Abstract
We have previously described oncolytic adenovirus (Ad) vectors KD3 and KD3–interferon (IFN) that were rendered cancer-specific by mutations in the E1A region of Ad; these mutations abolish binding of E1A proteins to p300/CBP and pRB. The antitumor activity of the vectors was enhanced by overexpression of the Adenovirus Death Protein (ADP, E3-11.6K) and by replication-linked expression of IFN-α. We hypothesized that the anticancer efficacy of the KD3–IFN vector could be further improved by expression of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). E1-deleted Ad vectors were constructed carrying reporter genes for enhanced green fluorescent protein or secreted placental alkaline phosphatase (SEAP) and a therapeutic gene for TRAIL under control of the TetON system. Expression of the genes was increased in the presence of a helper virus and the inducer doxycycline such that up to 231-fold activation of expression for the TetON–SEAP vector was obtained. Coinfection with TetON–TRAIL augmented oncolytic activity of KD3 and KD3–IFN in vitro. Induction of TRAIL expression did not reduce the yield of progeny virus. Combination of TetON–TRAIL and KD3–IFN produced superior antitumor activity in vivo as compared with either vector alone demonstrating the efficacy of a four-pronged cancer gene therapy approach, which includes Ad oncolysis, ADP overexpression, IFN-α-mediated immunotherapy, and pharmacologically controlled TRAIL activity.
Keywords: adenovirus, interferon-α, adenovirus death protein, TNF-related apoptosis-inducing ligand, gene expression regulation
Introduction
Oncolytic adenoviruses (Ad) represent a novel class of therapeutic agents that are being developed for the treatment of cancer. The oncolytic Ad ONYX-015, carrying a deletion of the e1b-55k gene, was evaluated in clinical trials and was found to be inefficient when used as a single modality.1,2 At the same time, ONYX-015 had significant anticancer activity in combination with chemotherapy in head and neck cancers.1,3 Recently, the oncolytic vector H101, which is similar to ONYX-015, was approved in China for clinical use in combination with chemotherapy in patients with head and neck cancers.4 Both ONYX-015 and H101 are deletion mutants of human Ad serotype 5 (Ad5) without the addition of an exogenous genetic payload. These vectors exert anticancer activity by killing infected cancer cells as part of the process of Ad replication. Expression of therapeutic transgenes represents an approach to improving the anticancer activity of oncolytic Ad vectors in single-agent treatments and to increasing the antitumor potency of the vectors in multimodal regimens.2
We have previously described the conditionally replicative Ad vector KD3–interferon (IFN) that has demonstrated antitumor activity both in immunodeficient and immunocompetent animal models.5 Replication of KD3–IFN was rendered cancer-specific by the dl1101/1107 mutation in the e1a gene; this mutation abolishes binding of E1A proteins to the cellular p300/CBP and pRB proteins.5,6 The antitumor activity of KD3–IFN was mediated by Ad oncolysis enhanced by overexpression of the Adenovirus Death Protein (ADP; E3-11.6K), which is essential for maximizing vector spread inside the tumor,6 and by the anticancer activity of IFN-α expressed from the vector. IFN-α is a pleiotropic cytokine that has antiproliferative, cytotoxic, antiangiogenic and immunomodulating activities, which are the mechanisms of clinical antitumor activity of recombinant IFN-α protein. The combination of the dl1101/1107 mutation with IFN-α expression provided additional cancer selectivity to the vector rendering replication of KD3–IFN sensitive to IFN-α-mediated antiviral activity in normal cells and consequently improving the therapeutic index of the vector.5
We hypothesized that the combination of KD3–IFN treatment with the proapoptotic activity of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) would produce improved anticancer activity in vitro and in vivo. TRAIL induces apoptosis in a variety of cancer cells via activation of specific death receptors.7 Normal cells were shown to suppress TRAIL-mediated apoptosis by decoy receptors and by activation of nuclear factor κB (NF-κB). 8,9 Replication-deficient (RD) and replication-competent (RC) Ad vectors expressing TRAIL were reported to mediate the production of TRAIL in vivo, and they demonstrated antitumor activity in animal cancer models. 10–15 However, high concentrations of TRAIL were found to be toxic for normal human hepatocytes16,17 and produced severe hepatitis in mice.18
TRAIL-mediated hepatotoxicity can be controlled by pharmacologically regulated expression of TRAIL.18 The TetON regulatable expression system allows for induction of gene expression by tetracycline or its analogue doxycycline (Dox); withdrawal of the inducer switches the expression off.19 We constructed the RD Ad vector TetON–TRAIL, which expresses human TRAIL under the control of the TetON system. We hypothesized that a binary Ad vector system consisting of RD TetON– TRAIL, expressing TRAIL in a pharmacologically controlled mode, and RC KD3–IFN, expressing ADP and IFN-α, could produce improved anticancer activity. It is known that RC Ad can trans-complement replication of an RD vector resulting in amplification of transgene expression.20–23 Therefore, we expected that KD3–IFN would serve as a helper for replication of TetON–TRAIL leading to increased TRAIL expression. IFN-activated signaling pathways were shown to sensitize tumor cells to apoptosis mediated by TRAIL;24 synergistic apoptotic activity of the combination of type I IFN (IFN-α or IFN-β) and TRAIL was demonstrated in various cancer cells in vitro.25–29
In this report, we describe the kinetics and pharmacological control of transgene expression mediated by RD Ad in the presence of trans-complementing oncolytic Ad, and we evaluate the in vitro and in vivo anticancer activity of a binary Ad system including oncolytic Ad, armed with ADP and IFN-α, and RD Ad expressing TRAIL in a pharmacologically controlled manner.
Materials and methods
Cell lines
Human cancer cell lines A549 (lung adenocarcinoma), Hep3B (hepatocellular carcinoma) and DLD-1 (colon adenocarcinoma) were purchased from the American Type Culture Collection (ATCC; Manassas, VA). Human embryonic kidney cells (HEK 293) were from Microbix (Toronto, ON, Canada). All cell lines were maintained in Dulbecco’s modified Eagle’s medium (JRH Biosciences, Lenexa, KS) supplemented with 10% fetal bovine serum (HyClone, Logan, UT). For Ad infection experiments, Tet system-approved fetal bovine serum was used (Clontech Laboratories, Mountain View, CA). RC Ads were propagated in suspension KB cells maintained in Joklik-modified MEM supplemented with 5% equine serum (HyClone).
Virus vectors
The KD3 and KD3–IFN RC Ad vectors were described previously.5,6 KD3 contains the dl1101/1107 mutation in the e1a gene and overexpresses ADP. KD3–IFN is a derivative of KD3 that has the gene for human IFN-α2b inserted downstream of adp. TetON–TRAIL, TetON– enhanced green fluorescent protein (EGFP) and TetON– secreted placental alkaline phosphatase (SEAP) are RD Ads carrying the cDNA for human TRAIL (Invivogen, San Diego, CA), or reporter genes for EGFP (BD Biosciences, San Jose, CA) or SEAP (Invivogen), respectively, in the place of the deletion of the E1 region and under the control of the TetON system (Clontech Laboratories). The levels of SEAP expression driven by the TetON system in the presence of Dox were equal to the levels of expression driven by the cytomegalovirus promoter as measured by quantification of SEAP reporter gene expression in transient plasmid transfection experiments (data not shown). The viruses were propagated in 293 cells or suspension KB cells, purified by CsCl banding, and titered on 293 and A549 cells by limiting dilution assay.
