Abstract
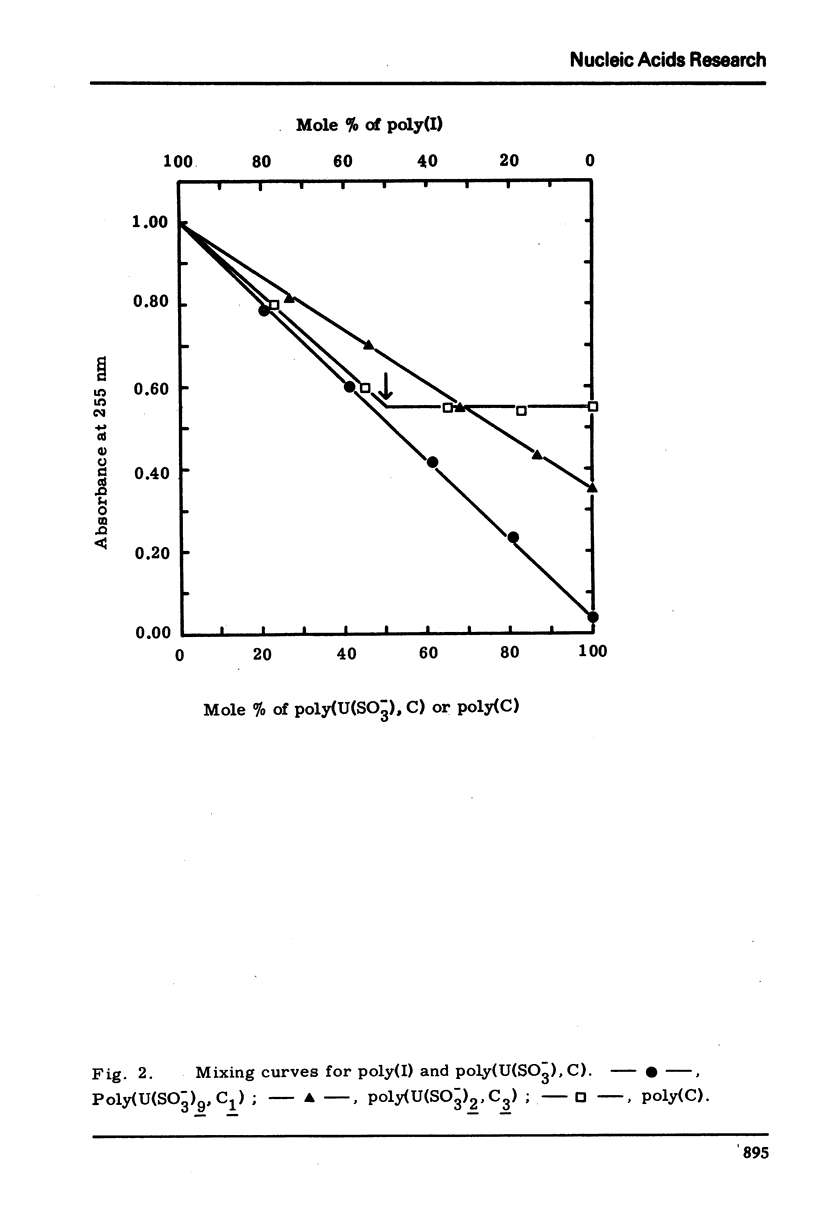
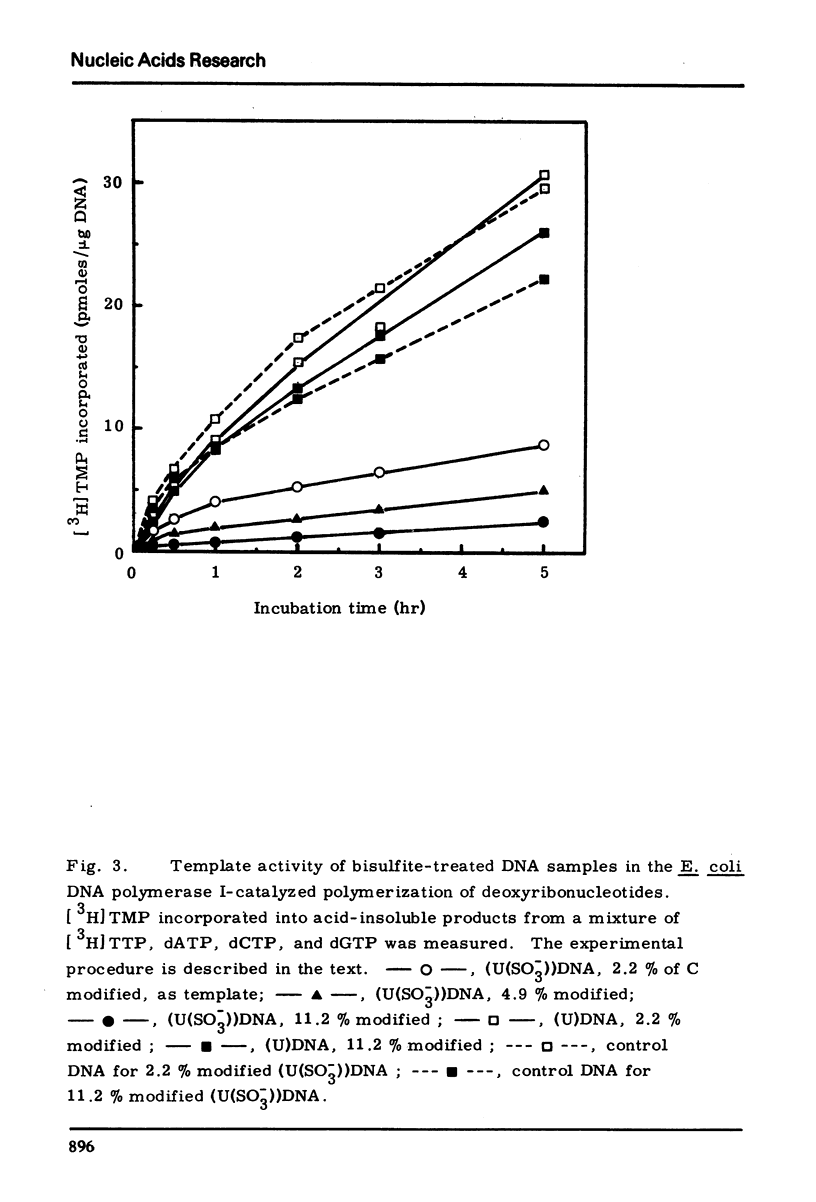
Cytosine residues of poly(C) and heat-denatured calf thymus DNA were transformed into 5,6-dihydrouracil-6-sulfonate (U(SO−3)) residues by treatment with bisulfite. The poly(U(SO−3)2, C3) and poly(U(SO−3)9, C1) prepared did not form inter-base binding with either poly(A) or poly(I) as judged by the absence of hypochromicity in ultraviolet absorbance. U(SO−3) residues in the DNA inactivated it to serve as template for E.coli DNA polymerase I, while the template activity was restored by conversion of the U(SO−3) residues into U.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorange J. L., Dupuy P. Mise en évidence d'une action mutagène du sulfite de sodium sur la levure. C R Acad Sci Hebd Seances Acad Sci D. 1972 May 15;274(20):2798–2800. [PubMed] [Google Scholar]
- Hayatsu H., Miller R. C., Jr The cleavage of DNA by the oxygen-dependent reaction of bisulfite. Biochem Biophys Res Commun. 1972 Jan 14;46(1):120–124. doi: 10.1016/0006-291x(72)90638-9. [DOI] [PubMed] [Google Scholar]
- Hayatsu H., Miura A. The mutagenic action of sodium bisulfite. Biochem Biophys Res Commun. 1970 Apr 8;39(1):156–160. doi: 10.1016/0006-291x(70)90771-0. [DOI] [PubMed] [Google Scholar]
- Hayatsu H., Wataya Y., Kai K., Iida S. Reaction of sodium bisulfite with uracil, cytosine, and their derivatives. Biochemistry. 1970 Jul 7;9(14):2858–2865. doi: 10.1021/bi00816a016. [DOI] [PubMed] [Google Scholar]
- Hayatsu H., Wataya Y., Kazushige K. The addition of sodium bisulfite to uracil and to cytosine. J Am Chem Soc. 1970 Feb 11;92(3):724–726. doi: 10.1021/ja00706a062. [DOI] [PubMed] [Google Scholar]
- Inoue M., Hayatsu H., Tanooka H. Concentration effect of bisulfite on the inactivation of transforming activity of DNA. Chem Biol Interact. 1972 Jul;5(2):85–95. doi: 10.1016/0009-2797(72)90035-x. [DOI] [PubMed] [Google Scholar]
- Jovin T. M., Englund P. T., Bertsch L. L. Enzymatic synthesis of deoxyribonucleic acid. XXVI. Physical and chemical studies of a homogeneous deoxyribonucleic acid polymerase. J Biol Chem. 1969 Jun 10;244(11):2996–3008. [PubMed] [Google Scholar]
