Abstract
Oncolytic (replication-competent) adenoviruses as anticancer agents provide new, promising tools to fight cancer. In support of a Phase I clinical trial, here we report safety data with INGN 007 (VRX-007), an oncolytic adenovirus with increased anti-tumor efficacy due to overexpression of the adenovirus-encoded ADP protein. Wild-type adenovirus type 5 (Ad5) and a replication-defective version of Ad5 were also studied as controls. A parallel study investigating the biodistribution of these viruses is described elsewhere in this issue. The toxicology experiments were conducted in two species, the Syrian hamster, which is permissive for INGN 007 and Ad5 replication and the poorly permissive mouse. The studies demonstrated that the safety profile of INGN 007 is similar to Ad5. Both viruses caused transient liver damage upon intravenous injection that resolved by 28 days post-infection. The No-Observable-Adverse-Effect-Level (NOAEL) for INGN 007 in hamsters was 3 × 1010 viral particles per kg. In hamsters, the replication-defective vector caused less toxicity, indicating that replication of Ad vectors in the host is an important factor in pathogenesis. With mice, INGN 007 and Ad5 caused toxicity comparable to the replication-defective adenovirus vector. Partially based on these results, the FDA granted permission to enter into a Phase I clinical trial with INGN 007.
Keywords: adenovirus, toxicology, oncolytic, Syrian hamster, mice, preclinical
Introduction
Cancer is one of the leading causes of mortality worldwide. Although great advances have been made in the treatment of certain types of cancer, there is still a critical need for novel cancer therapies. A new treatment approach has been developed recently using viral vectors, among them vectors based on human adenovirus (Ad) serotype 5(Ad5 ). Ad5 generally causes a mild respiratory illness in young children, resulting in life-long immunity.1 A subset of these Ad5-based vectors, so-called oncolytic (replication-competent (RC)) Ad vectors, kills cancer cells as part of the natural Ad life cycle.2–5 Oncolytic Ads replicate in and lyse the infected cancer cell, and then release progeny virions in the process that are able to infect neighboring tumor cells. Numerous laboratories have developed such vectors and have tested them in vitro and in vivo, and several vectors have reached human clinical trials. Thus far, no dose-limiting toxicities were reported in any of these clinical trials.6–11
We have developed a family of replicating Ad vectors for the treatment of cancer.12–20 In preparation for a Phase I clinical trial, we evaluated the safety and biodistribution of INGN 007 (VRX-007), one of our oncolytic Ad vectors. The data resulting from the safety study are presented below, whereas the results of the biodistribution experiments are discussed elsewhere. INGN 007 is a fully RC Ad vector with enhanced oncolytic capabilities due to overexpression of the Adenovirus Death Protein (ADP) (Figure 1b).13,15,16,18–20 We have tested the safety of INGN 007 in two rodent species. The first model, the C57BL/6 mouse, is the animal traditionally used for assessing the safety of Ad vectors.21–26 This model allowed us to make comparisons with published data. However, because mice are poorly permissive for human Ads, we also have performed a safety study in a second species, the Syrian hamster (Mesocricetus auratus). Syrian hamsters are immunocompetent, permissive for the replication of Ad5, support tumors in which human Ads replicate and mimic human disease with replicating Ads.18,19,27,28 These features of the Syrian hamster model allowed us to assess all facets of the interactions of the vector and the host animal.
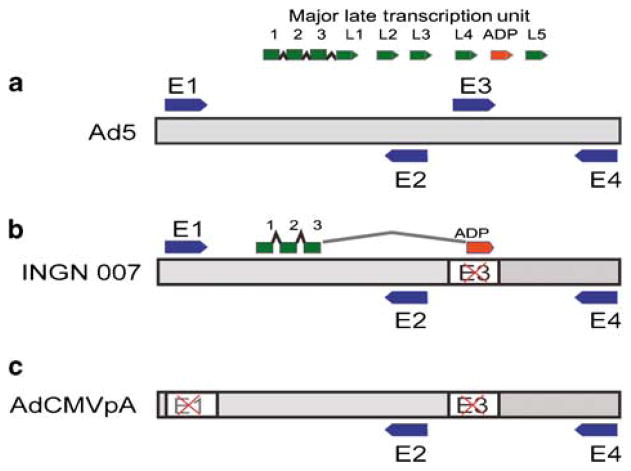
Figure 1.
Schematics of the genomes of viruses used in the study. (a) The gray bar represents the double-stranded DNA genome of Ad5. The early transcription units are depicted as blue arrows, the major late transcription unit is shown as green arrows. (b) In INGN 007, the early three (E3) transcription unit, which is responsible for host immune response evasion, is deleted and replaced with the adp gene. Except for the E3 and ADP proteins, all other early and late proteins are expressed by INGN 007 as they are in Ad5. (c) The RD AdCMVpA lacks both the early one and part of the early three (E1 and E3) transcription units. There is no transgene.
To our knowledge, this is the first published comprehensive safety study with an oncolytic Ad vector performed in both inadequately permissive and permissive species. A replication-defective (RD) Ad vector and wt Ad5were used as controls in the experiments to further broaden the range of data generated and allow for comparisons among the three vectors/viruses. The results indicate that the safety profile of INGN 007 is very similar to that of wild-type (wt) Ad5in the permissive Syrian hamster and that it is comparable to both Ad5and the RD vector in the mouse model.
Materials and methods
Study location
The experiments using Syrian hamsters were carried out at Saint Louis University, whereas the mouse experiments were performed by the Southern Research Institute (Birmingham, AL).
