Abstract
Objective
To determine whether patterns of functional connectivity of cortical regions responsible for auditory processing and executive functions differ in children with unilateral hearing loss (UHL) versus their normal hearing (NH) siblings.
Study Design
Prospective observational study
Setting
Academic medical center
Subjects and Methods
Children with severe-to-profound UHL (9 right UHL, 7 left UHL) and 10 NH sibling controls were imaged using resting state functional connectivity MRI (rs-fcMRI). All MRI images were transformed to a single common atlas; regions of interest (ROI) were chosen based on previous literature and unpublished results. Mean region-wise correlations and conjunction analyses were performed across 34 seed ROIs to identify temporally synchronized, low-frequency spontaneous fluctuations in the resting state blood oxygenation level-dependent (BOLD) signal that reveal functionally related regions.
Results
Mean region-wise T-tests found a left posterior opercular region with more correlated resting state activity with the inferior parietal lobule seed in the children with both left and right UHL than NH. In conjunction analysis, 4 regions showed different resting-state functional interactions between the NH and both UHL groups. These differences were in left medial globus pallidus, left middle temporal gyrus, right parahippocampal gyrus, and in mid-cingulate cortex. These regions include areas associated with auditory processing, executive function and memory formation.
Conclusions
Resting state fcMRI identified differences in brain network interconnections between children with UHL and NH and may inform further investigation into the educational and behavioral difficulties experienced by children with UHL.
Keywords: unilateral hearing loss, children, auditory processing, executive function
INTRODUCTION
In the past, unilateral hearing loss (UHL) in children was presumed to be of little consequence because speech and language skills were thought to develop appropriately with one normal hearing ear. Some studies reported that a significantly increased proportion (22–59%) of children with UHL may have educational and/or behavioral problems, compared to their normal hearing (NH) peers,1–4 as well as speech-language delays, increased rate of grade failures, and increased need for educational assistance.5 A recent case-control study comparing children with UHL with their NH siblings has shown that UHL is associated with a significant negative effect on scores in standardized language tests in elementary school children.6 Children with UHL also demonstrated increased rates of Individualized Education Plans (IEPs) and receipt of extra educational assistance compared to their siblings. These studies suggest that UHL affects not only auditory functions such as sound localization and listening in noise, but also may affect speech and language development. Furthermore, the academic and behavioral problems documented in many children with UHL may be related to the development of executive functions.
Resting state functional connectivity MRI (rs-fcMRI) measures low frequency (<0.1 Hz) blood oxygen level-dependent (BOLD) signal fluctuations of the brain at rest which are thought to be consequences of spontaneous neural activity.7–9 Correlations between brain regions in these low frequency BOLD fluctuations are presumed to reflect a long history of those regions “firing together,” hence being “functionally connected.”9, 10 Because rs-fcMRI methods are independent from experimental design, subject compliance, attention-to-task, and the training demands of learning a particular task, these imaging studies are ideal for populations of children and clinical groups who may be less able to comply with complex, attention-demanding tasks in the MRI scanner.11
In this study, we sought to investigate inter-regional functional connections in the brains of children with UHL using rs-fcMRI. We compared functional connectivity in hearing-related and executive function-related regions between children with UHL and their age-matched NH siblings. The auditory regions of interest were in primary auditory cortex (Brodmann's area [BA] 41), and secondary auditory (association) cortex, known as the planum polare and the planum temporale.12 We also examined two other sets of cortical regions shown to be functionally connected to the auditory cortex.13 These are in temporo-parieto-fronto network (Heschl's gyrus, planum temporale, posterior superior temporal sulcus, parietal lobe) which is thought to be responsible for perceptual integration of auditory signals, and dorsolateral- and medial-prefrontal cortex, which are thought to be related to attentional control. The other regions of interest included locations thought to be responsible for executive functions, such as task set maintenance and error monitoring; a default mode network thought to be required for “internally directed mental activity,” and whose activity decreases during goal-oriented tasks; as well as phonological and sensorimotor regions.9, 14–16 We hypothesized that children with UHL may possess different patterns of functional connectivity in brain regions responsible for executive functions, thus explaining some of the behavioral and educational difficulties experienced by these children.
MATERIALS AND METHODS
The Washington University Medical Center Human Research Protection Office gave institutional review board approval prior to the initiation of this study. All parents gave written informed consent and all participants gave pediatric assent for this study.
Participants
Children age 7–17 years with UHL were recruited from the Unilateral Hearing Loss in Children study.6 Inclusion criterion was severe-to-profound sensorineural UHL (defined as pure tone average [PTA] (500, 1000, 2000, 4000 Hz) at least 70 dB hearing level (HL) in the affected ear; and PTA [500, 1000, and 2000 Hz) less than 20 dB HL in the better hearing ear, with a threshold at 4000 Hz less than 30 dB). Exclusion criteria were any cognitive or neurodevelopmental delay or syndrome, such as cerebral palsy, Down syndrome, chromosomal abnormality, or congenital cytomegalovirus infection; temporary or conductive hearing loss; and contraindication to MRI scanning (e.g., metallic implant).
