Abstract
Centrosomes are composed of a centriole pair surrounded by an intricate proteinaceous matrix referred to as pericentriolar material. Although the mechanisms underpinning the control of centriole duplication are now well understood, we know relatively little about the control of centrosome size and shape. Here we used interaction proteomics to identify the E3 ligase HERC2 and the neuralized homologue NEURL4 as novel interaction partners of the centrosomal protein CP110. Using high resolution imaging, we find that HERC2 and NEURL4 localize to the centrosome and that interfering with their function alters centrosome morphology through the appearance of aberrant filamentous structures that stain for a subset of pericentriolar material proteins including pericentrin and CEP135. Using an RNA interference-resistant transgene approach in combination with structure-function analyses, we show that the association between CP110 and HERC2 depends on nonoverlapping regions of NEURL4. Whereas CP110 binding to NEURL4 is dispensable for the regulation of pericentriolar material architecture, its association with HERC2 is required to maintain normal centrosome integrity. NEURL4 is a substrate of HERC2, and together these results indicate that the NEURL4-HERC2 complex participates in the ubiquitin-dependent regulation of centrosome architecture.
Centrosomes are composed of a pair of barrel-shaped, 9-fold symmetric centrioles, surrounded by a proteinaceous matrix collectively referred to as pericentriolar material (PCM).1 Centrosomes are the major microtubule organizing centers in mammalian cells and participate in various cellular processes that include cell motility, mitotic cell division, and ciliogenesis. Proteomic characterization and protein correlation profiling established that the human centrosome is composed of over 100 proteins (1). Understanding how this intricate array of proteins is organized into functionally distinct protein complexes that regulate various aspects of centrosome biogenesis, function, and architecture constitutes a major challenge.
Centrosome biogenesis and function is tightly regulated during the cell cycle. Indeed, the single interphase centrosome must duplicate once and only once during the cell cycle such that two centrosomes are present in mitosis, with each centrosome organizing one pole of the bipolar mitotic spindle apparatus (2). At the G2/M transition, the centrosome must undergo a maturation process where they dramatically increase in size and microtubule nucleation capacity to effectively organize both poles of the mitotic spindle (3). Centrosome size and morphology varies surprisingly little from cell to cell within a given cell type. Recent work has provided much needed insight into the regulation of centrosome size. Indeed, centrosomin and SPD-2, two upstream regulators of centrosome biogenesis implicated in the recruitment of PCM, are limiting with respect to centrosome size, and their depletion results in a general and proportional decrease in centrosome size (4, 5). However, it remains unclear how these and other proteins are regulated to impart centrosome size homeostasis.
One pervasive mode of regulation in cellular biology is via protein ubiquitylation (6). Ubiquitylation is a reversible post-translational modification where the small polypeptide ubiquitin is attached to proteins, most often via an isopeptide bond between the C terminus of ubiquitin and the ε-amino group of lysine residues on the target protein (6). Ubiquitylation is a multi-step enzymatic process involving activation of ubiquitin by an E1 that promotes the transfer of ubiquitin onto an E2 through the formation of a thioester-linked intermediate. Ubiquitin is then conjugated to substrates via the E3 ligase, which acts primarily as a substrate adaptor (6). There are two large classes of E3 ligases, the HECT (homologous to the E6-AP C terminus) domain class and RING-type ligases. HECT-type ligases form a thioester intermediate with ubiquitin, which they receive from the E2, and then transfer ubiquitin to substrates. In contrast, RING ligases act as adaptors that link substrates to the E2, without the formation of a RING-ubiquitin intermediate (6). Following the transfer of ubiquitin onto the substrates, the cycle of ubiquitin conjugation can be repeated to build ubiquitin chains. Because ubiquitin itself contains at least eight ubiquitin acceptor sites, multiple chain topologies can be formed (7). Interestingly, ubiquitin linkage plays an important part in dictating the outcome of ubiquitylation. For example, lysine 48 (K48)- and K11-linked ubiquitin chains target proteins for degradation by the 26 S proteasome, whereas K63-linked chains are nondegradative and instead act as scaffolds to organize signaling pathways such as the NF-κB network or the response to DNA damage (7, 8).
Several aspects of centrosome biogenesis are controlled through ubiquitylation. The anaphase-promoting complex/cyclosome and the SCF complex (SKP1, CUL1, and F-box protein) are the two major E3 ligases involved in centrosome biology. First, the anaphase-promoting complex/cyclosome, together with its cofactor CDH1, induces the degradation of SASS6 upon exit from mitosis, thereby preventing centriole duplication until normal levels of SASS6 are restored (9). It has been initially observed that the SKP1 and CUL1 subunits of the SCF localize to the centrosome and that they control its duplication (10). Several F-box proteins including FBXW7, SKP2, FBXW5, and cyclin F have also been shown to specifically regulate centriole duplication through targeted degradation of their substrates cyclin E, p27, SASS6, and CP110, respectively (10–13). However, members of the HECT family of E3 ligases do not have any previously described function in the regulation of centrosome biogenesis. These examples serve to highlight the complexity of the regulatory network in place that is driven by ubiquitylation to effectively control the number of centrosomes present in the cell.
Here we used interaction proteomics to identify novel proteins that associate with CP110, a centrosome component implicated in various facets of centrosome biogenesis (14–18). We reveal the existence of a novel CP110-interacting protein complex composed of HERC2, a giant HECT-type E3 ligase, and NEURL4. HERC2 and NEURL4 are both associated with centrosomes, and depletion of either factor results in aberrant PCM morphology. These results identify new components of the CP110 interaction network and highlight a novel role for HERC2 ligase in the modulation of centrosome architecture.
EXPERIMENTAL PROCEDURES
Cell Culture
U2OS and MG63 cells were grown at 37 °C in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum, 2 mm l-glutamine under 5% CO2 in a humidified atmosphere and maintained using standard procedures. SaoS2 and HOS cells were grown at 37 °C in McCoy's 5A medium containing 10% fetal bovine serum, 2 mm l-glutamine. All of the tissue culture reagents were from Invitrogen unless otherwise mentioned. Flp-InTM T-RExTM U2OS cells were generated as described previously (19). The cells were cultured in media containing 1 μg/ml blasticidin and 250 μg/ml zeocin. Stable cell lines were maintained in presence of 1 μg/ml blasticidin and 250 μg/ml hygromycin. Exogenous protein expression was induced by 24 h of treatment with 1 μg/ml tetracycline. U2OS cells expressing doxycycline-inducible HERC2 small hairpin RNA were cultured in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum and supplemented with 1 μg/ml puromycin and 5 μg/ml blasticidin as described previously (20).
RNA Interference and Production of Endoribonuclease-prepared siRNA
esiRNA was prepared as described previously using the primer pairs indicated in supplemental Table S4 (21). The cells were transfected using Lipofectamine RNAiMAX (Invitrogen), according to manufacturer's recommendation (22). 40,000 cells (U2OS, SaoS2, MG63, and HEK293T) per well were seeded into a 12-well plate and transfected the following day. Transfection complexes were prepared by combining 1.5 μl of transfection reagent and 600 ng of esiRNA or 50 pmol of siRNA in 250 μl of OptiMEM (Invitrogen). The final volume of transfection mix and plating medium was 1.5 ml. Firefly luciferase esiRNA and siGENOME nontargeting siRNA #1 (Dharmacon) were used as negative controls, depending on whether esiRNA or siRNA SMARTpools from Dharmacon were used for transfection (22, 23).
Immunofluorescence and Microscopy
The cells grown on #1.5 glass coverslips were fixed in ice-cold methanol at −20 °C for 10 min. To visualize F-actin, the cells were fixed in 4% paraformaldehyde in PBS for 10 min. After blocking in 0.2% (w/v) gelatin from cold water fish skin (Sigma) in PBS (PBS/FSG) for 15 min, the coverslips were incubated with primary antibodies in PBS/FSG at the following concentrations for 60 min: 1:1000 rabbit anti-NEURL4, raised against the N-terminal part of the protein (amino acids 1–484); 625 ng/ml mouse anti-HERC2 (BD Transduction Laboratories, 612366); 1000 ng/ml rabbit anti-pericentrin (Abcam, ab4448); 500 ng/ml mouse anti-NEDD1 (Abcam, ab57336); 1:500 rabbit anti-CENPJ (CPAP) (Proteintech, 11517-1-AP); 1:500 rabbit anti-CEP164 (Novus Biologicals, NBP1-77006); 1:2000 mouse anti-centrin-2 clone 20H5 from Dr. Jeff Salisbury (24); 1:2000 rabbit anti-centrin-2 from Erich Nigg (25); 1:5000 rabbit anti-CEP135 from Dr. Alex Bird (26); and 1:1000 sheep anti-GFP from Dr. David Drechsel (22). Following a 15-min wash with 0.2% PBS/FSG, the cells were incubated in 4 μg/ml of secondary antibodies for 30 min. The secondary antibodies used were: donkey anti-mouse, -rabbit, or -sheep IgG, conjugated to Alexa 488, Alexa 594, or Alexa 647 (Invitrogen) or donkey anti-sheep IgG conjugated to DyLight649 (Jackson Immunoresearch). The cells were subsequently stained with 50 μg/ml DAPI to visualize DNA. To visualize F-actin, the cells were stained with 66 nm phalloidin, conjugated to Alexa647 (Invitrogen). After 20 min of washing with 0.2% FSG/PBS, the coverslips were mounted on glass slides by inverting them into mounting solution (ProLong Gold antifade; Invitrogen).
Sequential staining was performed for immunostaining with two rabbit antibodies (NEURL4 and CPAP, NEURL4 and CEP164). The cells were fixed and blocked as described, following by 60 min of incubation with anti-NEURL4 antibodies (1:1000). After a 15-min wash with 0.2% PBS/FSG, the cells were incubated in 8 μg/ml of donkey anti-rabbit, conjugated to Alexa 594 for 60 min. After a 30-min wash with 0.2% PBS/FSG, the cells were incubated with mouse anti-centrin antibodies (1:2000) and either rabbit anti-Cep164 (1:500) or rabbit anti-CPAP (1:500) or PBS/FSG (negative control) for 30 min. After a 15-min wash with 0.2% PBS/FSG, the cells were incubated in 4 μg/ml of donkey anti-rabbit conjugated to Alexa488 and 4 μg/ml of donkey anti-mouse conjugated to Alexa647 for 30 min. The cells were stained with DAPI and mounted as described above.