Animals
Male and female golden Syrian hamsters were purchased from Harlan Sprague Dawley (Indianapolis, IN). The hamsters were 5–6 weeks of age and weighed 89–105 g (females) and 84–103 g (males) at the initiation of the experiment. Male and female C57BL/6N (C57BL/6NCrlBR) mice were received from Charles River Laboratories Inc. (Raleigh, NC). The mice were approximately 6 weeks of age and weighed between 14.9–24.3 g (males) and 12.6–19.8 g (females) at the initiation of the study. All husbandry conditions were maintained as described in the Guide for the Care and Use of Laboratory Animals (National Research Council, Institute of Laboratory Animal Resources, 1996).
Viruses
INGN 007, AdCMVpA, an RD Ad vector with no transgene (Figure 1c) and wt Ad5were provided by Introgen Therapeutics Inc., at titers of 1.2–1. 4 × 1012 virus particles (vp) per ml in ca. 1ml aliquots. Vectors and virus were propagated on HEK-293 cells. Particle titers were established by column chromatography, and the infectious titers were determined by 50% tissue culture infective dose assay on HEK-293 cells. The physical to infectious particle ratio is ca. six for all three virus stocks. The aliquots were stored at −80 °C and kept on ice during dose preparation; Ads are stable under these conditions. The vehicle consisted of an aqueous solution of 10mM Tris-HCl pH 8.2, 10% glycerol. All viral dilutions were made in vehicle, kept on ice and used within 4 h following removal of the aliquot from the freezer.
Randomization and dosing
The week before dosing, animals were randomized by weight and assigned to one of six treatment groups. Each group consisted of 15female and 15 male hamsters (Table 1) or 27 female and 27 male mice (Table 2) for the hamster and mouse experiments, respectively. On day 1, each animal received a single intravenous dose of the test article (INGN 007) at one of three dose levels, control article (Ad5or AdCMVpA), or vehicle (see Tables 1 and 2 for the dose levels). Before dosing, hamsters were anesthetized with 45mg ml−1 ketamine plus 5mg ml−1 xylazine anesthetic cocktail. The dose volume was 200 μl injected through the jugular vein for all hamsters except for vehicle and AdCMVpA males, which received the articles in a 100 μl bolus. Mice were injected into the tail vein in a single 100 μl bolus, without anesthesia.
Table 1.
Experimental design, hamsters
| Group | Vector | Dose (vp per kg) | Sexes | N |
|---|---|---|---|---|
| 1 | INGN 007 | 3.0 × 109 | 2 | 30 |
| 2 | INGN 007 | 3.0 × 1010 | 2 | 30 |
| 3 | INGN 007 | 1.9 × 1012 | 2 | 30 |
| 4 | Vehicle | — | 2 | 30 |
| 5 | Ad5 | 1.9 × 1012 | 2 | 30 |
| 6 | AdCMVpA | 1.9 × 1012 | 2 | 30 |
Table 2.
Experimental design, mice
| Group | Vector | Dose (vp per kg) | Sexes | N |
|---|---|---|---|---|
| 1 | INGN 007 | 3.0 × 109 | 2 | 54 |
| 2 | INGN 007 | 3.0 × 1010 | 2 | 54 |
| 3 | INGN 007 | 1.5 × 1011 | 2 | 54 |
| 4 | Vehicle | — | 2 | 54 |
| 5 | Ad5 | 1.5 × 1011 | 2 | 54 |
| 6 | AdCMVpA | 1.5 × 1011 | 2 | 54 |
Data collection
The animals were checked daily for mortality, moribundity and for availability of food and water. Body weights were measured on specified days and before killing. At these times, animals were examined for clinical signs of toxicity. On days 2, 7 and 29, five female and five male hamsters or nine female and nine male mice were killed from each group. Necropsies were completed, select organs were weighed and a comprehensive panel of organs plus the injection site were fixed in 10% neutral-buffered formalin for histopathological evaluation. At necropsy, whole blood and serum samples were collected through cardiac puncture of all animals. In the case of hamsters, hematological, serum chemistry and electrolyte analyses were performed for each of the 10 animals killed at each time point. For mice, six animals were chosen for hematology, six for serum chemistry/electrolyte assay and three to assess coagulation parameters.
Histopathology
Histopathological analysis of the hamster samples was performed by Seventh Wave Pathology and Biotechnical Solutions, LLC (Chesterfield, MO); the mouse tissues were analyzed by the Southern Research Institute. All the collected tissues from animals in the vehicle control, high-dose INGN 007, Ad5and AdCMVpA groups were examined; based on the findings in these groups, potential target tissues were examined from low- and mid-dose groups of INGN 007-treated animals. Findings were characterized using standardized nomenclature. A five-step grading system of minimal, mild, moderate, marked and severe was used to characterize the severity of microscopic lesions.
Immunofluorescence
Human A549 cells were infected with 25PFU per cell INGN 007. At 24 h post-infection, cells were fixed with 3.7% paraformaldehyde in PBS and permeabilized with methanol. Following incubation with the primary (hamster or mouse) antiserum (at an initial dilution of 1:100), the cells were washed and incubated with a fluorochrome-conjugated secondary (anti-hamster or anti-mouse IgG) antibody. Uninfected A549 cells were used as a control.
Statistical analysis
Group means were calculated for body weight, absolute and relative organ weights and clinical pathology parameters. As the distribution of data did not satisfy requirements for parametric analysis, nonparametric tests were used. The Kruskal–Wallis test was used to detect overall treatment effect and the Mann–Whitney U-test was utilized to perform pairwise comparisons. In all cases, differences with P ≤ 0.05were considered significant.