Controls with normal hearing (NH), ages 7–17 years, were recruited from among the siblings of the children with UHL. The inclusion criterion for the controls was NH in both ears. Exclusion criteria were the same as for the participants with UHL. Although 31 children were recruited and participated, data from 26 were usable: 9 children with right-sided UHL, 7 children with left-sided UHL, and 10 normal-hearing controls.
MRI Data Acquisition and Preprocessing
Scanning Protocol and Image Acquisition
All images were obtained using the same Siemens TRIO 3.0 Tesla scanner (Erlangen, Germany) in the same scanning session. A high-resolution T1-weighted sagittal MPRAGE structural image (TE = 3.08 ms, TR (partition) =2.4 sec, TI = 1000 ms, flip angle = 8 degrees, 176 slices with 1×1×1 mm voxels) was obtained. This image was used to compute atlas transformation.
Functional imaging was performed using a blood oxygenation level-dependent (BOLD) contrast sensitive asymmetric spin-echo echo-planar sequence (volume TR=2.5 s, in-plane resolution 4×4 mm, T2* evolution time=27 ms, α=90°). Whole brain coverage was obtained with 32 contiguous, 4 mm-thick axial slices presented parallel to the anterior commissure-posterior commissure plane. Resting state fcMRI scanning data were collected in multiple 3-minute runs while subjects maintained gaze on a white fixation cross on a black background. At least 9 minutes of resting state data was obtained for each subject.
fcMRI Data Preprocessing
The BOLD images produced in each run were first combined into a 4D (x, y, z, time) time series. Next, timing offsets among slices within the same frame were compensated through sinc interpolation. Then slice intensity differences due to contiguous interleaved slice acquisition were removed. Finally, a six-parameter rigid body alignment registered all frames in all runs and was used for motion correction in each subject.17 Reslicing was by 3D cubic spline interpolation, and all image data were transformed to Talairach18 atlas space using a single common atlas19 derived from adult and child brains14 via a warping mechanism. The first four image acquisitions (10 s) of each run were discarded to allow for normalization of longitudinal magnetization. For each fMRI run the mode voxel intensity value was normalized to 1000.
Functional Connectivity Preprocessing
Preprocessing for the functional connectivity analyses was carried out as previously described to optimize the time-series data and to remove spurious variance.9 The steps include removal of the linear trend, temporal band-pass filtering (0.009 Hz < f < 0.08 Hz) and spatial smoothing at 6 mm full width at half maximum, as well as regression of six motion parameters, signals from whole brain, ventricle, and white matter, and the time-based derivatives of each. For six of the participants, frames with excessive movement (a total RMS>1.5 mm) were deleted from the analysis.
Region of Interest (ROI) Definition
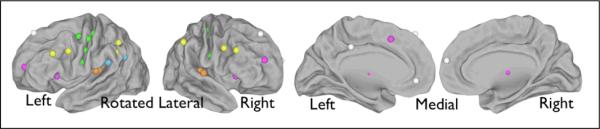
Hearing-related ROIs as well as cognitive control, default and sensorimotor regions were chosen based on previous literature9, 14, 15, 20, 21 and unpublished results; 10 mm diameter spheres centered on the coordinates were used. Seed ROIs used in this study, their coordinates, and their sources, are shown in Figure 1 and listed in Table 1.
Figure 1.
Seed regions from Table 1 projected to a brain surface [Van Essen, 2005 #6469]. Auditory regions are shown in red, cingulo-opercular in pink, default-mode in white, frontoparietal in yellow, sensorimotor in green, and language in blue. The lateral surfaces have been rotated slightly toward an anterior view to show regions in the central sulcus, and the regions are shown larger than their actual 10 mm diameter for ease of visualization.
Table 1.