Three-dimensional image data sets were acquired on an imaging system (DeltaVision Core, Applied Precision) equipped with an IX71 microscope (Olympus), a CCD camera (CoolSNAP 1024 × 1024; Roper Scientific), and 60×/1.42 NA or 100/1.4 NA Plan Apochromat oil immersion objectives (Olympus) using 1 × 1 binning. Z stacks (0.2 μm apart for each optical section) were collected, computationally deconvolved using the softWoRx software package (v4.0; Applied Precision) and shown as two-dimensional maximum intensity projections. Three-dimensional structured illumination microscopy was performed using DeltaVision OMX v3 (Applied Precision), equipped with 100×/1.40 NA Plan Apochromat oil immersion objective (Olympus), EMCCD cameras (Cascade II 512 × 512; Photometrics) and 405-, 488-, and 592.5-nm diode lasers. Images were collected and computationally reconstructed (27). Maximum projected images for individual channels were aligned using Acapella software (version 2.18; PerkinElmer Life Sciences).
Quantitative Analysis of Digital Images
Fluorescence intensity of centrosome-associated proteins and pericentrin-containing filaments were carried out. Quantification of fluorescence intensity was performed on deconvolved, maximum projected Z-stacks acquired under nonsaturating conditions. Identical exposure times were used between different samples to ensure the linearity of the fluorescence measurements. Fluorescence intensities were measured using Volocity software (version 5.5.1 64 bit; PerkinElmer Life Sciences) in the pericentriolar region. A centriole mask was created using centrin as a marker of centrioles. To obtain a mask for the pericentriolar region, the centriole mask was expanded using two-pixel dilation. Fluorescence intensity values were measured and corrected for background intensity. Pericentrin-positive structures were segmented based on an adaptive intensity-based threshold. The border perimeter and bounding circle diameter of each detected object was then analyzed supplemental Fig. S1. Segmentation was performed using Volocity software (version 6.0 64 bit; PerkinElmer Life Sciences).
Transfection and Generation of Lysates
10-cm diameter dishes of HEK293T or U2OS cells were cultured, and each dish was transfected with a total of 10 μg of the indicated plasmids using the polyethylenimine method (28). The cells were cultured for a further 36 h and lysed in 0.5 ml of ice-cold lysis buffer (50 mm HEPES, pH 7.5, 1 mm EGTA, 1 mm EDTA, 1% (w/v) Nonidet P-40, 10 mm sodium-β-glycerophosphate, 50 mm sodium fluoride, 5 mm sodium pyrophosphate, 10% glycerol, 1 mm DTT, and complete protease inhibitor mixture. The lysis buffer conditions used for affinity purification followed by mass spectrometry (AP/MS) were described previously (29, 30). For experiments investigating protein ubiquitylation, 5 mm N-ethylmaleimide (Sigma, E1271) was added to lysis buffer. Cell lysates were clarified by centrifugation at 4 °C for 20 min at 26,000 × g.
Generation of Stable Cell Lines
All of the inducible expression cell lines were generated using the Flp-In T-REx system (Invitrogen). These constructs were derived from the pcDNA5-FRT-TO-FLAG and pcDNA5-FRT-TO-eGFP vectors described previously (31). Cells were co-transfected with these constructs and the pOG44 vector (expressing FLP recombinase) into the U2OS or HEK293 Flp-In T-REx host cell lines. Cells that had integrated the expression plasmid were selected with 200 μg/ml hygromycin B for 2–3 weeks, and the colonies were pooled and maintained as described above.
Immunoprecipitation
0.5–1 mg of clarified cell lysates were subjected to immunoprecipitation (IP) with 2 μg of CEP97, CP110, HERC2, or GFP antibody, which was conjugated to 5 μl of protein G-Sepharose. For the FLAG IP, we used 5 μl of FLAG-M2-agarose resin for each IP. The control reactions were performed in parallel, and we used protein G-Sepharose resin coupled to 2 μg of either a mouse or rabbit IgG (depending on the antibody used). For the FLAG IP, the control IP was performed in parallel with 5 μl of FLAG-M2-agarose resin in cells transfected with an empty vector. The IPs were carried out for 1 h at 4 °C on a vibrating platform, the immunoprecipitates were washed twice with lysis buffer followed by two washes with in 50 mm Tris/HCl, pH 7.5, 0.1 mm EGTA, and 1 mm DTT. The immunoprecipitates were analyzed by immunoblotting using standard procedures.
Affinity Purification and Tandem Mass Spectrometry
Inducible FLAG-tagged NEURL4, CEP97, and CP110 and empty FLAG control HEK293 cell lines were generated using the Flp-In T-REx system (Invitrogen) as per the manufacturer's protocol and used for FLAG affinity purification. For HERC2, the affinity purification was performed with HERC2-specific antibody coupled to protein G-Sepharose resin as described above. In all cases, the purification was performed in biological replicates from ∼100 mg of protein (harvested from 5 × 150-mm confluent plates), essentially as described previously (32), with the following modifications: the lysates were precleared with 150 μl of packed protein G-Sepharose beads for 3 h at 4 °C; 125 units (total amount) of benzonase was added to lysis buffer prior to harvesting the cells; and the IP was performed using 75 μl of packed FLAG-M2-Sepharose beads for 3 h at 4 °C. The baits and associated proteins were eluted with ammonium hydroxide, lyophilized in a SpeedVac, resuspended in 50 mm ammonium bicarbonate (pH 8–8.3), and incubated at 37 °C with 100 μg of trypsin overnight. The ammonium bicarbonate was evaporated, and the samples were resuspended in HPLC buffer A (3% ACN, 0.1% formic acid) and directly loaded onto capillary columns (75-micron inner diameter; 13-cm length) packed in-house with Agilent Zorbax C18 reversed phase, 3.5 μm particle size, and 300 angstrom pore size. MS/MS data were acquired in data-dependent mode (over a 2-h acetonitrile 2–40% gradient) on a ThermoFinnigan LTQ equipped with a Proxeon NanoSource and an Agilent 1100 capillary pump. Acquisition parameters were: one MS survey scan, followed by four MS/MS scans on the most abundant ions. Each species was sequenced twice (within 30 s) before being placed on a dynamic exclusion list for up to 90 s (exclusion list contains 60 species). Acquired RAW files were converted to mgf format using the recommended parameter from Thermo in the ProteoWizard tool (33) and searched with the Mascot search engine (version 2.2; Matrix Sciences, London, UK) against the human RefSeq database (release 42; 39125 entries searched) with a precursor ion mass tolerance of 3.0 and a fragment ion mass tolerance of 0.6. Methionine oxidation was allowed as a variable modification, and trypsin specificity (with two missed cleavages allowed) was selected. Search results were parsed into our ProHits LIMS system for further analysis (34). Entries were parsed if they had a minimum peptide Mascot score of 35 and a significance threshold of p < 0.05; the option “require red bold” was also selected. Using these parameters yields an estimated false discovery rate of 2–3% against a target decoy database.
Scoring high confidence interaction partners was performed as follows: proteins detected in any of the six negative control runs were first eliminated from any subsequent analysis. Three of the control runs included data from control IP in HEK293 cells where rabbit IgG coupled to protein G-Sepharose resin was used. The other remaining control runs included data from IPs in HEK293 Flp-In T-REx cell lines expressing empty vector where FLAG-M2-agarose resin was used. Next, proteins that have been detected in ≥20% of all AP/MS samples analyzed in our laboratories (n = 2080) were deemed frequent fliers and also eliminated (taken together, these filters removed ∼400 proteins). The n = 2080 is the total number of AP/MS samples analyzed that had their resulting hits deposited in the database of our institute. The AP/MS samples in the database were generated by multiple users and cover a number of different cell lines and experimental procedures. The resulting hits were further filtered by requiring a minimal protein Mascot score of 60 and at least two unique peptides per protein. We required the protein to have been detected in at least two of the three biological replicates with at least one of the baits to be included in the data set. Lastly, to increase the stringency of the data set, we further required that at least a total of 40 total peptides was detected for a given hit across the three biological replicates. Hits that passed these two last criteria were included in Table I (highlighted in bold font) and Fig. 1A. All of the interactions involving these hits were reported in supplemental Table S1, whether they passed these last two filters with each bait or not. A list of unfiltered interactors as well as list of control contaminants can be found in supplemental Tables S2 and S3, respectively. Our entire data set is available from the ProteomeCommons Tranche network (using hash key e0kXD9iSyxImyy/jdW4ACc5IirbFXnLhWoh7GvDi+z74DB3z3AUH3CYlMqQkmL1Pos+yKsN9aoSjA4kIoWPkpytxiKEAAAAAAAANmg==).
Table I. Filtered list of protein interactions detected by AP/MS.
The parameters used for the CP110, CEP97, NEURL4, and HERC2 interactions were: minimum number of unique peptides = 2; maximum frequency across the database = 20; minimum Mascot score = 60; removed all hits detected in any one of control samples; included proteins detected in at least two-thirds of the biological replicates for each bait. The numbers listed in the columns for each bait represent the total number of peptides number for each hit. The bold font highlights those hits containing a sum of ≥40 total peptides number across the biological triplicates (labeled E1, E2, and E3 for experiments 1, 2, and 3) and were used to create Fig. 1A. The italic text highlights the total peptide number associated with the bait protein. Gene names are from NCBI Entrez Gene. Protein identifier is the NCBI gi identifier for the protein. The hits that were detected from the control samples were removed from this list. The control samples were as follows: three of the control runs included data from control IPs in HEK293 cells where rabbit IgG coupled to protein G-Sepharose resin was used. The other remaining control runs included data from IPs in HEK293 Flp-In T-REx cell lines expressing empty vector and FLAG-M2-agarose resin.
