Abstract
Nrf2 gene encodes a transcription factor that regulates the expression of a cluster of antioxidant and detoxification genes. Recent works from our laboratory indicate that oxidative stress causes rapid de novo synthesis of Nrf2 protein. We have found that 5′ Untranslated Region (5′UTR) of Nrf2 allows the mRNA to undergo an Internal Ribosomal Entry Site (IRES) mediated protein translation. Using liquid chromatography tandem MS, we have discovered that La/SSB protein bound to Nrf2 5′UTR in response to oxidative stress. In vitro RNA binding and in vivo ribonucleoprotein immunoprecipitation showed H2O2 dose and time dependent increases of La/SSB binding to Nrf2 5′UTR. La/SSB protein translocated from the nuclei to cytoplasm and distributed in the perinuclear space in cells treated with H2O2. Isolation of ribosomal fractions indicated that oxidants caused an association of La/SSB with ribosomes. Physical interaction of La/SSB with representative proteins from the small or large subunits of ribosomes was found to increase in cells responding to H2O2 treatment. Knocking down La/SSB gene with siRNA prevented Nrf2 protein elevation or Nrf2 5′UTR activation by oxidants. In contrast, overexpression of La/SSB gene was able to enhance Nrf2 5′UTR activation and Nrf2 protein increase. Our data suggest that oxidants cause nuclear export of La/SSB protein and subsequent association of La/SSB with Nrf2 5′UTR and ribosomes. These events contribute to de novo Nrf2 protein translation because of oxidative stress.
Nuclear factor erythroid-2 related factor 2 (Nrf2)1 is a transcription factor that regulates the expression of a cluster of antioxidant and detoxification genes. As a bZIP transcription factor, Nrf2 can bind and activate the antioxidant response element (ARE) in the promoters of target genes, including NAD(P)H:Quinone Oxidoreductase 1, glutathione S-transferases, epoxide hydrolase-1, hemeoxygenase-1, superoxide dismutases, thioredoxin reductase, and peroxiredoxin 1 (1–3). Whereas Nrf2 controls the antioxidant response, oxidants are a major inducer of Nrf2 protein and activity. This paradox influences the fate of cells under oxidative stress: to survive or to die.
As a transcription factor, the activity of Nrf2 is regulated at multiple levels. It is commonly known that under normal physiological conditions, Nrf2 activity is kept low through the ubiquitin E3 ligase complex Keap1/Cul3/Rbx1 mediated sequestration and proteolytic degradation in the cytosol (4). Oxidants cause Nrf2 protein to dissociate from Keap1/Cul3/Rbx1, resulting in an inhibition of Nrf2 protein degradation (1). When Nrf2 is free from the sequester Keap1, it translocates to the nucleus where it dimerizes with sMaf, ATF4 or JunD to promote transcription of target genes containing ARE in the promoters (1, 5). Recent works from our laboratory indicate that de novo protein translation comprises an important mechanism of rapid elevation of Nrf2 protein by oxidative stress (3).
Little is known about how proteins are translated under stress conditions. Whereas stress causes an overall inhibition of protein synthesis, increasing evidence suggests that genes containing an Internal Ribosomal Entry Site (IRES) in the 5′ Untranslated Region (5′UTR) of mRNA can bypass the mechanism of general protein synthesis, which is dependent on 7-methyl guanine cap at the 5′ end of mRNA. Although the sequences of IRES are not conserved between genes, a common feature of IRESs is GC rich and forms “stems and loops” secondary structures. Human Nrf2 gene encodes an mRNA strand with 555-nucleotides of 5′UTR containing 70% G and C. A stable secondary structure containing “stems and loops” has been predicted via Zucker's MFold algorithm (Xu et al., 2011 manuscript submitted). This suggests that Nrf2 5′UTR contains an IRES, allowing Nrf2 protein being translated under oxidative stress.
IRES mediated protein translation requires IRES trans-acting factors (ITAFs). When interacting with IRES, ITAFs appear to recruit eIFs and ribosomes to initiate translation (6, 7). Several RNA binding proteins have been characterized as functional ITAFs, including the polypyrimidine tract binding protein (PTB), poly r(C) binding protein 1/2 (PCBP1/2), La autoantigen, Upstream of N-ras (unr), heterogeneous nuclear ribonucleoproteins C1 and C2 (hnRNPC1/C2), death associated protein 5 (DAP5), embryonic lethal abnormal vision/protein HuR (ELAV/HuR), and nucleolin (8, 9). One or several ITAFs can bind to an IRES to regulate protein translation. Each gene recruits its own set of ITAFs for IRES binding and translation initiation. For example although unr and PTB occupy the IRES of Apaf-1 mRNA, Bag-1 IRES interacts with PCBP and PTBs (7). The IRES of XIAP has been linked to binding of La autoantigen, hnRNPC1/2 and DAP5 (7). A recent study using proteomic approaches found four proteins: G-rich RNA sequence binding factor 1 (GRSF-1), Y-box binding protein 1 (YB-1), PTB associated splicing factor (PSF), and its binding partner p54nrb, as ITAFs for regulating translation of myc family genes (10). In this study, we report the finding of La autoantigen as an ITAF regulating Nrf2 protein translation under oxidative stress.
EXPERIMENTAL PROCEDURES
Plasmids
The 555-nucleotide sequence of Nrf2 5′UTR (NM_006164.3) was PCR amplified using cDNA from Hela cells and Phusion DNA polymerase (Fermentas) with the primer set of AAATCAGGGAGGCGCAGCTC (forward) and GATGAGCTGTGGACCGTGTG (reverse). This blunt end PCR product was gel purified and ligated into pJet 1.2 (Fermentas) downstream of a T7 promoter, designated as pJet hsa Nrf2 5′UTR, or into pRL bicistronic vector for measurements of Nrf2 5′UTR activity (11). Human La autoantigen open reading frame (NM_003142.3) was cloned into pEGFPC1 vector (Clontech) after PCR with Phusion DNA polymerase and the primer set of CCGGGATCCATGGCTGAAAATGGTGATAATG (forward) and CGGTGGATCCCTACTGGTCTCCAGCACCAT(reverse).
In Vitro Transcription
The pJet hsa Nrf2 5′UTR plasmid DNA (1 μg) was linearized with XbaI and extracted with phenol/chloroform for in vitro transcription using MEGAscript T7 kit (Ambion) in the presence of Biotin-11-UTP (Invitrogen). The reaction was carried out at 37 °C for 2 h and the product was extracted with phenol/chloroform/isoamyl alcohol. The RNA probe was precipitated with isopropanol and washed with 70% ethanol. After air-drying, the RNA pellet was resuspended in nuclease free water.
RNA Affinity Chromatography
HeLa cell lysates were used for RNA affinity chromatography as described by Cok et al. (12). Briefly, cells harvested in nucleic acids binding buffer [10 mm HEPES (pH 7.6), 5 mm MgCl2, 40 mm KCl, 1 mm dithiothreitol, 5% glycerol, 5 mg/ml heparin] were lysed by sonication 3 times for 5 s each on ice. After centrifugation at 14,000 rpm at 4 °C to remove debris, the supernatant containing 500 μg proteins was used for binding to 5 μg of biotinylated RNA probe on ice for 1 h. Following binding reaction, 0.2 ml of streptavidin Sepharose beads (GE Healthcare) was added for overnight incubation at 4 °C with rotation. The beads were loaded on a 2 ml centrifugation column (Pierce) and washed three times with 2 ml of 1 m NaCl in the nucleic acids binding buffer. The captured proteins were released by boiling in SDS-PAGE loading buffer and were resolved in 10% SDS-PAGE. The gel was silver-stained with a mass spectrometry compatible kit (BioRad) and bands of interest were excised for LC-MS/MS analyses. Alternatively, the proteins on the gel were transferred to PVDF membrane for Western blot.