Western blotting
Hep3B cells grown in six-well plates (2 × 105 cells per well) were infected with TetON–TRAIL, KD3, KD3–IFN or coinfected with KD3 and TetON–TRAIL or KD3–IFN and TetON–TRAIL; the multiplicity of infection (MOI) was 50 plaque forming units (PFUs) per cell for each virus in serum-free medium. After 1 h adsorption, the medium was replaced with medium containing 10% fetal bovine serum and 1 μgml−1 of Dox (Sigma-Aldrich, St Louis, MO). Cells were collected at day 2 postinfection. The protein concentration was determined with the Bio-Rad DC protein assay kit (Bio-Rad Laboratories, Hercules, CA). Ten micrograms of protein per sample were separated on 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto an Immobilon polyvinylidene difluoride membrane. After overnight incubation at 4 °C in Tris-buffered saline containing 10% nonfat dry milk, the membrane was probed with anti-human TRAIL antibody (PeproTech, Rocky Hill, NJ). Secondary antibody was goat anti-rabbit horseradish peroxidase (HRPO; Cappel Organon Teknika, Durham, NC). Specific bands were visualized by the enhanced chemiluminescence system (Amersham Biosciences, Piscataway, NJ).
Cell culture studies
To study the kinetics of transgene expression, 293 cells grown in six-well plates (2 × 106 cells per well) were infected in triplicate with TetON–SEAP at an MOI of 10PFU per cell in the presence or absence of Dox (1 μgml−1). Hep3B (2 × 105 cells per well), A549 (2 × 105 cells per well) and DLD-1 (1 × 106 cells per well) cells grown in six-well plates were infected in triplicate with TetON–SEAP or coinfected with TetON–SEAP and KD3 or TetON–SEAP and KD3–IFN at an MOI of 10PFU per cell for each virus (total of 20PFU per cell for coinfection groups) in the presence or absence of Dox (1 μgml−1). SEAP activity in the medium at varying time points was quantified by OD405 measurement of p-nitrophenyl phosphate conversion (Sigma-Aldrich).30
To study the effect of inducible TRAIL expression amplified by vector replication on cell viability, Hep3B cells grown in six-well plates (3 × 105 cells per well) were coinfected with KD3 and TetON–TRAIL or TetON– EGFP at an MOI of 10PFU per cell for each virus (total of 20PFU per cell) in the absence or presence of Dox (1 μgml−1). Cells were microphotographed at the indicated time points.
To quantify cell viability, Hep3B and DLD-1 cells grown in 48-well plates (3 × 104 cells per well and 1 × 105 cells per well, respectively), were infected in triplicate with KD3 or KD3–IFN, or coinfected with KD3 and TetON– TRAIL, KD3 and TetON–EGFP, KD3–IFN and TetON– TRAIL, or KD3–IFN and TetON–EGFP at an MOI of 10PFU per cell for each virus in the presence or absence of Dox (1 μgml−1). At day 2 (DLD-1) or day 3 (Hep3B) after infection, cell viability was quantified by an MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay (Sigma-Aldrich). The viability of mock-infected cells at the end of the experiment was considered to be 100%. 293 cells grown in 96-well plate (8 × 103 cells per well) were infected in triplicate at day 0 with TetON–TRAIL or TetON–EGFP at an MOI ranging from 100 to 0.001PFU per cell in the presence or absence of Dox (1 μgml−1). Cell viability was quantified at day 6 postinfection using the MTT assay.
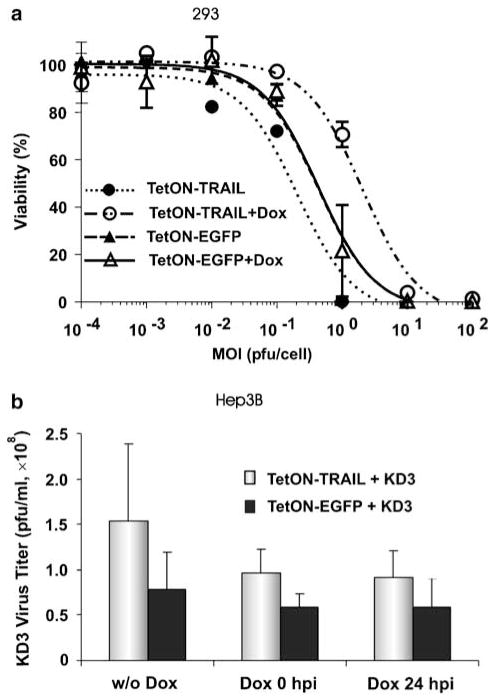
To study the effect of TRAIL expression on the extracellular yields of an RC vector, Hep3B cells grown in six-well plates (3 × 105 cells per well) were coinfected in triplicate with KD3 and TetON–TRAIL, or KD3 and TetON–EGFP at an MOI of 10PFU per cell for each virus. Dox (1 μgml−1) was added to the medium at the time of infection or 24 h postinfection. Samples of the medium were collected at days 1, 2 and 3 and viral titers were determined by limiting dilution assay on A549 cells.
In vivo study
The study was approved by the Saint Louis University Animal Care Committee and was performed in accordance with institutional and federal regulations. Subcutaneous Hep3B tumors were established in the hind flank of female nude mice (4- to 6-week-old, Harlan Sprague–Dawley, Indianapolis, IN) by injecting 5 × 106 cells in 100 μl of Dulbecco’s modified Eagle’s medium containing 50% Matrigel (BD Biosciences). At 18 days later the mice were randomized and assigned into groups with an average tumor volume of 235mm3. The animals were injected intratumorally with PBS (n = 5), 1 × 108PFU of TetON–TRAIL (n = 5), 1 × 109PFU of KD3–IFN (n = 11) or 1 × 108PFU of TetON–TRAIL plus 1 × 109PFU of KD3–IFN (n = 11) in 100 μl of PBS. The injections were repeated every second day for a total of five injections per tumor. Starting from day 9 after the first injection (the next day after the last virus injection) and until day 24 (15 days total), mice received Dox (2mg ml−1) with drinking water containing 5% sucrose. Control mice received water with 5% sucrose. Tumor dimensions were measured every second day, and tumor volumes were calculated as length × (width)2 × 0.5. Mice were euthanized when the tumor volume reached 10% bodyweight (2000mm3; uncensored event); when animals became cachexic (censored event) or when tumors became ulcerated (censored event).
Statistical analysis
The data are presented as mean ± s.d. for in vitro experiments, and mean ± s.e.m. for in vivo experiments. Statistical analyses were performed using SPSS (SPSS, Chicago, IL). The statistical significance of in vitro data was assessed with the General Linear Model followed by Tukey’s HSD test for pairwise comparisons between groups. In vivo tumor volume data were analyzed with the Mann–Whitney U-test and survival rates were analyzed with the log-rank test. P<0.05 was considered to be significant.
Results
Design of the vectors
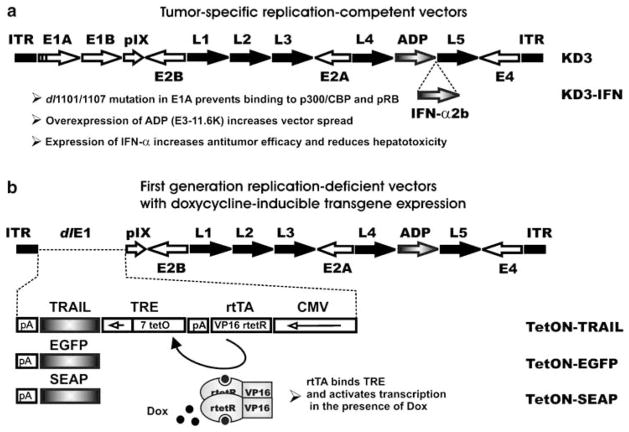
KD3 (Figure 1a) is an oncolytic Ad vector that replicates selectively in cancer cells due to the dl1101/1107 mutation in the e1a gene which abolishes binding of E1A proteins to cellular p300/CBP and pRb, and it has improved virus release and spread due to overexpression of ADP.6,31 The KD3–IFN vector (Figure 1a) is a derivative of KD3, which expresses human IFN-α late in infection, and it has superior anticancer activity in vivo relative to KD3.5 In addition, the combination of the dl1101/1107 mutation and IFN-α expression resulted in reduced hepatotoxicity of KD3–IFN in mice as compared to KD3.5
Figure 1.
