Abstract
Kidney dysfunction is a common clinical feature of symptomatic multiple myeloma. Some degree of renal insufficiency or renal failure is present at diagnosis or will occur during the course of the disease, and which, if not reversed, will adversely effect overall survival and quality of life. Chronic insults to the kidneys from other illnesses, treatment, or multiple myeloma itself can further damage renal function and increase the risk for additional complications, such as anemia. Patients with multiple myeloma who have light chain (Bence Jones protein) proteinuria may experience renal failure or progress to end-stage renal disease (ESRD) and require dialysis due to light chain cast nephropathy. Kidney failure in patients with presumed multiple myeloma may also result from amyloidosis, light chain deposition disease, or acute tubular necrosis caused by nephrotoxic agents; therefore identification of patients at risk for kidney damage is essential. The International Myeloma Foundation’s Nurse Leadership Board have developed these practice recommendations for screening for renal function, identifying positive and negative contributing risk and environmental factors, selecting appropriate therapies and supportive care measures to decrease progression to ESRD and dialysis, and reducing and managing renal complications in patients with multiple myeloma.
Renal dysfunction is one of the common clinical features of symptomatic multiple myeloma at presentation or throughout the course of the disease. In addition to renal dysfunction or some degree of renal insufficiency, other clinical features of multiple myeloma include bone pain, fatigue, recurrent infections, anemia, and hypercalcemia. (Rajkumar & Dispenzieri, 2008; Rajkumar & Kyle, 2005) Studies have shown that the presence of renal failure indicates a higher tumor burden and consequently more aggressive disease. (Dimopoulos, Kastritis, Rosinol, Blade, & Ludwig, 2008) Thus, patients who are diagnosed with renal insufficiency should be treated aggressively because reversal of this condition results in survival outcomes similar to those of patients who have normal renal function at diagnosis. (Tariman & Faiman, 2011; Rajkumar & Kyle, 2005)
Patients may experience several types of renal failure. The National Kidney Foundation (NKF) Clinical Practice Guidelines (NKF 2002) define renal failure as serum creatinine levels of greater that 3.0 mg/dL. At least 20% to 60% of patients will present with renal insufficiency or renal failure throughout their disease, (Tariman & Faiman, 2011; Blade et al., 1998) which in turn may negatively affect survival if not reversed. In addition, chronic insults to the kidneys from other illnesses, treatment, or the myeloma itself may have further negative impact on renal function.
The cost of care for patients with multiple myeloma is enormous. (van Agthoven et al., 2004; Cook, 2008) Costs associated with patients in the United States who require dialysis are difficult to quantify; however, an in-house audit of patients requiring chronic dialysis indicated that treatment of patients with myeloma who have kidney dysfunction is 5% to 33% more expensive than treatment of those patients without myeloma. The extra cost is attributed primarily to the greater number and longer duration of hospital admissions for infection. (Coward, 1989)
Conditions that complicate the treatment of patients with multiple myeloma include older age at presentation and multiple coexisting morbidities, such as hypertension, diabetes, or other chronic health conditions. (Rajkumar & Dispenzieri, 2008) Patients may experience renal failure or progress to end-stage renal disease (ESRD) and dialysis due to light chain cast nephropathy. Kidney failure in patients with a presumptive diagnosis of multiple myeloma may also be a result of light chain amyloidosis (AL), light chain deposition disease (LCDD), monoclonal immunoglobulin deposition disease (MIDD), or acute tubular necrosis (ATN) from the use of nephrotoxic agents in the setting of monoclonal gammopathy. (Leung et al., 2008) Although renal disease in patients with multiple myeloma is heterogeneous, careful attention must be paid when selecting an appropriate treatment to decrease progression to ESRD and dialysis, which are associated with shortened overall survival. (Blade et al., 1998; Blade & Rosinol, 2005).
Clinicians have the ability to identify patients at risk for kidney damage as a result of multiple myeloma and to institute preventive and therapeutic interventions; however, long-term negative effects may still result. Our goal is to describe the impact of and screening for kidney disease, examine contributing risk and environmental factors that may affect renal function, and provide recommendations to reduce and manage renal complications in patients with multiple myeloma. Adequate screening for acute or insidious development of kidney dysfunction is essential to these patients for early intervention to prevent long-term complications.
Clinical Presentations of Renal Insufficiency
Renal insufficiency is characterized by an elevated serum creatinine. Other associated signs and symptoms include anemia, fatigue, fluid and electrolyte imbalances, and light chain proteinuria. In most studies, a creatinine of 2 mg/dL is used to define the presence of renal dysfunction in newly diagnosed patients with multiple myeloma. (Rajkumar & Dispenzieri, 2008) Reduced glomerular filtration rate (GFR) of less than 60 mL/min per 1.73 m2 of body surface area calculated using the Modification of Diet in Renal Disease formula is indicative of renal dysfunction and is considered a clinically acceptable method of measurement (Dimopoulos et al., 2010; Kooman, 2009). Temporary elevations in serum creatinine or decline in GFR may be seen in patients experiencing acute dehydration as a result of nausea and vomiting, infection, hypercalcemia or non-steroidal anti-inflammatory drugs (Knudsen, Hjorth, Hippe, & The Nordic Myeloma Study Group, 2000).
Clinical presentations largely depend on the pathogenesis of renal dysfunction. For example, nephrotic range proteinuria without significant renal impairment, orthostatic hypotension, and thickening of cardiac walls may indicate systemic amyloidosis. (Rajkumar & Dispenzieri, 2008) Immunofixation showing a monoclonal protein is strong evidence of AL or LCDD in the presence of nephrotic syndrome. (Gertz, 2002; Leung et al., 2008)
In recent years a nephelometric assay for serum free light chains (sFLC) has been used as a quantitative marker in the diagnosis and evaluation of patients with non- secretory multiple myeloma. In patients with non-secretory multiple myeloma, measurable amounts of monoclonal protein are not secreted in the serum and/or urine, which provides a challenge for monitoring disease status. The sFLC assay relies on an imbalance between kappa and lambda light chains and is a surrogate marker for monoclonality. (Bradwell et al., 2009; Dispenzieri et al, 2009) Several studies have evaluated the validity of the sFLC assay since its inception. One group was able to identify a light chain imbalance in 19 out of 28 patients with non-secretory myeloma. (Drayson et al., 2001) In addition, abnormal sFLC ratios have been linked to a higher risk of progression from smoldering or asymptomatic myeloma to active multiple myeloma. (Dispenzieri et al., 2008) A recent report described 10 patients who appeared to have stable multiple myeloma as judged by conventional monitoring of intact immunoglobulin levels. However, when followed over a period of 4 years, these patients developed severe organ dysfunction as a consequence of initially undetected light chain progression; this is termed free light chain escape. (Kuhnemund et al., 2009) Classic diagnostics, such as electrophoresis and quantitative immunoglobulin measurement, proved futile to detect light chain progression, whereas sFLC were reliable markers. (Kuhnemund et al., 2009)
LCDD is characterized by renal failure with nephrotic range proteinuria and usually kappa light chains. Lambda light chain proteinuria is commonly seen in amyloidosis. (Rajkumar & Kyle, 2005) Fanconi syndrome is a disorder of proximal tubular transport dysfunction, leading to urinary excretion of amino acids, glucose, bicarbonate, uric acid, phosphate, potassium, and low molecular weight proteins. The presence of hypophosphatemia, hypokalemia, hypouricemia, and glycosuria in a patient with normal serum glucose is strongly suggestive of Fanconi syndrome (Bridoux et al., 2005).