Results
For simplicity’s sake, findings other than in-life observations will be presented grouped according to the organ systems affected. Results obtained from hamsters will be shown first, followed by data generated with mice. The effect of the viruses/vectors on the examined organ systems is summarized in Tables 3 and 4 for hamsters and mice, respectively. Figures depict pooled data from both sexes. Comprehensive tables describing clinical pathology and histopathology findings in both mice and hamsters are provided in Supplementary Information.
Table 3.
Treatment-related effects in hamsters
| Organ/organ system | Effect
|
||
|---|---|---|---|
| AdCMVpA | Ad5 | INGN 007 (high dose) | |
| Liver | None | Elevated serum transaminase level, hepatocellular necrosis, inflammation | Elevated serum transaminase level, hepatocellular necrosis, inflammation |
| Hematopoietic | None | Decreased blood erythrocyte numbers, bone marrow degeneration | Decreased blood erythrocyte numbers, bone marrow degeneration |
| Immune | Increased blood neutrophil numbers | Increased blood neutrophil and lymphocyte numbers | Increased blood neutrophil and lymphocyte numbers |
| Brain | None | None | None |
| Muscle (heart and skeletal) | None | None | None |
| Lung/bronchi | None | None | None |
| Urinary | None | None | None |
| Reproductive | None | None | None |
| Digestive (colon, pancreas) | None | None | None |
| Thymus | None | None | None |
| Adrenal glands | None | None | None |
Table 4.
Treatment-related effects in mice
| Organ/organ system | Effect
|
||
|---|---|---|---|
| AdCMVpA | Ad5 | INGN 007 (high dose) | |
| Liver | Elevated serum transaminase level, Individual hepatocellular necrosis, inflammation | Elevated serum transaminase level, Individual hepatocellular necrosis, inflammation | Individual hepatocellular necrosis, Inflammation |
| Immune | Lymphopenia at day 2, increased blood lymphocyte numbers by day 29 | Lymphopenia at day 2, increased blood lymphocyte numbers by day 29 | Lymphopenia at day 2, increased blood lymphocyte numbers by day 29 |
| Hematopoietic | None | None | None |
| Brain | None | None | None |
| Muscle (heart and skeletal) | None | None | None |
| Lung/bronchi | None | None | None |
| Urinary | None | None | None |
| Reproductive | None | None | None |
| Digestive (colon, pancreas) | None | None | None |
| Thymus | None | None | None |
| Adrenal glands | None | None | None |
In-life observations
Two male hamsters, one in the INGN 007 high-dose group (Table 1, group 3) and the other in the Ad5group (Table 1, group 5), died on day 6. Another animal in the high-dose INGN 007 group was mildly dehydrated, showed hunched posture and looked generally unthrifty at day 7. None of the animals in the other groups showed signs of morbidity, and there were no statistically significant changes in body weight. A male hamster in the INGN 007 low-dose group died on day 21; this death was deemed non-drug-related. No necropsy was performed on any of the animals that were found dead.
All virus-injected mice became slightly lethargic approximately 10 min after virus injection; the symptoms resolved in about 30 min. No other clinical signs were observed with mice.
Liver effects
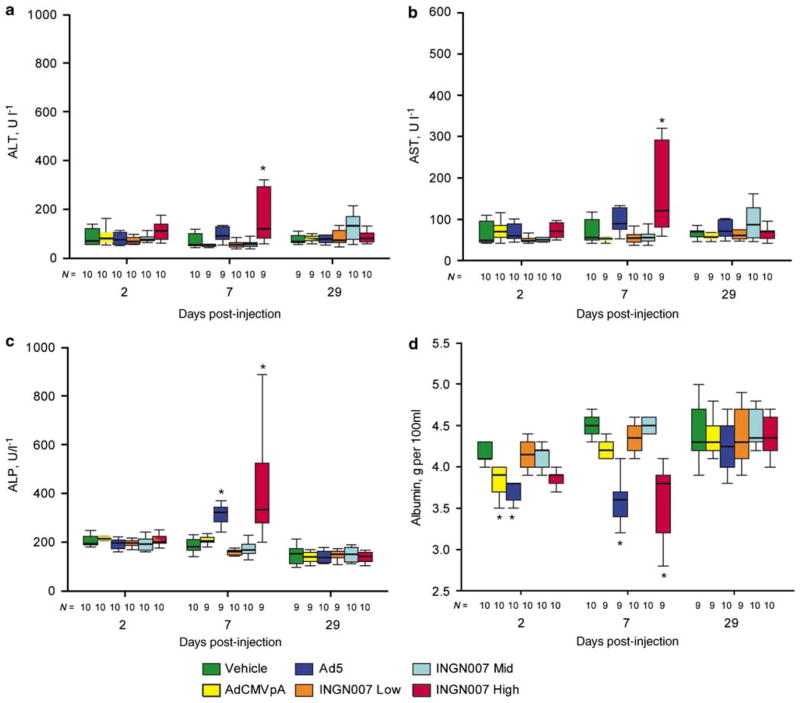
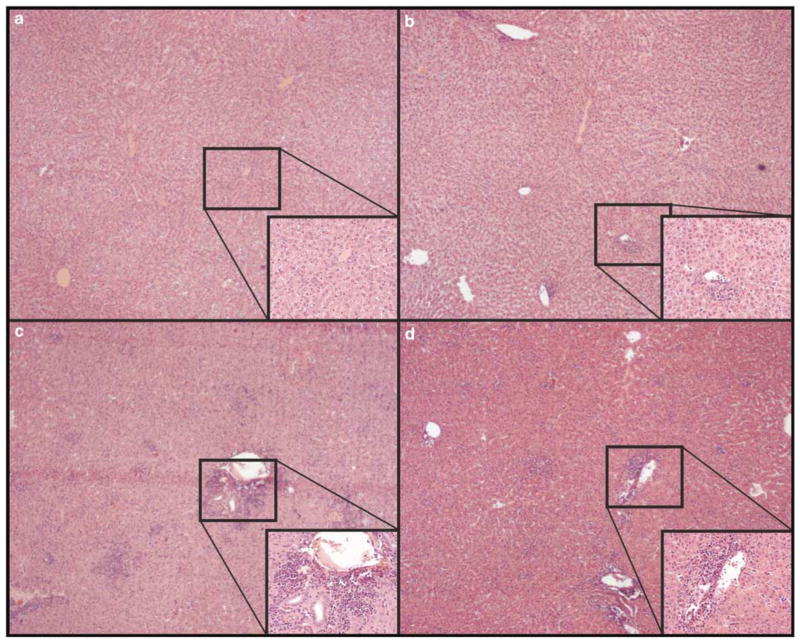