Tandem Mass Spectrometry Coupled Liquid Chromatography (LC-MS/MS)
Excised silver-stained protein bands following SDS-PAGE were digested in gel with trypsin (10 μg/ml) at 37 °C overnight (13). LC-MS/MS analyses were carried out using a linear quadrupole ion trap ThermoFinnigan LTQ mass spectrometer (San Jose, CA) equipped with a Michrom Paradigm MS4 HPLC, a SpectraSystems AS3000 autosampler, and a nanoelectrospray source. Peptides were eluted from a 15 cm pulled tip capillary column (100 μm I.D. × 360 μm O.D; 3–5 μm tip opening) packed with 7 cm Vydac C18 (5 μ, 300Å pore size, Hesperia, CA), using a 0–65% gradient of solvent B (98% methanol/2% water/0.5% formic acid/0.01% triflouroacetic acid) over a 60-min period at a flow rate of 350 nl/min. The LTQ electrospray positive mode spray voltage was set at 1.6 kV with the capillary temperature of 180 °C. Dependent data scanning was performed by the Xcalibur version 1.4 software (14) with a default charge of 2, an isolation width of 1.5 amu, an activation amplitude of 35%, activation time of 30 msec, and a minimal signal of 100 ion counts. Global dependent data settings were as follows: reject mass width of 1.5 amu, dynamic exclusion enabled, exclusion mass width of 1.5 amu, repeat count of 1, repeat duration of 1 min, and exclusion duration of 5 min. Scan event series included one full scan with mass range 350 - 2000 Da, followed by three dependent MS/MS scan of the most intense ion. Tandem MS spectra of peptides were analyzed with SEQUEST™ (version 27, rev. 12, ThermonFinnigan, San Jose, CA), a program that allows the correlation of experimental tandem MS data with theoretical spectra generated from known protein sequences (15). The peak list (dta files) for the search was generated by Bioworks 3.1. Parent peptide mass error tolerance was set at 2.0 amu and fragment ion mass tolerance was set at 1.0 amu during the search. The criteria used for a preliminary positive peptide identification are the same as previously described, namely a dCn score > 0.08, and Xcorr >1.8 for +1, Xcorr >2.5 for +2, and Xcorr >3.5 for +3 charged peptide precursor ions (16). Peptides matching proteins of interest were confirmed by visual examination of the spectra. All spectra were searched against a human protein database downloaded from IPI October 30, 2009 (ipihuman version 3.65; http://www.ebi.ac.uk/IPI), based on semitryptic peptides between 700–4500 amu with up to two missed cleavages. At the time of the search, the ipi human version 3.65 protein database contained 86,379 entries. The results were also validated using X!Tandem, another search engine (17) and visualized with Scaffold (version Scaffold_3_00_02, Proteome Software Inc., Portland, OR), a program that relies on various search engine results (i.e. Sequest, X!Tandem, MASCOT) and uses Bayesian statistics to reliably identify more spectra (18, 19). X!Tandem identifications required at least -Log(Expect Scores) of greater than 3.0. Protein identifications were accepted if they contained at least two identified peptides. Oxidation of methionine and iodoacetamide derivative of cysteine were specified in Sequest and X!Tandem as variable modifications. The protein false discovery rate (FDR) was calculated at 0.1% by the Scaffold software using the ProteinProfit based probabilities [(sum of probabilities of proteins/sum of maximum probabilities for each protein) = A; 1.00-A = B*100 = FDR].
RNA- Protein Complex Immunoprecipitation and qPCR Analysis
HeLa cells were lysed on ice with nucleic acid binding buffer containing RNase and protease inhibitors. Antibodies (2 μg) were first incubated with 100 μl of Protein A/G Plus beads (Santa Cruz Biotechnology, Santa Cruz, CA) for 1 h at 25 °C. After washing away unbound antibodies, the beads were incubated at 4 °C with 500 μg cell lysates for 4 h. Unbound proteins were removed by 5 times washes with nucleic acid binding buffer. RNA was extracted with Trizol (Invitrogen, Carlsbad, CA) from the beads and was converted to cDNA for real-time PCR with MMLV reverse transcriptase (Fermentas, Hanover, MD) and oligo d(T) primer. The primer sets for real-time PCR were designed with an online tool provided by IDTDNA and synthesized by Signosis as 5′-CAGGTTGCCCACATTCCCAAATCA-3′ and 5′-AGCAATGAAGACTGGGCTCTCGAT-3′ for human Nrf2 (NM_006164); 5′-TCGACAGTCAGCCGCATCTTCTTT-3′ and 5′-ACCAAATCCGTTGACTCCGACCTT-3′ for human GAPDH (NM_002046.3). Nrf2 mRNA abundance was determined with SYBR Green Master Mix (Fermentas) and BioRad CFX Manager Software after normalization to GAPDH.
Transfection and Luciferase Assay
The DNA plasmid or siRNA was transfected with Fugene 6 (Roche) or LipofectAMINE 2000 (Invitrogen) respectively. HeLa cells transfected with pRL bicistronic vector of Nrf2 5′UTR in 24-well plates were treated with H2O2 24 h post-transfection. Luciferase activity was measured using a Dual-Luciferase Assay Kit (Promega, Madison, WI) and luminometer.
Immunocytochemistry
HeLa cells were seeded onto glass coverslips in 35-mm dishes and grown to 30% confluency. After H2O2 treatment, the cells were washed twice with phosphate-buffered saline (PBS) before fixation in methanol. Following blockage with 2% bovine serum albumin in PBS, the coverslips were incubated with primary antibodies (1:400 dilution) for 2 h. The coverslips were washed 3 times with PBS before incubation with Alexa conjugated secondary antibodies (Invitrogen) for 1 h. After PBS washes, the coverslips were mounted onto a microscope slide with Anti-fade Mounting Medium containing DAPI (Invitrogen). The images were acquired with a 60X lens on an Olympus BX53 Microscope and analyzed with CellSens software from Olympus.
Isolation of Ribosomes
HeLa cells were treated with 5 μg/ml cyclohexamide 10 min before harvesting. The cytoplasm was extracted with lysis buffer after cells being washed twice with cold PBS containing 0.5 μg/ml cyclohexamide. The lysate was centrifuged at 400 × g for 5 min to remove nuclei and cell debris. The supernatant was transferred to an Eppendorf tube and centrifuged at 14,000 × g for 10 min to remove postmitochondrial fractions. The supernatant was loaded on 10 and 35% sucrose cushions, and centrifuged at 30,000 rpm with a SW41 rotor for 1.5 h at 4 °C to collect the ribosomal pellet (20). For ribosomal profiling, the postmitochondrial fraction was loaded on a linear sucrose gradient (15–50% w/v) and centrifuged at 40,000 rpm with a SW40 rotor for 1.5 h at 4 °C. The gradient was displaced upright with 60% sucrose and the distribution of ribosome was recorded at absorbance of OD 254 nm with a BioLogic LC System (BioRad). The fractions were collected at a flow rate of 1 ml per min. Proteins in each fraction were precipitated with 10% trichloroacetic acid.
Statistics
Two-tailed Student's t test was used when two means were compared between each other. When means from multiple groups were compared, 1-way ANOVA was used. In either case, p ≤ 0.05 was considered significant.