Both LCDD and renal amyloidosis have a poor prognosis; however, newer therapies such as bortezomib (Velcade®, Millennium Pharmaceuticals, Cambridge, MA) and lenalidomide (Revlimid®, Celgene Corporation, Summit, NJ) in combination with dexamethasone or prednisone have demonstrated efficacy in the treatment and maintenance setting. Autologous stem cell transplant may also be considered in patients with amyloidosis or LCDD. (Rajkumar & Dispenzieri, 2008; Dimopoulos et al., 2008)
The Long-Term Effects of Multiple Myeloma on Renal Function
Pathogenesis of Renal Insufficiency in Myeloma
Cast nephropathy is the most common cause of myeloma kidney. The casts are surrounded by multinucleated giant cells located in the distal and collecting tubules. These large, dense, tubular casts can precipitate in the tubules and obstruct and rupture the tubular epithelium. Tubulointerstitial damage may occur in the form of flattened tubular cells, degeneration with necrosis, and stripping away of the tubular basement membrane, leading to tubuloepithelial cell atrophy and interstitial fibrosis. (Leung et al., 2008; Dimopoulos et al., 2008).
The glomerulus is responsible for filtering immunoglobulin light chains before the light chains are catabolized or excreted in the urine. Light chains present in the urine may overwhelm proximal tubules’ ability to catabolize the proteins. As proteins reach the nephrons, light chains may combine with Tamm–Horsfall mucoprotein, leading to cast formation. (Dimopoulos et al., 2008) This is manifested as large casts obstructing the tubules, which may lead to increased serum creatinine levels, decreased glomerular filtration rate (GFR), and increased risk for further deterioration of renal function, as described by Gertz (2005).
Acute Tubular Necrosis (ATN)
Acute tubular necrosis (ATN) can be precipitated by dehydration in the presence of kappa or lambda light chains that may deposit in the kidney, as described above. The use of loop diuretics may also contribute to cast formation and increased serum creatinine levels. Vasoconstriction as a result of hypercalcemia, and decreased blood flow from the kidneys as a result of the use of non-steroidal anti-inflammatory drugs (NSAIDs) or aminoglycosides, may also damage the kidneys. (Tariman & Faiman, 2011)
Amyloidosis
Amyloidosis is a disease characterized by the deposition of amyloid fibrils, which consist of monoclonal light chains, in various tissues of the body; it often leads to organ dysfunction. Amyloid is a fibrillar structure that most commonly deposits in the heart, kidneys, nervous system, or gastrointestinal tract, as described by Gertz (2002)
Symptoms at presentation can be vague and depend on the affected organ, which often makes this disease difficult to diagnose. Clinical presentation may include nephrotic syndrome, congestive heart failure, peripheral neuropathy, macroglossia, periorbital purpura, or hepatomegaly. (Gertz 2002) A diagnosis of amyloidosis depends upon the presence of amyloid in a tissue biopsy or subcutaneous fat aspirate by Congo red staining. A bone marrow aspirate and biopsy should be performed to demonstrate the presence of monoclonal plasma cells. (Gertz 2002)
In the kidney, amyloid deposits are predominantly found within the glomeruli. (Dimopoulos et al., 2008) Patients usually present with significant proteinuria, but not always with renal failure. Progression to renal failure can be slow. (Rajkumar & Dispenzieri, 2008)
Light Chain Deposition Disease (LCDD)
In LCDD, diagnosis is supported by immunofluorescence and by electron microscopy. Linear peritubular deposits of monotypic light chains are usually found, but these deposits are also found along the basement membrane, mesangial nodules, Bowman’s capsule, and vascular structures, and in the interstitium. (Dimopoulos et al., 2008) In addition to the glomerular findings, the presence of interstitial fibrosis is a frequent finding. If amyloidosis is suspected, a biopsy needs to be done to confirm the diagnosis. Subcutaneous fat (fat pad biopsy), rectal, renal, heart, or liver biopsy with positive Congo red staining confirms the diagnosis of amyloidosis. (Haroun et al., 2003)
The pathology of light chain deposition disease differs from amyloid; these deposits have a granular rather than fibrillar structure. LCDD usually consists of kappa light chains, which do not stain for Congo red, (Herrera, Poblet, Cabrera, Pedrero, & Alonso, 2008) and the deposits are typically found in the renal tubular basement membranes. (Gertz 2005)
Clinical presentation includes nephrotic syndrome, anemia, and eventually, in almost all cases, renal failure. In LCDD, kidney involvement is frequently part of a more diverse and complex clinicopathologic picture when compared with amyloidosis. Diagnosis of LCDD requires a renal biopsy. (Dimopoulos et al., 2008)
Diagnosis of Renal Insufficiency
Renal insufficiency is evidenced by serum creatinine levels greater than 2.0 mg/dL; it may improve and even resolve in some patients if it is detected early and treated appropriately. Electrophoresis and immunofixation of serum and urine (an aliquot from a 24-hour urine collection) are necessary. Electrolytes and serum creatinine should be measured. Complete blood count (CBC) testing may identify anemia secondary to renal failure. Baseline and periodic monitoring of serum free light chain assays in patients with free light chain escape is important (Dispenzieri et al., 2008).