In hamsters, INGN 007 and Ad5caused more serious liver damage than did AdCMVpA, whereas in mice the injury caused by INGN 007 or Ad5did not exceed that caused by AdCMVpA. Syrian hamster serum chemistry parameters and histopathology sections were analyzed to identify possible vector-induced toxicity. With hamsters, all three viruses at the high dose caused a similar drop in serum albumin levels at day 2 (Figure 2d). At day 7, however, only Ad5and the high dose of INGN 007 caused a significant elevation of serum transaminases (Figures 2a and b), an increase in serum alkaline phosphatase levels (Figure 2c) and a significant decrease in serum albumin levels (Figure 2d), indicating hepatocellular dysfunction. The extent of the changes was similar for animals treated with the high dose of INGN 007 or Ad5. At this time, no changes were seen with the mid or low dose INGN 007- or AdCMVpA-treated hamsters. Histopathological examination of the liver of Ad5- or high-dose INGN 007-treated hamsters killed on day 7 revealed discrete necrotic foci that ranged in severity from mild to marked (Figures 3c and d). These lesions were densely scattered throughout the parenchyma, preferentially localizing in periportal regions. The necrotic areas were infiltrated primarily by mononuclear cells. These lesions mostly resolved by day 29: only minimal to mild lesions were detected in male hamsters receiving Ad5or high-dose INGN 007, and no lesions were seen in female hamsters (data not shown). No treatment-related lesions were observed with the mid- or low-dose INGN 007- or AdCMVpA-treated hamsters (Figure 3b).
Figure 2.
With hamsters, RC Ads cause liver injury. Serum transaminase and alkaline phosphatase levels were elevated (a–c) and serum albumin levels were decreased (d), indicating liver damage and impaired function. For this figure and other figures containing box graphs, the boxes represent the interquartile range; the whiskers show the largest and the smallest values, excluding outliers (values more than 1.5 box lengths from the box edge). The horizontal line inside each box signifies the median. N, population size, *P<0.05.
Figure 3.
With hamsters, high doses of RC but not RD Ads cause liver lesions. Hematoxylin–eosin staining of liver sections from (a) vehicle-, (b) AdCMVpA-, (c) Ad5- or (d) INGN 007-injected animals at day 7. The insets show an expanded view of the periportal areas. Original magnifications were × 40 for the large pictures and × 200 for the insets.
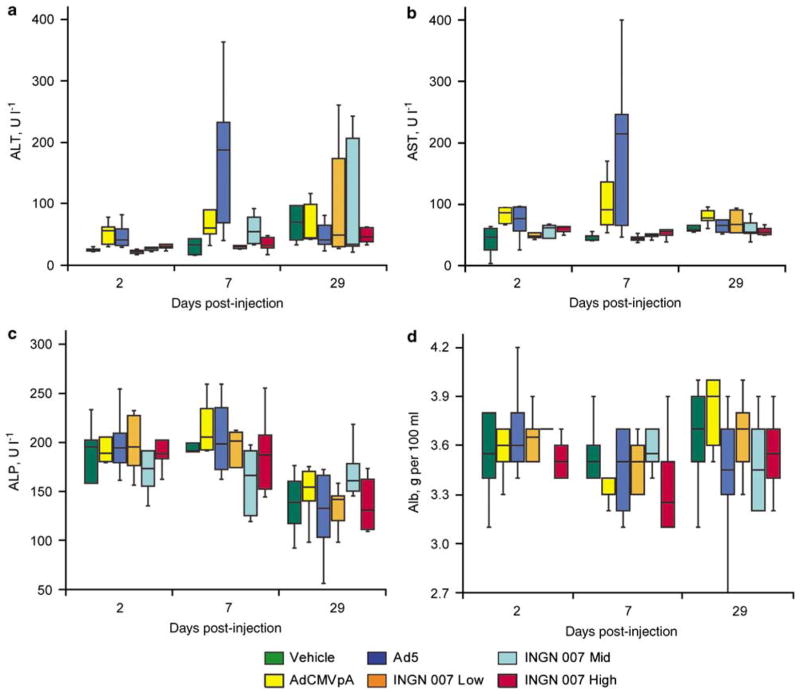
Vector-induced toxicity in mice was investigated similarly to that in hamsters. Minimal increases in alanine aminotransferase and aspartate aminotransferase serum concentrations were observed for mice treated with Ad5and AdCMVpA at days 2 and 7 (Figures 4a and b). No significant changes were detected in serum alkaline phosphatase levels (Figure 4c). INGN 007-injected mice did not develop treatment-related elevation in serum transaminases; however, a minimal decrease of serum albumin levels was detected with Ad5- and high-dose INGN 007-treated animals at day 7 (Figure 4d). Histopathological examination of liver sections showed minimal individual cell necrosis with accompanying minimal to moderate chronic inflammation. Individual cell necrosis was more prominent in animals treated with AdCMVpA or Ad5, whereas chronic inflammation was more accentuated in animals treated with the high dose of INGN 007 (data not shown).