RESULTS
LC-MS/MS Identification of Nrf2 5′UTR Binding Proteins
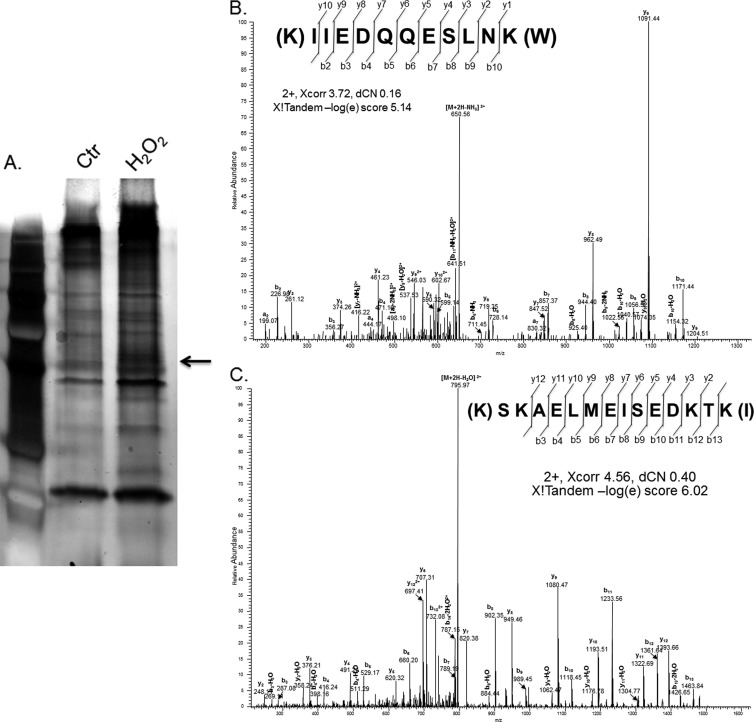
LC-MS/MS based protein identification has been proven useful in identifying proteins bound to RNA. We cloned the full length human Nrf2 5′UTR downstream of T7 promoter for in vitro transcription to generate biotinylated RNA bait for isolation of binding proteins from HeLa cells following H2O2 treatment. As shown in Fig. 1, a band corresponding to the molecular weight of 50 kDa appeared specific from H2O2 treated cells. Excision of the band for LC-MS/MS analysis revealed two peptides belonging to the human La autoantigen (Accession number IPI009032, 6% sequence coverage, Figs. 1A–1C).
Fig. 1.
Identification of Human La/SSB as a binding protein of Nrf2 5′UTR. Full length biotinylated human Nrf2 5′UTR was used as a bait for pulling down binding proteins from HeLa cell lysates harvested 1 h after 10 min treatment with 100 μm H2O2. A, Bound proteins were separated by SDS-PAGE and the band of 50 kDa specific to the H2O2 treated cells, as indicated by an arrow, was excised for LC-MS/MS analyses. B–C, Tandem mass spectra of two unique peptides were found matching human La/SSB (IPI00009032, 6% sequence coverage).
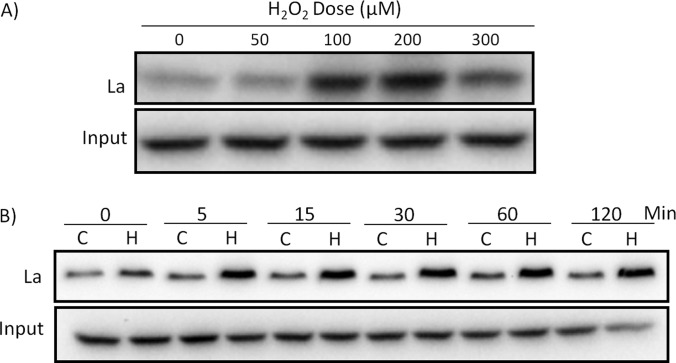
La autoantigen is a 52-kDa protein being targeted by antibodies in patients with Sjögren's Syndrome, and therefore also named as Sjögren Syndrome Antigen B (SSB). To verify La/SSB protein binding to Nrf2 5′UTR, we used RNA affinity chromatography with biotinylated Nrf2 5′UTR as a bait to pull down binding proteins for Western blot to detect La/SSB, a technique named Far Western. Fig. 2A shows that the binding of La/SSB to Nrf2 5′UTR increased with increasing dose of H2O2 treatment in HeLa cells. This binding reached the maximum in cells treated with 100 to 200 μm H2O2. The time course study with 100 μm H2O2 indicated that the binding was detectable right after 10 min H2O2 treatment and significantly increased after 5 min incubation. The binding reached the highest level after 15 min and maintained a high level for 1–2 h following H2O2 treatment (Fig. 2B). These results confirmed that H2O2 treatment indeed caused La/SSB binding to Nrf2 5′UTR.
Fig. 2.
In vitro binding of La/SSB protein to Nrf2 5′UTR. HeLa cells were harvested 1 h later (A) or at indicated time (B) after 10 min treatment of H2O2 at indicated dose (A) or 100 μm (B). Biotinylated human Nrf2 5′UTR was used as a bait for pulling down La/SSB from the cell lysates. La/SSB associated with Nrf2 5′UTR was measured by Western blot analysis.
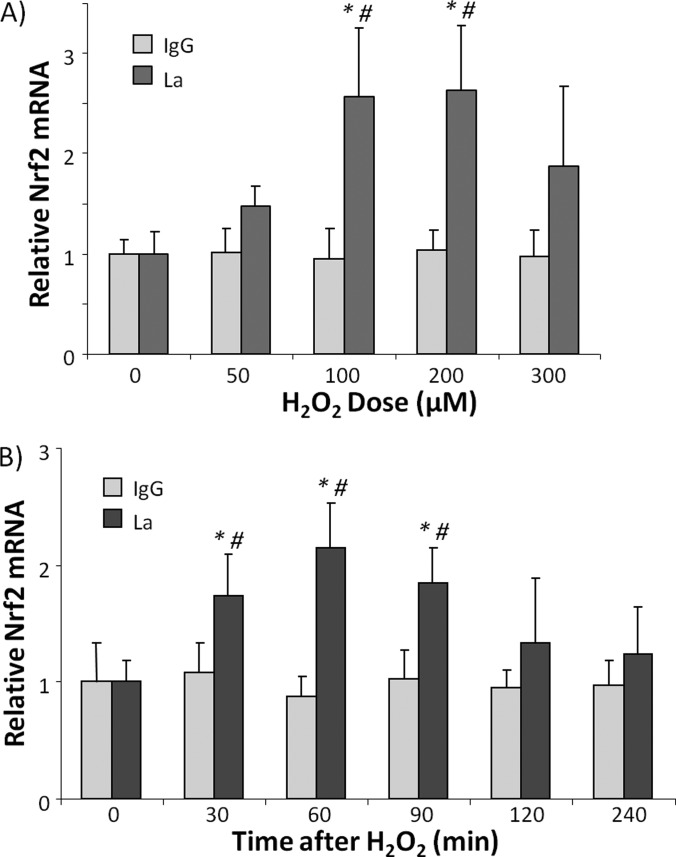
For LC-MS/MS and Far Western experiments, the binding of La/SSB from cell lysates to Nrf2 5′UTR occurred in vitro. To demonstrate the binding of La/SSB to Nrf2 5′UTR occurring in cells, we used RNA-protein complex immunoprecipitation to examine the association of Nrf2 mRNA with La/SSB protein. With cell lysates harvested after H2O2 treatment, the La/SSB antibody was able to pull down Nrf2 mRNA (Fig. 3). H2O2 treatment induced a dose and time-dependent increase of Nrf2 mRNA in La/SSB immunoprecipitates (Fig. 3). The dose response and time course of Nrf2 mRNA binding to La/SSB were consistent with that of Far Western data.
Fig. 3.
La/SSB binds to Nrf2 mRNA in response to H2O2 In Hela cells. HeLa cells were treated with H2O2 of indicated doses (A) or 100 μm (B) for 10 min, and harvested at 1 h (A) or indicated time (B). The cells were harvested for immunoprecipitation with anti-La/SSB antibody. RNA was extracted from the immunoprecipitates for reverse transcription. The abundance of Nrf2 mRNA was quantitated with qPCR. Rabbit IgG was used as a negative control for immunoprecipitation. The graph represents the average ± standard deviations from three independent experiments with 0 dose or 0 time point being set to 1. * denotes significant difference (p < 0.05) from 0 μm or 0 time point value. # indicates significant difference (p < 0.05) when levels of Nrf2 mRNA from anti-La/SSB antibody pull down samples were compared with that of corresponding IgG negative control pull down samples.