Schematics of vector genomes. Open arrows, early Ad regions. Closed arrows, late Ad regions. Gray arrows or boxes, inserted transgenes. Closed boxes, inverted terminal repeats (ITR). Short arrow in an open box, minimal CMV promoter. TRE, tetracycline-response element; rtetR, reverse tetracycline repressor; tetO, tetracycline operator; rtTA, reverse tetracycline transcriptional activator; pA, polyadenylation signals. (a) KD3 and KD3–IFN are conditionally replicative Ad vectors carrying two small deletions in the E1A region rendering virus replication cancer-specific. The vectors overexpress ADP for improved spread. KD3–IFN expresses human IFN-α2b strictly late in infection for increased anticancer activity and reduced hepatotoxicity of the vector. (b) TetON–TRAIL, TetON–EGFP and TetON–SEAP are replication-defective Ad vectors carrying TRAIL, EGFP or SEAP expression cassettes, respectively, in place of the E1 region deletion. The CMV promoter in the expression cassette drives expression of rtTA which consists of rtetR fused to VP16, a transcriptional activator derived from herpes simplex virus. rtTa binds to tetO in the presence of Dox, activating a minimal CMV promoter and leading to transgene expression. CMV, cytomegalovirus. EGFP, enhanced green fluorescent protein; IFN, interferon; SEAP, secreted placental alkaline phosphatase; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand.
Due to the potential toxicity of TRAIL,17,18 control of TRAIL expression might be required for prevention of side effects during treatment. The TetON expression system allows for induction of transgene expression in the presence of tetracycline or its derivative (for example, Dox); withdrawal of the inducer inhibits transgene expression.19 We constructed the RD Ad vectors TetON–TRAIL, TetON–EGFP and TetON–SEAP expressing TRAIL, EGFP and SEAP, respectively, under control of the TetON system (Figure 1b). The vectors carry expression cassettes in place of the E1 deletion. The E3 region in these vectors is identical to the E3 region in KD3 allowing for overexpression of ADP in the absence of immunomodulatory E3 genes.32 The TetON–EGFP and TetON–SEAP vectors were constructed for visualization 33 and quantification30 of transgene expression, respectively.
Kinetics and regulation of transgene expression from RD Ad coinfected with helper RC Ad
We sought to evaluate the effect of trans-complementation on pharmacological control of transgene expression driven by the inducible TetON system. It has been reported previously that oncolytic Ad supports the replication of RD Ad when two viruses coinfect the cell.6,20,31,34,35 We have previously demonstrated that each coinfected A549 cell produced 300 infectious units of RD Ad-βgal vector upon coinfection with RC KD3 vector.6 Trans-complementation allows for amplification of transgene expression from a non-replicative virus. We sought to study the kinetics of transgene expression mediated by RD Ad alone or in coinfection with an oncolytic virus.
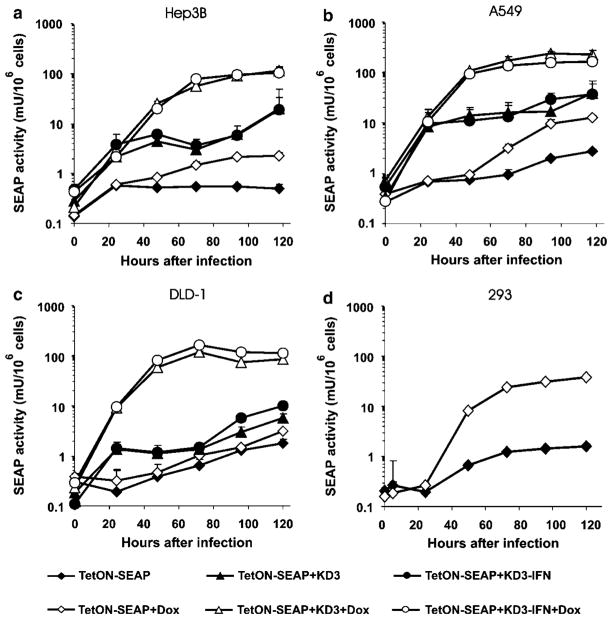
To study the kinetics of transgene expression in cancer cell lines that do not support replication of RD Ad, we infected Hep3B, A549 and DLD-1 cells with the TetON– SEAP vector alone, or in combination with the RC vectors KD3 or KD3-IFN. SEAP activity in the medium was measured at the indicated time points after infection. Addition of Dox increased SEAP activity in all cell lines (Figures 2a–c). Activation of SEAP expression by the addition of Dox to the TetON–SEAP-infected cells was in the range of 1.7- to 4.7-fold at day 5 postinfection. Coinfection of the cells with TetON–SEAP and either RC vector produced higher levels of SEAP expression as compared to the levels produced by infection with the TetON–SEAP vector alone (Table 1). Pharmacological control of SEAP expression was sustained in TetON– SEAP and RC Ad-coinfected cells, with Dox inducing 4.3- to 14.8-fold activation.
Figure 2.
Coinfection with oncolytic Ad allows for regulatable expression of SEAP from the RD Ad TetON–SEAP. (a) Hep3B, (b) A549, (c) DLD-1 and (d) 293 cells were infected with TetON–SEAP, TetON–SEAP+KD3 or TetON–SEAP+KD3–IFN at an MOI of 10PFU per cell for each virus in the presence or absence of Dox. SEAP enzymatic activity in the medium was determined at various time points. MOI, multiplicity of infection; PFU, plaque forming unit; RD, replication deficient; SEAP, secreted placental alkaline phosphatase.
Table 1.
Activation levels of SEAP expression in cancer cells infected with RD Ad TetON–SEAP or coinfected with TetON–SEAP and RC Ad KD3 or KD3–IFN (fold increase)
| Dox |
TetON–SEAP
|
TetON–SEAP +KD3
|
TetON–SEAP +KD3–IFN
|
|||
|---|---|---|---|---|---|---|
| − | + | − | + | − | + | |
| A549 | 1 | 4.7±0.4 | 14.1±7.9 | 82.4±18.5 | 13.8±7.6 | 59.3±8.6 |
| Hep3B | 1 | 4.5±0.7 | 39.5±23.4 | 231.9±48.7 | 37.5±58.0 | 205.1±54.4 |
| DLD-1 | 1 | 1.7±0.1 | 3.2±1.1 | 47.2±4.2 | 5.7±0.9 | 63.8±3.8 |
| 293 | 1 | 23.7±1.7a | ND | ND | ND | ND |
Abbreviation: ND, not determined; IFN, interferon; RC, replication competent; SEAP, secreted placental alkaline phosphatase.
TetON–SEAP replicates in 293 cells.
There was no statistically significant difference in the levels of SEAP activity in Hep3B and A549 cells regardless of the helper virus used (P>0.14). In DLD-1 cells, the KD3–IFN helper provided slightly but significantly higher SEAP expression than did KD3 (P<0.001). An IFN-α-stimulated response element-like sequence within the TRE of the TetON system has been reported,36 suggesting that the expression of IFN-α by KD3–IFN could mediate various levels of SEAP expression in different cell lines.
We also studied the kinetics, levels and regulation of gene expression mediated by TetON–SEAP in 293 cells, which support replication of the vector (Figure 2d). In the absence of Dox, we could detect low SEAP activity in the medium of infected cells at late time points resulting from leakiness of the TetON system. A progressive increase of SEAP activity was observed in the presence of Dox, reaching a cumulative 23.7-fold activation of expression at day 5 postinfection (Table 1).