Figure 4.
RC and RD Ads caused comparable liver damage in mice. AdCMVpA and Ad5 induced a transient elevation of serum transaminase levels at days 2 and 7 (a and b). No changes were observed in alkaline phosphatase levels (c). Ad5 and INGN 007 caused a decrease in serum albumin levels at day 7 (d).
Hematopoietic effects
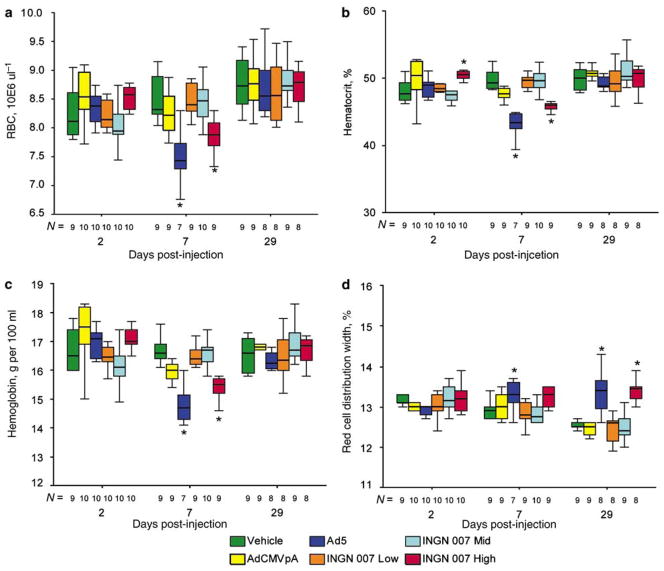
INGN 007 and Ad5caused mild disruption of erythropoiesis in hamsters. At day 7, in hamsters, erythrocyte parameters showed a statistically significant decrease in the number of circulating erythrocytes with the Ad5- and the high-dose INGN 007-injected animals, which normalized by day 29 (Figures 5a–c). The increase in the red cell distribution width values at day 29 may indicate a compensatory increase in erythropoiesis (Figure 5d). Furthermore, degeneration/loss of cells was observed in the bone marrow of the high-dose INGN 007 group on day 7 (males only). These aberrations in erythrocyte parameters at day 7 and day 29 might indicate decreased bone marrow function resulting from the bone marrow degeneration observed at day 7. No similar changes were observed in mice.
Figure 5.
Ad5 and the high dose of INGN 007 caused bone marrow damage in hamsters. With hamsters injected with the high dose of RC viruses, changes in hematological parameters indicate damage to the bone marrow. At day 7, erythrocyte counts (a), hematocrit (b) and hemoglobin concentration (c) decreased significantly in Ad5- or high-dose INGN 007-injected hamsters, whereas red cell distribution width (d) increased at day 29 in these animals. N: population size, *P<0.05.
Immune response
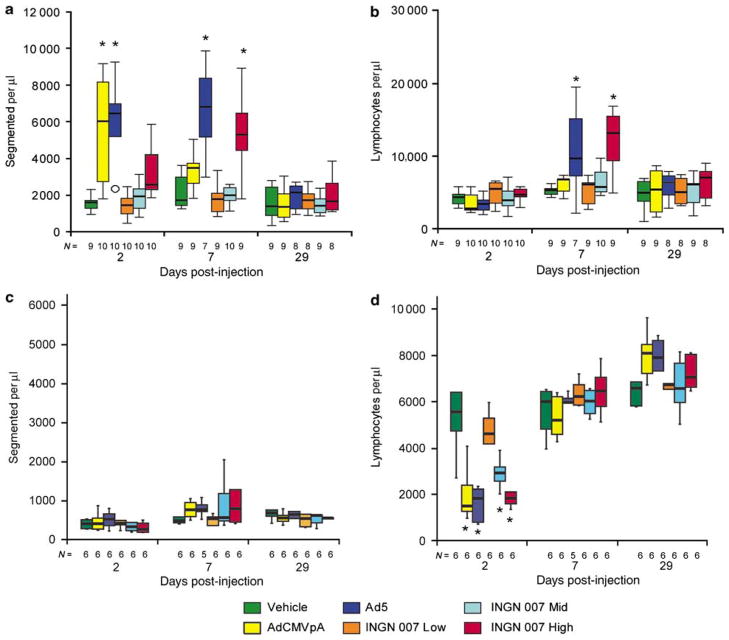
Adenoviruses induced distinct immune responses in hamsters and mice. Immune responses in both species were examined by determining blood leukocyte levels and investigating the development of anti-Ad antibodies. With hamsters, white blood cell counts were increased from the Ad5, AdCMVpA and high-dose INGN 007 groups on day 2, primarily due to increases in neutrophils (Figure 6a). By day 7, lymphocyte (Figure 6b) and monocyte (not shown) counts were also elevated in animals in the high-dose INGN 007 group and in the Ad5group. Only a minimal increase in lymphocyte levels was seen with the AdCMVpA- and the low- and mid-dose INGN 007-injected hamsters. Splenic weights were increased in the AdCMVpA, Ad5and high-dose INGN 007 groups on days 2 and 7, and marginally increased on day 29 in the Ad5- and high-dose INGN 007-injected hamsters.