Impact on Multiple Myeloma Therapeutics and Risk Factors
Reversal of hypercalcemia with hydration, corticosteroids, and bisphosphonates is essential. Reducing tumor burden by initiating myeloma treatment, such bortezomib with or without high-dose dexamethasone pulses (40 mg on days 1 through 4, 9 through 12, and 17 through 20 of a 28-day cycle) may be effective if renal insufficiency is related to myeloma. (Rajkumar & Dispenzieri, 2008)
The efficacy of plasmapheresis has not been conclusively demonstrated in the few small-scale studies that have been conducted. (Clark et al., 2005; Clark & Garg, 2008) Dialysis is indicated if the patient’s GFR is critically low and if symptomatic uremia is present. Extended hemodialysis using high-cutoff dialyzers may effectively remove sFLC and can lead to improved renal function. (Hutchison et al., 2009)
Factors to Consider in Relapsed Myeloma
Despite therapy advancements in the last decade that have increased survival rates, multiple myeloma remains incurable. (NCCN 2010b) A malignant clone will eventually re-emerge, and relapse may occur along with renal insufficiency. As a result, it is important for nurses to consider the safety of novel agents in patients with renal insufficiency. Agents such as bortezomib, thalidomide (Thalomid®, Celgene Corporation, Summit, NJ), and doxorubicin alone or in combination with steroids are generally safe. (Chanan-Khan et al., 2007; Chanan-Khan et al., 2009) Lenalidomide can be given to patients with renal insufficiency or renal failure with dose modifications as discussed further below. Other agents such as cyclophosphamide and melphalan are also safe to use in patients with renal insufficiency, but the dose of melphalan may be reduced based on the clinician’s judgment. (Celgene Corporation, 2004)
Patients with myeloma often experience renal insufficiency due to disease progression. While dexamethasone pulses may assist in reversing renal failure due to hypercalcemia or disease progression, the overall benefit often is not sustained. Most practitioners will use bortezomib in combination with dexamethasone (Kastritis et al., 2008). The advent of novel treatments, using combination therapies to improve progression-free survival, fortunately offers many available options to treat patients at the time of relapse even in the setting of decreased renal function. (NCCN, 2010b)
Additional Risk Factors
The National Kidney Foundation identifies persons at increased risk for chronic renal disease, including those with diabetes, cardiovascular disease, hypertension, age greater than 60, US racial or ethnic minority status, and those with a family history of chronic kidney disease. (NKF 2010) Patients with myeloma are at an increased risk of renal failure not only from their myeloma, but also from age and other risk factors. Diabetes and hypertension are the leading causes of ESRD, with diabetes mellitus as the number one cause of kidney failure. Almost half of all new dialysis patients have diabetes, making it the fastest growing risk factor for kidney disease. Blood pressure and blood sugar control can help prevent progression to ESRD (O’Seaghdha et al., 2009; Firestone & Mold, 2006). Common risk factors for kidney dysfunction in patients with myeloma are listed in Figure 2.
Figure 2.
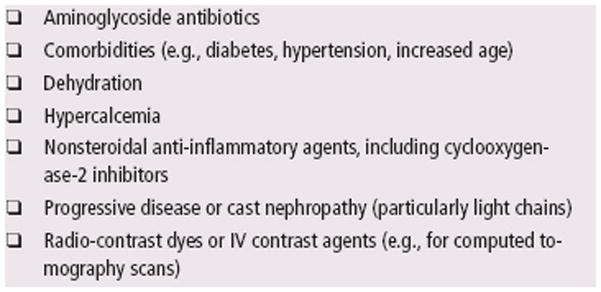
Drugs or Conditions That May Contribute to Kidney Disease in Myeloma
Hypertension is both a cause and complication of chronic kidney disease and should be carefully treated and controlled in all patients. Penfield (2006) discusses how weight control, exercise, smoking cessation, and medications for controlling blood pressure may prevent or slow the progression to kidney failure in patients with multiple myeloma.
Unlike risk factors that may be prevented or minimized with lifestyle modifications, other risk factors, such as age and race/ethnicity, cannot be altered. Since kidney function is reduced in older people, the older the patient, the greater the risk of developing renal insufficiency. Additionally, patients with myeloma are at a higher risk due to diabetes or steroid side effects and may require close monitoring and tighter control of their diabetes. (Faiman et al., 2008) Some ethnic groups also are at an increased risk of renal disease compared with whites (NIH 2010).
Bone Complications
Bone loss associated with chronic renal disease is an osseous complication of multiple myeloma, and bone changes can begin in adults many years before symptoms actually appear. Older patients, post-menopausal women, and patients with multiple myeloma in general are at increased risk for osteoporosis, which progresses as renal function worsens. Patients, in turn, experience increased risk of bone fractures and resultant joint and bone pain as a result of renal osteodystrophy and hyperparathyroidism secondary to chronic renal disease (Malluche, Koszewski, Monier-Faugere, Williams, & Mawad, 2006; Malluche, Mawad, & Monier-Faugere, 2010).
The kidneys play an important role in maintaining healthy bone mass throughout life by maintaining calcium and phosphorus levels in the blood. Normally, kidneys remove excess phosphorus from the blood. When the kidneys fail, though, serum phosphorus increases and combines with serum calcium, leading to lower circulating levels of calcium in the blood. This resultant hypocalcemia stimulates the parathyroid glands to release parathyroid hormone (PTH), which draws calcium from the bones to raise blood calcium levels. This results in osteopenia with weakening of the bones. Nurses should be aware that patients with CKD should have routine monitoring for serum PTH and vitamin D. (Levey et al., 2003) The IMF’s Nurse Leadership Board have addressed maintaining bone health as part of their survivorship care plan. (Miceli, Colson, Faiman, Tariman, Miller, & IMF NLB. 2011)
Immunomodulatory Agents
Thalidomide and lenalidomide are both immunomodulatory agents and responses in myeloma have been demonstrated in randomized and controlled clinical trials. Thalidomide is considered safe in renal insufficiency, and dosage adjustments are not needed. (Celgene 2010b) Lenalidomide is FDA-approved for treatment of myeloma in patients who have received at least 1 prior therapy; however, caution must be exercised when using this agent, as it is mainly excreted through the urine. (Celgene 2010a) Renal insufficiency has been linked to increased myelosuppression in patients receiving lenalidomide therapy. (Celgene 2010a) Despite the absence of clear recommendations, CBC and chemistry panels should be monitored carefully in any patient with renal insufficiency who is receiving lenalidomide. Dose modifications for patients with lenalidomide based on renal insufficiency must be followed.
Bortezomib is a proteasome inhibitor that is FDA-approved for treatment of multiple myeloma with no dosage reductions for patients with renal failure. (Millennium Pharmaceuticals 2010) A subset analysis of data from two key clinical trials in patients with relapsed myeloma demonstrated a response rate of 30% in patients with severe renal impairment (creatinine clearance <30 mL/min), and 25% in those with moderate renal impairment. In addition, patients with creatinine clearance values as low as 13.8 mL/min have been included in clinical trials. The pharmacokinetics of bortezomib have been studied in patients with normal renal function and in patients with varying degrees of renal impairment from moderate to severe, including patients on dialysis (doses after dialysis). There were no difference in bortezomib exposure among the patient groups. (Millennium Pharmaceuticals 2010)
Chanan-Khan, et al. (2007) conducted a multicenter retrospective study on the safety and efficacy of bortezomib in patients with multiple myeloma with renal failure requiring dialysis. The median serum creatinine level in this study was 6.8 mg/dL. Patients were treated with bortezomib alone or in combination with other agents (dexamethasone, liposomal doxorubicin [Doxil®, OrthoBiotech, Raritan, NJ], or thalidomide). The overall response rate was 75% among the 20 patients with response data. Three of four patients had improved renal function following bortezomib-based therapy. This included one patient who was spared dialysis and two patients who no longer required dialysis support after complete response (CR). These findings suggest that bortezomib is a safe and useful agent in renal failure and dialysis-dependent patients. (Chanan-Khan et al., 2007)
Newly Diagnosed Patients Eligible for Transplant
Frontline therapy for the transplant-eligible patient with decreased renal function includes regimens that contain thalidomide, dexamethasone, vincristine, doxorubicin, liposomal doxorubicin, cyclophosphamide, and bortezomib. Alkylating agents, such as melphalan, may be administered to patients who are not considered candidates for transplant. (NCCN 2010b) The combinations of lenalidomide and dexamethasone (Rajkumar, et al., 2010) or bortezomib and pegylated liposomal doxorubicin (Jakubowiak et al., 2009) have shown promising results. Clinical trials suggest that bortezomib and liposomal doxorubicin is a safe combination in the presence of renal insufficiency. (Blade 2008)
Autologous stem cell transplant has been well-documented as an effective treatment for patients with newly diagnosed myeloma as well as for those with relapsed or refractory disease. While this is true, few experts agree concerning the type of induction regimen, use of single or tandem transplant, and the role, if any, of allogeneic transplant. Transplant may be considered in patients receiving dialysis, but morbidity and mortality are higher. In addition, the timing of transplant, whether at diagnosis or relapse, is uncertain. Novel agents such as thalidomide, lenalidomide, and bortezomib must be considered for induction therapy.