La/SSB Mediates Nrf2 Translation
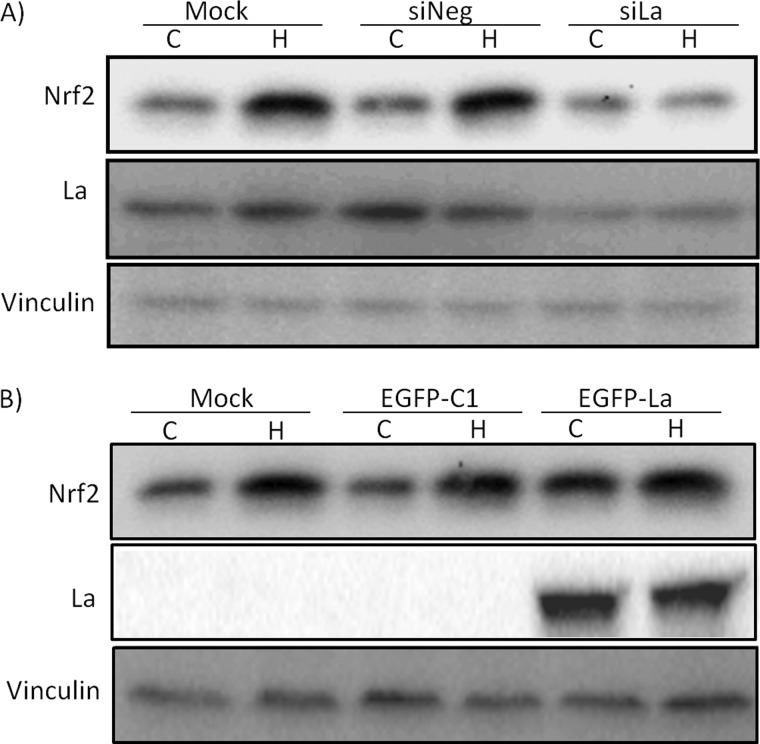
To address whether La/SSB binding to Nrf2 5′UTR is critical for Nrf2 translation under oxidative stress, we measured Nrf2 induction by modulating the level of La/SSB protein. When siRNA was transfected into HeLa cells, it was able to reduce the level of La/SSB protein. La/SSB siRNA was able to inhibit H2O2 from inducing Nrf2 protein (Fig. 4A). We cloned a construct for constitutive expression of La/SSB fused with a Green Fluorescent Protein tag. Ectopic expression of GFP-La/SSB was sufficient for increasing Nrf2 protein (Fig. 4B). Measurements of Nrf2 transcripts excluded the possibility that La/SSB siRNA or GFP-La/SSB modulated Nrf2 gene transcription (data not shown).
Fig. 4.
Modulation of La/SSB expression affects Nrf2 protein level. HeLa cells were transfected with siRNA against La/SSB (A), negative control siRNA (A) or GFP-La/SSB expression vector (B) before treatment with 100 μm H2O2 for 10 min at 24 h after transfection. The cells were harvested 1 h later for Western blot analysis of La/SSB and Nrf2 with vinculin as a loading control.
To demonstrate that La/SSB indeed regulated Nrf2 protein translation via Nrf2 5′UTR, we cloned Nrf2 5′UTR into a dicistronic reporter construct, a system commonly used for measurements of IRES mediated protein translation (21). Such dicistronic reporter vector contains SV40 promoter for transcription of both Renilla and Firefly luciferases, with Renilla luciferase representing 5′ 7-methyl guanine cap dependent translation and Firefly luciferase under the control of Nrf2 5′UTR. The ratio of Firefly luciferase over Renilla luciferase reflects Nrf2 5′UTR mediated protein translation. After HeLa cells were transfected with the dicistronic construct and treated with H2O2, we found that H2O2 treatment caused an increase in Nrf2 5′UTR activity (Fig. 5A). Reducing La/SSB expression using siRNA resulted in a reduction of H2O2 induced Nrf2 5′UTR activity (Fig. 5A). Conversely, overexpression of La/SSB alone was sufficient to induce Nrf2 5′UTR activity (Fig. 5B). These results support that La/SSB up-regulated Nrf2 protein translation via interaction with IRES in Nrf2 5′UTR.
Fig. 5.
Modulation of La/SSB expression affects Nrf2 5′UTR activity. HeLa cells were transfected with bicistronic reporter construct of Nrf2 5′UTR along with siRNA against La/SSB (A), negative control siRNA (A) or GFP-La/SSB expression vector (B). After 24 h, the cells were treated with 100 μm H2O2 for 10 min and harvested 1 h later for dual luciferase assays to determine the ratios of Firefly versus Renilla luciferases (F/R). The graph represents the average ± standard deviations of three independent experiment. * denotes significant difference (p < 0.05) from the corresponding control untreated group. # indicates significant difference (p < 0.05) when siRNA against La/SSB treated groups were compared with negative control siRNA groups (A).
Subcellular Relocalization of La/SSB under Oxidative Stress
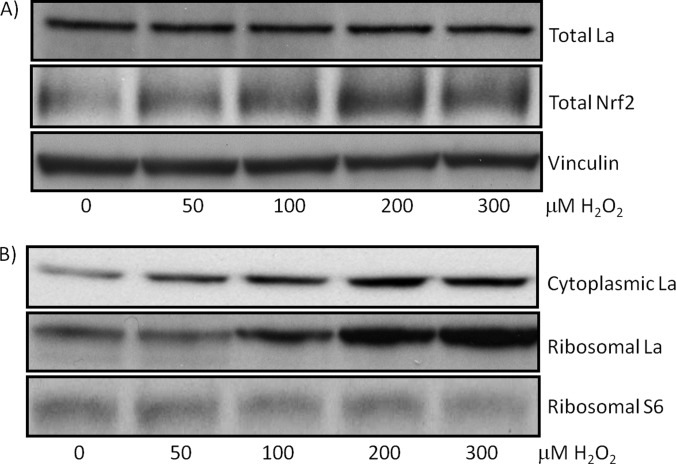
An important question is how oxidants activate La/SSB, which in turn regulates Nrf2 protein translation. Given the fact that overexpression of La/SSB was sufficient to induce Nrf2 protein, a simplest explanation would be that oxidants induced elevation of La/SSB protein. Western blot analyses of the whole cell lysates did not reveal changes in La/SSB protein levels upon H2O2 treatment, whereas the same set of samples showed a dose dependent elevation of Nrf2 protein (Fig. 6A). Oxidants initiate a cascade of signaling events. Measurements of La/SSB phosphorylation by antibodies against phospho Serine, Threonine, or Tyrosine failed to find positive results (data not shown). Two dimensional Western blot did not show changes in the pattern of La/SSB protein (data not shown), arguing against post-translational modifications.
Fig. 6.
Subcellular distribution of La/SSB protein. HeLa cells were treated with H2O2 at indicated doses and harvested 1 h later. Levels of La/SSB or Nrf2 protein in total cell lysates were measured using Western blot with vinculin as the loading control (A). Cytoplasmic and ribosomal fractions were isolated as described in the Method for measurements of La/SSB protein levels by Western blot (B). Ribosomal protein L36a was used as the loading control for ribosomal fractions.