| Gene name | Protein identifier | CP110 |
CEP97 |
NEURL4 |
HERC2 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E1 | E2 | E3 | E1 | E2 | E3 | E1 | E2 | E3 | E1 | E2 | E3 | ||
| CP110 | 190341065 | 88 | 34 | 74 | 10 | 12 | 16 | 18 | 12 | 13 | |||
| CEP97 | 31377705 | 59 | 20 | 48 | 453 | 274 | 370 | 16 | 5 | 4 | |||
| HERC2 | 126032348 | 46 | 11 | 31 | 161 | 150 | 108 | 218 | 71 | 208 | |||
| ARPC2 | 5031599 | 12 | 8 | 2 | |||||||||
| CEP290 | 109255234 | 9 | 2 | 7 | 3 | ||||||||
| SNAP23 | 18765729 | 4 | 6 | 4 | 9 | 3 | |||||||
| LYN | 4505055 | 10 | 3 | 6 | 6 | 6 | |||||||
| ACTR3 | 5031573 | 9 | 3 | 4 | 3 | 3 | |||||||
| NEURL4 | 53829368 | 19 | 4 | 157 | 103 | 102 | 19 | 27 | |||||
| FLOT2 | 94538362 | 6 | 4 | ||||||||||
| GPI | 18201905 | 2 | 3 | 3 | 4 | 4 | |||||||
| SSSCA1 | 5453838 | 5 | 2 | 4 | 3 | 6 | 10 | 11 | |||||
| CAND1 | 21361794 | 4 | 3 | 3 | |||||||||
| CEP76 | 21314728 | 5 | 3 | ||||||||||
| SUGT1 | 5730041 | 21 | 7 | 10 | |||||||||
| GNB4 | 11055998 | 2 | 2 | ||||||||||
| KTN1 | 118498362 | 68 | 37 | 62 | 3 | ||||||||
| PECI | 45643119 | 36 | 14 | 21 | 6 | 10 | |||||||
| PCM1 | 134142826 | 7 | 6 | ||||||||||
| RPA3 | 4506587 | 11 | 9 | 5 | 3 | 4 | |||||||
| RPA1 | 4506583 | 24 | 7 | 5 | 2 | 8 | |||||||
| MAP7D1 | 78042577 | 8 | 4 | 4 | |||||||||
| CEP170 | 109255230 | 5 | 4 | 4 | 7 | 2 | 14 | ||||||
| MAPK6 | 4506091 | 3 | 3 | 3 | |||||||||
| VAC14 | 39780552 | 14 | 3 | ||||||||||
| RPA2 | 4506585 | 8 | 2 | ||||||||||
| POLR2A | 4505939 | 9 | 2 | ||||||||||
| SRGAP2 | 112363100 | 201 | 88 | 146 | |||||||||
| TBK1 | 7019547 | 21 | 3 | 17 | |||||||||
| TANK | 19743569 | 5 | 5 | 7 | |||||||||
| SEC23IP | 6005824 | 7 | 6 | ||||||||||
Fig. 1.
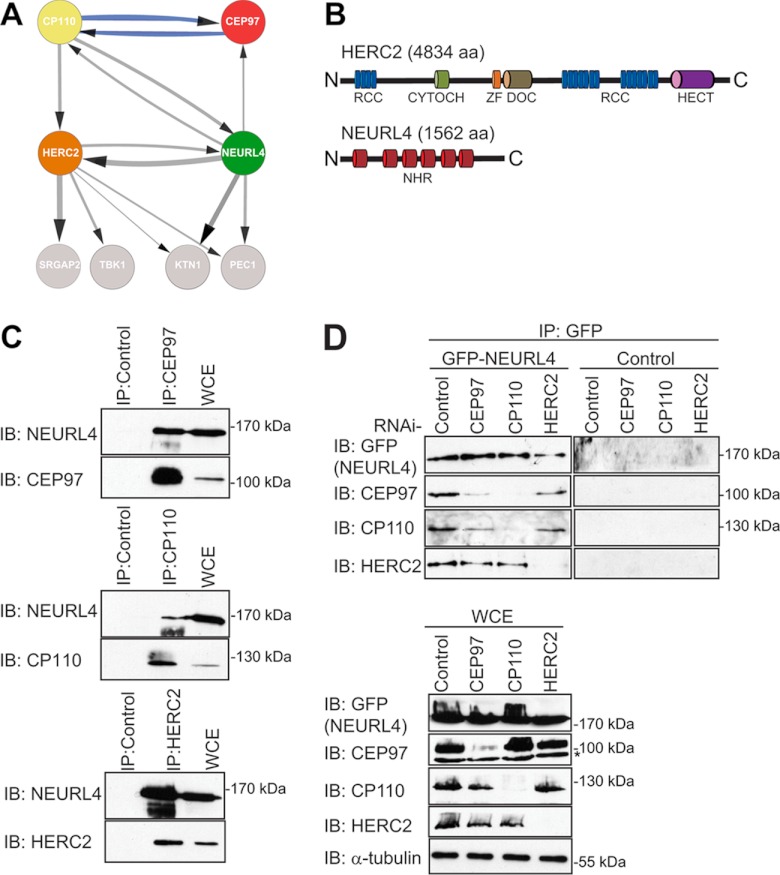
Identification of novel CP110 interacting proteins. A, the interaction network for NEURL4, HERC2, CP110, and CEP97 was prepared using Cytoscape software (56–58). The parameters used for the indicated interactions were: minimum number of unique peptides = 2; maximum frequency across the database = 20; minimum Mascot score = 60; ≥40 total peptide numbers; remove all hits detected in any one of control samples; included proteins detected in at least two-thirds of the biological replicates for each bait. The control samples were as follows: three of the control runs included data from control IP in HEK293 cells where rabbit IgG coupled to protein G-Sepharose resin was used. The other remaining control runs included data from IPs in HEK293 Flp-In T-REx cell lines expressing empty vector with FLAG-M2-agarose resin. The thicknesses of the connecting lines are proportional to the average number of total peptides associated with the interaction. The gray connecting lines indicate new interactions, whereas the blue connecting lines indicate previously reported interactions. B, domain structure of HERC2 and NEURL4. RCC, regulator of chromosome condensation (amino acids 514–727, 2959–3327, and 3952–4319); CYTOCH, cytochrome b5 heme-binding domain (amino acids 1207–1283); ZF, zinc finger (amino acids 2702–2749); DOC, Anaphase-promoting complex, subunit 10 (amino acids 2759–2930); HECT, homologous to the E6-AP C terminus (amino acids 4457–2794); NHR, neuralized homology region (amino acids 41–207, 317–484, 520–686, 716–884, 913–1086, and 1131–1294). C, endogenously expressed CEP97, CP110, and HERC2 were immunoprecipitated from HEK293T WCE with their own respective antibodies. As a control, IP was performed in parallel with a nonspecific rabbit IgG. Immunoprecipitated proteins were subjected to immunoblot analysis using the CEP97, CP110, HERC2, and NEURL4 antibodies. D, U2OS cells stably expressing GFP-NEURL4 were transfected with esiRNA targeting CEP97, CP110, HERC2, or luciferase (negative control). 72 h post-transfection, cells were harvested, and GFP-NEURL4 was immunoprecipitated using anti-GFP antibody (top panel). Right top panel, a GFP control IP was performed in U2OS cells expressing an empty vector and immunoblotted with GFP, CEP97, CP110, and HERC2 antibodies. The immune complexes were subjected to immunoblot analysis with CEP97, CP110, HERC2, and GFP antibodies. WCE were blotted as a loading control (bottom panel). The protein size (kDa) is indicated for each immunoblot. The nonspecific cross-reactive bands are indicated by an asterisk. C and D, HERC2 is a 528-kDa protein and runs as a very high molecular mass band, and there is no molecular mass standard in that region of the gel. IB, immunoblot.
Generation of NEURL4 Antibodies and Other Antibodies
Rabbit polyclonal antibodies to NEURL4 were raised against GST-NEURL4521–883 and GST-NEURL41–484 fusion proteins and were affinity-purified against MBP-NEURL4521–883 or MBP-NEURL41–484, respectively, as described previously (35). Antibody production services were purchased from Covance (Denver, PA). All of the following antibodies were used at a concentration of 1 μg/ml for immunoblotting unless otherwise indicated. NEURL4 (rabbit, generated in house; for immunoblotting anti-NEURL4521–883 was used; for immunofluorescence anti-NEURL41–484 was used), CEP97 (rabbit, Bethyl Laboratories, A301–345A), CP110 (rabbit, Bethyl Laboratories A301–344A), HERC2 (rabbit, Bethyl Laboratories, A301–905A), ubiquitin (rabbit, Dako, Z0458), K48-ubiquitin (rabbit, Millipore, 05-1307), K63-ubiquitin (rabbit, Millipore, 05-1308), K11-ubiquitin (human, kind gift from Genentech) (36), α-tubulin (mouse, Calbiochem, CP06), FLAG (rabbit, Sigma, F7425), and GFP (goat) as previously described (22).
Ubiquitylation Assays
The reaction was carried out as previously described (19). Briefly, the assays were in a total volume of 25 μl containing 50 mm Tris/HCl, pH 8.0, 1 mm DTT, immunoprecipitated HERC2 (from 1 mg of HEK293T WCE) still conjugated to protein G-agarose beads, 0.4 μm UBCH5C (Boston Biochem), 0.0125 μm E1, and 16 μm ubiquitin (Boston Biochem). The reactions were initiated by the addition of 2 mm ATP, 5 mm MgCl2 and performed on a vibrating platform at 30 °C for 2 h. The reactions were terminated by the addition of 1× SDS-PAGE sample buffer. The ubiquitylation reactions were assessed by subjecting the samples to immunoblot analysis with different ubiquitin antibodies.
RESULTS
HERC2 and NEURL4 Are Novel Interactors of CP110
Through its association with CEP97, CEP76, or CEP290, CP110 is a critical regulator of centrosome biogenesis (13, 15–18). An interesting feature of CP110 function appears to be its association in different subcomplexes with distinct functions. Indeed, although CP110 in a complex with CEP97 negatively regulates centriole length, CP110 in a complex with CEP290 is required for ciliogenesis (15, 16). These observations led us to search for additional CP110-associated proteins by AP/MS (22, 30, 32). We generated a human embryonic kidney cell line (HEK293) stably expressing tetracycline-inducible FLAG-tagged CP110. Our mass spectrometry analyses confirmed previously known interactors of CP110 including CEP97, CEP290m and CEP76 (Table I, Fig. 1A, and supplemental Tables S1–S3) (15, 16). Importantly, we detected several putative novel interactors including the HECT domain and RLD 2 protein (HERC2) neuralized-like protein 4 (NEURL4), which were selected for further study (Table I, Fig. 1A, and supplemental Table S1). We did not follow up any further on the other interacting proteins detected, including SRGAP2, TBK1, PEC1, and KTN1, because our analysis revealed that they were not shared among the network of NEURL4-CEP97-CP110-HERC2. HERC2 is a 528-kDa protein that contains three RCC1-like domains and a C-terminal HECT domain required for its E3 ubiquitin ligase activity (37). RCC1-like domains have been implicated in Ran-mediated nuclear transport and chromatin-based microtubule-nucleation (38, 39) (Fig. 1B, top panel). Although the biological function of HERC2 has long remained elusive, HERC2 was recently shown to modulate the activity of the RNF8 ubiquitin ligase during the DNA damage response, using a noncatalytic mechanism (20). In addition, HERC2 also regulates the levels of BRCA1 and XPA through ubiquitylation and proteasomal degradation (40, 41). Most recently HERC2 was reported to be a member of the DNA replication fork complex (42). NEURL4 belongs to a family of neuralized-like proteins whose function is not understood in mammalian cells. NEURL4 is composed of six tandem neuralized homology regions (NHR) that span almost the entire length of the protein (Fig. 1B, bottom panel). We note, however, that many of the mammalian proteins bearing NHR domains also contain an E3 ligase domain or suppressor of cytokines signaling domain (43) linking them to putative ubiquitylation-related functions in cells. However, NEURL4 does not appear to have an E3 ligase domain, making it unlikely that it functions as an E3 ligase. Furthermore, to this date the specific role of the mammalian and Drosophila NEURL4 and the function of its NHR domains remain uncharacterized. The presence of HERC2 and NEURL4 in our CP110 interaction network raised the possibility that centrosome biogenesis could be regulated through HERC2-dependent ubiquitylation.