Figure 6.
The hamster and the mouse immune systems reacted differently to Ad infection. High doses of Ads caused an elevation in neutrophil counts in hamsters (a) but not in mice (c). With hamsters, RC Ads induced more pronounced elevation of lymphocyte counts than AdCMVpA (b), whereas in mice all Ads caused transient lymphopenia (d). N: population size, *P<0.05.
Blood leukocyte levels changed differently with mice. There was no increase in granulocyte numbers (Figure 6c), and a significant drop in lymphocyte numbers was observed at day 2 (Figure 6d). These numbers returned to normal by day 7, and they were marginally elevated by day 29 with the AdCMVpA-, Ad5- and high-dose INGN 007-treated groups. At day 7, splenic weights were increased in mice in all these groups, and by day 29, the mice had mounted a humoral immune response (Figure 7).
Figure 7.
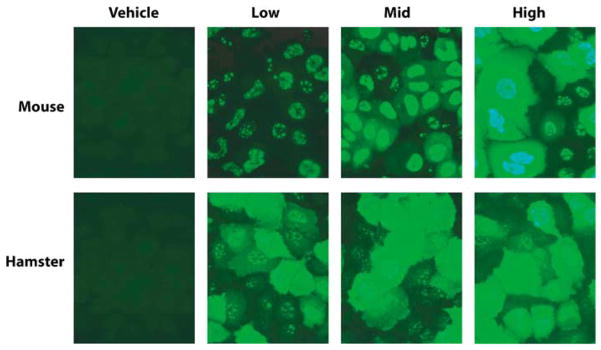
The humoral immune response is dependent on the input dose of RC Ads in mice but not in hamsters. Sera collected from mice and hamsters infected with increasing doses of INGN 007 were used as a primary antibody in indirect immunofluorescence assays against cultured human cells that were infected with Ad5.
Interestingly, the immune response observed in the mice was dose-dependent, whereas the low, mid and high-dose INGN 007 elicited similar response in hamsters (Figure 7). In both species, Ad5and AdCMVpA induced approximately the same level of humoral immune response as the high dose of INGN 007 (not shown).
Other organ systems
There were no treatment-related lesions detected with the following organs: brain, kidneys, urinary bladder, colon, pancreas, heart, skeletal muscle, lungs/bronchi, ovaries, testes, prostate, seminal vesicles, spleen, lymph nodes, thymus and adrenal glands.
Discussion
Replication-competent Ad vectors for cancer therapy have been developed by numerous laboratories. Although these vectors might have an advantage over RD Ad vectors, they also require more rigorous safety testing before they can be used in clinical trials. Here, we present the findings of two toxicological studies with INGN 007 that were performed in two rodent species, the mouse and the Syrian hamster, in preparation for a clinical trial. Based partially on the data presented here, the FDA granted permission to enter into a Phase I clinical trial with INGN 007.
As expected, in both species the liver was the primary target organ. The hepatocellular necrosis observed in both species is in agreement with data previously published with mice.21 With mice, which are poorly permissive for the replication of human Ads,29 toxicity results mainly from the input virus and possibly from the expression of viral genes.30 This was verified in our experiments, as the toxicity of INGN 007 (and Ad5) matched that of the RD vector AdCMVpA in mice. At day 2, similar results were obtained with hamsters, with no difference seen between the RD and RC viruses. At day 7, however, in the permissive hamster model, AdCMVpA induced less damage than INGN 007 or Ad5. Therefore, we conclude that with hamsters, viral replication played a role in the extent of liver damage. As the dose levels in the two animal models differed, the severity of the lesions cannot be compared directly. The differences between the replication of Ads in the two species were borne out in the accompanying paper that describes the biodistribution of Ads in hamsters and mice. Although a large amount of infectious Ad5 and INGN 007 (but no AdCMVpA) was recovered from the liver of hamsters, no infectious Ads were detected in mice.
INGN 007 and Ad5seemed to cause a defect in bone marrow functions in hamsters as evidenced by histopathological lesions and anomalous erythrocyte parameters. As all aberrations were in the minimal to mild range, the clinical relevance of this finding is unknown. Why these changes were not observed in mice is unknown. The fact that AdCMVpA-treated hamsters did not exhibit bone marrow-related pathology indicates that Ad replication or late gene expression is necessary to induce these lesions.
Further differences between the two species’ response to Ad were observed in the immune response to the virus. With mice, a transient, dose-dependent lymphopenia was observed at day 2. This was described earlier for mice25 and rhesus macaques31,32 infected with high doses of RD Ads. This phenomenon was not seen with hamsters; rather, a significant elevation in the neutrophil count was detected that persisted until day 7. At the beginning of the infection, this difference is more likely to be attributed to differences between the immune system of the two rodent species than to differences between Ad replication in these animals, as at day 2 the white blood cell counts of the RD AdCMVpA-infected hamsters were similar to those of hamsters infected with the same dose of Ad5or INGN 007. By day 7, however, both neutrophil and lymphocyte counts were higher in the high-dose RC virus infected hamsters than in the AdCMVpA group, indicating that replication induced a stronger immune response. With mice, no such differences were seen; these animals reacted equally to RC and RD Ads.