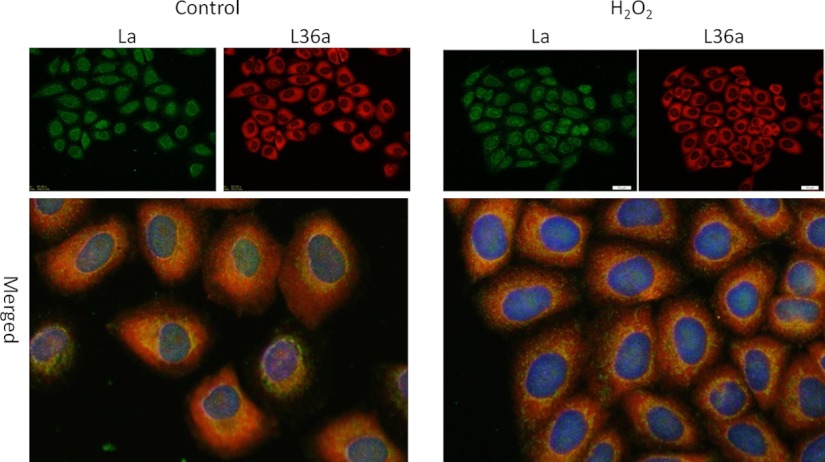
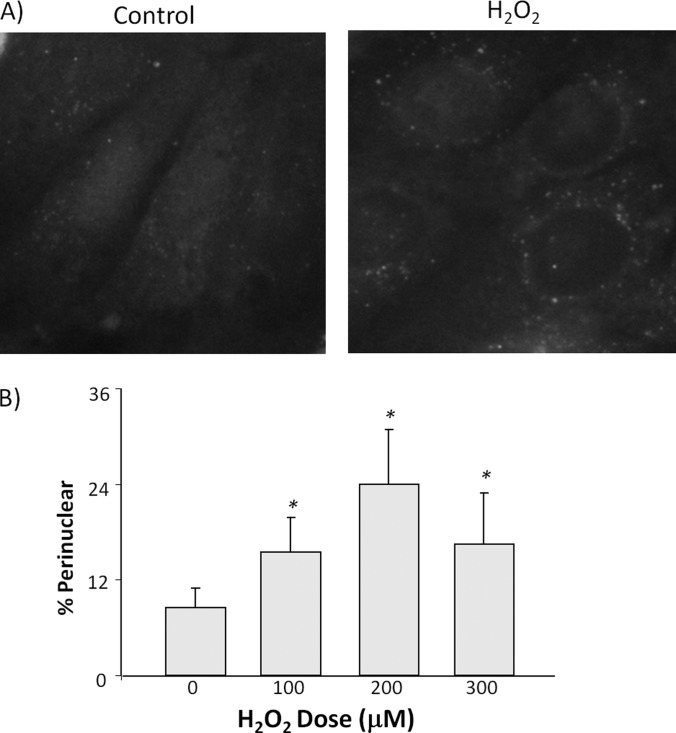
It has been shown that La/SSB is primarily a nuclear protein and can translocate to the cytoplasm (22–25). When cytosolic proteins were collected for Western blot, a H2O2 dose dependent increase of La/SSB was observed (Fig. 6B). To demonstrate La/SSB translocation upon H2O2 treatment, we measured subcellular localization of La/SSB by immunocytochemical staining and found reduced nuclear La/SSB staining in H2O2 treated cells (Fig. 7). Instead, an increased portion of cells showed perinuclear distribution (Fig. 7). Such perinuclear distribution overlapped with that of ribosomal protein L36a (Fig. 7). To further demonstrate that H2O2 treatment caused subcellular relocalization of La/SSB, we transfected cells with an expression vector for GFP-La/SSB fusion protein. Tracking GFP fluorescence confirmed that H2O2 caused nuclear export and perinuclear redistribution of La/SSB (Fig. 8A). Counting for GFP under a fluorescent microscope indicated that 100 μm H2O2 treatment caused threefold increase in the number of cells containing perinuclear La/SSB.
Fig. 7.
Colocalization of La/SSB with L36a. HeLa cells were treated with 100 μm H2O2 for 10 min and fixed 1 h later for immunocytochemistry to stain for La/SSB (green) or L36a (red). The images were acquired under an Olympus fluorescence microscope with a 60x lens and merged using CellSens software (Olympus).
Fig. 8.
Relocalization of EGFP-La/SSB because of H2O2 treatment. HeLa cells were transfected with EGFP-La/SSB for 24 h before treatment with 100 μm H2O2 for 10 min. Cells were fixed 1 h after for examination under an Olympus fluorescence microscopy. Representative images were acquired under a 60 × lens. About 200 cells were counted under a microscope with a 40 × lens for the portion of cells containing perinuclear distribution of EGFP-La/SSB. The graph represents the average ± standard deviations of three independent experiment. * denotes significant difference (p < 0.05) from the portion of cells showing perinuclear distribution with 0 μm H2O2 treatment.
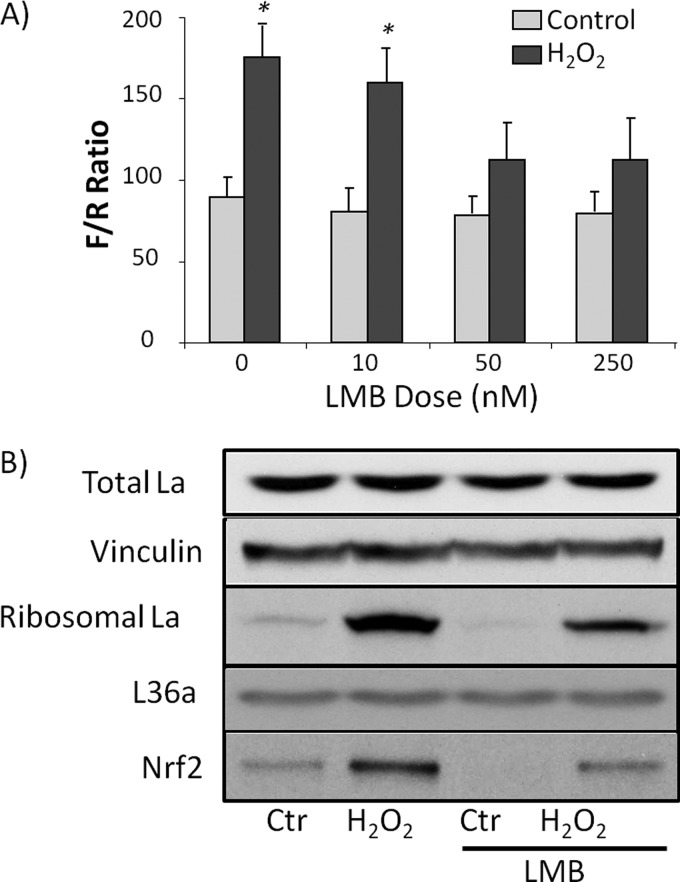
If nuclear export of La/SSB is important for Nrf2 protein translation, inhibition of La/SSB export should abolish Nrf2 protein elevation or Nrf2 5′UTR activation by H2O2. We treated cells with Leptomycin B (LMB), an exportin 1 inhibitor that has been widely used to block nuclear export of proteins (26). Leptomycin B was able to prevent H2O2 from activating Nrf2 5′UTR in a dose dependent manner (Fig. 9A). The optimal dose 50 nm of Leptomycin B was able to reduce the level of La/SSB in ribosomal fractions (Fig. 9B). Such treatment reduced the level of Nrf2 protein induction by H2O2 treatment (Fig. 9B).
Fig. 9.
Leptomycin B (LMB) reduced Nrf2 induction by H2O2. HeLa cells were transfected with Nrf2 5′UTR bicistronic reporter and treated with 100 μm H2O2 at indicated doses for 10 min. The cells were incubated 1 h in the presence of LMB at indicated doses before being harvested for dual luciferase assay (A), isolation of ribosomes or Western blot (B). Vinculin was used as a loading control for measurement of La/SSB from total cell lysates, whereas ribosomal S6 protein was used as a loading control for ribosomal La/SSB Western blot (B). * denotes significant difference (p < 0.05) from control without H2O2 treatment.