The overall levels of SEAP expression in A549 and Hep3B cells coinfected with TetON–SEAP and KD3 or KD3–IFN, or in 293 cells infected with TetON–SEAP in the presence of Dox were 23- to 53-fold lower when compared to the expression levels produced by the RC Ad vector expressing SEAP under control of the major late promoter.5,30
Trans-complementing binary vector system allows for regulatable expression of functionally active TRAIL
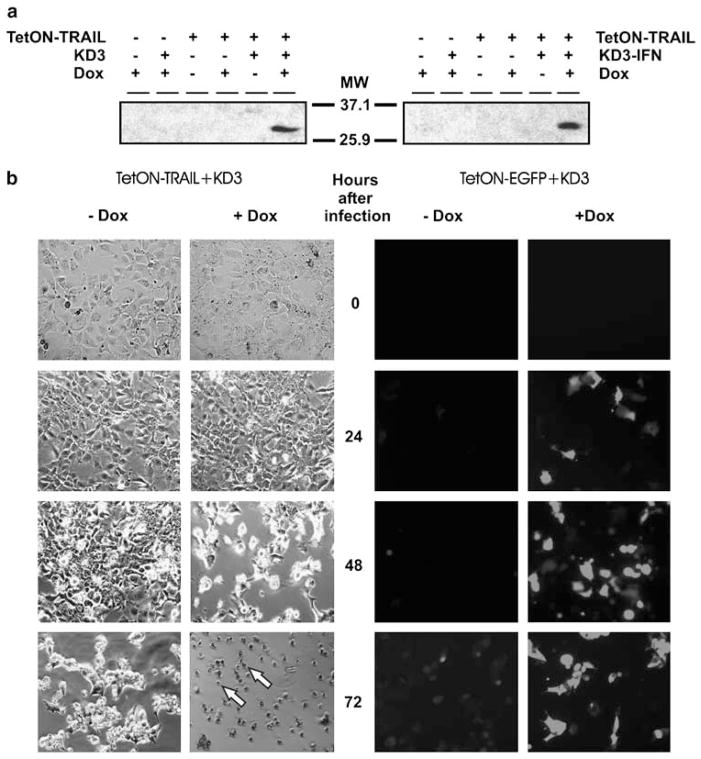
We studied the expression of TRAIL in Hep3B cells infected with the TetON–TRAIL vector alone or in combination with KD3 or KD3–IFN. TRAIL was detected by western blotting only in cells coinfected with TetON–TRAIL and either RC vector, and only in the presence of Dox (Figure 3a) implying that the antibody against TRAIL had a sensitivity threshold preventing detection of TRAIL at lower concentrations. Coinfection with either KD3 or KD3–IFN resulted in similar levels of TRAIL. To confirm that the TRAIL produced by TetON–TRAIL was functionally active, we coinfected Hep3B cells with TetON–TRAIL and KD3 and monitored the morphology of the cells (Figure 3b, left). Dox caused an augmented cytopathic effect, beginning from 48 h, compared to cells without Dox, in which cytopathic effect began to manifest at 72 h after infection. Cells infected with TetON–TRAIL alone in the absence of an RC helper and in the presence of Dox also developed cytopathic effect which was delayed by 1–2 days as compared with cells coinfected with TetON–TRAIL and KD3 in the presence of Dox (data not shown). The levels of cytopathic effect depending on the presence or absence of Dox correlated with the levels of transgene expression as monitored by fluorescence microscopy of Hep3B cells coinfected with TetON–EGFP and KD3 in the presence or absence of Dox (Figure 3b, right). These results indicate that TetON–TRAIL expresses functionally active TRAIL in Hep3B cells and that pharmacological control of TRAIL expression is maintained in the presence of trans-complementing helper virus.
Figure 3.
TetON–TRAIL expresses TRAIL. (a) Detection of TRAIL expression by TetON–TRAIL after coinfection with RC Ad in the presence of Dox. Hep3B cells were infected with the indicated vectors at an MOI of 50PFU per cell for each virus in the presence or absence of Dox. TRAIL expression in the cells at day 2 postinfection was detected by western blotting. (b) The functional activity of TRAIL and EGFP is regulated by Dox in cells coinfected with TetON–TRAIL or TetON–EGFP and helper RC Ad. Hep3B cells were coinfected with TetON–TRAIL and KD3 or with TetON–EGFP and KD3 at an MOI of 10PFU per cell in the presence or absence of Dox. Phase-contrast or fluorescence microphotographs were taken at indicated time points. Original magnification × 100. Arrows indicate the apoptotic morphology of cells. EGFP, enhanced green fluorescent protein; MOI, multiplicity of infection; PFU, plaque forming unit; RC, replication competent; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand.
TRAIL expression increases the oncolytic efficacy of cancer-specific RC Ad in cancer cells
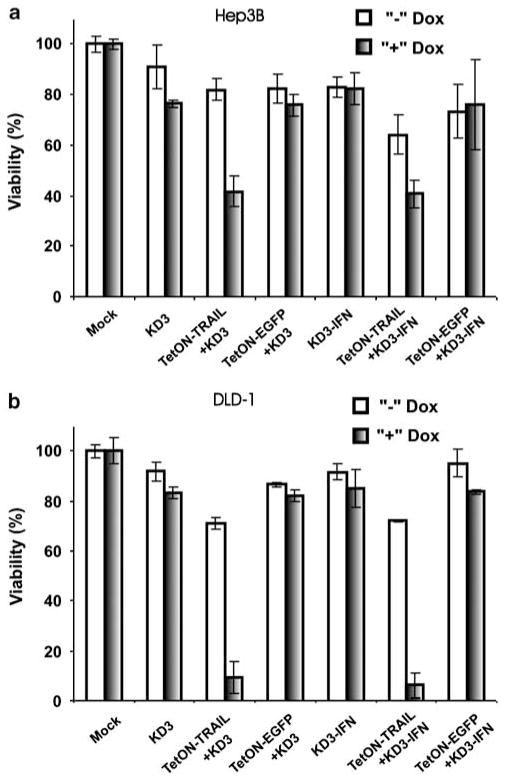
The combination of RC Ad oncolytic activity with the expression of TRAIL was shown to be superior to either modality alone in suppressing breast cancer cells in vitro and in vivo,23 and it revealed synergistic caspase activation in hepatoma cells in vitro.37 To assess whether regulatable expression of TRAIL from TetON–TRAIL improves the oncolytic activity of the conditionally replicative Ads KD3 and KD3–IFN, Hep3B and DLD-1 cells were infected with KD3 or KD3–IFN, or coinfected with TetON–TRAIL or TetON–EGFP and either of the RC vectors (Figure 4). In both cell lines, Dox-induced TRAIL expression but not EGFP expression (P<0.002) increased cell death when compared with either RC vector alone (P<0.001). DLD-1 cells were more sensitive to TRAIL, and the combination of TetON–TRAIL with an RC vector caused more cell death in this cell line at day 2 after infection relative to the level of cell death in Hep3B cells at day 3 after infection. The KD3 and KD3–IFN vectors were equally effective in killing cancer cells (P>0.45 for Hep3B, P>0.96 for DLD-1).
Figure 4.
TRAIL enhances the oncolytic effect of tumor-specific, RC Ad in cancer cell lines. (a) Hep3B and (b) DLD-1 cells were infected with KD3, TetON–TRAIL+KD3, TetON–EGFP+KD3, KD3–IFN, TetON–TRAIL+KD3–IFN or TetON–EGFP+KD3–IFN at an MOI of 10PFU per cell for each virus in the presence or absence of Dox. Cell viability was quantified at day 3 (Hep3B) or day 2 (DLD-1) postinfection. EGFP, enhanced green fluorescent protein; IFN, interferon; MOI, multiplicity of infection; PFU, plaque forming unit; RC, replication competent; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand.
High induced level of TRAIL expression impairs spread of TetON–TRAIL in 293 cells; low level of expression improves the spread
293 cells express E1A and E1B proteins allowing for replication of E1-deleted Ad vectors. We sought to study the effect of regulated TRAIL expression on the replication and spread of Ad vectors in this complementing cell line. For this purpose, we infected 293 cells with TetON– TRAIL or TetON–EGFP at various MOIs (Figure 5a). Induction of EGFP expression by Dox did not change the MOI–response curve for the TetON–EGFP vector (EC50 = 0.4PFU per cell for both groups). There was no statistically significant difference between the levels of cell death mediated by TetON–EGFP depending on the presence or absence of Dox (P = 0.9). In contrast, the induction of TRAIL expression significantly changed the profile of the MOI–response curve for the TetON– TRAIL vector (P<0.001 for TetON–TRAIL vs TetON– TRAIL+Dox). Interestingly, induction of TRAIL expression resulted in markedly reduced vector spread and cell death mediated by TetON–TRAIL relative to cells infected with TetON–TRAIL in the absence of Dox (EC50 = 0.2PFU per cell for TetON–TRAIL, EC50 = 2PFU per cell for TetON–TRAIL+Dox). TetON–TRAIL in the absence of Dox killed cells at low MOIs significantly better than did TetON–EGFP both in the presence and absence of Dox (P<0.02), whereas TetON– TRAIL+Dox produced reduced levels of cell death in comparison to both the TetON–EGFP and TetON– EGFP+Dox groups (P<0.001).