Brain system, regions and coordinates examined in this study
| Coordinates |
|||||
|---|---|---|---|---|---|
| System | Brain Regions | Source | X | y | z |
|
Auditory
|
L Heschl | unpublished | −39 | −35 | 12 |
| L BA41 | WOROI20 | −51 | −28 | 14 | |
| R BA41 | WOROI | 52 | −23 | 10 | |
| R Heschl | Jancke et al21 | 61 | −11 | 8 | |
|
| |||||
|
Cingulo-Opercular
|
dACC/msFC | Dosenbach15 | −1 | 10 | 46 |
| L aPFC | Dosenbach | −28 | 51 | 15 | |
| R aPFC | Dosenbach | 27 | 50 | 23 | |
| L anterior thalamus | Dosenbach | −12 | −15 | 7 | |
| R anterior thalamus | Dosenbach | 10 | −15 | 8 | |
| L aIfO | Dosenbach | −35 | 14 | 5 | |
| R aIfO | Dosenbach | 36 | 16 | 4 | |
|
| |||||
|
Default Mode
|
amPFC | Fox et al9 | 1 | 54 | 21 |
| vmPFC | Fox et al | −3 | 39 | −2 | |
| L superior frontal | Fox et al | −14 | 38 | 52 | |
| R superior frontal | Fox et al | 17 | 37 | 52 | |
| PCC | Fox et al | −2 | −36 | 37 | |
|
| |||||
|
Fronto-Parietal
|
L frontal | Dosenbach | −41 | 3 | 36 |
| R frontal | Dosenbach | 41 | 3 | 36 | |
| L IPL | Dosenbach | −51 | −51 | 36 | |
| R IPL | Dosenbach | 51 | −47 | 42 | |
| L IPS | Dosenbach | −31 | −59 | 42 | |
| R IPS | Dosenbach | 30 | −61 | 39 | |
| L dlPFC | Dosenbach | −43 | 22 | 34 | |
| R dlPFC | Dosenbach | 43 | 22 | 34 | |
|
| |||||
|
Phonological
|
Angular gyrus | Church et al14 | −49 | −62 | 29 |
| Supramarginal | Church et al | −52 | −42 | 24 | |
|
| |||||
|
Sensorimotor
|
L motor hand a | unpublished | −32 | −11 | 51 |
| L motor handb | unpublished | −36 | −28 | 55 | |
| L sensory cortex | unpublished | −41 | −20 | 50 | |
| L motor mouth a | unpublished | −45 | −15 | 38 | |
| L motor mouth b | unpublished | −51 | −10 | 25 | |
| R sensory cortex | unpublished | 33 | −29 | 55 | |
| R motor hand | unpublished | 44 | −21 | 48 | |
| R motor mouth | unpublished | 50 | −9 | 27 | |
BA41, Brodmann's Area 41; dACC/msFC, dorsal anterior cingulate cortex/medial superior frontal cortex; PFC, prefrontal cortex; aPFC, anterior PFC; aIfO, anterior insula/frontal operculum; amPFC, anterior medial PFC; vmPFC, ventromedial PFC; PCC, posterior cingulate cortex; IPL, inferior parietal lobule; IPS, intraparietal sulcus; dlPFC, dorsolateral PFC.
Brain regions discovered in our analyses were identified through direct visualization of the region on the subjects' average structural anatomy.
Statistical Analysis
Computation of Mean Regionwise Correlations
For each subject, a resting state BOLD time series was calculated for each of the 34 seeds regions. For each seed region, a correlation of this time series with the time series in each voxel of the brain was calculated to produce a correlation seed map. T-tests were performed on the Fisher Z transformed seed maps from each region to compare the brain correlation patterns of the control subjects to those of the UHL subjects. A z-score of greater than 3 (P < 0.001 uncorrected) with a cluster size greater than 810 cubic mm (thirty 3×3×3 mm voxels) was used to determine statistical significance, in an attempt to correct for multiple comparisons.
Conjunction Analysis
To identify brain locations in which subjects with both right and left UHL differed from controls, a conjunction analysis was performed across all 34 seed regions using the following procedures. First, masks were created from the z-transformed t-test image for each seed region, assigning each voxel with a statistical value of z ≥1.75 a mask value of 1, and all other voxels a value of 0. Second, these masks were summed across seeds to create conjunction images whose voxel values were the number of seeds showing differences between UHL and control subjects at that voxel. Four conjunction images were created, separating right and left UHL groups as well as positive (controls more correlated than UHL) and negative t-test differences (UHL more correlated than controls). Third, masks of these conjunction images were made, requiring the contribution of at least 5 seed regions at each voxel. Finally, the centers of mass and contributing voxels of these overlap regions were determined using an automated algorithm.14 The seed regions contributing to each of these overlap regions for each UHL group were identified.
RESULTS
Demographics
Demographic characteristics, educational and medical history of the study participants are summarized in Table 2. There were no significant differences in age, sex, or handedness between the subjects with UHL and the control subjects. Of note, the UHL children were found to have higher rates of speech and language problems than their NH siblings. They were more likely to have required speech therapy, and were also more likely to have received an Individualized Educational Plan at school.
Table 2.