Bortezomib is an effective treatment option for patients with renal insufficiency who are eligible for transplant. In a phase 2 open label intergroup trial, patients (N=48) received a combination of bortezomib and dexamethasone prior to an autologous stem cell transplant (ASCT). The pre-transplant response rates were a CR rate of 21% and a 10% very good partial remission (VGPR, defined as >90% reduction of the M-component). Investigators concluded that the bortezomib plus dexamethasone regimen appeared effective and well-tolerated in newly diagnosed myeloma patients. (Harousseau et al., 2006)
Newly Diagnosed Patients Not Eligible for Transplant
Thalidomide and dexamethasone have been studied in elderly patients with myeloma. The use of high-dose dexamethasone is toxic for elderly patients and not well-tolerated. (Palumbo & Rajkumar 2009) Recently, results of a clinical trial reported that a combination of melphalan, prednisone, and bortezomib was superior to melphalan and prednisone (MP) in patients with newly diagnosed myeloma, including those with renal impairment. In the international clinical trial of Velcade as Initial Standard Therapy in Multiple Myeloma (VISTA) (San Miguel et al., 2008), 682 patients were randomly assigned to receive either MP given every 6 weeks or V-MP (bortezomib plus MP) given on a 6-week cycle with two 3-week cycles of bortezomib and one cycle of MP. V-MP was superior to MP in time to progression, overall survival, CR, progression-free survival, and time to next therapy. The immunofixation-negative CR rate was an unprecedented 35% for V-MP compared with only 5% for MP, and there was a 52% reduction in risk of progression for the group that received V-MP. In the 185 patients with renal impairment (defined as creatinine clearance less than 60 mL/min), there was no significant difference in CR rates, time to progression, or overall rate of survival compared with the 159 patients with normal renal function (defined as creatinine clearance ≥60 mL/min). These data suggest that adding bortezomib to melphalan and prednisone is an effective upfront therapy for patients with multiple myeloma, including those with impaired renal function. (San Miguel et al., 2008).
Supportive Care Recommendations
Bisphosphonates, such as zoledronic acid and pamidronate, are potent inhibitors of bone resorption that promote bone formation. However, bisphosphonates may be toxic to the kidneys in patients with renal insufficiency or chronic kidney disease. (Perazella & Markowitz 2008; Miceli et al., 2011). It is also important to note that both pamidronate and zoledronic acid are indicated for the purpose of decreasing skeletal-related events and fractures in patients with multiple myeloma, but their effects on patients differ. Pamidronate is less nephrotoxic but more likely to cause tubular injury and nephrotic syndrome. This is generally seen with very prolonged use and/or higher doses. Zoledronic acid is more nephrotoxic than pamidronate, especially in patients with uncontrolled myeloma, and is associated with toxic ATN. Clinicians need to be especially careful with baseline elevation in serum creatinine, as zoledronic acid can induce renal failure that may or may not be reversible. (Perazella & Markowitz 2008)
Caution must be exercised when using these drugs, both of which are FDA-approved for patients with hypercalcemia of malignancy (pamidronate) and multiple myeloma (pamidronate and zoledronic acid). (Kyle et al., 2007) Once renal function has stabilized, serum creatinine levels should be evaluated at baseline and prior to each infusion of bisphosphonates. Dose reductions of zoledronic acid and pamidronate also may be needed, and longer infusion times of pamidronate are currently recommended for patients with reduced creatinine clearance. Patients who are receiving bisphosphonates should also be given calcium 1000 mg/day and vitamin D 400 IU per day. However, calcium supplementation is contraindicated in patients with hypercalcemia (Faiman et al 2008).
Anemia, defined in myeloma as a hemoglobin concentration 2 g/dL below the institutional limits of normal, is often present in patients with moderate to severe renal dysfunction, but may also be due to blood loss, cytotoxic therapy, or increased disease activity. If blood loss is not found, and the anemia is not considered to be related to treatment or disease progression, then assessing serum erythropoietin, iron, folic acid, and vitamin B12 levels may identify the type of anemia. Erythropoiesis stimulating agents (ESAs, e.g., erythropoietins such as epoetin alfa or darbepoetin alfa) may be used in managing anemia. (NCCN 2010a)
The use of ESAs in renal disease is accepted; however, their use in myeloma remains controversial. In addition, increasing concerns about ESA use abound, as multiple studies show decreased survival in patients with CKD and other cancers. (Tariman & Faiman, 2011) Currently, the National Comprehensive Cancer Network practice guidelines in oncology recommend that darbepoetin can be initiated at a dose of 2.25 μg/kg2 weekly, while 500 μg every 3 weeks is an appropriate fixed dose. (NCCN 2010a) In addition, epoetin alfa can be given at a dose of 150 units 3 times weekly or up to 40,000 units weekly subcutaneously. It is important to note long-term erythropoietin therapy may be associated with a functional iron deficiency; therefore, it is recommended that serum iron, ferritin, and serum total iron binding capacity (TIBC) be assessed prior to initiating oral iron therapy.