Figure 5.
Influence of TRAIL on spread and extracellular yields of oncolytic Ad. (a) High level of TRAIL expression impairs TetON–TRAIL spread in 293 cells; low basal expression improves the spread. 293 cells were infected with TetON–TRAIL or TetON–EGFP at various MOI. Transgene expression was induced by Dox. Cell viability was quantified at day 6 postinfection. (b) Expression of TRAIL increases the extracellular titers of the tumor-specific oncolytic Ad in Hep3B cells. Cells were coinfected with TetON–TRAIL and KD3, or TetON–EGFP and KD3, at an MOI of 10PFU per cell. Transgene expression was induced by addition of Dox to the medium at 0 or 24 h postinfection. Virus titers were determined in the medium at 48 h postinfection. EGFP, enhanced green fluorescent protein; MOI, multiplicity of infection; PFU, plaque forming unit; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand.
TRAIL expression from TetON–TRAIL does not compromise replication of cancer-specific oncolytic Ad in Hep3B cells
It has been shown that premature apoptosis of Ad-infected cells can reduce Ad yields and spread.38–41 TRAIL-mediated induction of apoptosis, therefore, could potentially decrease oncolytic Ad yields when these two modalities are combined. In contrast, apoptosis induced at late stages of Ad infection can increase the fraction of virus progeny released from infected cells and result in improved spread of virus.38 We evaluated the impact of TRAIL on the titers of cancer-specific RC Ad in the supernatants of Hep3B cells infected with TetON–TRAIL and KD3. To study the effect of TRAIL expression timing on KD3 replication, Dox was added to the cells at the time of infection or 24 h postinfection (Figure 5b). We found that KD3 titers were increased in the medium of cells coinfected with TetON–TRAIL as compared to TetON–EGFP coinfected cells at 24 h (P = 0.019) (data not shown) and 48 h (P = 0.033) (Figure 5b) but not at 72 h postinfection (P = 0.221) (data not shown). Dox did not significantly influence KD3 titers at any time point although there was a trend toward significance with increase in time after infection (24 h: P = 0.515; 48 h: P = 0.103; 72 h: P = 0.076).
Combination of regulatable TRAIL expression with KD3–IFN provides enhanced antitumor activity in vivo
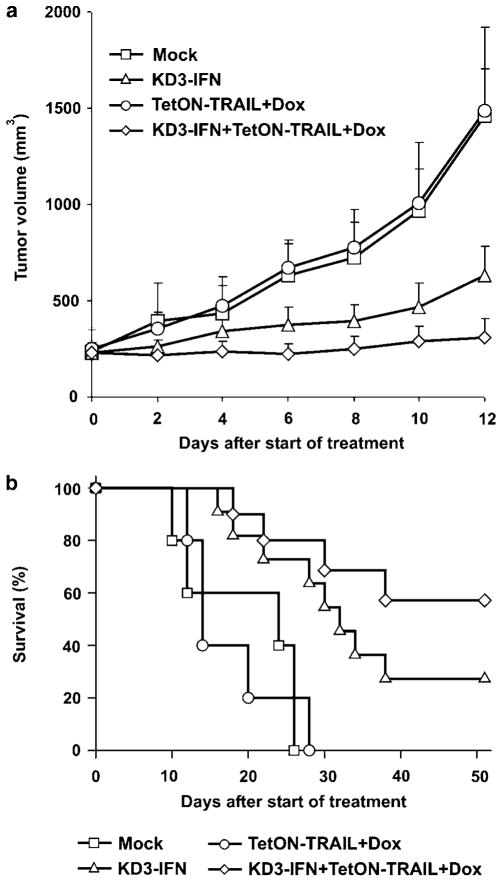
We have previously shown that the cancer-specific oncolytic Ad vector KD3–IFN had superior antitumor activity in large established xenografts of human hepatocellular carcinoma in nude mice relative to the parental KD3 vector.5 In this study, we sought to evaluate whether regulatable expression of TRAIL could further improve the antitumor activity of KD3–IFN in this model. Subcutaneous xenografts of Hep3B cells established in nude mice were injected intratumorally with TetON– TRAIL, KD3–IFN or a combination of the vectors; the injections were repeated every second day for a total of 5 × 108 or 5 × 109 PFU per tumor for TetON–TRAIL or KD3–IFN, respectively. Expression of TRAIL was induced by addition of Dox to drinking water. The TetON–TRAIL+Dox-treated group was not different from the buffer-treated group on day 12 after the start of the treatment (P = 0.59) (Figure 6a). In contrast, both the KD3–IFN and KD3–IFN+TetON–TRAIL+Dox-treated groups were significantly different from the buffer and TetON–TRAIL+Dox groups (P<0.037). The combination treatment produced superior tumor suppression in comparison with KD3–IFN (P = 0.049). Survival rates of the mice treated with buffer or TetON–TRAIL+Dox were not statistically different (P = 0.96) (Figure 6b). Both the KD3–IFN- and KD3–IFN+TetON–TRAIL+Dox-treated groups had significantly prolonged survival relative to buffer and TetON–TRAIL+Dox-treated groups (P ≤ 0.01). Three mice in the coinfection group were euthanized at days 16 or 40 due to cachexia and at day 22 due to tumor ulceration and were analyzed as censored events. The difference in survival between the KD3–IFN- and KD3–IFN+TetON–TRAIL+Dox-treated groups did not reach statistical significance in this experiment (P = 0.18). However, all four surviving mice in the combination treatment group were either tumor-free or had residual tumors with less than 10mm3 volume at the end of the experiment (day 51 after start of treatment). In the KD3–IFN-treated group there was one tumor-free mouse and two mice with tumors of volume 32 and 800mm3 at the end of the experiment, respectively.
Figure 6.
Antitumor activity of KD3–IFN combined with regulatable expression of TRAIL. (a) Expression of TRAIL increases the antitumor efficacy of the cancer-specific Ad armed with ADP and IFN-α. Established subcutaneous Hep3B tumors were injected intratumorally with PBS (n = 5), 1 × 109PFU of KD3–IFN (n = 11), 1 × 108PFU of TetON–TRAIL (n = 5) or 1 × 109PFU of KD3–IFN plus 1 × 108PFU of TetON–TRAIL (n = 11). The injections were repeated with 1-day intervals for a total of five injections. Mice injected with TetON–TRAIL or TetON–TRAIL+KD3–IFN received Dox with the drinking water. (b) For animals from (a) Kaplan–Meier survival curves were plotted. ADP, Adenovirus Death Protein; IFN, interferon; PFU, plaque forming unit; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand.
Discussion
New generations of oncolytic Ad vectors with increased anticancer potency and specificity are being developed for anticancer treatment. 2 We have recently described the cancer-specific RC Ad vector KD3–IFN which demonstrated antitumor activity mediated by Ad oncolysis that was improved by overexpression of ADP and by expression of functionally active IFN-α.5 Importantly, the combination of a specific mutation in the E1A region of the vector with IFN-α expression resulted in higher cancer specificity of KD3–IFN as compared with the parental KD3 vector. In the present study, we tested the hypothesis that the proapoptotic activity of TRAIL could further enhance the antitumor efficacy of KD3– IFN.