Demographic and socioeconomic information about the participants.
| Characteristic | NH (n=10) | All UHL (n=16) | P Value |
|---|---|---|---|
| Male sex, n (%) | 6 (60) | 8 (50) | .62 |
| Age in years, mean, (SD) | 11.8 (2.6) | 11.0 (3.5) | .50 |
| Race/ethnicity, n (%) | .83 | ||
| African American | 2 (20) | 2 (13) | |
| Caucasian | 7 (70) | 11 (69) | |
| Asian | 0 | 1 (6) | |
| Other | 1 (10) | 2 (13) | |
| Hispanic/Latino | 0 | 1 (6) | |
| Number of siblings, n (%) | .56 | ||
| 1 | 4 (40) | 4 (25) | |
| 2 | 5 (50) | 8 (50) | |
| 3 or more | 1 (10) | 4 (25) | |
| Type of Insurance, n (%) | .24 | ||
| Medicaid | 0 | 2 (13) | |
| Private | 10 (100) | 14 (88) | |
| Premature, n (%) | 3 (30) | 2 (13) | .27 |
| Speech Delays, n (%) | 2 (20) | 4 (25) | .47 |
| Language Delays, n (%) | 0 | 3 (19) | .35 |
| Speech/Language Evaluation, n (%) | 2 (20) | 10 (63) | .03 |
| Speech Problems, n (%) | 2 (20) | 9 (56) | .07 |
| Speech Therapy, n (%) | 0 | 7 (44) | .03 |
| Repeat Grade, n (%) | 0 | 3 (19) | .15 |
| Individualized education plan | 1 (10) | 8 (50) | .04 |
| Asthma, n (%) | 1 (10) | 2 (13) | .85 |
| Recurrent Otitis Media, n (%) | 2 (20) | 2 (13) | .61 |
| Chronic condition, n (%) | 3 (30) | 8 (50) | .32 |
| Tympanostomy tubes, n (%) | 6 (60) | 6 (38) | .26 |
| Other Ear Surgeries, n (%) | 1 (10) | 4 (25) | .35 |
| Dominant Hand, n (%) | .80 | ||
| Left | 2 (20) | 2 (13) | |
| Right | 7 (70) | 13 (81) | |
| Both | 1 (10) | 1 (6) | |
| Wears Glasses, n (%) | 2 (20) | 6 (31) | .35 |
NH, children with normal-hearing; UHL, children with unilateral hearing loss; SD, standard deviation
fcMRI Data Analysis
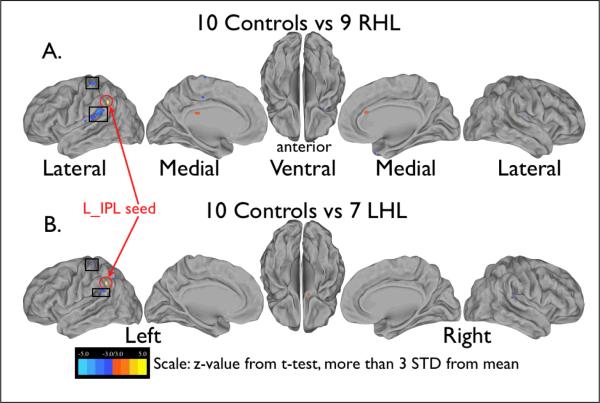
The correlation maps for each group of subjects (left UHL, right UHL, NH controls) were compared using groupwise t-tests to identify voxels whose correlations with the timecourse of the seed region differed significantly between groups. The t-test images in Figure 2 show the only seed (left inferior parietal lobule, L_IPL) which had overlapping significant differences for NH versus both right and left UHL. This fronto-parietal seed showed greater correlation with an auditory region in posterior operculum (box inferior to L_IPL seed) for both UHL groups compared to controls, with an overlap of 0.135 cc between the regions selected from the two t-tests. Another small region of overlap in postcentral gyrus (box superior to L_IPL seed) in the left UHL comparison did not meet the correction for multiple comparisons.
Figure 2.
Images showing the results of the t-test comparison of left inferior parietal lobule (L_IPL) seedmaps for the group of 10 normal hearing controls with both A) the group of 9 children with right UHL and B) the group of 7 children with left UHL. Orange indicates regions where the control group showed greater correlation with the L_IPL seed (shown in yellow and circled, coordinates −57, −43, 23) than the right UHL group, and the blue indicates regions where right UHL group showed greater correlation with the seed than the control group. Boxes indicate regions where both left and right UHL groups showed greater correlation with the L_IPL seed than controls.