Results of a study suggested that ESAs may have a negative effect on the survival of patients with myeloma. (Katodritou et al., 2008) In this study, 323 Greek patients with multiple myeloma were evaluated over a 20-year period from 1988 to 2007. The median survival was 31 months for patients who received ESAs, compared with 67 months for those who were not exposed to ESAs. The median progression-free survival for patients in the ESA group was 14 months versus 30 months for those without ESA exposure. (Katodritou 2008) While these results suggest that using ESAs could lead to disease progression, the use of these drugs is recommended in National Comprehensive Cancer Network guidelines for management of anemia (NCCN 2010a). Due to the increased risk for thrombus, particularly in patients with multiple myeloma who are treated with ESAs, and the potential of decreased survival, NCCN recommends that the severity of anemia be assessed and risks of ESA therapy versus blood transfusion be balanced with the benefits of therapy. Some clinicians have called for reevaluation of the use of ESAs in patients with cancer. (Unger, Thompson, Blank, & Temple, 2010)
Supportive care considerations in the dialysis-dependent patient differ from those of the non-dialysis dependent patient with chronic renal insufficiency. Individuals undergoing dialysis can benefit from many of the available novel treatment strategies, but doses of bortezomib, thalidomide, and lenalidomide should be given after dialysis. Dose reductions for bortezomib are not required (Millennium Pharmaceuticals, 2010), but modifications for patients receiving lenalidomide must be followed. (Celgene Corporation 2010a)
Monitoring
It is essential for nurses and clinicians to closely monitor patients’ myeloma and renal insufficiency by blood chemistry and CBC testing. Additional assessment of myeloma parameters, such as serum and urine protein electrophoresis, serum beta-2 microglobulin, 24-hour urine for protein electrophoresis, and sFLC assay is also necessary. While the frequency of this testing depends on the degree of renal failure, as well as patients’ response to therapy, it is reasonable to test CBC and chemistry lab parameters on a monthly basis. The nurse may use the Chronic Kidney Disease Staging system outlined in Table 2 to monitor patients with renal dysfunction.
Table 2.
Chronic Kidney Disease Staging System for Patients With Renal Disease (Stage I—IV)
| STAGE | DESCRIPTION | KIDNEY FUNCTIONa |
|---|---|---|
| I | Kidney damage with normal or increasing GFR | 90 or higher |
| II | Kidney damage with mild decreasing GFR | 60–89 |
| T | For transplant | |
| III | Moderate decreasing GFR | 30–59 |
| IV | Severe decreasing GFR | 15–29 |
| V | Kidney failure | Less than 15 (or dialysis) |
| D | For dialysis |
GFR ml per minute per 173 m2
GFR—glomerular filtration rate
Note. T for transplant represents all kidney transplant recipients; D for dialysis is at stage V for people being treated with dialysis.
Note. From “About Chronic Kidney Disease,” by the National Kidney Foundation, 2011. Retrieved from http://www.kidney.org/professionals/KLS/aboutCKD.cfm. Copyright 2011 by the National Kidney Foundation. Reprinted with permission.
Special Considerations in Patients with Multiple Myeloma on Dialysis
Approximately 20% of patients with multiple myeloma are currently receiving dialysis. (Blade & Rosinol, 2005) The clinicians must be cognizant of the following potential issues and concerns surrounding patients requiring hemodialysis: (Blade & Rosinol; Cook 2008; Penfield; Finkelstein, Wuerth, & Finkelstein, 2009)
Finances
Dialysis and supportive care associated with end-stage renal disease add to the cumulative costs of treating the patient with multiple myeloma. This includes additional expenses when plasma exchanges are utilized to restore normal renal function.
Quality of Life
There is no study examining the quality of life (QoL) of myeloma patients on dialysis. However, research findings from QoL studies in chronic renal disease have consistently shown that the patients’ overall health-related QoL is compromised.
During Stem Cell Transplantation
Toxic deaths were not different between patients with low and normal GFRs, although patients with reduced GFR had more morbidity from mucositis, diarrhea, and infections. (Blade et al., 2005; Finkelstein et al.)
Renal Transplant in the Setting of Multiple Myeloma
Although renal transplantation is not generally considered for patients with multiple myeloma, it has been performed, and could be considered for patients with ESRD whose myeloma is in remission. (Taheri, et al., 2007).
Conclusion
Renal insufficiency in patients with myeloma should be assessed at initial diagnosis and throughout therapy. Preventive measures should be initiated at diagnosis and throughout the course of the disease. Nurses have the unique ability to play a key role in early identification of renal insufficiency and to provide patient education on preventive interventions, such as liberal oral hydration and avoiding NSAID therapy. In addition, by ensuring patients undergo routine laboratory evaluation with attention to serum calcium and creatinine levels, nurses may help patients avoid acute renal failure. Prompt intervention with hydration and identification of the underlying cause of renal failure may allow a patient’s renal function to improve, provide patients with more therapeutic options, and offer the potential for a longer and improved quality of life.
Figure 1. Cross Section of a Human Kidney.
Note. Copyright 2011 by BSIP/Photo Researchers, Inc. Used with permission.
Figure 3.
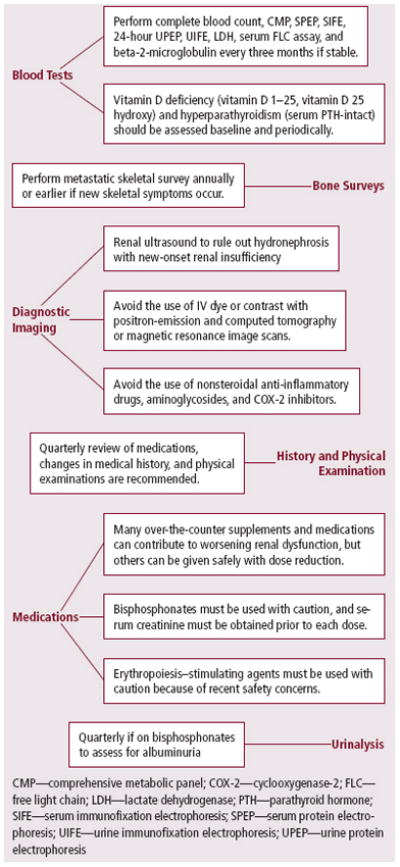
Long-Term Survivor Renal Care Plan for Clinicians
Figure 4.
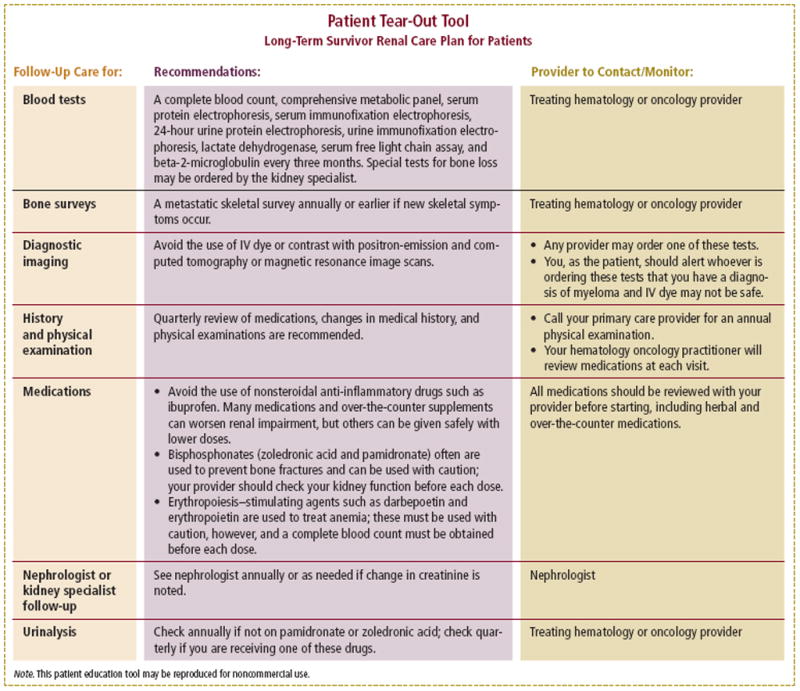
Patient Tear-Out Tool
Table 1.