To expand the CP110 interaction network, we subjected FLAG-tagged CEP97 and NEURL4 to AP/MS. In agreement with our CP110 interaction data, we identified CP110, CEP97, and HERC2 as NEURL4-interacting proteins (Fig. 1A, Table I, and supplemental Table S1). In contrast, CEP97 pulled down CP110 but we failed to retrieve NEURL4 or HERC2 in these immunopurifications (Fig. 1A, Table I, and supplemental Table S1). Because we were unable to produce full-length tagged HERC2 for AP/MS, we tested two commercial HERC2 antibodies for their ability to immunoprecipitate HERC2 (supplemental Fig. S2A). Using the A301–905A anti-HERC2 antibodies, we immunopurified endogenous HERC2 from HEK293T lysates and analyzed HERC2 and associated proteins by mass spectrometry. Whereas we could readily detect NEURL4 peptides in the HERC2 immunoprecipitate, we were unable to detect peptides corresponding to CEP97 or CP110 (Fig. 1A, Table I, and supplemental Table S1). Collectively, these results suggested a tentative model where HERC2-NEURL4 and CP110-CEP97 form stable complexes that interact via NEURL4 and CP110. To validate the AP/MS results using an orthogonal approach, we generated antibodies against NEURL4 (supplemental Fig. S2B) and used them to analyze CEP97, CP110, and HERC2 immunoprecipitates. We confirmed the interaction between endogenous NEURL4, CEP97, CP110, and HERC2 (Fig. 1C). Identical results were obtained when transiently expressing FLAG-NEURL4 (supplemental Fig. S3A).
To probe the dependences in this interaction network, we depleted CP110, CEP97, and HERC2 using esiRNA and assessed protein interactions in U2OS cells stably expressing inducible GFP-NEURL4 (Fig. 1D). Consistent with our AP/MS results, we found that treatment with either CEP97 or CP110 esiRNA had little impact on the ability of HERC2 to associate with NEURL4 (Fig. 1D). Furthermore, knockdown of CP110 severely impaired binding of CEP97 to NEURL4, confirming that CEP97 is bridged to NEURL4 via CP110. As previously reported, we also found that CEP97 depletion led to a decrease in CP110 and in turn decreased binding to NEURL4 (15). None of the NEURL4-interacting proteins were detected in control immunoprecipitation with an empty vector (Fig. 1D). Taken together, these data suggest that the HERC2-NEURL4 complex interacts with CP110-CEP97 via a NEURL4-CP110 interaction.
HERC2 and NEURL4 Localize to the Centrosome
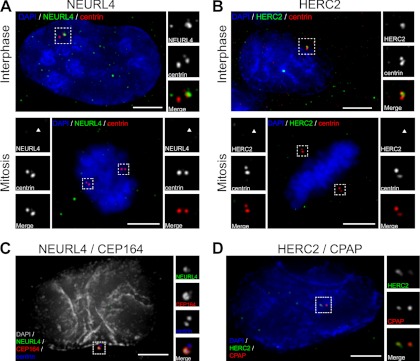
The interaction between NEURL4, HERC2, and the known centrosome proteins CP110 and CEP97 prompted us to investigate whether NEURL4 and HERC2 also associate with centrosomes (14, 15). By immunofluorescence, we observed distinct NEURL4 foci that co-localized in the vicinity of centrin positive structures, a core subdistal component of centrioles (24) (Fig. 2A, top panel). Further co-localization analysis with other centrosome markers revealed that NEURL4 overlaps more with CEP164 (Fig. 2C), a component of distal appendages of mature centrioles (44), suggesting that NEURL4 is predominantly localized to the mother centriole. A similar localization pattern was observed using GFP-tagged NEURL4 (supplemental Fig. S3, E and F). NEURL4 labeling at centrosomes decreased upon NEURL4 RNAi, suggesting that the labeling observed was specific (supplemental Fig. S5, A and C). We also observed noncentrosomal NEURL4 foci (Fig. 2A, top panel), suggesting that NEURL4 not only localizes to the centrosome. We note that some of these noncentrosomal foci were still present after NEURL4 depletion, indicating that a subset of these structures are due to nonspecific labeling or that they are stable enough to still be present after RNAi. Analysis of NEURL4 distribution at different stages of the cell cycle revealed that it associates with centrosomes during interphase but not during mitosis (Fig. 2A, bottom panel). We analyzed NEURL4 protein levels at different stages of the cell cycle and found them to be slightly increased during mitosis (supplemental Fig. S3B). This suggests that the observed change in localization in mitosis is caused by the dissociation of NEURL4 from the centrosome. Similarly, we observed that HERC2 also associates with centrosomes, as judged by its localization in the vicinity of centrin structures (Fig. 2B, top panel). Further co-localization analyses with additional centrosome markers showed that HERC2 appears to predominantly label the proximal region of centrioles, based on its co-localization with CPAP (Fig. 2D) (25, 45). HERC2 labeling intensity at centrosomes was reduced upon HERC2 esiRNA treatment, indicating that the centrosomal localization observed was specific (supplemental Fig. S5, B and D). Similarly to NEURL4, immunostaining with HERC2 antibodies was characterized by noncentrosomal foci. Notably, some of those foci do not represent HERC2-specific signal, based on immunostaining in HERC2-depleted cells (supplemental Fig. S5B). Much akin to what we observed with NEURL4, HERC2 labeling at centrosomes was noticeably reduced during mitosis (Fig. 2B, bottom panel). In over 100 cells in three independent experiments, 92 and 85% of cells display NEURL4 and HERC2 labeling at centrosomes, respectively. Taken together, these results suggest that a portion of NEURL4 and HERC2 localize to interphase centrosomes and that they appear to predominantly accumulate at the distal and proximal region, respectively. Furthermore, both proteins appear to lose their association with centrosomes during mitosis.
Fig. 2.
NEURL4 and HERC2 are novel centrosomal proteins. A and B, U2OS cells were labeled for DNA (blue; DAPI), centrin (red), and NEURL4 (green) (A) or DNA (blue), centrin (red), and HERC2 (green) (B). Interphase cells are shown in the top panels. The images show co-localization of NEURL4 (A) and HERC2 (B) with centriolar marker centrin (scale bar, 5 μm). Both NEURL4 and HERC2 are absent from the centrosomes in mitosis (bottom panels). The insets are 2-fold magnifications of the outlined areas to better visualize the centrosomal regions. C, U2OS cells were sequentially labeled for NEURL4 (green) and CEP164 (red), followed by labeling for centrin (blue) and DNA (gray, DAPI). NEURL4 co-localizes with Cep164 to the distal appendages of mature centriole. D, U2OS cells were labeled for DNA (blue; DAPI), CPAP (red), and HERC2 (green). HERC2 co-localization with CPAP, a marker of the proximal region of centrioles (scale bar, 5 μm). The insets are 2-fold magnifications of the outlined areas to better visualize the centrosomal regions. DAPI, 4′,6′-diamino-2-phenylindole.
NEURL4 Knockdown Alters Centrosome Morphology
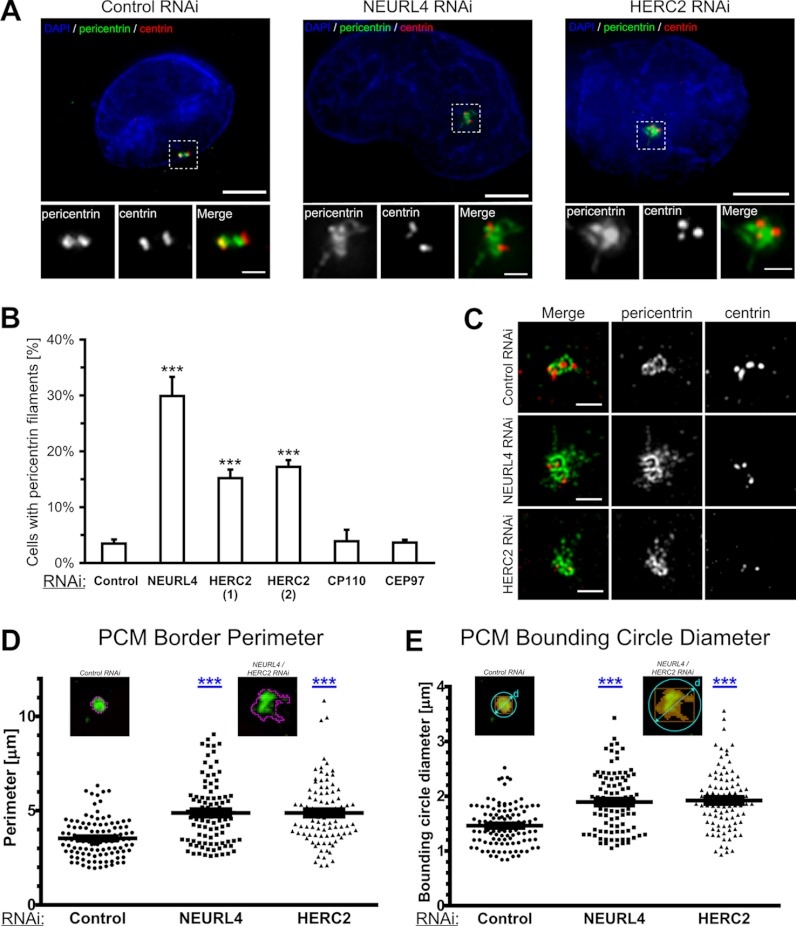
The CEP97-CP110 protein complex caps the distal ends of centrioles, physically restricting centriole length (15, 25, 45). The depletion of either protein induces the formation of overly long centrioles (15). Our finding that CP110 associates with a NEURL4, which in turn associates with HERC2, and the fact that both NEURL4 and HERC2 localize to centrosome led us to investigate whether they were also implicated in the regulation of centriole length. Consistent with previous results, we observed a high frequency of long centrin-containing structures in CEP97 or CP110 esiRNA-treated cells (supplemental Fig. S3, C and D). However, no such increase was observed in cells depleted of NEURL4 and HERC2 (supplemental Fig. S3D). Notably, both NEURL4 and HERC2 were localized to centrosome in cells depleted of CEP97 or CP110, suggesting that they are not required for the localization of NEURL4 and HERC2 to centrosome (supplemental Fig. S5, A and B). Strikingly, however, we observed that NEURL4 and HERC2 depletion altered the morphology of pericentriolar material (Fig. 3A). In particular, both NEURL4- and HERC2-depleted U2OS cells exhibited a high frequency of pericentrin-bearing filamentous structures, in sharp contrast to control-transfected cells that harbor more punctate centrosomes (Fig. 3, A and B). Additionally these filamentous structures contained CEP135 but were devoid of γ-tubulin, NEDD1, CPAP4, and centrin (Fig. 3A and supplemental Fig. S4, C and D). Filamentous structures were observed in ∼30% of NEURL4-depleted cells and ∼17% of HERC2-depleted cells, as opposed to <5% of control, CP110, or CEP97 esiRNA-treated cells (Fig. 3B). To further characterize the pericentrin-containing filaments, we imaged the cells using structured illumination microscopy (27). This allowed us to observe with higher spatial resolution fine details of the modifications in pericentriolar material architecture (Fig. 3C). We have performed detailed morphometric measurements on centrosomes from control, NEURL4, and HERC2 RNAi-treated cells. We found that the border perimeter and bounding circle diameter are both significantly increased upon NEURL4 and HERC2 knockdown (Fig. 3, D and E). We investigated the effect of depleting NEURL4 in different cell lines including SaoS2, HOS, and MG63 and found that the frequency of pericentrin-bearing filaments was higher in SaoS2 cells (supplemental Fig. S4A). These results suggest that different cell types have developed varying sensitivities toward NEURL4 depletion in terms of its impact on centrosome morphology.