H2O2 Enhances La/SSB Interaction with Ribosomes
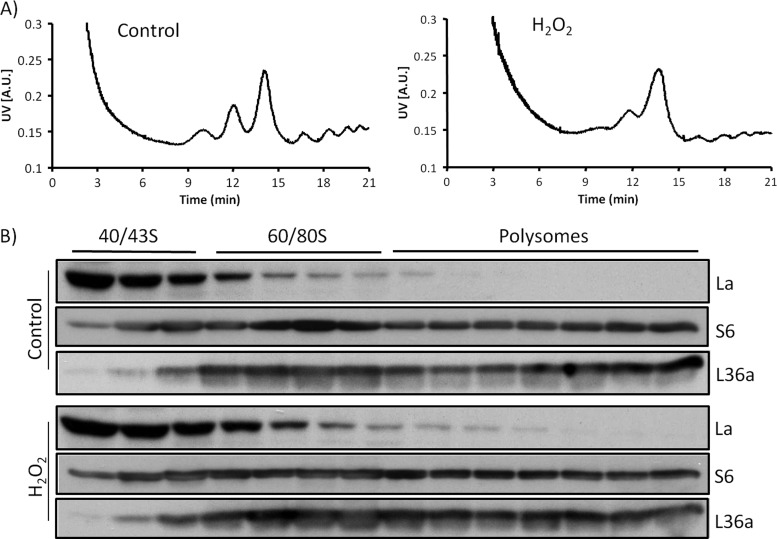
Several lines of evidence suggest H2O2 caused an association of La/SSB with ribosomes: perinuclear distribution, colocalization with L36a, and dose dependent increase of La/SSB protein levels in ribosomal fractions. Ribosomes containing 40S and 60S subunits, have a sedimentation coefficiency of 80S and form polysomes when multiple ribosomes bind to the same strand of mRNA during the process of translation elongation (Fig. 10A). When ribosomes were separated based on their sizes, we found La/SSB was mainly associated with 40S ribosomal fractions, although H2O2 treatment caused an increase of La/SSB in 60/80S ribosomal fractions (Fig. 10B). To test the physical interaction of La/SSB with ribosomes upon H2O2 treatment, we used immunoprecipitation to pull down La/SSB protein and measured its interaction with a representative ribosomal small or large subunit protein, S6 or L36a. We found that H2O2 treatment enhanced La/SSB interaction with the small or large subunit protein S6 or L36a (Fig. 11). This result indicates the physical interaction of La/SSB with ribosomes in cells treated with H2O2.
Fig. 10.
Association of La/SSB with 40/43S ribosomal subunits. HeLa cells were treated with 100 μm H2O2 for 10 min and harvested 1 h later for isolation of ribosomes and separation of ribosomal subunits by a fractionator as described in Materials and Methods. The proteins in each fraction were analyzed for the presence of La/SSB, S6 and L36a by Western blot.
Fig. 11.
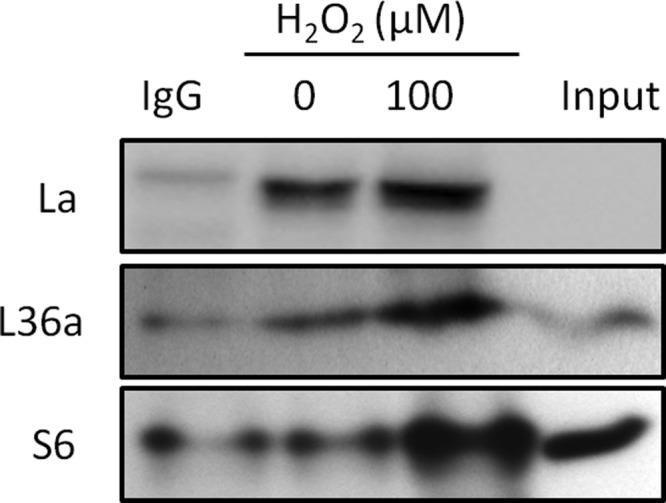
La/SSB interacts with ribosomal proteins. HeLa cells were treated with 100 μm H2O2 for 10 min and harvested 1 h later for immunoprecipitation using La/SSB antibodies. The immunoprecipitates were used for Western blot to detect La/SSB, S6 and L36 protein.
DISCUSSION
We present here a novel mechanism of de novo Nrf2 protein translation under oxidative stress. A proteomic approach led to the discovery of La/SSB as a binding partner to Nrf2 5′UTR. H2O2 induced La/SSB binding to Nrf2 5′UTR was confirmed by Far Western and RNA-protein complex immunoprecipitation approaches. We found that oxidants caused nuclear export of La/SSB and such subcellular translocation was important for Nrf2 protein translation. Three lines of evidence indicate increased interaction of La/SSB with ribosomes because of oxidative stress: (1) colocalization of La/SSB with ribosomal protein L36a; (2) H2O2 dose dependent elevation of La/SSB in ribosomal fractions; and (3) binding of La/SSB with L36a or S6 as detected by immunoprecipitation. We have demonstrated that La/SSB played an important role in driving de novo Nrf2 protein translation, because siRNA against La/SSB prevented H2O2 from inducing Nrf2 protein or activity of Nrf2 5′UTR, whereas overexpression of La/SSB was sufficient for inducing Nrf2 protein or activating Nrf2 5′UTR. Therefore the interactions of La/SSB with Nrf2 mRNA and with ribosomes are key events underlying oxidant induced de novo Nrf2 protein translation.
Nrf2 5′UTR contains a highly conserved 18S rRNA binding site required for internal initiation and a hairpin structure within 103 nt upstream of Nrf2 coding sequence (27). H2O2 treatment was able to activate Nrf2 5′UTR as tested by a bicistronic reporter containing the 103 nt sequence (27), consistent with our findings of Nrf2 5′UTR activation as reported here and predicted previously (28). The 103 nt Nrf2 5′UTR sequence did not interact with the ribosome, nor facilitated the assembly of 48S initiation complex (29). Li et al. (29) postulated that ITAF(s) are required for Nrf2 protein translation through IRES. Indeed we present evidence here that La/SSB acts as an ITAF regulating Nrf2 protein translation under oxidative stress.
La/SSB gene is well conserved in functional domains among mammalian species (30). The protein contains two RNA Recognition Motifs (RRMs) and a nuclear retention element in the C terminus (30, 31). These features are important for its functions from tRNA maturation to stabilization and nuclear export of nascent mRNA (30, 31). La/SSB was the first ITAF identified for promoting translation of poliovirus (32), and later found to mediate translation of HBV, HCV, HIV, encephalomyocarditis, and giardia viruses (30). For cellular proteins, La/SSB functions as an ITAF for translation of cyclin D1 (33), XIAP (6), BiP (34), and MDM2 (35). In addition to being an ITAF, La/SSB also binds to mRNAs with 5′UTR terminal oligopyrimidine track (5′-TOP), the products of RNA polymerase III (30). Many of rRNA species and mRNAs encoding certain components of translational machinery contain 5′-TOP and therefore are subject of La/SSB regulation (36). In the cytoplasm, binding of La/SSB to 5′-TOP targets the mRNA species for translation (31). Although Nrf2 mRNA does not contain 5′-TOP, it is possible that La/SSB may indirectly increase Nrf2 protein translation through regulation of genes of translational machinery encoding 5′-TOP. Nevertheless, our data are consistent with the finding that La/SSB interacts with ribosomes and promotes recruitment of IRES containing mRNAs onto the ribosomes (37, 38).