Both species produced abundant antibodies to INGN 007. However, while a distinct dose–response was observed with mice (that is, higher doses of INGN 007 elicited a stronger immune response), with hamsters this was less apparent. One possible explanation for this is that virus replication in hamsters may have overcome the original differences in input virus amounts. Differences in the immune response to Ad5and INGN 007 were not observed (not shown), despite the fact that INGN 007 lacks the immunomodulatory E3 genes. At this point, however, it is uncertain if any of the E3 proteins function properly in hamsters; further research is needed to elucidate this.
For the purposes of a clinical trial, we conclude that the No-Observable-Adverse-Effect-Level (NOAEL) in hamsters for INGN 007 is the mid dose (3 × 1010 vp per kg). All changes were reversed by day 29 with the possible exception of a slight increase in the severity of hepatic necrosis/inflammation in males from the high-dose INGN 007 group. Although there were changes in the AdCVMpA group that may have been associated with treatment, those effects were minimal and clearly distinct from changes that were present in the high-dose INGN 007 and Ad5groups. The NOAEL in the mouse study was not established for INGN 007 because chronic inflammation was detected in the liver even in the low-dose INGN 007 group.
Further, changes in the Ad5group were often similar to those of the high-dose INGN 007 hamsters. As INGN 007 does not contain any genetic modification that would limit its replication to cancer cells per se, and it is made more lytic by the overexpression of ADP, it is noteworthy that the toxicity of the vector did not increase compared to wt Ad5. Ad5 causes a mild, self-resolving infection in most immunocompetent adults; therefore, we expect that INGN 007 would have a similar safety profile in human patients.
Supplementary Material
Acknowledgments
This research was supported by Grants CA118022, CA108335and CA81829 to WSMW.
Footnotes
Supplementary Information accompanies the paper on Cancer Gene Therapy website (http://www.nature.com/cgt)
References
- 1.Wold WSM, Horwitz MS. Adenoviruses. In: Knipe DM, Howley PM, editors. Field’s Virology. 5. Lippincott, Williams, & Wilkins; Philadelphia, PA: 2007. pp. 2395–2436. [Google Scholar]
- 2.Jiang H, McCormick F, Lang FF, Gomez-Manzano C, Fueyo J. Oncolytic adenoviruses as antiglioma agents. Expert Rev Anticancer Ther. 2006;6:697–708. doi: 10.1586/14737140.6.5.697. [DOI] [PubMed] [Google Scholar]
- 3.Jounaidi Y, Doloff JC, Waxman DJ. Conditionally replicating adenoviruses for cancer treatment. Curr Cancer Drug Targets. 2007;7:285–301. doi: 10.2174/156800907780618301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thorne SH, Hermiston T, Kirn D. Oncolytic virotherapy: approaches to tumor targeting and enhancing antitumor effects. Semin Oncol. 2005;32:537–548. doi: 10.1053/j.seminoncol.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Young LS, Searle PF, Onion D, Mautner V. Viral gene therapy strategies: from basic science to clinical application. J Pathol. 2006;208:299–318. doi: 10.1002/path.1896. [DOI] [PubMed] [Google Scholar]
- 6.Cattaneo R, Miest T, Shashkova EV, Barry MA. Reprogrammed viruses as cancer therapeutics: targeted, armed and shielded. Nat Rev Microbiol. 2008;6:529–540. doi: 10.1038/nrmicro1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shirakawa T. The current status of adenovirus-based cancer gene therapy. Mol Cells. 2008;25:462–466. [PubMed] [Google Scholar]
- 8.Nemunaitis J, Edelman J. Selectively replicating viral vectors. Cancer Gene Ther. 2002;9:987–1000. doi: 10.1038/sj.cgt.7700547. [DOI] [PubMed] [Google Scholar]
- 9.Nemunaitis J, Vorhies JS, Pappen B, Senzer N. 10-year follow-up of gene-modified adenoviral-based therapy in 146 non-small-cell lung cancer patients. Cancer Gene Ther. 2007;14:762–763. doi: 10.1038/sj.cgt.7701048. [DOI] [PubMed] [Google Scholar]
- 10.Post LE. Selectively replicating adenoviruses for cancer therapy: an update on clinical development. Curr Opin Invest Drugs. 2002;3:1768–1772. [PubMed] [Google Scholar]
- 11.Reid T, Warren R, Kirn D. Intravascular adenoviral agents in cancer patients: Lessons from clinical trials. Cancer Gene Ther. 2002;9:979–986. doi: 10.1038/sj.cgt.7700539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doronin K, Toth K, Kuppuswamy M, Ward P, Tollefson AE, Wold WSM. Tumor-specific, replication-competent adenovirus vectors overexpressing the Adenovirus Death Protein. J Virol. 2000;74:6147–6155. doi: 10.1128/jvi.74.13.6147-6155.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doronin K, Toth K, Kuppuswamy M, Krajcsi P, Tollefson AE, Wold WSM. Overexpression of the ADP (E3-11.6K) protein increases cell lysis and spread of adenovirus. Virology. 2003;305:378–387. doi: 10.1006/viro.2002.1772. [DOI] [PubMed] [Google Scholar]