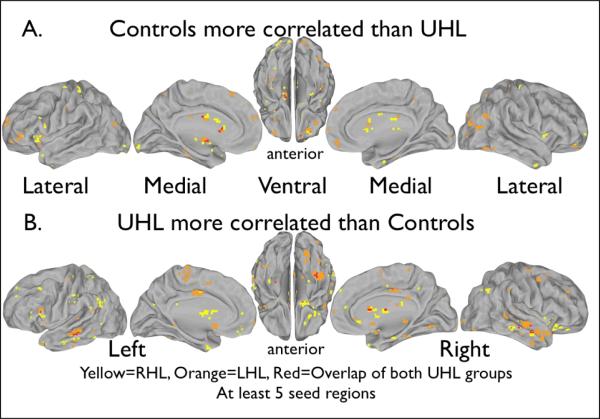
The conjunction analysis showed differences in patterns of BOLD signal fluctuation correlations between the UHL and control groups. Summing the positive conjunction masks (Figure 3A) shows the overlap in red where control subjects showed greater correlations than both groups of UHL subjects between those voxels. Similarly, Figure 3B shows the sum of the negative conjunction masks, indicating in red voxels where both groups of UHL subjects showed greater correlation with seed regions than controls. While there were many brain regions in which the right UHL or the left UHL group alone differed significantly from controls (yellow and orange), we focused on the four regions (in red) which showed correlation differences between both UHL groups and the control group for at least five seed regions. Table 3 lists the coordinates and contributing seed regions for these regions, while Figure 4 represents the relationships graphically. More than 5 seed contributors show up for each of our conjunction regions since different seeds may contribute to different parts of the region.
Figure 3.
Conjunction analysis maps showing locations of BOLD signal fluctuation correlations that differ between the UHL groups and the control group. Red indicates locations that differed from controls in both the right and left UHL groups. Yellow indicates differences from controls in the right UHL group alone, and orange shows differences from controls in the left UHL group. At least five seed regions contributed to the differences shown. Table 3 shows the seed regions that were found to contribute to each location. (A) The control group had higher correlations with the seed regions than the UHL groups. (B) The UHL groups had a higher correlation with the seeds regions than the control group.
Table 3.
Comparisons of differences across children with right unilateral hearing loss (RHL) and left unilateral hearing loss (LHL) with 10 controls.
| Region Coordinates | Contributing Seeds,LHL | Contributing Seeds, RHL | Contributing Seeds, both LHL and RHL | ||
|---|---|---|---|---|---|
| Controls > UHL | |||||
|
| |||||
| (−15, −7, m5) | R BA41 | L Heschl | L motor mouth a | R BA41 | |
| R Heschl | L BA41 | L motor mouth b | R Heschl | ||
| dACC/msFC | R BA41 | L motor handb | dACC/msFC | ||
| PCC | R Heschl | R motor hand | PCC | ||
| L motor handb | dACC/msFC | R motor mouth | L motor hand b | ||
| L motor mouth a | PCC | L motor mouth a | |||
| L motor mouth b | R frontal | L motor mouth b | |||
| L sensory cortex | L IPL | L sensory cortex | |||
| R motor mouth | Supramarginal gyrus | R motor hand | |||
| R motor hand | L sensory cortex | R motor mouth | |||
|
| |||||
| UHL > Controls | |||||
|
| |||||
| (−55, −30, −10) | L Heschl | L motor handb | L Heschl | L Heschl | |
| L BA41 | L sensory cortex | L BA41 | L BA41 | ||
| R BA41 | L motor mouth b | L frontal | L frontal | ||
| R Heschl | R motor hand | R IPL | L dlPFC | ||
| L anterior thalamus | L dlPFC | L motor hand b | |||
| R anterior thalamus | Supramarginal gyrus | R motor hand | |||
| L alfO | L motor hand b | ||||
| L frontal | R motor hand | ||||
| L dlPFC | R motor mouth | ||||
| L motor hand a | |||||
|
| |||||
| (38, −32, −25) | L Heschl | L motor mouth a | L Heschl | R motor hand | L Heschl |
| L BA41 L | motor mouth b | dACC/msFC | dACC/msFC | ||
| R BA41 | L sensory cortex | LaPFC | L IPL | ||
| R Heschl | R sensory cortex | L frontal | Supramarginal gyrus | ||
| dACC/msFC | R motor hand | L IPL | L motor hand a | ||
| R aPFC | R motor mouth | Supramarginal gyrus | L motor mouth b | ||
| L dlPFC | R sensory cortex | L motor hand a | L motor mouth a | ||
| L IPL | R frontal | L motor hand b | L motor mouth b | ||
| L anterior thalamus | L motor mouth a | L sensory cortex | |||
| Supramarginal gyrus | L motor mouth b | R sensory cortex | |||
| L motor hand a | L sensory cortex | R motor hand | |||
| L motor hand b | R sensory cortex | ||||
|
| |||||
| (0, −15, 39) | PCC | L motor mouth a | PCC | PCC | |
| L dlPFC | L motor mouth b | L frontal | L frontal | ||
| L frontal | L sensory cortex | R frontal | R frontal | ||
| L IPL | R motor hand | L dlPFC | L dlPFC | ||
| L IPS | R motor mouth | L motor hand b | L motor hand b | ||
| R frontal | L sensory cortex | L sensory cortex | |||
| R IPS | L motor mouth a | L motor mouth a | |||
| L motor hand a | L motor mouth b | L motor mouth b | |||
| L motor hand b | R sensory cortx | ||||
| Key for contributing regions: | Auditory | CingOpercular | Default | FrontoParietal | Sensorimotor | Phonological |
BA41, Brodmann's Area 41; dACC/msFC, dorsal anterior cingulate cortex/medial superior frontal cortex; PFC, prefrontal cortex; PCC, posterior cingulate cortex; IPL, inferior parietal lobule; aIfO, anterior insula/frontal operculum; dlPFC, dorsolateral PFC; aPFC, anterior PFC.