Dose and Modification Guidelines for Lenalidomide in Patients With Renal Insufficiency
| DEGREE OF RENAL IMPAIRMENT | RENAL FUNCTION (COCKCROFT-GAULT) | MODIFIED DOSE FOR MULTIPLE MYELOMA |
|---|---|---|
| Moderate | CLcr 30–60a ml per minute | 10 mg every 24 hours |
| Severe (not requiring dialysis) | CLcr less than 30 ml per minute | 15 mg every 48 hours |
| End-stage renal disease (requiring dialysis) | CLcr less than 30 ml per minute | 5 mg once daily. On days of dialysis, the dose should be administered following dialysis. |
The definition of moderate renal impairment in the prescribing information approved by the European Medicines Agency for use in the European Union is 30 ≤ CLcr < 50 ml per minute.
CLcr—creatinine clearance
Note. The normal CLcr range for men is 97–137 ml per minute and 88–128 ml per minute for women.
Note. Based on information from Celgene Corp., 2010a.
At a Glance.
Renal dysfunction occurs commonly in patients with symptomatic multiple myeloma at diagnosis or during the course of the disease, and can negatively affect overall survival and quality of life. Renal dysfunction may be exacerbated by concomitant illnesses, treatment, or multiple myeloma itself, putting patients at higher risk for additional complications.
Patients with myeloma who have light chain (Bence Jones protein) proteinuria may experience renal failure or progress to end-stage renal disease (ESRD) and require dialysis due to light chain cast nephropathy. Renal failure in patients with a presumptive diagnosis of multiple myeloma may also result from amyloidosis, light chain deposition disease, or acute tubular necrosis caused by nephrotoxic agents.
SThe International Myeloma Foundation’s Nurse Leadership Board have developed practice recommendations for screening for renal function, identifying risk factors, selecting appropriate preventative, therapeutic, and supportive care measures to reduce renal dysfunction and decrease progression to ESRD and dialysis, and managing renal complications in patients with multiple myeloma.
Acknowledgments
The authors thank Brian G. M. Durie, MD, and Robert A. Kyle, MD, for critical review of the manuscript; Lynne Lederman, PhD, for preparation of the manuscript; and Lakshmi Kamath, PhD, Valerie Sudakin, PhD, and Sallie Glomb, PhD, for assistance in preparation of the manuscript.
Footnotes
Author Disclosures:
- Speakers bureau: Celgene Corporation, Millennium Pharmaceuticals, Medtronic
- Consultant: Millennium Pharmaceuticals
- Speakers bureau: Celgene Corporation, Millennium Pharmaceuticals
- None
Contributor Information
Beth Faiman, Email: faimanb@ccf.org.
Joseph D. Tariman, Email: phdinseattle@yahoo.com.
Patricia A. Mangan, Email: patricia.mangan@uphs.upenn.edu.
Jacy Spong, Email: spong.jacy@mayo.edu.
References
- Attal M, Harousseau JL, Facon T, Guilhot F, Doyen C, Fuzibet JG InterGroupe Francophone du Myelome. Single versus double autologous stem-cell transplantation for multiple myeloma. New England Journal of Medicine. 2003;349(26):2495–2502. doi: 10.1056/NEJMoa032290. [DOI] [PubMed] [Google Scholar]
- Blade J, Fernandez-Llama P, Bosch F, Montoliu J, Lens XM, Montoto S, Monserrat E. Renal failure in multiple myeloma: presenting features and predictors of outcome in 94 patients from a single institution. Archives of Internal Medicine. 1998;158(17):1889–1893. doi: 10.1001/archinte.158.17.1889. [DOI] [PubMed] [Google Scholar]
- Blade J, Rosinol L. Renal, hematologic and infectious complications in multiple myeloma. Best Practice & Research Clinical Haematology. 2005;18(4):635–652. doi: 10.1016/j.beha.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Blade J, Sonneveld P, San Miguel JF, Sutherland HJ, Hajek R, Nagler A DOXYL-MMY-3001 Study Investigators. Pegylated liposomal doxorubicin plus bortezomib in relapsed or refractory multiple myeloma: efficacy and safety in patients with renal function impairment. Clinical Lymphoma & Myeloma. 2008;8(6):352–355. doi: 10.3816/CLM.2008.n.051. [DOI] [PubMed] [Google Scholar]
- Bradwell AR, Harding SJ, Fourrier NJ, Wallis GLF, Drayson MT, Carr-Smith HD, Mead GP. Assessment of monoclonal gammopathies by nephelometric measurement of individual immunoglobulin kappa/lambda ratios. Clinical Chemistry. 2009;55(9):1646–1655. doi: 10.1373/clinchem.2009.123828. [DOI] [PubMed] [Google Scholar]
- Bridoux F, Sirac C, Hugue V, Decourt C, Thierry A, Quellard N, Touchard G. Fanconi’s syndrome induced by a monoclonal Vκ3 light chain in Waldenström’s macroglobulinemia. American Journal of Kidney Diseases. 2005;45(4):749–757. doi: 10.1053/j.ajkd.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Celgene Corporation. Alkeran® (melphalan) [Product information] Warren, NJ: Author; 2004. [Google Scholar]
- Celgene Corporation. Revlimid® (lenalidomide) [Product information] Summit, NJ: Author; 2010a. [Google Scholar]
- Celgene Corporation. Thalomid® (thalidomide) [Product information] Summit, NJ: Author; 2010b. [Google Scholar]
- Chanan-Khan AA, Kaufman JL, Mehta J, Richardson PG, Miller KC, Lonial S, Singhal S. Activity and safety of bortezomib in multiple myeloma patients with advanced renal failure: a multicenter retrospective study. Blood. 2007;109:2604–2606. doi: 10.1182/blood-2006-09-046409. [DOI] [PubMed] [Google Scholar]
- Chanan-Khan AA, Miller KC, Musial L, Padmanabhan S, Yu J, Ailawadhi S, Czuczman MS. Bortezomib in combination with pegylated liposomal doxorubicin and thalidomide is an effective steroid independent salvage regimen for patients with relapsed or refractory multiple myeloma: Results of a phase II clinical trial. Leukemia & Lymphoma. 2009;50(7):1096–1101. doi: 10.1080/10428190902912460. [DOI] [PubMed] [Google Scholar]
- Clark WF, Garg AX. Plasma exchange for myeloma kidney: cast(s) away? Kidney International. 2008;73(11):1211–1213. doi: 10.1038/ki.2008.117. [DOI] [PubMed] [Google Scholar]
