K. M., DiMeco F., Vescovi A. L., Fan X. (2011) Endothelial cells create a stem cell niche in glioblastoma by providing NOTCH ligands that nurture self-renewal of cancer stem-like cells. Cancer Res. 71, 6061–6072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bao S., Wu Q., Li Z., Sathornsumetee S., Wang H., McLendon R. E., Hjelmeland A. B., Rich J. N. (2008) Targeting cancer stem cells through L1CAM suppresses glioma growth. Cancer Res. 68, 6043–6048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eyler C. E., Foo W. C., LaFiura K. M., McLendon R. E., Hjelmeland A. B., Rich J. N. (2008) Brain cancer stem cells display preferential sensitivity to Akt inhibition. Stem Cells 26, 3027–3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li Z., Bao S., Wu Q., Wang H., Eyler C., Sathornsumetee S., Shi Q., Cao Y., Lathia J., McLendon R. E., Hjelmeland A. B., Rich J. N. (2009) Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell 15, 501–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang H., Lathia J. D., Wu Q., Wang J., Li Z., Heddleston J. M., Eyler C. E., Elderbroom J., Gallagher J., Schuschu J., MacSwords J., Cao Y., McLendon R. E., Wang X. F., Hjelmeland A. B., Rich J. N. (2009) Targeting interleukin 6 signaling suppresses glioma stem cell survival and tumor growth. Stem Cells 27, 2393–2404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guryanova O. A., Wu Q., Cheng L., Lathia J. D., Huang Z., Yang J., MacSwords J., Eyler C. E., McLendon R. E., Heddleston J. M., Shou W., Hambardzumyan D., Lee J., Hjelmeland A. B., Sloan A. E., Bredel M., Stark G. R., Rich J. N., Bao S. (2011) Nonreceptor tyrosine kinase BMX maintains self-renewal and tumorigenic potential of glioblastoma stem cells by activating STAT3. Cancer Cell 19, 498–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eyler C. E., Wu Q., Yan K., MacSwords J. M., Chandler-Militello D., Misuraca K. L., Lathia J. D., Forrester M. T., Lee J., Stamler J. S., Goldman S. A., Bredel M., McLendon R. E., Sloan A. E., Hjelmeland A. B., Rich J. N. (2011) Glioma stem cell proliferation and tumor growth are promoted by nitric oxide synthase-2. Cell 146, 53–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Giltnane J. M., Rimm D. L. (2004) Technology insight: Identification of biomarkers with tissue microarray technology. Nat. Clin. Pract. Oncol. 1, 104–111 [DOI] [PubMed] [Google Scholar]
- 23. Leth-Larsen R., Lund R. R., Ditzel H. J. (2010) Plasma membrane proteomics and its application in clinical cancer biomarker discovery. Mol. Cell. Proteomics 9, 1369–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Beier D., Hau P., Proescholdt M., Lohmeier A., Wischhusen J., Oefner P. J., Aigner L., Brawanski A., Bogdahn U., Beier C. P. (2007) CD133(+) and CD133(-) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 67, 4010–4015 [DOI] [PubMed] [Google Scholar]
- 25. Wang J., Sakariassen P. Ø., Tsinkalovsky O., Immervoll H., Boe S. O., Svendsen A., Prestegarden L., Rosland G., Thorsen F., Stuhr L., Molven A., Bjerkvig R., Enger P. O. (2008) CD133 negative glioma cells form tumors in nude rats and give rise to CD133 positive cells. Int. J. Cancer 122, 761–768 [DOI] [PubMed] [Google Scholar]
- 26. Pfenninger C. V., Roschupkina T., Hertwig F., Kottwitz D., Englund E., Bengzon J., Jacobsen S. E., Nuber U. A. (2007) CD133 is not present on neurogenic astrocytes in the adult subventricular zone, but on embryonic neural stem cells, ependymal cells, and glioblastoma cells. Cancer Res. 67, 5727–5736 [DOI] [PubMed] [Google Scholar]
- 27. Joo K. M., Kim S. Y., Jin X., Song S. Y., Kong D. S., Lee J. I., Jeon J. W., Kim M. H., Kang B. G., Jung Y., Jin J., Hong S. C., Park W. Y., Lee D. S., Kim H., Nam D. H. (2008) Clinical and biological implications of CD133-positive and CD133-negative cells in glioblastomas. Lab. Invest. 88, 808–815 [DOI] [PubMed] [Google Scholar]
- 28. He J., Liu Y., Xie X., Zhu T., Soules M., DiMeco F., Vescovi A. L., Fan X., Lubman D. M. (2010) Identification of cell surface glycoprotein markers for glioblastoma-derived stem-like cells using a lectin microarray and LC-MS/MS approach. J. Proteome Res. 9, 2565–2572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baum C. M., Weissman I. L., Tsukamoto A. S., Buckle A. M., Peault B. (1992) Isolation of a candidate human hematopoietic stem-cell population. Proc. Natl. Acad. Sci. U.S.A. 89, 2804–2808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dennis J. E., Esterly K., Awadallah A., Parrish C. R., Poynter G. M., Goltry K. L. (2007) Clinical-scale expansion of a mixed population of bone-marrow-derived stem and progenitor cells for potential use in bone-tissue regeneration. Stem Cells 25, 2575–2582 [DOI] [PubMed] [Google Scholar]
- 31. Yang Z. F., Ho D. W., Ng M. N., Lau C. K., Yu W. C., Ngai P., Chu P. W., Lam C. T., Poon R. T., Fan S. T. (2008) Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell 13, 153–166 [DOI] [PubMed] [Google Scholar]
- 32. He J., Liu Y., He S., Wang Q., Pu H., Ji J. (2007) Proteomic analysis of a membrane skeleton fraction from human liver. J. Proteome Res. 6, 3509–3518 [DOI] [PubMed] [Google Scholar]
- 33. Bar E. E., Chaudhry A., Lin A., Fan X., Schreck K., Matsui W., Piccirillo S., Vescovi A. L., DiMeco F., Olivi A., Eberhart C. G. (2007) Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells 25, 2524–2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. He J., Liu Y., Zhu T. S., Xie X., Costello M. A., Talsma C. E., Flack C. G., Crowley J. G., Dimeco F., Vescovi A. L., Fan X., Lubman D. M. (2011) Glycoproteomic analysis of glioblastoma stem cell differentiation. J. Proteome Res. 10, 330–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Masson N. M., Currie I. S., Terrace J. D., Garden O. J., Parks R. W., Ross J. A. (2006) Hepatic progenitor cells in human fetal liver express the oval cell marker Thy-1. Am. J. Physiol. Gastrointest. Liver Physiol. 291, G45–54 [DOI] [PubMed] [Google Scholar]
- 36. Wang R., Chadalavada K., Wilshire J., Kowalik U., Hovinga K. E., Geber A., Fligelman B., Leversha M., Brennan C., Tabar V. (2010) Glioblastoma stem-like cells give rise to tumour endothelium. Nature 468, 829–833 [DOI] [PubMed] [Google Scholar]
- 37. Ricci-Vitiani L., Pallini R., Biffoni M., Todaro M., Invernici G., Cenci T., Maira G., Parati E. A., Stassi G., Larocca L. M., De Maria R. (2010) Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature 468, 824–828 [DOI] [PubMed] [Google Scholar]