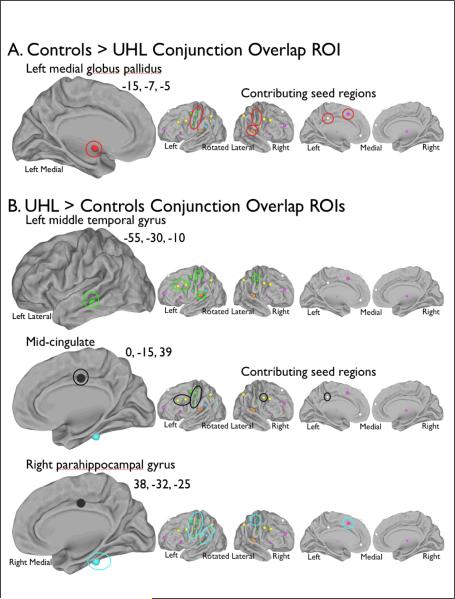
Figure 4.
Seeds contributing to differences from controls in both UHL groups. Regions discovered in the conjunction analyses are circled on the left larger image, while the regions circled on the right smaller images are the seed regions which contributed to the differences in both left and right UHL groups from NH controls. (A) The control group had higher correlations with the seed regions than the UHL groups. (B) The UHL groups had a higher correlation with the seeds regions than the control group.
Figure 4A shows the one region where BOLD signal fluctuations were more correlated for controls than the UHL groups. For this left medial globus pallidus region with coordinates (−15, −7, −5), 10 seed regions were associated in the left UHL group and 15 in the right UHL group. Ten of these seeds were common to both UHL groups: two auditory seeds, one cingulo-opercular (task level control15) seed, one default (reduced activation during tasks9) seed, and six sensorimotor seeds.
Figure 4B highlights three regions where BOLD signal fluctuations were more correlated in the subjects with UHL than in controls. In the left middle temporal gyrus region (−55, −30, −10), 14 seeds were associated with differences in BOLD signal fluctuation in the left UHL group and nine in the right UHL group. Six seeds were common to both left and right UHL groups; two were auditory seeds, two were frontoparietal (trial level control15) seeds, and two were sensorimotor seeds.
The mid-cingulate region (0, −15, 39) was associated with eight common seed regions, one default, three frontoparietal, and four sensorimotor seeds. Fourteen seeds were associated with the left UHL group and nine with the right UHL group.
Finally, in the parahippocampal gyrus region with coordinates (38, −32, −25), 19 seed regions were associated with the left UHL group and 13 with the right UHL group; 11 were common to both UHL groups. Of the common seeds, one was auditory, one cingulo-opercular, one frontoparietal, seven sensorimotor, and one phonological.
DISCUSSION
With the establishment of newborn hearing screening programs to evaluate hearing status at birth, the number of infants identified with UHL is on the rise and the need to understand the impact of UHL in children has gained recent attention.22 In this pilot study, we have begun to investigate whether patterns of functional connectivity associated with central auditory processes and executive function in children with UHL differ from NH siblings using rs-fcMRI. Resting state fcMRI measures offer the advantage of independence from task protocols, so results are not dependent on subjects' attention or performance. These methods also carry the promise of demonstrating how the brain's functional architecture, as revealed by functional connectivity, is modified in the presence of UHL.10
The results of this pilot study show that rs-fcMRI can identify differences in the brain's functional network architecture when comparing children with UHL and children with NH. Because the degree to which UHL might alter auditory processing networks is unknown, we chose to examine children with severe-to-profound UHL rather than those with less severe UHL. We found differences between children with UHL and NH in multiple networks. The left IPL seed, the only seed to show consistent mean regionwise t-test differences between controls and both UHL groups in this study, is a fronto-parietal region (trial level control) involved in sustained task set maintenance activity.8 The posterior operculum region, which is more correlated to left IPL in UHL subjects than controls, is close to a region found by Julie Fiez and colleagues to be related to echoic memory (personal communication, Steve Petersen). This same region found by Feiz and colleagues is thought to be involved with domain-general working memory.23 A possible interpretation is that UHL subjects need more subvocal rehearsal to stay on task than controls, so have strengthened this functional connection.