A number of reports have indicated a role of nuclear export of ITAFs in protein translation. Lewis et al. proposed that ITAFs in the nucleus may bind to their target IRES and sequester the mRNA species from accessible to translational machinery (38). Upon changes in cellular environment, such as viral infection or chemical stress, these ITAFs translocate to the cytoplasm along with their targeted mRNA species. Alternatively, these ITAFs are confined in the nucleus until appropriate signals are received (38). For example, in HeLa cells treated with vincristine, translation of the antiapoptotic protein BAG1 increases through an IRES mediated mechanism (39). PTB and PCBP1 as ITAFs of BAG1 translocate from the nuclei to the cytoplasm upon vincristine treatment. Similarly, hnRNP A1 translocates to the cytoplasm and stimulates IRES mediated translation of fibroblast growth factor 2 in cells challenged with high osmolarity (40, 41). ITAF translocation promotes the accessibility to the translational machinery and is essential for translation of the targeted mRNA species.
We reported here that La/SSB protein undergoes subcellular relocalization upon H2O2 treatment. Previous reports show that La/SSB protein is primarily localized in the nucleus and translocates to the cytoplasm upon viral infection (37) or growth factor stimulation (23). The La/SSB protein is subject to phosphorylation at multiple residues, leading to its nuclear export (22–25). Intine et al. reported that dephosphorylation of Ser366 resulted in cytoplasmic localization of La/SSB (22). Conversely, phosphorylation of Thr301 by AKT in mouse glial progenitor cells promotes its cytoplasmic translocation (23). With immunoprecipitated La/SSB, we failed to observe any changes in phospho-serine, phospho-threonine, or phospho-tyrosine (data not shown). Furthermore, inhibiting Akt with LY294002 did not affect H2O2 from inducing Nrf2 either (data not shown). Thus, how La/SSB translocates from the nuclei to the cytoplasm in the H2O2 treated cells remains to be investigated. Two-dimensional Western blot analyses indicate that La/SSB may not undergo post-translational modifications because of H2O2 treatment, suggesting the possibility of a carrier protein in mediating nuclear export of La/SSB.
It has been shown that La/SSB protein is associated with small ribosomal subunits through its binding to 18S rRNA in HeLa cells (42). We have observed an association of La/SSB with 40S ribosomal fractions in HeLa cells (Figs. 10 and 11). Because 40S small subunit together with eIF1/1A/3/2/5 forms the 43S pre-initiation complex that is important for binding to mRNA for translation initiation (43), it is possible that La/SSB binding to Nrf2 mRNA facilitates the landing of 43S Pre-Initiation Complex. An earlier study by Costa-Mattioli et al. (44) supports such a function of La/SSB. Regardless, the physical interaction of La/SSB with ribosomal proteins was detected with immunoprecipitation experiments (Figs. 10 and 11). The presence of La/SSB in 40 to 80S ribosomal fractions (Fig. 11B) supports the role of La/SSB as a key factor for initiating Nrf2 protein translation. Importantly, La/SSB mediated Nrf2 protein translation is not confined to HeLa cells, because similar results were observed in rat primary cultured cardiomyocytes in response to oxidative stress or mouse myocardium in response to ischemic stress (Xu B et al., 2011 manuscript under revision). Since both La/SSB protein and Nrf2 mRNA are ubiquitously expressed in various tissues (30), it is possible that La/SSB mediated de novo Nrf2 protein translation is universal in cells responding to chemical stress.
Footnotes
* This work has been supported by National Institutes of Health grants R01 HL 076530, R01 HL089958, R21ES017473, T32 ES007091, Arizona Biomedical Research Commission (QMC), and Marjorie Hornbeck Estate Research Award (JZ) from University of Arizona Sarver Heart Center. Mass spectrometric data were acquired by the Arizona Proteomics Consortium supported by NIEHS grant ES06694 to the SWEHSC, NIH/NCI grant CA023074 to the AZCC and by the BIO5 Institute of the University of Arizona.
1 The abbreviations used are:
- Nrf2
- nuclear factor erythroid-2 related factor 2
- UTR
- untranslated region
- IRES
- Internal Ribosomal Entry Site
- ARE
- Antioxidant Response Element
- ITAF
- IRES trans-acting factor.
REFERENCES
- 1. Taguchi K., Motohashi H., Yamamoto M. (2011) Molecular mechanisms of the Keap1-Nrf2 pathway in stress response and cancer evolution. Genes Cells 16, 123–140 [DOI] [PubMed] [Google Scholar]
- 2. Lee J. M., Anderson P. C., Padgitt J. K., Hanson J. M., Waters C. M., Johnson J. A. (2003) Nrf2, not the estrogen receptor, mediates catechol estrogen-induced activation of the antioxidant responsive element. Biochim. Biophys. Acta 1629, 92–101 [DOI] [PubMed] [Google Scholar]
- 3. Purdom-Dickinson S. E., Lin Y., Dedek M., Morrissy S., Johnson J., Chen Q. M. (2007) Induction of antioxidant and detoxification response by oxidants in cardiomyocytes: evidence from gene expression profiling and activation of Nrf2 transcription factor. J. Mol. Cell. Cardiol. 42, 159–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang D. D., Lo S. C., Cross J. V., Templeton D. J., Hannink M. (2004) Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol. Cell. Biol. 24, 10941–10953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Giudice A., Arra C., Turco M. C. (2010) Review of molecular mechanisms involved in the activation of the Nrf2-ARE signaling pathway by chemopreventive agents. Methods Mol. Biol. 647, 37–74 [DOI] [PubMed] [Google Scholar]
- 6. Holcik M., Korneluk R. G. (2000) Functional characterization of the X-linked inhibitor of apoptosis (XIAP) internal ribosome entry site element: role of La autoantigen in XIAP translation. Mol. Cell. Biol. 20, 4648–4657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Spriggs K. A., Bushell M., Mitchell S. A., Willis A. E. (2005) Internal ribosome entry segment-mediated translation during apoptosis: the role of IRES-trans-acting factors. Cell Death Differ. 12, 585–591 [DOI] [PubMed] [Google Scholar]
- 8. Babitzke P., Baker C. S., Romeo T. (2009) Regulation of translation initiation by RNA binding proteins. Ann. Rev. Microbiol. 63, 27–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hamilton T. L., Stoneley M., Spriggs K. A., Bushell M. (2006) TOPs and their regulation. Biochem. Soc. Trans. 34, 12–16 [DOI] [PubMed] [Google Scholar]
- 10. Cobbold L. C., Spriggs K. A., Haines S. J., Dobbyn H. C., Hayes C., de Moor C. H., Lilley K. S., Bushell M., Willis A. E. (2008) Identification of internal ribosome entry segment (IRES)-trans-acting factors for the Myc family of IRESs. Mol. Cell. Biol. 28, 40–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Han B., Zhang J. T. (2002) Regulation of gene expression by internal ribosome entry sites or cryptic promoters: the eIF4G story. Mol. Cell. Biol. 22, 7372–7384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cok S. J., Acton S. J., Sexton A. E., Morrison A. R. (2004) Identification of RNA-binding proteins in RAW 264.7 cells that recognize a lipopolysaccharide-responsive element in the 3-untranslated region of the murine cyclooxygenase-2 mRNA. J. Biol. Chem. 279, 8196–8205 [DOI] [PubMed] [Google Scholar]