- 14.Doronin K, Kuppuswamy M, Toth K, Tollefson AE, Krajcsi P, Krougliak V, et al. Tissue-specific, tumor-selective, replication-competent adenovirus vector for cancer gene therapy. J Virol. 2001;75:3314–3324. doi: 10.1128/JVI.75.7.3314-3324.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuppuswamy M, Spencer JF, Doronin K, Tollefson AE, Wold WS, Toth K. Oncolytic adenovirus that overproduces ADP and replicates selectively in tumors due to hTERT promoter-regulated E4 gene expression. Gene Therapy. 2005;12:1608–1617. doi: 10.1038/sj.gt.3302581. [DOI] [PubMed] [Google Scholar]
- 16.Toth K, Djeha H, Ying BL, Tollefson AE, Kuppuswamy M, Doronin K, et al. An oncolytic adenovirus vector combining enhanced cell-to-cell spreading, mediated by the ADP cytolytic protein, with selective replication in cancer cells with deregulated Wnt signaling. Cancer Res. 2004;64:3638–3644. doi: 10.1158/0008-5472.CAN-03-3882. [DOI] [PubMed] [Google Scholar]
- 17.Shashkova EV, Spencer JF, Wold WSM, Doronin K. Human interferon-alpha expression increases antitumor efficacy and reduces hepatotoxicity of E1A-mutated spread-enhanced oncolytic adenovirus. Mol Ther. 2007;15:598–607. doi: 10.1038/sj.mt.6300064. [DOI] [PubMed] [Google Scholar]
- 18.Thomas MA, Spencer JF, La Regina MC, Dhar D, Tollefson AE, Toth K, et al. Syrian hamster as a permissive immunocompetent animal model for the study of oncolytic adenovirus vectors. Cancer Res. 2006;66:1270–1276. doi: 10.1158/0008-5472.CAN-05-3497. [DOI] [PubMed] [Google Scholar]
- 19.Thomas MA, Spencer JF, Toth K, Sagartz JE, Phillips N, Wold WSM. Immunosuppression enhances oncolytic adenovirus replication and anti tumor efficacy in the Syrian hamster model. Mol Ther. 2008;16:1665–1673. doi: 10.1038/mt.2008.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toth K, Spencer JF, Tollefson AE, Kuppuswamy M, Doronin K, Lichtenstein DL, et al. Cotton rat tumor model for the evaluation of oncolytic adenoviruses. Hum Gene Ther. 2005;16:139–146. doi: 10.1089/hum.2005.16.139. [DOI] [PubMed] [Google Scholar]
- 21.Heise CC, Williams AM, Xue S, Propst M, Kirn DH. Intravenous administration of ONYX-015, a selectively replicating adenovirus, induces antitumoral efficacy. Cancer Res. 1999;59:2623–2628. [PubMed] [Google Scholar]
- 22.Lieber A, He CY, Meuse L, Schowalter D, Kirillova I, Winther B, et al. The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. J Virol. 1997;71:8798–8807. doi: 10.1128/jvi.71.11.8798-8807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Q, Muruve DA. Molecular basis of the inflammatory response to adenovirus vectors. Gene Therapy. 2003;10:935–940. doi: 10.1038/sj.gt.3302036. [DOI] [PubMed] [Google Scholar]
- 24.Sauthoff H, Hu J, Maca C, Goldman M, Heitner S, Yee H, et al. Intratumoral spread of wild-type adenovirus is limited after local injection of human xenograft tumors: virus persists and spreads systemically at late time points. Hum Gene Ther. 2003;14:425–433. doi: 10.1089/104303403321467199. [DOI] [PubMed] [Google Scholar]
- 25.Varnavski AN, Calcedo R, Bove M, Gao G, Wilson JM. Evaluation of toxicity from high-dose systemic administration of recombinant adenovirus vector in vector-naive and pre-immunized mice. Gene Therapy. 2005;12:427–436. doi: 10.1038/sj.gt.3302347. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Chirmule N, Gao GP, Qian R, Croyle M, Joshi B, et al. Acute cytokine response to systemic adenoviral vectors in mice is mediated by dendritic cells and macrophages. Mol Ther. 2001;3:697–707. doi: 10.1006/mthe.2001.0329. [DOI] [PubMed] [Google Scholar]
- 27.Hjorth RN, Bonde GM, Pierzchala WA, Vernon SK, Wiener FP, Levner MH, et al. A new hamster model for adenoviral vaccination. Arch Virol. 1988;100:279–283. doi: 10.1007/BF01487691. [DOI] [PubMed] [Google Scholar]
- 28.Toth K, Spencer JF, Dhar D, Sagartz JE, Buller RM, Painter GR, et al. Hexadecyloxypropyl-cidofovir, CMX001, prevents adenovirus-induced mortality in a permissive, immunosuppressed animal model. Proc Natl AcadSci USA. 2008;105:7293–7297. doi: 10.1073/pnas.0800200105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jogler C, Hoffmann D, Theegarten D, Grunwald T, Uberla K, Wildner O. Replication properties of human adenovirus in vivo and in cultures of primary cells from different animal species. J Virol. 2006;80:3549–3558. doi: 10.1128/JVI.80.7.3549-3558.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mccoy RD, Davidson BL, Roessler BJ, Huffnagle GB, Janich SL, Laing TJ, et al. Pulmonary inflammation induced by incomplete or inactivated adenoviral particles. Human Gene Ther. 1995;6:1553–1560. doi: 10.1089/hum.1995.6.12-1553. [DOI] [PubMed] [Google Scholar]
- 31.Schnell MA, Zhang Y, Tazelaar J, Gao GP, Yu QC, Qian R, et al. Activation of innate immunity in nonhuman primates following intraportal administration of adenoviral vectors. Mol Ther. 2001;3:708–722. doi: 10.1006/mthe.2001.0330. [DOI] [PubMed] [Google Scholar]
- 32.Varnavski AN, Zhang Y, Schnell M, Tazelaar J, Louboutin JP, Yu QC, et al. Preexisting immunity to adenovirus in rhesus monkeys fails to prevent vector-induced toxicity. J Virol. 2002;76:5711–5719. doi: 10.1128/JVI.76.11.5711-5719.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.