The conjunction analysis identified seed regions associated with auditory, sensorimotor, default mode, and phonological networks, as well as cingular opercular and frontoparietal task control networks, whose patterns of correlation differed between children with UHL and NH. Particularly noteworthy are the differences in the frontoparietal and cingulo-opercular networks, which are related to task level control, a type of executive function, involving rapid/adaptive and sustained/maintenance control, respectively.15, 24 Differences in brain networks responsible for these functions could explain some of the educational and behavioral problems experienced by children with UHL, and are further explicated below.
Regions from Figure 4B show correlations that are stronger in subjects with UHL than in controls. The middle temporal gyrus has been shown to be involved in auditory sentence comprehension in children.25 Atypical functional connectivity with the parahippocampal gyrus might handicap children with UHL since it has been widely implicated in both place processing and episodic memory, while Aminoff et al. argue that parahippocampal cortex plays a central role in contextual associative processing.26 The mid-cingulate region has been associated with cognitive processes such as error detection, salience, decision-making, attention to stimuli and anticipation.27
Sensorimotor seeds showed atypical connectivity with all of our conjunction analysis regions, including but not limited to motor mouth regions. This finding may help explain the high rates of speech problems and need for speech therapy among children with UHL in this study.
The implications of decreased functional connectivity in the medial globus pallidus (Figure 4A), an output nucleus of the basal ganglia, are unclear. Globus pallidus has been implicated in disorders of attention and impulsivity,28, 29 so perhaps future studies should quantify even subclinical attention and impulsivity issues in UHL patients and controls.
A recent fMRI study comparing children with UHL to children with NH using narrowband noise and speech-in-noise tasks found that children with UHL had less activation of auditory, auditory association, and attention areas than NH controls.30 Our study adds to Propst et al.'s results since the design differs in two primary ways. First, our study looked at functional connections in subjects at rest instead of using tasks to identify brain activations. Second, we identified possible differences in connections to executive function, default, and sensorimotor regions in addition to auditory pathways. Both studies found differences between NH controls and children with UHL that warrant further study.
A major limitation of our study is the small sample size. As this was a pilot study, our primary goal was to demonstrate that it is possible to identify differing patterns of inter-regional brain connectivity in children with UHL versus siblings with NH. Now that we have demonstrated the feasibility of our methods and generated preliminary data, we will expand the study to include more participants as well as examine differences in the language regions of the brain such as Broca's and Wernicke's areas, and those involved in visual processing.
In addition to performing rs-fcMRI scans on the subjects we have included thus far, we performed cognitive, achievement, language, and audiologic testing on each subject scanned, which we will correlate with the rs-fcMRI data. We will use these data to identify relationships between patterns of brain network activity and specific education-related difficulties in children with UHL. Understanding these networks could lead to targeted interventions, beyond amplification of sound to the impaired ear, and possibly better educational outcomes. Collectively, these findings could ultimately affect the provision of sensory devices and rehabilitation strategies specific to UHL.
Acknowledgements
This work was funded by the American Hearing Research Foundation Wiley H. Harrison Memorial Research Award. Dr. Lieu was also supported by NIH grant K23DC006638.
Abbreviations
- BA
Brodmann's area
- IPL
inferior parietal lobule
- NH
normal hearing
- PTA
pure tone thresholds
- UHL
unilateral hearing loss
REFERENCES
- (1).Bess FH, Tharpe AM. Unilateral hearing impairment in children. Pediatrics. 1984;74(2):206–216. [PubMed] [Google Scholar]
- (2).Bovo R, Martini A, Agnoletto M, et al. Auditory and academic performance of children with unilateral hearing loss. Scand Audiol Suppl. 1988;30:71–74. [PubMed] [Google Scholar]
- (3).Brookhouser PE, Worthington DW, Kelly WJ. Unilateral hearing loss in children. Laryngoscope. 1991;101(12 Pt 1):1264–1272. doi: 10.1002/lary.5541011202. [DOI] [PubMed] [Google Scholar]
- (4).Oyler RF, Oyler AL, Matkin ND. Unilateral hearing loss: demographics and educational impact. Lang Speech Hear Serv Sch. 1988;19:201–210. [Google Scholar]
- (5).Lieu JE. Speech-language and educational consequences of unilateral hearing loss in children. Arch Otolaryngol Head Neck Surg. 2004;130(5):524–530. doi: 10.1001/archotol.130.5.524. [DOI] [PubMed] [Google Scholar]