- 13. Shevchenko A., Wilm M., Vorm O., Mann M. (1996) Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 68, 850–858 [DOI] [PubMed] [Google Scholar]
- 14. Andon N. L., Hollingworth S., Koller A., Greenland A. J., Yates J. R., 3rd, Haynes P. A. (2002) Proteomic characterization of wheat amyloplasts using identification of proteins by tandem mass spectrometry. Proteomics 2, 1156–1168 [DOI] [PubMed] [Google Scholar]
- 15. Eng J. K., Mccormack A. L., Yates J. R. (1994) An Approach to Correlate Tandem Mass-Spectral Data of Peptides with Amino-Acid-Sequences in a Protein Database. J. Am. Soc. Mass Spectr. 5, 976–989 [DOI] [PubMed] [Google Scholar]
- 16. Cooper B., Eckert D., Andon N. L., Yates J. R., Haynes P. A. (2003) Investigative proteomics: identification of an unknown plant virus from infected plants using mass spectrometry. J. Am. Soc. Mass Spectrom. 14, 736–741 [DOI] [PubMed] [Google Scholar]
- 17. Craig R., Beavis R. C. (2004) TANDEM: matching proteins with tandem mass spectra. Bioinformatics 20, 1466–1467 [DOI] [PubMed] [Google Scholar]
- 18. Keller A., Nesvizhskii A. I., Kolker E., Aebersold R. (2002) Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem. 74, 5383–5392 [DOI] [PubMed] [Google Scholar]
- 19. Nesvizhskii A. I., Keller A., Kolker E., Aebersold R. (2003) A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem. 75, 4646–4658 [DOI] [PubMed] [Google Scholar]
- 20. Hresko R. C., Mueckler M. (2002) Identification of pp68 as the Tyrosine-phosphorylated Form of SYNCRIP/NSAP1. A cytoplasmic RNA-binding protein. J. Biol. Chem. 277, 25233–25238 [DOI] [PubMed] [Google Scholar]
- 21. Balvay L., Soto Rifo R., Ricci E. P., Decimo D., Ohlmann T. (2009) Structural and functional diversity of viral IRESes. Biochim. Biophys. Acta 1789, 542–557 [DOI] [PubMed] [Google Scholar]
- 22. Intine R. V., Tenenbaum S. A., Sakulich A. L., Keene J. D., Maraia R. J. (2003) Differential phosphorylation and subcellular localization of La RNPs associated with precursor tRNAs and translation-related mRNAs. Mol. Cell 12, 1301–1307 [DOI] [PubMed] [Google Scholar]
- 23. Brenet F., Socci N. D., Sonenberg N., Holland E. C. (2009) Akt phosphorylation of La regulates specific mRNA translation in glial progenitors. Oncogene 28, 128–139 [DOI] [PubMed] [Google Scholar]
- 24. Broekhuis C. H., Neubauer G., van der Heijden A., Mann M., Proud C. G., van Venrooij W. J., Pruijn G. J. (2000) Detailed analysis of the phosphorylation of the human La (SS-B) autoantigen. (De)phosphorylation does not affect its subcellular distribution. Biochemistry 39, 3023–3033 [DOI] [PubMed] [Google Scholar]
- 25. Fan H., Sakulich A. L., Goodier J. L., Zhang X., Qin J., Maraia R. J. (1997) Phosphorylation of the human La antigen on serine 366 can regulate recycling of RNA polymerase III transcription complexes. Cell 88, 707–715 [DOI] [PubMed] [Google Scholar]
- 26. Kudo N., Wolff B., Sekimoto T., Schreiner E. P., Yoneda Y., Yanagida M., Horinouchi S., Yoshida M. (1998) Leptomycin B inhibition of signal-mediated nuclear export by direct binding to CRM1. Exp. Cell Res. 242, 540–547 [DOI] [PubMed] [Google Scholar]
- 27. Li W., Thakor N., Xu E. Y., Huang Y., Chen C., Yu R., Holcik M., Kong A. N. (2010) An internal ribosomal entry site mediates redox-sensitive translation of Nrf2. Nucleic Acids Res. 38, 778–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Purdom-Dickinson S. E., Sheveleva E. V., Sun H., Chen Q. M. (2007) Translational control of nrf2 protein in activation of antioxidant response by oxidants. Mol. Pharmacol. 72, 1074–1081 [DOI] [PubMed] [Google Scholar]
- 29. Li W., Thakor N., Xu E. Y., Huang Y., Chen C., Yu R., Holcik M., Kong A. N. (2010) An internal ribosomal entry site mediates redox-sensitive translation of Nrf2. Nucleic Acids Res 38, 778–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wolin S. L., Cedervall T. (2002) The La protein. Ann. Rev. Biochem. 71, 375–403 [DOI] [PubMed] [Google Scholar]
- 31. Jacks A., Babon J., Kelly G., Manolaridis I., Cary P. D., Curry S., Conte M. R. (2003) Structure of the C-terminal domain of human La protein reveals a novel RNA recognition motif coupled to a helical nuclear retention element. Structure 11, 833–843 [DOI] [PubMed] [Google Scholar]
- 32. Meerovitch K., Pelletier J., Sonenberg N. (1989) A cellular protein that binds to the 5′-noncoding region of poliovirus RNA: implications for internal translation initiation. Genes Dev. 3, 1026–1034 [DOI] [PubMed] [Google Scholar]
- 33. Sommer G., Dittmann J., Kuehnert J., Reumann K., Schwartz P. E., Will H., Coulter B. L., Smith M. T., Heise T. (2011) The RNA-binding protein La contributes to cell proliferation and CCND1 expression. Oncogene 30, 434–444 [DOI] [PubMed] [Google Scholar]
- 34. Kim Y. K., Back S. H., Rho J., Lee S. H., Jang S. K. (2001) La autoantigen enhances translation of BiP mRNA. Nucleic Acids Res. 29, 5009–5016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nakatake M., Monte-Mor B., Debili N., Casadevall N., Ribrag V., Solary E., Vainchenker W., Plo I. (2011) JAK2(V617F) negatively regulates p53 stabilization by enhancing MDM2 via La expression in myeloproliferative neoplasms. Oncogene (in press) doi:10.1038/onc.2011.313 [DOI] [PubMed] [Google Scholar]
- 36. Meyuhas O. (2000) Synthesis of the translational apparatus is regulated at the translational level. Eur. J. Biochem. 267, 6321–6330 [DOI] [PubMed] [Google Scholar]
- 37. Meerovitch K., Svitkin Y. V., Lee H. S., Lejbkowicz F., Kenan D. J., Chan E. K., Agol V. I., Keene J. D., Sonenberg N. (1993) La autoantigen enhances and corrects aberrant translation of poliovirus RNA in reticulocyte lysate. J. Virol. 67, 3798–3807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lewis S. M., Holcik M. (2008) For IRES trans-acting factors, it is all about location. Oncogene 27, 1033–1035 [DOI] [PubMed] [Google Scholar]
- 39. Dobbyn H. C., Hill K., Hamilton T. L., Spriggs K. A., Pickering B. M., Coldwell M. J., de Moor C. H., Bushell M., Willis A. E. (2008) Regulation of BAG-1 IRES-mediated translation following chemotoxic stress. Oncogene 27, 1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bonnal S., Pileur F., Orsini C., Parker F., Pujol F., Prats A. C., Vagner S. (2005) Heterogeneous nuclear ribonucleoprotein A1 is a novel internal ribosome entry site trans-acting factor that modulates alternative initiation of translation of the fibroblast growth factor 2 mRNA. J. Biol. Chem. 280, 4144–4153 [DOI] [PubMed] [Google Scholar]
- 41. Lewis S. M., Veyrier A., Hosszu Ungureanu N., Bonnal S., Vagner S., Holcik M. (2007) Subcellular relocalization of a trans-acting factor regulates XIAP IRES-dependent translation. Mol. Biol. Cell 18, 1302–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Peek R., Pruijn G. J., Van Venrooij W. J. (1996) Interaction of the La (SS-B) autoantigen with small ribosomal subunits. Eur. J. Biochem. 236, 649–655 [DOI] [PubMed] [Google Scholar]
- 43. Jackson R. J., Hellen C. U., Pestova T. V. (2010) The mechanism of eukaryotic translation initiation and principles of its regulation. Nat. Rev. Mol. Cell Biol. 11, 113–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Costa-Mattioli M., Svitkin Y., Sonenberg N. (2004) La autoantigen is necessary for optimal function of the poliovirus and hepatitis C virus internal ribosome entry site in vivo and in vitro. Mol. Cell. Biol. 24, 6861–6870 [DOI] [PMC free article] [PubMed] [Google Scholar]