Fig. 3.
NEURL4 and HERC2 regulate centrosome morphology. A, U2OS cells were transfected with siGENOME nontargeting siRNA #1, NEURL4 siRNA, or HERC2 esiRNA-2 and labeled for DNA (blue; DAPI), centrin (red), and pericentrin (green). The cells were imaged using conventional deconvolution microscopy. NEURL4 depletion caused abnormalities in pericentriolar material, shown in more detail in the insets. The insets are magnifications of the outlined centrosomal regions. Scale bars, 5 μm. B, U2OS cells were transfected with luciferase (control), HERC2(1), HERC2(2), CP110, CEP97 esiRNA, or NEURL4 siRNA. The presence of pericentrin filaments was quantified 72 h post-transfection. The graph shows the averages ± S.E. of the number of cells with pericentrin filaments, obtained in three independent experiments. At least 100 cells were analyzed under each condition. The increase in the number of pericentrin filaments is statistically significant. ***, p value < 0.0001. C, cells treated as described in A were imaged on a structured-illumination microscope. High resolution images of the centrosome regions reveal fine details of the filamentous structures, induced by NEURL4 or HERC2 depletion. Scale bars, 1 μm. D and E, wild type and filamentous centrosomes were segmented, based on an adaptive threshold. The PCM border perimeter (D) and the PCM bounding circle diameter (E) of each detected object (see insets) were then analyzed. The increase in both parameters was highly significant. ***, p value < 0.0001. Cut-off for considering a centrosome as filamentous was set at 5 μm for border perimeter and 2 μm for bounding circle diameter. At least 30 centrosomes were measured in three independent experiments for each condition. DAPI, 4′,6′-diamino-2-phenylindole.
Structure-function Analysis of NEURL4
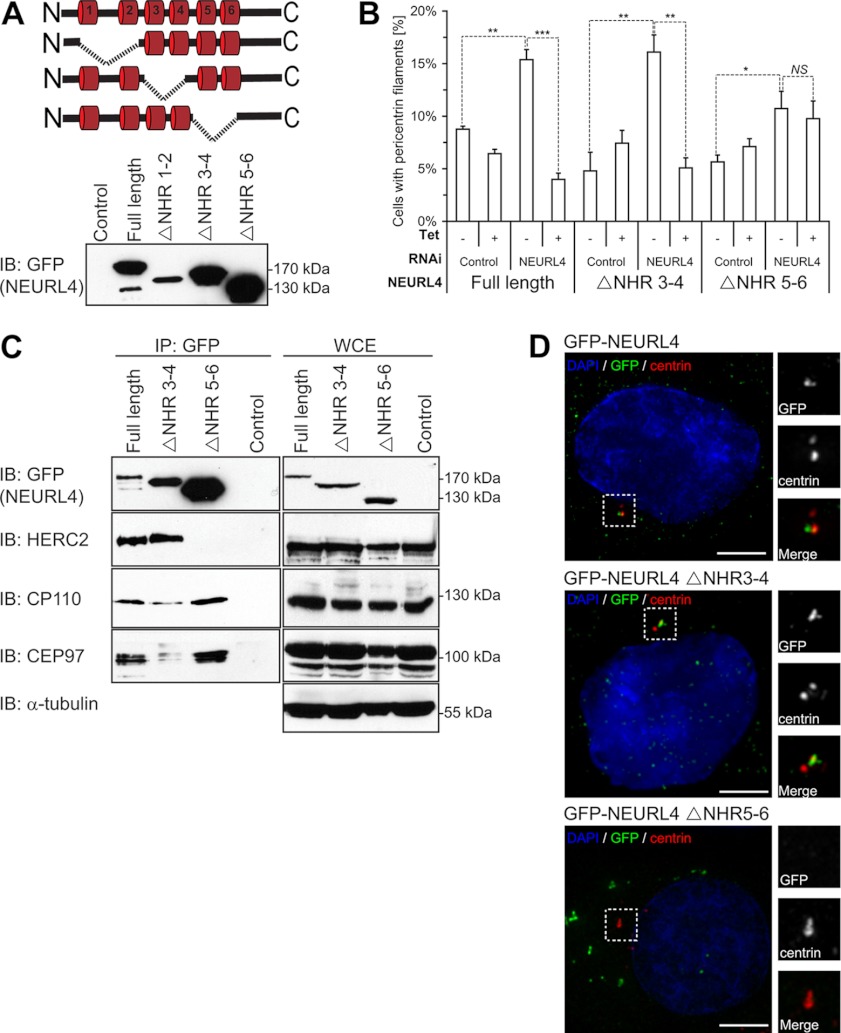
As a first step toward understanding the molecular basis of the role of NEURL4 in the regulation of PCM morphology, we generated tetracycline-inducible cell lines expressing GFP-tagged NEURL4 mutants lacking different pairs of NHR domains (Fig. 4A). Protein expression analysis revealed that although GFP-NEURL4 ΔNHR3–4 and GFP-NEURL4 ΔNHR5–6 were expressed at levels that either approached or exceeded those of wild type, the NEURL4 mutant lacking the NHR1–2 region was expressed at much lower levels (Fig. 4A, bottom panel), suggesting that the NHR1–2 region is necessary for protein stability. For this reason, we selected the ΔNHR3–4 and ΔNHR5–6 mutants for further analysis.
Fig. 4.
Structure-function analysis of NEURL4. A, schematic representation of the different deletion constructs generated for GFP-NEURL4 protein (top panel). Stable U2OS cell lines were generated that expressed each of these deletion constructs, and protein expression was confirmed by immunoblot analysis with the GFP antibody (bottom panel). B, U2OS cell expressing inducible GFP-tagged NEURL4 or NHR deletion mutants were transfected with luciferase esiRNA or esiRNA targeting the 3′-UTR region of NEURL4. Presence of pericentrin filaments was quantified 48 h after induction with 1 μg/ml tetracycline (where indicated) and 72 h after RNAi transfection. The graph shows the averages ± S.E. of the number of cells with pericentrin filaments, obtained in three independent experiments. At least 100 cells were analyzed under each condition. t test results comparing the indicated data sets are shown in the graph. *, p value < 0.05; **, p value < 0.001; ***, p value < 0.0001. NS, nonsignificant difference. C, U2OS stable cell lines expressing the GFP-tagged NEURL4, NEURL4 ΔNHR3–4, and NEURL4 ΔNHR5–6 constructs were induced for expression with 1 μg/ml tetracycline for 16–18 h before harvesting. The exogenously expressed GFP-NEURL4 was immunoprecipitated using GFP antibodies, and the immune complexes were subjected to immunoblot analysis with antibodies against GFP, HERC2, CP110, and CEP97 (left panel). A control IP was performed in parallel in U2OS stable cell lines expressing an empty vector. WCEs were immunoblotted with antibodies to the same proteins as well as the α-tubulin as a loading control (right panel). The control samples were WCEs from U2OS cell line expressing empty vector. HERC2 is a 528-kDa protein and runs as a very high molecular mass band, and there is no molecular mass standard in that region of the gel. D, U2OS cells expressing inducible GFP-tagged NEURL4, NEURL4 ΔNHR3–4, and NEURL4 ΔNHR5–6 were treated with 1 μg/ml tetracycline for 48 h to induce expression. The cells were labeled for DNA (blue; DAPI), pericentrin (red), and GFP (green). Scale bars, 5 μm. GFP-NEURL4 ΔNHR3–4 is co-localized with pericentrin, whereas GFP-NEURL4 ΔNHR5–6 is excluded from the centrosomal region. The insets are magnifications of the outlined regions. The protein size (kDa) is indicated for each immunoblot. DAPI, 4′,6′-diamino-2-phenylindole; IB, immunoblot.
Importantly, the abnormal centrosome morphology imparted by depletion of NEURL4, using an esiRNA that targets the 3′-UTR of the NEURL4 mRNA, could be reversed by expression of an esiRNA-resistant GFP-NEURL4 transcript (Fig. 4B). This latter result not only ensured specificity of the NEURL4 depletion phenotype but also enabled us to determine which regions of NEURL4 modulate the morphology of pericentriolar material. Using the same strategy as above, we found that although GFP-NEURL4 ΔNHR3–4 rescued the phenotype caused by NEURL4 depletion, GFP-NEURL4 ΔNHR5–6 did not. These results indicated that the NHR5–6 region is critical for the maintenance of centrosome architecture (Fig. 4B). Considering our lack of efficient 3′-UTR reagents and full-length cDNA for HERC2, we instead validated the phenotype using two nonoverlapping esiRNAs (Fig. 3C). Together these results suggest that the depletion of NEURL4 and HERC2 leads to a specific change in the organization of PCM.