High concentrations of TRAIL were found to induce hepatotoxicity.16–18 However, pharmacological regulation of Ad-mediated TRAIL expression can allow for control over potential side effects of TRAIL treatment.18 The limited cloning capacity of RC Ad vectors prevents the use of large two-step transcription amplification systems such as TetON. Accordingly, we constructed the RD Ad vector TetON–TRAIL which expresses TRAIL under control of the TetON system to be used as an RD component of a binary Ad vector system.
We hypothesized that a binary vector system including RC KD3–IFN and RD TetON–TRAIL could produce improved anticancer efficacy. RC Ad vectors support replication of coinfected RD Ad leading to amplification of transgene expression from the RD vector.6,20,22,23,31,42 rtTA, used in the TetON system, is known to have some affinity for TetO sequences within the TRE in the absence of Dox, leading to low basal expression of the transgene in the absence of inducer.19,43 Increased expression of rtTA due to replication of the RD vector in the presence of helper virus, therefore, could theoretically diminish the pharmacological control of TetON-driven transgene expression. Using the TetON–SEAP vector, expressing the reporter gene under control of the TetON system, we demonstrated that regulatability of SEAP expression is maintained in cells coinfected with TetON–SEAP and RC Ad KD3 or KD3–IFN. Addition of Dox to the coinfected cells produced a 4.3- to 14.8-fold increase in SEAP activity in various cancer cell lines as compared to cells without Dox. Further, as expected, Dox induced up to 58-fold higher SEAP activity in coinfected cells relative to cells infected with the TetON–SEAP vector alone. Together, the presence of the helper virus plus Dox mediated up to 231-fold activation of SEAP expression from the TetON–SEAP vector. However, the complementing functions from the RC Ad also increased the basal expression of SEAP in the absence of Dox; these were found to be from 3- to 39-fold higher relative to the basal expression detected in the absence of helper virus. New versions of tetracycline-inducible promoters with tighter regulation of transgene expression should also be tested experimentally in combination with RC Ad to find an optimal regulatable system for use in trans-complementing infections.
We found that TetON–TRAIL improved the oncolytic activity of KD3 and KD3–IFN in Hep3B and DLD-1 cells in the presence of Dox. Notably, the activity of TRAIL was regulated in a trans-complementing infection. As the induction of apoptosis in Ad-infected cells can influence the yields and spread of RC Ad,38 we examined the effect of regulatable TRAIL expression on the spread of TetON–TRAIL in 293 cells, which support replication of RD Ad vectors. Interestingly, induced TRAIL expression impaired spread of the vector in this cell line when compared to spread of the TetON–EGFP vector. However, TetON–TRAIL spread was improved in the absence of Dox as compared with TetON–EGFP spread, resulting in higher cell killing at low MOI. TRAIL was shown to activate the transcription factor NF-κB in 293 cells, leading to overexpression of the TRAIL death receptor DR5 and thereby amplifying the apoptotic response to TRAIL.44 This might explain the deleterious effect of induced high levels of TRAIL on the replication of TetON–TRAIL in 293 cells; that is, the early apoptosis would kill infected cells before the virus matures and is released from the cells.38 In contrast, low levels of TRAIL might induce apoptosis later in infection and facilitate the release and spread of virus progeny. Taken together, these results suggest that the effect of TRAIL expression on the anticancer activity of replicative Ad is dependent on the sensitivity of the infected cells to TRAIL-induced apoptosis; therefore, different levels of TRAIL expression might be required for various cancer cells when these two modalities are combined.
The Ad E3 proteins 6.7K, RIDα/β and 14.7K, whose genes are deleted from the TetON–TRAIL, were shown to inhibit TRAIL-induced apoptosis in Ad-infected cells.45–47 The retention of these genes in TetON–TRAIL might be necessary for combining this vector with RC Ad in cancer cells that are highly sensitive to TRAIL-mediated apoptosis to prevent early death of the infected cells and to allow for a completion of the RC Ad infection cycle. Alternatively, the use of a weak promoter instead of an inducible promoter system for driving TRAIL expression might be sufficient for improvement of the oncolytic activity of an RC Ad in TRAIL-sensitive tumor cells.
The release from the infected cells and consequent dissemination of virus progeny are important determinants of oncolytic activity of RC viruses.6,31 We studied the effect of TRAIL-induced apoptosis on the release of KD3 from Hep3B cells infected with KD3 and TetON– TRAIL. The extracellular titers of KD3 from TetON– TRAIL coinfected cells were increased as compared to those from TetON–EGFP coinfected cells at early time points but not at a late time point. Induction of TRAIL expression did not reduce the KD3 titers whether Dox was added early or late in infection cycle suggesting that the relative resistance of Hep3B cells to TRAIL-induced apoptosis25,48 allows for the combination of RC Ad with high induced levels of TRAIL expression without a deleterious effect on Ad replication.
The combination treatment including TetON– TRAIL+Dox and KD3–IFN produced superior anti-tumor activity in vivo relative to either modality alone, significantly suppressing the growth of Hep3B tumor xenografts in nude mice. This result could be explained by a combination of several mechanisms including a paracrine effect of TRAIL amplified by the RC virus, an autocrine effect of TRAIL on oncolytic virus release, and IFN-mediated sensitization of cancer cells to TRAIL-induced apoptosis. Further detailed study is warranted to investigate the role of each mechanism in the overall antitumor activity of the binary vector system. Additionally, further studies may reveal an important role for the timing of the induction of TRAIL expression on the antitumor efficacy of the treatment.
Binary Ad systems represent a flexible approach in improving the antitumor efficacy of existing or novel oncolytic Ad vectors, and they provide the necessary cloning capacity for additional therapeutic transgenes and the ability to regulate the expression of toxic genes. The recently described exploitation of infected cells as carriers for the systemic delivery of oncolytic viruses to the tumor site49,50 represents an attractive strategy that could theoretically be readily applied to the delivery of binary Ad vector systems for the treatment of primary tumors and metastatic tumor nodules. Expression of Ad ‘stealth’ genes by RD Ad might also decrease immune-mediated clearance of the coinfected cell carriers in the immunocompetent host and lead to further improvement of antitumor efficacy.32,51,52
Ad genome modifications introduced with the goal of improving the toxicity profile can compromise the oncolytic potency of the vectors due to attenuation of their replication in cancer cells as compared with wild-type (wt) Ad5. However, wt Ad5 can cause lethal outcomes in immunocompromised patients.53 As cancer patients are often immunosuppressed, safety features might represent essential characteristics of recombinant Ad vectors that are being developed as oncolytic agents. It remains to be tested experimentally whether the therapeutic index of the binary system offers an advantage over other oncolytic agents including wt Ad5.
In conclusion, the RD Ad vector expressing TRAIL enhances the anticancer activity of RC Ad in cancer cell cultures, and the pharmacological control of TRAIL expression is maintained in a trans-complementing infection. Importantly, such combinations appear to require fine tuning of the levels of TRAIL expression depending on the sensitivity of the tumor cells to TRAIL-mediated apoptosis and the antiapoptotic properties of the Ad vector backbone to achieve optimal treatment efficacy. In large established Hep3B tumor xenografts, the binary Ad system produced improved antitumor efficacy as compared to the Ad components applied separately. These results demonstrate the efficacy of a four-pronged approach to anticancer therapy, which includes Ad oncolysis, ADP overexpression, IFN-α-mediated immunotherapy and pharmacologically controlled activity of TRAIL.
Acknowledgments
This work was supported by NIH Grants CA108335 and CA118022 to WSMW, and CA105841 to KD.