- Clark WF, Stewart AK, Rock GA, Sternbach M, Sutton DM, Barrett BJ the Canadian Apheresis Group. Plasma exchange when myeloma presents as acute renal failure: a randomized, controlled trial. Annals of Internal Medicine. 2005;143(11):777–784. doi: 10.7326/0003-4819-143-11-200512060-00005. [DOI] [PubMed] [Google Scholar]
- Coleman RE. Risks and benefits of bisphosphonates. British Journal of Cancer. 2008;98(11):1736–1740. doi: 10.1038/sj.bjc.6604382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook R. Economic and clinical impact of multiple myeloma to managed care. Journal of Managed Care Pharmacy. 2008;14(7 Suppl):S19–S25. doi: 10.18553/jmcp.2008.14.S7-A.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coward RA. The cost of chronic dialysis in multiple myeloma. Postgraduate Medicine Journal. 1989;65(763):302–306. doi: 10.1136/pgmj.65.763.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimopoulos MA, Kastritis E, Rosinol L, Blade J, Ludwig H. Pathogenesis and treatment of renal failure in multiple myeloma. Leukemia. 2008;22(8):1485–1493. doi: 10.1038/leu.2008.131. [DOI] [PubMed] [Google Scholar]
- Dimopoulos MA, Terpos E, Chanan-Khan A, Leung N, Ludwig H, Jagannath S, San Miguel J. Renal impairment in patients with multiple myeloma: a consensus statement on behalf of the International Myeloma Working Group. Journal of Clinical Oncology. 2010;28(33):4976–4984. doi: 10.1200/JCO.2010.30.8791. [DOI] [PubMed] [Google Scholar]
- Dispenzieri A, Kyle RA, Katzmann JA, Therneau TM, Larson D, Benson J, Rajkumar SV. Immunoglobulin free light chain ratio is an independent risk factor for progression of smoldering (asymptomatic) multiple myeloma. Blood. 2008;111(2):785–789. doi: 10.1182/blood-2007-08-108357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dispenzieri A, Kyle R, Merlini G, San Miguel J, Ludwig H, Hajek R International Myeloma Working Group. International Myeloma Working Group guidelines for serum-free light chain analysis in multiple myeloma and related disorders. Leukemia. 2009;23(2):215–224. doi: 10.1038/leu.2008.307. [DOI] [PubMed] [Google Scholar]
- Drayson M, Tang LX, Drew R, Mead GP, Carr-Smith H, Bradwell AR. Serum free light-chain measurements for identifying and monitoring patients with nonsecretory multiple myeloma. Blood. 2001;97(9):2900–2902. doi: 10.1182/blood.v97.9.2900. [DOI] [PubMed] [Google Scholar]
- Faiman B, Bilotti E, Mangan PA, Rogers K the IMF Nurse Leadership Board. Consensus statement for the management of steroid-associated side effects in patients with multiple myeloma. Clinical Journal of Oncology Nursing. 2008;12(3 Suppl):53–62. doi: 10.1188/08.CJON.S1.53-62. [DOI] [PubMed] [Google Scholar]
- Finkelstein FO, Wuerth D, Finkelstein SH. Health related quality of life and the CKD patient: challenges for the nephrology community. Kidney International. 2009;76:946–952. doi: 10.1038/ki.2009.307. [DOI] [PubMed] [Google Scholar]
- Firestone B, Mold JW. Type 2 diabetes: which interventions best reduce absolute risks of adverse events? Journal of Family Practice. 2009;58(6):E1–E10. [PubMed] [Google Scholar]
- Gertz MA. Diagnosing primary amyloidosis. Mayo Clinic Proceedings. 2002;77(12):1278–1279. doi: 10.4065/77.12.1278. [DOI] [PubMed] [Google Scholar]
- Gertz MA. Managing myeloma kidney. Annals of Internal Medicine. 2005;143(11):835–837. doi: 10.7326/0003-4819-143-11-200512060-00013. [DOI] [PubMed] [Google Scholar]
- Haroun MK, Jaar BG, Hoffman SC, Comstock GW, Klag MJ, Coresh J. Risk factors for chronic kidney disease: a prospective study of 23,534 men and women in Washington County, Maryland. Journal of the American Society of Nephrology. 2003;14(11):2934–2941. doi: 10.1097/01.asn.0000095249.99803.85. [DOI] [PubMed] [Google Scholar]
- Harousseau JL, Attal M, Leleu X, Troncy J, Pegourie B, Stoppa AM, Avet-Loiseau H. Bortezomib plus dexamethasone as induction treatment prior to autologous stem cell transplantation in patients with newly diagnosed multiple myeloma: results of an IFM phase II study. Haematologica. 2006;91(11):1498–1505. [PubMed] [Google Scholar]
- Herrera CM, Poblet MS, Cabrera R, Pedrero MD, Alonso JF. Light chain deposition disease. Experience in our environment. Nefrologia. 2008;28(5):539–542. [PubMed] [Google Scholar]
- Hutchison CA, Bradwell AR, Cook M, Basnayake K, Basu S, Harding S, Cockwell P. Treatment of acute renal failure secondary to multiple myeloma with chemotherapy and extended high cut-off hemodialysis. Clinical Journal of the American Society of Nephrology. 2009;4(4):745–754. doi: 10.2215/CJN.04590908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowiak AJ, Kendall T, Al-Zoubi A, Khaled Y, Mineishi S, Ahmed A, Kaminski MS. Phase II trial of combination therapy with bortezomib, pegylated liposomal doxorubicin, and dexamethasone in patients with newly diagnosed myeloma. Journal of Clinical Oncology. 2009;27(30):5015–5022. doi: 10.1200/JCO.2008.19.5370. [DOI] [PubMed] [Google Scholar]
- Kastritis E, Anagnostopoulos A, Roussou M, Gika D, Matsouka C, Barmparousi D, Dimopoulos A. Reversibility of renal failure in newly diagnosed multiple myeloma patients treated with high dose dexamethasone-containing regimens and the impact of novel agents. Haematologica. 2007;92(04):546–549. doi: 10.3324/haematol.10759. [DOI] [PubMed] [Google Scholar]
- Katodritou E, Verrou E, Hadjiaggelidou C, Gastari V, Laschos K, Kontovinis L, Zervas K. Erythropoiesis-stimulating agents are associated with reduced survival in patients with multiple myeloma. American Journal of Hematology. 2008;83(9):697–701. doi: 10.1002/ajh.21239. [DOI] [PubMed] [Google Scholar]
- Knudsen LM, Hjorth M, Hippe E The Nordic Myeloma Study Group. Renal failure in multiple myeloma: reversibility and impact on the prognosis. European Journal of Haematology. 2000;65(3):175–181. doi: 10.1034/j.1600-0609.2000.90221.x. [DOI] [PubMed] [Google Scholar]
- Kooman JP. Estimation of renal function in patients with chronic kidney disease. Journal of Magnetic Resonance Imaging. 2009;30(6):1341–1346. doi: 10.1002/jmri.21970. [DOI] [PubMed] [Google Scholar]