Next, we tested whether deletion of different NHRs impaired the ability of NEURL4 to interact with its partners in co-immunoprecipitation studies. The deletion of the NHR3–4 region impaired the interaction of NEURL4 with CP110 and CEP97 but did not affect its ability to interact with HERC2. In contrast, the deletion of the region encompassing NHR5–6 abrogated binding to HERC2 (Fig. 4C) while retaining some association of NEURL4 with CP110 or CEP97. These results indicate that NEURL4 utilizes different NHR-containing regions for binding to HERC2 and CP110/CEP97 and further suggest that the interaction between HERC2 and NEURL4 is critical for modulating centrosome morphology, because the GFP-NEURL4 ΔNHR5–6 mutant failed to rescue the NEURL4 depletion phenotype (Fig. 4B).
In parallel, we also examined the subcellular localization of GFP-tagged NEURL4 mutants. Consistent with the rescue experiments, we observed that GFP-tagged NEURL4 and NEURL4 ΔNHR3–4 both localized to centrosomes upon tetracycline induction in U2OS cells (Fig. 4D). By contrast, NEURL4 ΔNHR5–6 failed to localize to centrosomes and instead accumulated in discrete perinuclear structures (Fig. 4D). Taken together, these results suggest that NEURL4 localization to the centrosome is independent of its ability to bind CP110 and CEP97, but it requires its NHR5–6-containing region.
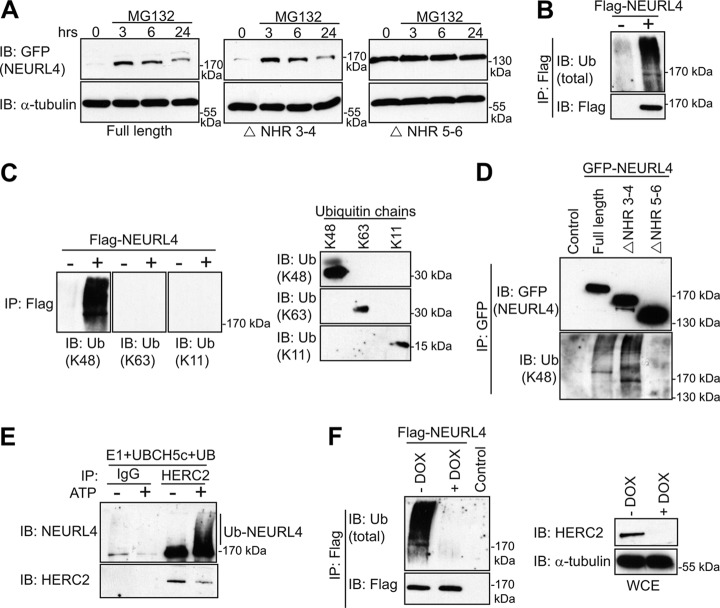
NEURL4 Is Ubiquitylated, and Protein Levels Are Regulated by the Proteasome
The observation that GFP-NEURL4 ΔNHR5–6 exhibited higher protein expression levels than wild type or NEURL4 ΔNHR3–4 (Fig. 4, A and C) was particularly intriguing because all of the proteins were expressed from the same genomic location in the U2OS Flp-In cell lines. We surmised that the elevated expression of the NEURL4 ΔNHR5–6 might be due to the loss of a regulatory mechanism that restricts its expression level. Given that the NHR5–6 region is necessary for the interaction with HERC2, an E3 ligase, we first examined whether NEURL4 steady-state levels were regulated by the proteasome. We treated the U2OS stable cell lines with the proteasome inhibitor MG132 (46) and monitored GFP-NEURL4 expression levels at various time points (Fig. 5A). Although MG132 treatment caused a rapid increase in GFP-NEURL4 and GFP-NEURL4 ΔNHR3–4 levels, followed by a decrease 24 h post-treatment, it had little to no effect on the levels of GFP-NEURL4 ΔNHR5–6, which remained high at each time point. This result suggested that the NEURL4 ΔNHR5–6 mutant might not be targeted to the proteasome. In further support of the possibility that NEURL4 is regulated by proteasomal degradation, we detected robust ubiquitylation of a FLAG-NEURL4 protein in HEK293T cells (Fig. 5B). FLAG-NEURL4 is solely modified by K48-linked ubiquitin chains because immunoprecipitated FLAG-NEURL4 was labeled with a linkage-specific antibody against K48-linked chains but not with antibodies against K11- or K63-linked ubiquitin chains (Fig. 5C, left panel) even though all three antibodies could recognize their cognate-purified chains (Fig. 5C, right panel). To determine which NHR domains of NEURL4 are required for ubiquitylation, we examined the ubiquitylation status of the various GFP-NEURL4 mutants. We observed that GFP-NEURL4 and GFP-NEURL4 ΔNHR3–4 were ubiquitylated with K48-linked ubiquitin chains, but we failed to detect any ubiquitylation on GFP-NEURL4 ΔNHR5–6 (Fig. 5D). Considering our previous observation that the NEURL4 interaction with HERC2 is mediated through binding to the NHR5–6 region (Fig. 4C), this result raised the possibility that NEURL4 is ubiquitylated in a HERC2-dependent manner. To test this hypothesis, we carried out in vitro ubiquitylation assays on the immunopurified NEURL4-HERC2 complex by adding E1, the E2 UBCH5c, and ATP. We observed robust ubiquitylation of NEURL4 as indicated by a characteristic ATP-dependent accumulation of higher molecular mass species of NEURL4 (Fig. 5E). Finally, to test whether HERC2 is necessary for NEURL4 ubiquitylation in vivo, we took advantage of a U2OS cell line containing a stably integrated doxycycline-inducible small hairpin RNA against HERC2 (shHERC2) developed by Bekker-Jensen et al. (20). Using this cell line, we observed that depletion of endogenous HERC2 results in a dramatic decrease in FLAG-NEURL4 ubiquitylation, specifically K48-linked chains (Fig. 5F and supplemental Fig. S4B). Together, these results indicate that NEURL4 is ubiquitylated in a HERC2-dependent manner.
Fig. 5.
NEURL4 is ubiquitylated in a HERC2-dependent manner. A, expression of the GFP-tagged NEURL4, NEURL4 ΔNHR3–4, and NEURL4 ΔNHR5–6 was induced with tetracycline in the U2OS stable cells lines. 24 h post-induction, the cells were treated with 10 μm MG132. The cells were lysed at 3, 6, and 24 h post-MG132 additions. The protein levels of NEURL4 were determined by immunoblotting with the GFP antibody, and α-tubulin immunoblot was performed as a loading control. B, FLAG-NEURL4 protein was immunoprecipitated from HEK293T cells and immunoblotted with total ubiquitin antibody. As a control, we performed the experiment in parallel with cells transfected with an empty vector (right panel). C, left panel, as in B but immunoblotted with antibodies specific to K11, K48, and K63 ubiquitin chains. Right panel, 250 ng each of tetra-ubiquitin K48, tetra-ubiquitin K63, or di-ubiquitin K11 were subjected to SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with ubiquitin antibodies specific for K48, K11, or K63 ubiquitin chains. D, WCEs from U2OS cell lines stably expressing different GFP-NEURL4 constructs were subjected to immunoprecipitation using the GFP antibody. Immunoprecipitated proteins were analyzed by SDS-PAGE and immunoblotted with GFP antibody or ubiquitin antibody specific to K48 polyubiquitin chains. E, endogenously expressed HERC2 was immunopurified from U2OS cells using HERC2 antibody conjugated to protein G-Sepharose. Although the protein was still on the resin, UBE1, UBCH5c, and ATP were added to initiate the ubiquitylation assay. The assay was terminated by the addition of SDS buffer, and the eluted proteins were subjected to immunoblot analysis with antibodies against NEURL4 and HERC2. As a control, IP was performed in parallel with a nonspecific rabbit IgG. F, U2OS cells stably expressing doxycycline-inducible shHERC2 were transfected with FLAG-NEURL4. 96 h post-shHERC2 induction, the cells were lysed, subjected to FLAG-IP, and immunoblotted with antibodies against FLAG and total ubiquitin. As a control, the IP was performed in parallel with cells transfected with an empty vector. The efficiency of knockdown and loading controls are shown in the lower panel. The protein size (kDa) is indicated for each immunoblot. E and F, HERC2 is a 528-kDa protein and runs as a very high molecular mass band, and there is no molecular mass standard in that region of the gel. IB, immunoblot; Ub, ubiquitin; DOX, doxycycline.
DISCUSSION
Using an AP/MS approach, we identified NEURL4 as a novel interactor of CP110 and confirmed previously known interactions between CP110, CEP290, CEP97, and, to a lesser degree, CEP76 (Fig. 1A, Table I, and supplemental Table S1). Although depletion of CP110 and CEP97 leads to the formation of overly long centrioles, depletion of different CP110-interacting proteins yield clearly distinguishable phenotypes. Indeed, whereas CEP76 depletion induces centriole overduplication, CEP290 depletion perturbs primary cilia assembly (16, 18). Here, we report that NEURL4 depletion does not cause the characteristic elongated centriole structures observed upon CP110 and CEP97 depletion in mammalian cells. Additionally, we did not detect major defects in PCM recruitment upon depletion of NEURL4, as was previously observed in Drosophila cells upon treatment with CEP97 and CP110 RNAi (47). In addition, NEURL4 knockdown does not appear to have any effect on the centriole duplication cycle (data not shown). Instead, NEURL4 depletion alters centrosome morphology by inducing the appearance of filamentous structures that label for pericentrin and CEP135. Together, these results suggest that we have identified another CP110-interacting protein that regulates an additional facet of centrosome biogenesis.