References
- 1.Kirn D. Clinical research results with dl1520 (Onyx-015), a replication-selective adenovirus for the treatment of cancer: what have we learned? Gene Therapy. 2001;8:89–98. doi: 10.1038/sj.gt.3301377. [DOI] [PubMed] [Google Scholar]
- 2.Hermiston T. A demand for next-generation oncolytic adenoviruses. Curr Opin Mol Ther. 2006;8:322–330. [PubMed] [Google Scholar]
- 3.Khuri FR, Nemunaitis J, Ganly I, Arseneau J, Tannock IF, Romel L, et al. A controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med. 2000;6:879–885. doi: 10.1038/78638. [DOI] [PubMed] [Google Scholar]
- 4.Garber K. China approves world’s first oncolytic virus therapy for cancer treatment. J Natl Cancer Inst. 2006;98:298–300. doi: 10.1093/jnci/djj111. [DOI] [PubMed] [Google Scholar]
- 5.Shashkova EV, Spencer JF, Wold WS, Doronin K. Targeting interferon-alpha increases antitumor efficacy and reduces hepatotoxicity of E1A-mutated spread-enhanced oncolytic adenovirus. Mol Ther. 2007;15:598–607. doi: 10.1038/sj.mt.6300064. [DOI] [PubMed] [Google Scholar]
- 6.Doronin K, Toth K, Kuppuswamy M, Ward P, Tollefson AE, Wold WS. Tumor-specific, replication-competent adenovirus vectors overexpressing the adenovirus death protein. J Virol. 2000;74:6147–6155. doi: 10.1128/jvi.74.13.6147-6155.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 8.Sheridan JP, Marsters SA, Pitti RM, Gurney A, Skubatch M, Baldwin D, et al. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science. 1997;277:818–821. doi: 10.1126/science.277.5327.818. [DOI] [PubMed] [Google Scholar]
- 9.Degli-Esposti MA, Dougall WC, Smolak PJ, Waugh JY, Smith CA, Goodwin RG. The novel receptor TRAIL-R4 induces NF-kappaB and protects against TRAIL-mediated apoptosis, yet retains an incomplete death domain. Immunity. 1997;7:813–820. doi: 10.1016/s1074-7613(00)80399-4. [DOI] [PubMed] [Google Scholar]
- 10.Kagawa S, He C, Gu J, Koch P, Rha SJ, Roth JA, et al. Antitumor activity and bystander effects of the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) gene. Cancer Res. 2001;61:3330–3338. [PubMed] [Google Scholar]
- 11.Sova P, Ren XW, Ni S, Bernt KM, Mi J, Kiviat N, et al. A tumor-targeted and conditionally replicating oncolytic adenovirus vector expressing TRAIL for treatment of liver metastases. Mol Ther. 2004;9:496–509. doi: 10.1016/j.ymthe.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Ren XW, Liang M, Meng X, Ye X, Ma H, Zhao Y, et al. A tumor-specific conditionally replicative adenovirus vector expressing TRAIL for gene therapy of hepatocellular carcinoma. Cancer Gene Ther. 2006;13:159–168. doi: 10.1038/sj.cgt.7700868. [DOI] [PubMed] [Google Scholar]
- 13.Dong F, Wang L, Davis JJ, Hu W, Zhang L, Guo W, et al. Eliminating established tumor in nu/nu nude mice by a tumor necrosis factor-{alpha}-related apoptosis-inducing ligand-armed oncolytic adenovirus. Clin Cancer Res. 2006;12:5224–5230. doi: 10.1158/1078-0432.CCR-06-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Gu J, Zhao L, He L, Qian W, Wang J, et al. Complete elimination of colorectal tumor xenograft by combined manganese superoxide dismutase with tumor necrosis factor-related apoptosis-inducing ligand gene virotherapy. Cancer Res. 2006;66:4291–4298. doi: 10.1158/0008-5472.CAN-05-1834. [DOI] [PubMed] [Google Scholar]
- 15.Griffith TS, Broghammer EL. Suppression of tumor growth following intralesional therapy with TRAIL recombinant adenovirus. Mol Ther. 2001;4:257–266. doi: 10.1006/mthe.2001.0439. [DOI] [PubMed] [Google Scholar]
- 16.Jo M, Kim TH, Seol DW, Esplen JE, Dorko K, Billiar TR, et al. Apoptosis induced in normal human hepatocytes by tumor necrosis factor-related apoptosis-inducing ligand. Nat Med. 2000;6:564–567. doi: 10.1038/75045. [DOI] [PubMed] [Google Scholar]
- 17.Armeanu S, Lauer UM, Smirnow I, Schenk M, Weiss TS, Gregor M, et al. Adenoviral gene transfer of tumor necrosis factor-related apoptosis-inducing ligand overcomes an impaired response of hepatoma cells but causes severe apoptosis in primary human hepatocytes. Cancer Res. 2003;63:2369–2372. [PubMed] [Google Scholar]
- 18.Ichikawa K, Liu W, Zhao L, Wang Z, Liu D, Ohtsuka T, et al. Tumoricidal activity of a novel anti-human DR5 monoclonal antibody without hepatocyte cytotoxicity. Nat Med. 2001;7:954–960. doi: 10.1038/91000. [DOI] [PubMed] [Google Scholar]
- 19.Goverdhana S, Puntel M, Xiong W, Zirger JM, Barcia C, Curtin JF, et al. Regulatable gene expression systems for gene therapy applications: progress and future challenges. Mol Ther. 2005;12:189–211. doi: 10.1016/j.ymthe.2005.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Habib NA, Mitry R, Seth P, Kuppuswamy M, Doronin K, Toth K, et al. Adenovirus replication-competent vectors (KD1, KD3) complement the cytotoxicity and transgene expression from replication-defective vectors (Ad-GFP, Ad-Luc) Cancer Gene Ther. 2002;9:651–654. doi: 10.1038/sj.cgt.7700481. [DOI] [PubMed] [Google Scholar]
- 21.Lee CT, Lee YJ, Kwon SY, Lee J, Kim KI, Park KH, et al. In vivo imaging of adenovirus transduction and enhanced therapeutic efficacy of combination therapy with conditionally replicating adenovirus and adenovirus-p27. Cancer Res. 2006;66:372–377. doi: 10.1158/0008-5472.CAN-05-1515. [DOI] [PubMed] [Google Scholar]
- 22.Thorne SH, Tam BY, Kirn DH, Contag CH, Kuo CJ. Selective intratumoral amplification of an antiangiogenic vector by an oncolytic virus produces enhanced antivascular and anti-tumor efficacy. Mol Ther. 2006;13:938–946. doi: 10.1016/j.ymthe.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 23.Guo W, Zhu H, Zhang L, Davis J, Teraishi F, Roth JA, et al. Combination effect of oncolytic adenovirotherapy and TRAIL gene therapy in syngeneic murine breast cancer models. Cancer Gene Ther. 2006;13:82–90. doi: 10.1038/sj.cgt.7700863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clemens MJ. Interferons and apoptosis. J Interferon Cytokine Res. 2003;23:277–292. doi: 10.1089/107999003766628124. [DOI] [PubMed] [Google Scholar]
- 25.Shigeno M, Nakao K, Ichikawa T, Suzuki K, Kawakami A, Abiru S, et al. Interferon-alpha sensitizes human hepatoma cells to TRAIL-induced apoptosis through DR5 upregulation and NF-kappa B inactivation. Oncogene. 2003;22:1653–1662. doi: 10.1038/sj.onc.1206139. [DOI] [PubMed] [Google Scholar]
- 26.Liedtke C, Groger N, Manns MP, Trautwein C. Interferon-alpha enhances TRAIL-mediated apoptosis by up-regulating caspase-8 transcription in human hepatoma cells. J Hepatol. 2006;44:342–349. doi: 10.1016/j.jhep.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 27.Kumar-Sinha C, Varambally S, Sreekumar A, Chinnaiyan AM. Molecular cross-talk between the TRAIL and interferon signaling pathways. J Biol Chem. 2002;277:575–585. doi: 10.1074/jbc.M107795200. [DOI] [PubMed] [Google Scholar]