- (6).Lieu JE, Tye-Murray N, Karzon RK, Piccirillo JF. Unilateral hearing loss is associated with worse speech-language scores in children. Pediatrics. 2010;125(6):e1348–e1355. doi: 10.1542/peds.2009-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- (8).Fair DA, Dosenbach NU, Church JA, et al. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci U S A. 2007;104(33):13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van E, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A. 2006;103(26):10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Fair DA, Schlaggar BL, Cohen AL, et al. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. Neuroimage. 2007;35(1):396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Caclin A, Fonlupt P. Functional and effective connectivity in an fMRI study of an auditory-related task. Eur J Neurosci. 2006;23(9):2531–2537. doi: 10.1111/j.1460-9568.2006.04773.x. [DOI] [PubMed] [Google Scholar]
- (13).Calvert GA, Bullmore ET, Brammer MJ, et al. Activation of auditory cortex during silent lipreading. Science. 1997;276(5312):593–596. doi: 10.1126/science.276.5312.593. [DOI] [PubMed] [Google Scholar]
- (14).Church JA, Coalson RS, Lugar HM, Petersen SE, Schlaggar BL. A developmental fMRI study of reading and repetition reveals changes in phonological and visual mechanisms over age. Cereb Cortex. 2008;18(9):2054–2065. doi: 10.1093/cercor/bhm228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Dosenbach NU, Fair DA, Miezin FM, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104(26):11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Fair DA, Cohen AL, Dosenbach NU, et al. The maturing architecture of the brain's default network. Proc Natl Acad Sci U S A. 2008;105(10):4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Miezin FM, Maccotta L, Ollinger JM, Petersen SE, Buckner RL. Characterizing the hemodynamic response: effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. Neuroimage. 2000;11(6 Pt 1):735–759. doi: 10.1006/nimg.2000.0568. [DOI] [PubMed] [Google Scholar]
- (18).Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Thieme Medical Publishers, Inc.; 1988. [Google Scholar]
- (19).Burgund ED, Kang HC, Kelly JE, et al. The feasibility of a common stereotactic space for children and adults in fMRI studies of development. Neuroimage. 2002;17(1):184–200. doi: 10.1006/nimg.2002.1174. [DOI] [PubMed] [Google Scholar]
- (20).WOROI: 347 - Brodmann Area 41. [Accessed 5-5-2009];The Human Brain Project - Denmark. http://neuro.imm.dtu.dk/services/jerne/brede/WOROI_347.html.
- (21).Jancke L, Buchanan TW, Lutz K, Shah NJ. Focused and nonfocused attention in verbal and emotional dichotic listening: an FMRI study. Brain Lang. 2001;78(3):349–363. doi: 10.1006/brln.2000.2476. [DOI] [PubMed] [Google Scholar]
- (22).Mild and unilateral hearing loss: National Workshop. Centers for Disease Control and Prevention; Beaver Run Resort, Breckenridge, Colorado: 2005. [Google Scholar]
- (23).Ravizza SM, Delgado MR, Chein JM, Becker JT, Fiez JA. Functional dissociations within the inferior parietal cortex in verbal working memory. Neuroimage. 2004;22(2):562–573. doi: 10.1016/j.neuroimage.2004.01.039. [DOI] [PubMed] [Google Scholar]
- (24).Dosenbach NU, Visscher KM, Palmer ED, et al. A core system for the implementation of task sets. Neuron. 2006;50(5):799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Yeatman JD, Ben-Shachar M, Glover GH, Feldman HM. Individual differences in auditory sentence comprehension in children: An exploratory event-related functional magnetic resonance imaging investigation. Brain Lang. 2010;114(2):72–79. doi: 10.1016/j.bandl.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Aminoff E, Gronau N, Bar M. The parahippocampal cortex mediates spatial and nonspatial associations. Cereb Cortex. 2007;17(7):1493–1503. doi: 10.1093/cercor/bhl078. [DOI] [PubMed] [Google Scholar]
- (27).Taylor KS, Seminowicz DA, Davis KD. Two systems of resting state connectivity between the insula and cingulate cortex. Hum Brain Mapp. 2009;30(9):2731–2745. doi: 10.1002/hbm.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Qiu A, Crocetti D, Adler M, et al. Basal ganglia volume and shape in children with attention deficit hyperactivity disorder. Am J Psychiatry. 2009;166(1):74–82. doi: 10.1176/appi.ajp.2008.08030426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Cao X, Cao Q, Long X, et al. Abnormal resting-state functional connectivity patterns of the putamen in medication-naive children with attention deficit hyperactivity disorder. Brain Res. 2009 Dec 15;1303:195–206. doi: 10.1016/j.brainres.2009.08.029. [DOI] [PubMed] [Google Scholar]
- (30).Propst EJ, Greinwald JH, Schmithorst V. Neuroanatomic differences in children with unilateral sensorineural hearing loss detected using functional magnetic resonance imaging. Arch Otolaryngol Head Neck Surg. 2010;136(1):22–26. doi: 10.1001/archoto.2009.208. [DOI] [PubMed] [Google Scholar]