- KOTAKA T., BALDWIN R. L. EFFECTS OF NITROUS ACID ON THE DAT COPOLYMER AS A TEMPLATE FOR DNA POLYMERASE. J Mol Biol. 1964 Aug;9:323–339. doi: 10.1016/s0022-2836(64)80210-2. [DOI] [PubMed] [Google Scholar]
- Kitamura N., Hayatsu H. Cleavage of the glycosidic linkage of pyrimidine ribonucleosides by the bisulfite-oxygen system. Nucleic Acids Res. 1974 Jan;1(1):75–86. doi: 10.1093/nar/1.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEHMAN I. R., BESSMAN M. J., SIMMS E. S., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. I. Preparation of substrates and partial purification of an enzyme from Escherichia coli. J Biol Chem. 1958 Jul;233(1):163–170. [PubMed] [Google Scholar]
- Mukai F., Hawryluk I., Shapiro R. The mutagenic specificity of sodium bisulfite. Biochem Biophys Res Commun. 1970 Jun 5;39(5):983–988. doi: 10.1016/0006-291x(70)90421-3. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Braverman B. Modification of polyuridylic acid by bisulfite: effect on double helix formation and coding properties. Biochem Biophys Res Commun. 1972 May 12;47(3):544–550. doi: 10.1016/0006-291x(72)90913-8. [DOI] [PubMed] [Google Scholar]
- Shapiro R., DiFate V., Welcher M. Deamination of cytosine derivatives by bisulfite. Mechanism of the reaction. J Am Chem Soc. 1974 Feb 6;96(3):906–912. doi: 10.1021/ja00810a043. [DOI] [PubMed] [Google Scholar]
- Sono M., Wataya Y., Hayatsu H. Role of bisulfite in the deamination and the hydrogen isotope exchange of cytidylic acid. J Am Chem Soc. 1973 Jul 11;95(14):4745–4749. doi: 10.1021/ja00795a044. [DOI] [PubMed] [Google Scholar]
- Summers G. A., Drake J. W. Bisulfite mutagenesis in bacteriophage T4. Genetics. 1971 Aug;68(4):603–607. doi: 10.1093/genetics/68.4.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRAUTNER T. A., SWARTZ M. N., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. X. Influence of bromouracil substitutions on replication. Proc Natl Acad Sci U S A. 1962 Mar 15;48:449–455. doi: 10.1073/pnas.48.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]