Interestingly, analysis of PCM morphology revealed that the filamentous structures observed upon NEURL4 depletion do not contain components of the γ-tubulin ring complex or its recruiting factor NEDD1 (supplemental Fig. S4, C and D) (48–52). Considering the well established role of NEDD1 and γ-tubulin ring complexes in microtubule nucleation, and our inability to detect major changes in the microtubule landscape of NEURL4 RNAi-treated cells (supplemental Fig. S6), it seems unlikely that the change in centrosome morphology observed following NEURL4 depletion affects the microtubule nucleation capacity of interphase centrosomes. The change in centrosome morphology also does not seem to noticeably alter the global architecture of the actin cytoskeleton (supplemental Fig. S6A). It will be interesting to determine whether, during mitosis, when centrosomes are under increased mechanical stress, the NEURL4-associated morphological changes translate into spindle assembly defects or genome instability. This would be consistent with previous work suggesting that pericentrin and its associated proteins act as a scaffold for other centrosomal proteins (53). Another possibility could be that NEURL4/HERC2 is implicated in the regulation of other centrosome-related processes. HERC2 has been implicated in the regulation of the DNA damage checkpoint and was shown to physically associate with ATM (Ataxia Telangiectasia Mutated), ATR (ATM- and RAD3-related), and DNA-PK (DNA-dependent protein kinase), three major regulators of the DNA damage response (20). Interestingly, mutations have been identified in pericentrin that can cause Seckel syndrome through defects in ATR signaling (54). It is therefore tempting to speculate that changes in centrosome architecture might modulate the kinetics of ATR-dependent DNA damage response.
The function of NEURL4 in modulating centrosome morphology appears to be dependent on its association with the giant HECT-type E3 ligase HERC2. Indeed, HERC2 depletion induced morphological changes in the PCM indistinguishable from those observed upon NEURL4 depletion. We have shown that both proteins localize to the centrosome, which is consistent with a role in maintaining centrosome architecture. Furthermore, the deletion of the NHR 5–6 region of NEURL4, which disrupts its interaction with HERC2, results in a protein that fails to promote wild type PCM morphology while at the same time uncoupling NEURL4 from its regulation by the proteasome. Together, the available data led us to propose a model by which the HERC2-NEURL4 complex regulates PCM architecture. Because it is possible that NEURL4 protein levels are key for this regulation, we tested the hypothesis that reducing HERC2 protein levels would stabilize NEURL4 protein levels by preventing its ubiquitylation and subsequent degradation. We found that HERC2 siRNA instead caused a reduction in NEURL4 protein levels (data not shown). Our interpretation of this result is that NEURL4 is more stable when present in a complex with HERC2 and that it is destabilized in absence of HERC2. This observation makes it difficult for us to unambiguously determine whether HERC2 ubiquitylation directly regulates NEURL4 protein level and suggests that HERC2-dependent NEURL4 ubiquitylation may serve a different purpose than regulating NEURL4 levels. One such possibility is that NEURL4 acts as a substrate adaptor for HERC2. This possibility is attractive given the presence of NHR domains in other E3 ligases and the observation that the levels of substrate adaptors of cullin-RING-ligases are also regulated by ubiquitylation (55). In the future, it will be interesting to further explore this possibility because our results indicate that CP110 or associated proteins are unlikely to be the centrosomal targets of HERC2 activity. Nevertheless, to our knowledge, HERC2 is the first HECT family E3 ligase implicated in the regulation of PCM architecture.
Acknowledgments
We thank Rachel Szilard and Christina Yeh for critical reading of the manuscript; Alex Bird, Erich Nigg, Jeff Salisbury, and David Drechsel for kindly providing crucial antibodies; and Neils Mailand for the kind gift of the shHERC2 stable cell line. We also thank Brett Larsen for providing technical help and advice with mass spectrometry and Christine Holley and Andrea Tagliaferro for help with esiRNA production.
Footnotes
* This work was funded by a grant-in-aid of the Krembil Foundation (to L. P. and D. D.), the Cancer Research Society (to L. P.), and the CIHR MOP-84314 (to A.-C. G). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
 This article contains supplemental material.
This article contains supplemental material.
1 The abbreviations used are:
- PCM
- pericentriolar material
- AP/MS
- affinity purification followed by mass spectrometry
- esiRNA
- endoribonuclease-prepared siRNA
- FSG
- gelatin from cold water fish skin
- GFP
- green fluorescent protein
- HEK293T
- human embryonic kidney 293T
- IP
- immunoprecipitation
- K11
- lysine 11
- K48
- lysine 48
- K63
- lysine 63
- NHR
- neuralized homology region
- RNAi
- RNA interference
- U2OS
- U2 osteosarcoma
- WCE
- whole cell extract
- E1
- ubiquitin-activating enzyme
- E2
- ubiquitin-conjugating enzyme
- E3
- ubiquitin-protein ligase.
REFERENCES
- 1. Andersen J. S., Wilkinson C. J., Mayor T., Mortensen P., Nigg E. A., Mann M. (2003) Proteomic characterization of the human centrosome by protein correlation profiling. Nature 426, 570–574 [DOI] [PubMed] [Google Scholar]
- 2. Nigg E. A., Raff J. W. (2009) Centrioles, centrosomes, and cilia in health and disease. Cell 139, 663–678 [DOI] [PubMed] [Google Scholar]
- 3. Palazzo R. E., Vogel J. M., Schnackenberg B. J., Hull D. R., Wu X. (2000) Centrosome maturation. Curr. Top. Dev. Biol. 49, 449–470 [DOI] [PubMed] [Google Scholar]
- 4. Conduit P. T., Brunk K., Dobbelaere J., Dix C. I., Lucas E. P., Raff J. W. (2010) Centrioles regulate centrosome size by controlling the rate of Cnn incorporation into the PCM. Curr. Biol. 20, 2178–2186 [DOI] [PubMed] [Google Scholar]
- 5. Decker M., Jaensch S., Pozniakovsky A., Zinke A., O'Connell K. F., Zachariae W., Myers E., Hyman A. A. (2011) Limiting amounts of centrosome material set centrosome size in C. elegans embryos. Curr. Biol. 21, 1259–1267 [DOI] [PubMed] [Google Scholar]
- 6. Pickart C. M. (2001) Mechanisms underlying ubiquitination. Annu. Rev. Biochem. 70, 503–533 [DOI] [PubMed] [Google Scholar]
- 7. Behrends C., Harper J. W. (2011) Constructing and decoding unconventional ubiquitin chains. Nat. Struct. Mol. Biol. 18, 520–528 [DOI] [PubMed] [Google Scholar]
- 8. Al-Hakim A., Escribano-Diaz C., Landry M. C., O'Donnell L., Panier S., Szilard R. K., Durocher D. (2010) The ubiquitous role of ubiquitin in the DNA damage response. DNA Repair 9, 1229–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Strnad P., Leidel S., Vinogradova T., Euteneuer U., Khodjakov A., Gönczy P. (2007) Regulated HsSAS-6 levels ensure formation of a single procentriole per centriole during the centrosome duplication cycle. Dev. Cell 13, 203–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Freed E., Lacey K. R., Huie P., Lyapina S. A., Deshaies R. J., Stearns T., Jackson P. K. (1999) Components of an SCF ubiquitin ligase localize to the centrosome and regulate the centrosome duplication cycle. Genes Dev. 13, 2242–2257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nakayama K., Nagahama H., Minamishima Y. A., Matsumoto M., Nakamichi I., Kitagawa K., Shirane M., Tsunematsu R., Tsukiyama T., Ishida N., Kitagawa M., Hatakeyama S. (2000) Targeted disruption of Skp2 results in accumulation of cyclin E and p27(Kip1), polyploidy and centrosome overduplication. EMBO J. 19, 2069–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Puklowski A., Homsi Y., Keller D., May M., Chauhan S., Kossatz U., Grünwald V., Kubicka S., Pich A., Manns M. P., Hoffmann I., Gönczy P., Malek N. P. (2011) The SCF-FBXW5 E3-ubiquitin ligase is regulated by PLK4 and targets HsSAS-6 to control centrosome duplication. Nat. Cell Biol. 13, 1004–1009 [DOI] [PubMed] [Google Scholar]
- 13. D'Angiolella V., Donato V., Vijayakumar S., Saraf A., Florens L., Washburn M. P., Dynlacht B., Pagano M. (2010) SCF(Cyclin F) controls centrosome homeostasis and mitotic fidelity through CP110 degradation. Nature 466, 138–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen Z., Indjeian V. B., McManus M., Wang L., Dynlacht B. D. (2002) CP110, a cell cycle-dependent CDK substrate, regulates centrosome duplication in human cells. Dev. Cell 3, 339–350 [DOI] [PubMed] [Google Scholar]
- 15. Spektor A., Tsang W. Y., Khoo D., Dynlacht B. D. (2007) Cep97 and CP110 suppress a cilia assembly program. Cell 130, 678–690 [DOI] [PubMed] [Google Scholar]
- 16. Tsang W. Y., Bossard C., Khanna H., Peränen J., Swaroop A., Malhotra V., Dynlacht B. D. (2008) CP110 suppresses primary cilia formation through its interaction with CEP290, a protein deficient in human ciliary disease. Dev. Cell 15, 187–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tsang W. Y., Spektor A., Luciano D. J., Indjeian V. B., Chen Z., Salisbury J. L., Sánchez I., Dynlacht B. D. (2006) CP110 cooperates with two calcium-binding proteins to regulate cytokinesis and genome stability. Mol. Biol. Cell 17, 3423–3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tsang W. Y., Spektor A., Vijayakumar S., Bista B. R., Li J., Sanchez I., Duensing S., Dynlacht B. D. (2009) Cep76, a centrosomal protein that specifically restrains centriole reduplication. Dev. Cell 16, 649–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stewart G. S., Panier S., Townsend K., Al-Hakim A. K., Kolas N. K., Miller E. S., Nakada S., Ylanko J., Olivarius S., Mendez M., Oldreive C., Wildenhain J., Tagliaferro A., Pelletier L., Taubenheim N., Durandy A., Byrd P. J., Stankovic T., Taylor A. M., Durocher D. (2009) The RIDDLE syndrome protein mediates a ubiquitin-dependent signaling cascade at sites of DNA damage. Cell 136, 420–434 [DOI] [PubMed] [Google Scholar]
- 20. Bekker-Jensen S., Rendtlew Danielsen J., Fugger K., Gromova I., Nerstedt A., Lukas C., Bartek J., Lukas J., Mailand N. (2010) HERC2 coordinates ubiquitin-dependent assembly of DNA repair factors on damaged chromosomes. Nat. Cell Biol. 12, 80–86 [DOI] [PubMed] [Google Scholar]