- 28.Chawla-Sarkar M, Leaman DW, Jacobs BS, Borden EC. IFN-beta pretreatment sensitizes human melanoma cells to TRAIL/Apo2 ligand-induced apoptosis. J Immunol. 2002;169:847–855. doi: 10.4049/jimmunol.169.2.847. [DOI] [PubMed] [Google Scholar]
- 29.Leaman DW, Chawla-Sarkar M, Vyas K, Reheman M, Tamai K, Toji S, et al. Identification of X-linked inhibitor of apoptosis-associated factor-1 as an interferon-stimulated gene that augments TRAIL Apo2L-induced apoptosis. J Biol Chem. 2002;277:28504–28511. doi: 10.1074/jbc.M204851200. [DOI] [PubMed] [Google Scholar]
- 30.Doronin KK, Zakharchuk AN, Grinenko NF, Yurov GK, Krougliak VA, Naroditsky BS. Expression of the gene encoding secreted placental alkaline phosphatase (SEAP) by a nondefective adenovirus vector. Gene. 1993;126:247–250. doi: 10.1016/0378-1119(93)90374-c. [DOI] [PubMed] [Google Scholar]
- 31.Doronin K, Toth K, Kuppuswamy M, Krajcsi P, Tollefson AE, Wold WS. Overexpression of the ADP (E3-11.6K) protein increases cell lysis and spread of adenovirus. Virology. 2003;305:378–387. doi: 10.1006/viro.2002.1772. [DOI] [PubMed] [Google Scholar]
- 32.Lichtenstein DL, Toth K, Doronin K, Tollefson AE, Wold WS. Functions and mechanisms of action of the adenovirus E3 proteins. Int Rev Immunol. 2004;23:75–111. doi: 10.1080/08830180490265556. [DOI] [PubMed] [Google Scholar]
- 33.Shashkova EV, Cherenova LV, Kazansky DB, Doronin K. Avian adenovirus vector CELO-TK displays anticancer activity in human cancer cells and suppresses established murine melanoma tumors. Cancer Gene Ther. 2005;12:617–626. doi: 10.1038/sj.cgt.7700822. [DOI] [PubMed] [Google Scholar]
- 34.Guse K, Dias JD, Bauerschmitz GJ, Hakkarainen T, Aavik E, Ranki T, et al. Luciferase imaging for evaluation of oncolytic adenovirus replication in vivo. Gene Therapy. 2007;14:902–911. doi: 10.1038/sj.gt.3302949. [DOI] [PubMed] [Google Scholar]
- 35.Alemany R, Lai S, Lou YC, Jan HY, Fang X, Zhang WW. Complementary adenoviral vectors for oncolysis. Cancer Gene Ther. 1999;6:21–25. doi: 10.1038/sj.cgt.7700001. [DOI] [PubMed] [Google Scholar]
- 36.Rang A, Will H. The tetracycline-responsive promoter contains functional interferon-inducible response elements. Nucleic Acids Res. 2000;28:1120–1125. doi: 10.1093/nar/28.5.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wirth T, Kuhnel F, Fleischmann-Mundt B, Woller N, Djojosubroto M, Rudolph KL, et al. Telomerase-dependent virotherapy overcomes resistance of hepatocellular carcinomas against chemotherapy and tumor necrosis factor-related apoptosis-inducing ligand by elimination of Mcl-1. Cancer Res. 2005;65:7393–7402. doi: 10.1158/0008-5472.CAN-04-3664. [DOI] [PubMed] [Google Scholar]
- 38.Mi J, Li ZY, Ni S, Steinwaerder D, Lieber A. Induced apoptosis supports spread of adenovirus vectors in tumors. Hum Gene Ther. 2001;12:1343–1352. doi: 10.1089/104303401750270995. [DOI] [PubMed] [Google Scholar]
- 39.Ganly I, Kim YT, Hann B, Balmain A, Brown R. Replication and cytolysis of an E1B-attenuated adenovirus in drug-resistant ovarian tumour cells is associated with reduced apoptosis. Gene Therapy. 2001;8:369–375. doi: 10.1038/sj.gt.3301402. [DOI] [PubMed] [Google Scholar]
- 40.Chiou SK, White E. Inhibition of ICE-like proteases inhibits apoptosis and increases virus production during adenovirus infection. Virology. 1998;244:108–118. doi: 10.1006/viro.1998.9077. [DOI] [PubMed] [Google Scholar]
- 41.Subramanian T, Vijayalingam S, Chinnadurai G. Genetic identification of adenovirus type 5 genes that influence viral spread. J Virol. 2006;80:2000–2012. doi: 10.1128/JVI.80.4.2000-2012.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jounaidi Y, Waxman DJ. Use of replication-conditional adenovirus as a helper system to enhance delivery of P450 prodrug-activation genes for cancer therapy. Cancer Res. 2004;64:292–303. doi: 10.1158/0008-5472.can-03-1798. [DOI] [PubMed] [Google Scholar]
- 43.Sipo I, Hurtado PA, Wang X, Eberle J, Petersen I, Weger S, et al. An improved Tet-On regulatable FasL-adenovirus vector system for lung cancer therapy. J Mol Med. 2006;84:215–225. doi: 10.1007/s00109-005-0009-1. [DOI] [PubMed] [Google Scholar]
- 44.Shetty S, Gladden JB, Henson ES, Hu X, Villanueva J, Haney N, et al. Tumor necrosis factor-related apoptosis inducing ligand (TRAIL) up-regulates death receptor 5 (DR5) mediated by NFkappaB activation in epithelial derived cell lines. Apoptosis. 2002;7:413–420. doi: 10.1023/a:1020031023947. [DOI] [PubMed] [Google Scholar]
- 45.Benedict CA, Norris PS, Prigozy TI, Bodmer JL, Mahr JA, Garnett CT, et al. Three adenovirus E3 proteins cooperate to evade apoptosis by tumor necrosis factor-related apoptosis-inducing ligand receptor-1 and -2. J Biol Chem. 2001;276:3270–3278. doi: 10.1074/jbc.M008218200. [DOI] [PubMed] [Google Scholar]
- 46.Tollefson AE, Toth K, Doronin K, Kuppuswamy M, Doronina OA, Lichtenstein DL, et al. Inhibition of TRAIL-induced apoptosis and forced internalization of TRAIL receptor 1 by adenovirus proteins. J Virol. 2001;75:8875–8887. doi: 10.1128/JVI.75.19.8875-8887.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lichtenstein DL, Doronin K, Toth K, Kuppuswamy M, Wold WS, Tollefson AE. Adenovirus E3-6. 7K protein is required in conjunction with the E3-RID protein complex for the internalization and degradation of TRAIL receptor 2. J Virol. 2004;78:12297–12307. doi: 10.1128/JVI.78.22.12297-12307.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamanaka T, Shiraki K, Sugimoto K, Ito T, Fujikawa K, Ito M, et al. Chemotherapeutic agents augment TRAIL-induced apoptosis in human hepatocellular carcinoma cell lines. Hepatology. 2000;32:482–490. doi: 10.1053/jhep.2000.16266. [DOI] [PubMed] [Google Scholar]
- 49.Power AT, Bell JC. Cell-based delivery of oncolytic viruses: a new strategic alliance for a biological strike against cancer. Mol Ther. 2007;15:660–665. doi: 10.1038/sj.mt.6300098. [DOI] [PubMed] [Google Scholar]
- 50.Pereboeva L, Curiel DT. Cellular vehicles for cancer gene therapy: current status and future potential. BioDrugs. 2004;18:361–385. doi: 10.2165/00063030-200418060-00003. [DOI] [PubMed] [Google Scholar]
- 51.Wang Y, Hallden G, Hill R, Anand A, Liu TC, Francis J, et al. E3 gene manipulations affect oncolytic adenovirus activity in immunocompetent tumor models. Nat Biotechnol. 2003;21:1328–1335. doi: 10.1038/nbt887. [DOI] [PubMed] [Google Scholar]
- 52.Toth K, Doronin K, Kuppuswamy M, Ward P, Tollefson AE, Wold WS. Adenovirus immunoregulatory E3 proteins prolong transplants of human cells in immunocompetent mice. Virus Res. 2005;108:149–159. doi: 10.1016/j.virusres.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 53.Kojaoghlanian T, Flomenberg P, Horwitz MS. The impact of adenovirus infection on the immunocompromised host. Rev Med Virol. 2003;13:155–171. doi: 10.1002/rmv.386. [DOI] [PubMed] [Google Scholar]