- Kuhnemund A, Liebisch P, Bauchmuller K, zur Hausen A, Veelken H, Wasch R, Engelhardt M. ‘Light-chain escape-multiple myeloma’—an escape phenomenon from plateau phase: report of the largest patient series using LC-monitoring. Journal of Cancer Research and Clinical Oncology. 2009;135(3):477–484. doi: 10.1007/s00432-008-0470-7. [DOI] [PubMed] [Google Scholar]
- Kyle RA, Yee GC, Somerfield MR, Flynn PJ, Halabi S, Jagannath S, Anderson K. American Society of Clinical Oncology 2007 clinical practice guideline update on the role of bisphosphonates in multiple myeloma. Journal of Clinical Oncology. 2007;25(17):2464–2672. doi: 10.1200/JCO.2007.12.1269. [DOI] [PubMed] [Google Scholar]
- Leung N, Gertz MA, Zeldenrust SR, Rajkumar SV, Dispenzieri A, Fervenza FC, Winters JL. Improvement of cast nephropathy with plasma exchange depends on the diagnosis and on reduction of serum free light chains. Kidney International. 2008;73(11):1282–1288. doi: 10.1038/ki.2008.108. [DOI] [PubMed] [Google Scholar]
- Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Eknoyan G. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Annals of Internal Medicine. 2003;139(2):137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- Malluche HH, Koszewski N, Monier-Faugere MC, Williams JP, Mawad H. Influence of the parathyroid glands on bone metabolism. European Journal of Clinical Investigation. 2006;36(Suppl 2):23–33. doi: 10.1111/j.1365-2362.2006.01664.x. [DOI] [PubMed] [Google Scholar]
- Malluche HH, Mawad HW, Monier-Faugere M-C. Renal osteodystrophy in the first decade of the new millennium-analysis of 630 bone biopsies in black and white patients. Journal of Bone and Mineral Research. 2010 doi: 10.1002/jbmr.309. published on line 2 December. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miceli T, Colson K, Faiman B, Tariman JD, Miller K & the IMF Nurse Leadership Board. Maintaining bone health in patients with multiple myeloma: Survivorship care plan of the IMF Nurse Leadership Board. doi: 10.1188/11.S1.CJON.9-23. [submitted to CJON as part of this supplement] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millennium Pharmaceuticals Inc. Bortezomib (Velcade) [Product information] Cambridge, MA: Author; 2010. [Google Scholar]
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: Cancer- and chemotherapy-induced anemia. Version 1.2011. Author; 2010a. Available at http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. [Google Scholar]
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: Multiple myeloma. Version 1.2011. Author; 2010b. Available at http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. [Google Scholar]
- National Institutes of Health. US Renal Data System, USRDS 2010 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2010. Available at http://www.usrds.org/atlas.htm. [Google Scholar]
- National Kidney Foundation. About chronic kidney disease. 2010 Available at http://www.kidney.org/professionals/KLS/aboutCKD.cfm.
- OrthoBiotech. Doxil® (doxorubicin HCl liposome injection). [Product information] Raritan, NJ: Author; 2008. [Google Scholar]
- O’Seaghdha CM, Perkovic V, Lam TH, McGinn S, Barzi F, Gu DF the Asia pacific Cohort Studies Collaboration. Blood pressure is a major risk factor for renal death: an analysis of 560 352 participants from the Asia-Pacific region. Hypertension. 2009;54(3):509–515. doi: 10.1161/HYPERTENSIONAHA.108.128413. [DOI] [PubMed] [Google Scholar]
- Palumbo A, Rajkumar SV. Treatment of newly diagnosed myeloma. Leukemia. 2009;23(3):449–456. doi: 10.1038/leu.2008.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield JG. Multiple myeloma in end-stage renal disease. Seminars in Dialysis. 2006;19(4):329–334. doi: 10.1111/j.1525-139X.2006.00181.x. [DOI] [PubMed] [Google Scholar]
- Perazella MA, Markowitz GS. Bisphosphonate nephrotoxicity. Kidney International. 2008;74(11):1385–1393. doi: 10.1038/ki.2008.356. [DOI] [PubMed] [Google Scholar]
- Rajkumar SV, Jacobus S, Callander NS, Fonseca R, Vesole DH, Williams MV the Eastern Cooperative Oncology Group. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed myeloma: an open-label randomised controlled trial. The Lancet Oncology. 2010;11(1):29–37. doi: 10.1016/S1470-2045(09)70284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkumar SV, Dispenzieri A. Multiple myeloma. In: Abeloff MD, Armitage JO, Niederhuber JE, Kastan ME, McKenna WG, editors. A beloff clinical oncology. 4. Philadelphia: W. B. Saunders Company; 2008. pp. 2323–2352. [Google Scholar]
- Rajkumar SV, Kyle RA. Multiple myeloma: diagnosis and treatment. Mayo Clinic Proceedings. 2005;80(10):1371–1382. doi: 10.4065/80.10.1371. [DOI] [PubMed] [Google Scholar]
- San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M the VISTA trial investigators. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. New England Journal of Medicine. 2008;359(9):906–917. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- Taheri D, Chehrei A, Fesharakizadeh M, Seyrafean S, Shahidi S, Emami A, et al. Recurrent multiple myeloma following renal transplantation: a case report. Transplantation Proceedings. 2007;39(4):1063–1065. doi: 10.1016/j.transproceed.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Tariman J, Faiman B. Multiple myeloma. In: Yarbro CH, Wujcik D, Gobel BH, editors. Cancer nursing principles and practice. 7. Massachusetts: Jones and Bartlett; 2011. pp. 1513–1545. [Google Scholar]
- Unger EF, Thompson AM, Blank MJ, Temple R. Erythropoiesis-stimulating agents -- time for a reevaluation. New England Journal of Medicine. 2010;362(3):189–192. doi: 10.1056/NEJMp0912328. [DOI] [PubMed] [Google Scholar]
- van Agthoven M, Segeren CM, Buijt I, Uyl-de Groot CA, van der Holt B, Lokhorst HM, Sonneveld P. A cost-utility analysis comparing intensive chemotherapy alone to intensive chemotherapy followed by myeloablative chemotherapy with autologous stem-cell rescue in newly diagnosed patients with stage II/III multiple myeloma; a prospective randomised phase III study. European Journal of Cancer. 2004;40(8):1159–1169. doi: 10.1016/j.ejca.2004.01.019. [DOI] [PubMed] [Google Scholar]