- 21. Kittler R., Heninger A. K., Franke K., Habermann B., Buchholz F. (2005) Production of endoribonuclease-prepared short interfering RNAs for gene silencing in mammalian cells. Nat. Methods 2, 779–784 [DOI] [PubMed] [Google Scholar]
- 22. Lawo S., Bashkurov M., Mullin M., Ferreria M. G., Kittler R., Habermann B., Tagliaferro A., Poser I., Hutchins J. R., Hegemann B., Pinchev D., Buchholz F., Peters J. M., Hyman A. A., Gingras A. C., Pelletier L. (2009) HAUS, the 8-subunit human Augmin complex, regulates centrosome and spindle integrity. Curr. Biol. 19, 816–826 [DOI] [PubMed] [Google Scholar]
- 23. Zhu F., Lawo S., Bird A., Pinchev D., Ralph A., Richter C., Müller-Reichert T., Kittler R., Hyman A. A., Pelletier L. (2008) The mammalian SPD-2 ortholog Cep192 regulates centrosome biogenesis. Curr. Biol. 18, 136–141 [DOI] [PubMed] [Google Scholar]
- 24. Sanders M. A., Salisbury J. L. (1994) Centrin plays an essential role in microtubule severing during flagellar excision in Chlamydomonas reinhardtii. J. Cell Biol. 124, 795–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kleylein-Sohn J., Westendorf J., Le Clech M., Habedanck R., Stierhof Y. D., Nigg E. A. (2007) Plk4-induced centriole biogenesis in human cells. Dev. Cell 13, 190–202 [DOI] [PubMed] [Google Scholar]
- 26. Bird A. W., Hyman A. A. (2008) Building a spindle of the correct length in human cells requires the interaction between TPX2 and Aurora A. J. Cell Biol. 182, 289–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schermelleh L., Carlton P. M., Haase S., Shao L., Winoto L., Kner P., Burke B., Cardoso M. C., Agard D. A., Gustafsson M. G., Leonhardt H., Sedat J. W. (2008) Subdiffraction multicolor imaging of the nuclear periphery with 3D structured illumination microscopy. Science 320, 1332–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Durocher Y., Perret S., Kamen A. (2002) High-level and high-throughput recombinant protein production by transient transfection of suspension-growing human 293-EBNA1 cells. Nucleic Acids Res. 30, E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nakada S., Tai I., Panier S., Al-Hakim A., Iemura S., Juang Y. C., O'Donnell L., Kumakubo A., Munro M., Sicheri F., Gingras A. C., Natsume T., Suda T., Durocher D. (2010) Non-canonical inhibition of DNA damage-dependent ubiquitination by OTUB1. Nature 466, 941–946 [DOI] [PubMed] [Google Scholar]
- 30. O'Donnell L., Panier S., Wildenhain J., Tkach J. M., Al-Hakim A., Landry M. C., Escribano-Diaz C., Szilard R. K., Young J. T., Munro M., Canny M. D., Kolas N. K., Zhang W., Harding S. M., Ylanko J., Mendez M., Mullin M., Sun T., Habermann B., Datti A., Bristow R. G., Gingras A. C., Tyers M. D., Brown G. W., Durocher D. (2010) The MMS22L-TONSL complex mediates recovery from replication stress and homologous recombination. Mol. Cell 40, 619–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kean M. J., Ceccarelli D. F., Goudreault M., Sanches M., Tate S., Larsen B., Gibson L. C., Derry W. B., Scott I. C., Pelletier L., Baillie G. S., Sicheri F., Gingras A. C. (2011) Structure-function analysis of core STRIPAK proteins: A signaling complex implicated in Golgi polarization. J. Biol. Chem. 286, 25065–25075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goudreault M., D'Ambrosio L. M., Kean M. J., Mullin M. J., Larsen B. G., Sanchez A., Chaudhry S., Chen G. I., Sicheri F., Nesvizhskii A. I., Aebersold R., Raught B., Gingras A. C. (2009) A PP2A phosphatase high density interaction network identifies a novel striatin-interacting phosphatase and kinase complex linked to the cerebral cavernous malformation 3 (CCM3) protein. Mol. Cell Proteomics 8, 157–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kessner D., Chambers M., Burke R., Agus D., Mallick P. (2008) ProteoWizard: Open source software for rapid proteomics tools development. Bioinformatics 24, 2534–2536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu G., Zhang J., Larsen B., Stark C., Breitkreutz A., Lin Z. Y., Breitkreutz B. J., Ding Y., Colwill K., Pasculescu A., Pawson T., Wrana J. L., Nesvizhskii A. I., Raught B., Tyers M., Gingras A. C. (2010) ProHits: Integrated software for mass spectrometry-based interaction proteomics. Nat. Biotechnol. 28, 1015–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lane H. (1988) Antibodies: A Laboratory Manual, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 36. Matsumoto M. L., Wickliffe K. E., Dong K. C., Yu C., Bosanac I., Bustos D., Phu L., Kirkpatrick D. S., Hymowitz S. G., Rape M., Kelley R. F., Dixit V. M. (2010) K11-linked polyubiquitination in cell cycle control revealed by a K11 linkage-specific antibody. Mol. Cell 39, 477–484 [DOI] [PubMed] [Google Scholar]
- 37. Garcia-Gonzalo F. R., Rosa J. L. (2005) The HERC proteins: Functional and evolutionary insights. Cell Mol. Life Sci. 62, 1826–1838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dasso M. (1993) RCC1 in the cell cycle: The regulator of chromosome condensation takes on new roles. Trends Biochem. Sci. 18, 96–101 [DOI] [PubMed] [Google Scholar]
- 39. Seki T., Hayashi N., Nishimoto T. (1996) RCC1 in the Ran pathway. J. Biochem. 120, 207–214 [DOI] [PubMed] [Google Scholar]
- 40. Kang T. H., Lindsey-Boltz L. A., Reardon J. T., Sancar A. (2010) Circadian control of XPA and excision repair of cisplatin-DNA damage by cryptochrome and HERC2 ubiquitin ligase. Proc. Natl. Acad. Sci. U.S.A. 107, 4890–4895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wu W., Sato K., Koike A., Nishikawa H., Koizumi H., Venkitaraman A. R., Ohta T. (2010) HERC2 is an E3 ligase that targets BRCA1 for degradation. Cancer Res. 70, 6384–6392 [DOI] [PubMed] [Google Scholar]
- 42. Izawa N., Wu W., Sato K., Nishikawa H., Kato A., Boku N., Itoh F., Ohta T. (2011) HERC2 interacts with claspin and regulates DNA origin firing and replication fork progression. Cancer Res. 71, 5621–5625 [DOI] [PubMed] [Google Scholar]
- 43. Kile B. T., Schulman B. A., Alexander W. S., Nicola N. A., Martin H. M., Hilton D. J. (2002) The SOCS box: A tale of destruction and degradation. Trends Biochem. Sci. 27, 235–241 [DOI] [PubMed] [Google Scholar]
- 44. Graser S., Stierhof Y. D., Lavoie S. B., Gassner O. S., Lamla S., Le Clech M., Nigg E. A. (2007) Cep164, a novel centriole appendage protein required for primary cilium formation. J. Cell Biol. 179, 321–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kohlmaier G., Loncarek J., Meng X., McEwen B. F., Mogensen M. M., Spektor A., Dynlacht B. D., Khodjakov A., Gönczy P. (2009) Overly long centrioles and defective cell division upon excess of the SAS-4-related protein CPAP. Curr. Biol. 19, 1012–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lee D. H., Goldberg A. L. (1998) Proteasome inhibitors: Valuable new tools for cell biologists. Trends Cell Biol. 8, 397–403 [DOI] [PubMed] [Google Scholar]
- 47. Dobbelaere J., Josué F., Suijkerbuijk S., Baum B., Tapon N., Raff J. (2008) A genome-wide RNAi screen to dissect centriole duplication and centrosome maturation in Drosophila. PLoS Biol. 6, e224–e224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Haren L., Remy M. H., Bazin I., Callebaut I., Wright M., Merdes A. (2006) NEDD1-dependent recruitment of the γ-tubulin ring complex to the centrosome is necessary for centriole duplication and spindle assembly. J. Cell Biol. 172, 505–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lüders J., Patel U. K., Stearns T. (2006) GCP-WD is a γ-tubulin targeting factor required for centrosomal and chromatin-mediated microtubule nucleation. Nat. Cell Biol. 8, 137–147 [DOI] [PubMed] [Google Scholar]
- 50. Fava F., Raynaud-Messina B., Leung-Tack J., Mazzolini L., Li M., Guillemot J. C., Cachot D., Tollon Y., Ferrara P., Wright M. (1999) Human 76p: A new member of the γ-tubulin-associated protein family. J. Cell Biol. 147, 857–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Murphy S. M., Preble A. M., Patel U. K., O'Connell K. L., Dias D. P., Moritz M., Agard D., Stults J. T., Stearns T. (2001) GCP5 and GCP6: Two new members of the human γ-tubulin complex. Mol. Biol. Cell 12, 3340–3352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Murphy S. M., Urbani L., Stearns T. (1998) The mammalian γ-tubulin complex contains homologues of the yeast spindle pole body components spc97p and spc98p. J. Cell Biol. 141, 663–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dictenberg J. B., Zimmerman W., Sparks C. A., Young A., Vidair C., Zheng Y., Carrington W., Fay F. S., Doxsey S. J. (1998) Pericentrin and γ-tubulin form a protein complex and are organized into a novel lattice at the centrosome. J. Cell Biol. 141, 163–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Griffith E., Walker S., Martin C. A., Vagnarelli P., Stiff T., Vernay B., Al Sanna N., Saggar A., Hamel B., Earnshaw W. C., Jeggo P. A., Jackson A. P., O'Driscoll M. (2008) Mutations in pericentrin cause Seckel syndrome with defective ATR-dependent DNA damage signaling. Nat. Genet. 40, 232–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Petroski M. D., Deshaies R. J. (2005) Function and regulation of cullin-RING ubiquitin ligases. Nat. Rev. Mol. Cell Biol. 6, 9–20 [DOI] [PubMed] [Google Scholar]
- 56. Smoot M. E., Ono K., Ruscheinski J., Wang P. L., Ideker T. (2011) Cytoscape 2.8: New features for data integration and network visualization. Bioinformatics 27, 431–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cline M. S., Smoot M., Cerami E., Kuchinsky A., Landys N., Workman C., Christmas R., Avila-Campilo I., Creech M., Gross B., Hanspers K., Isserlin R., Kelley R., Killcoyne S., Lotia S., Maere S., Morris J., Ono K., Pavlovic V., Pico A. R., Vailaya A., Wang P. L., Adler A., Conklin B. R., Hood L., Kuiper M., Sander C., Schmulevich I., Schwikowski B., Warner G. J., Ideker T., Bader G. D. (2007) Integration of biological networks and gene expression data using Cytoscape. Nat. Protoc. 2, 2366–2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Shannon P., Markiel A., Ozier O., Baliga N. S., Wang J. T., Ramage D., Amin N., Schwikowski B., Ideker T. (2003) Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 [DOI] [PMC free article] [PubMed] [Google Scholar]