Abstract
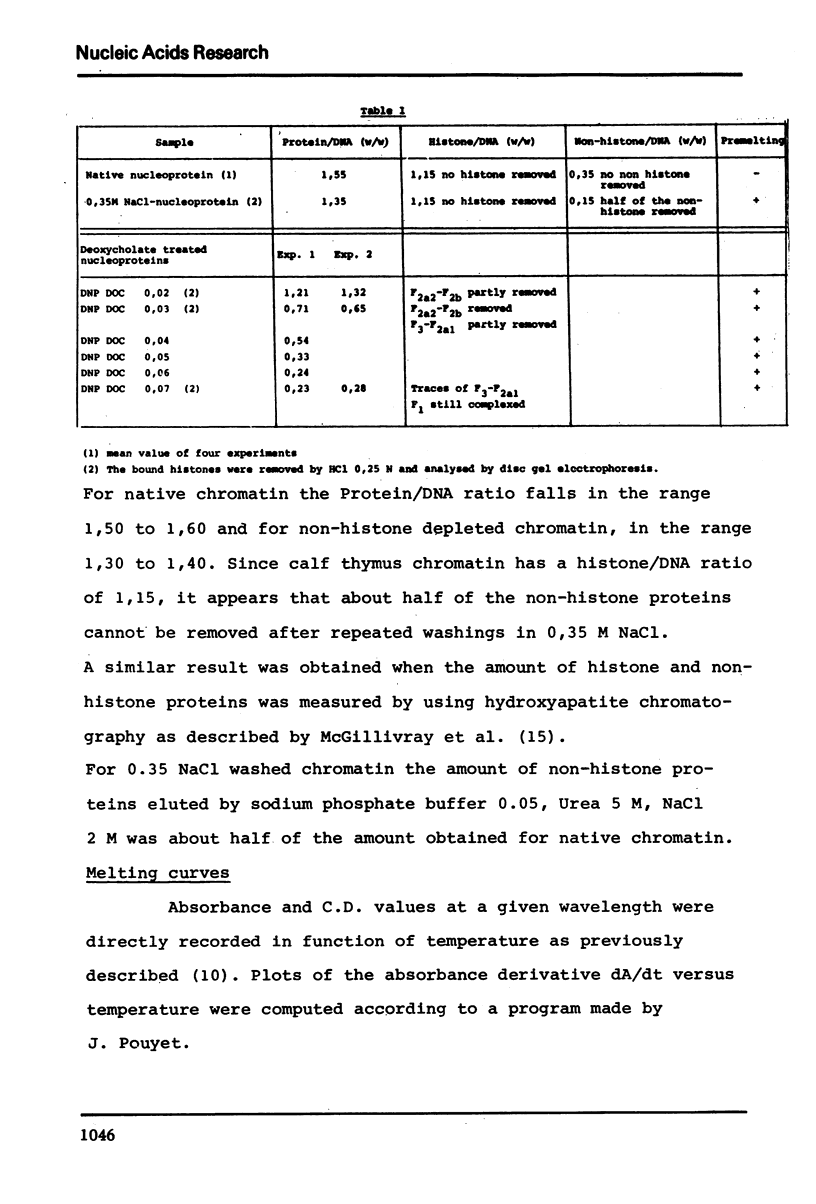
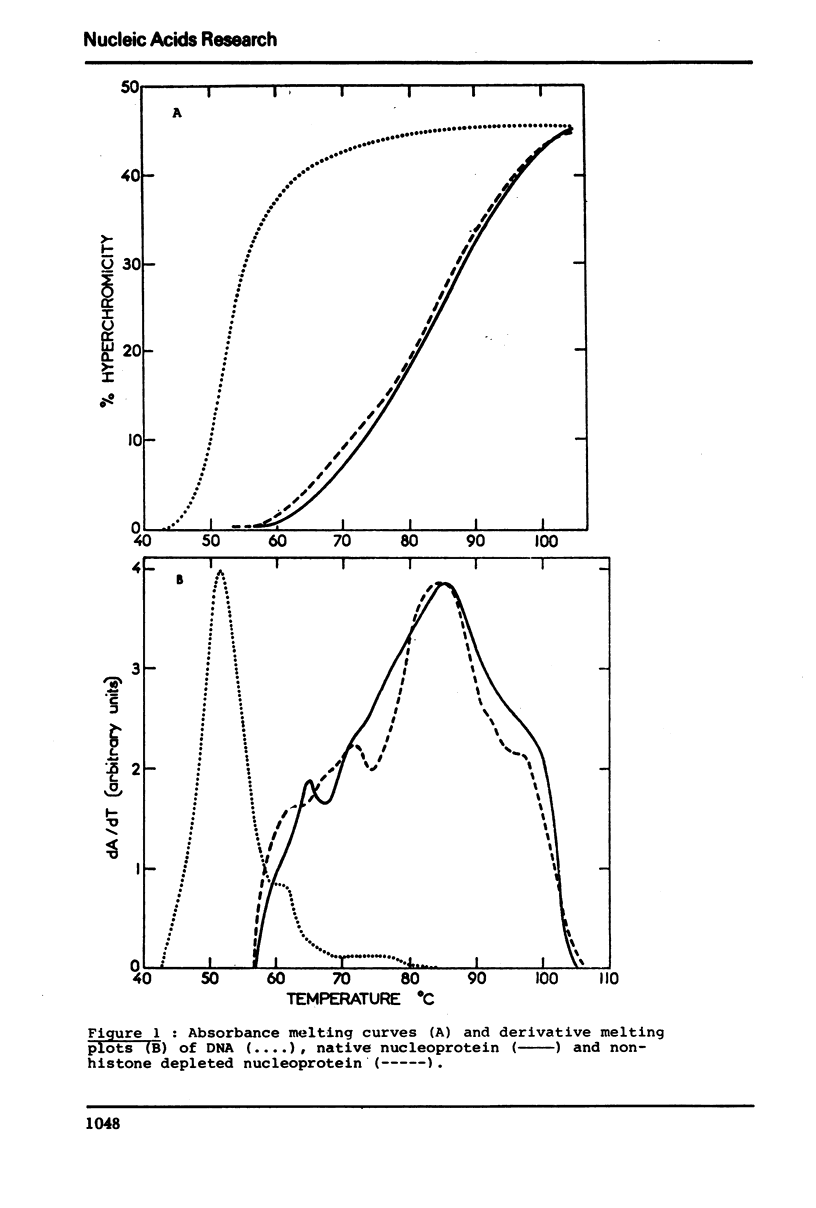
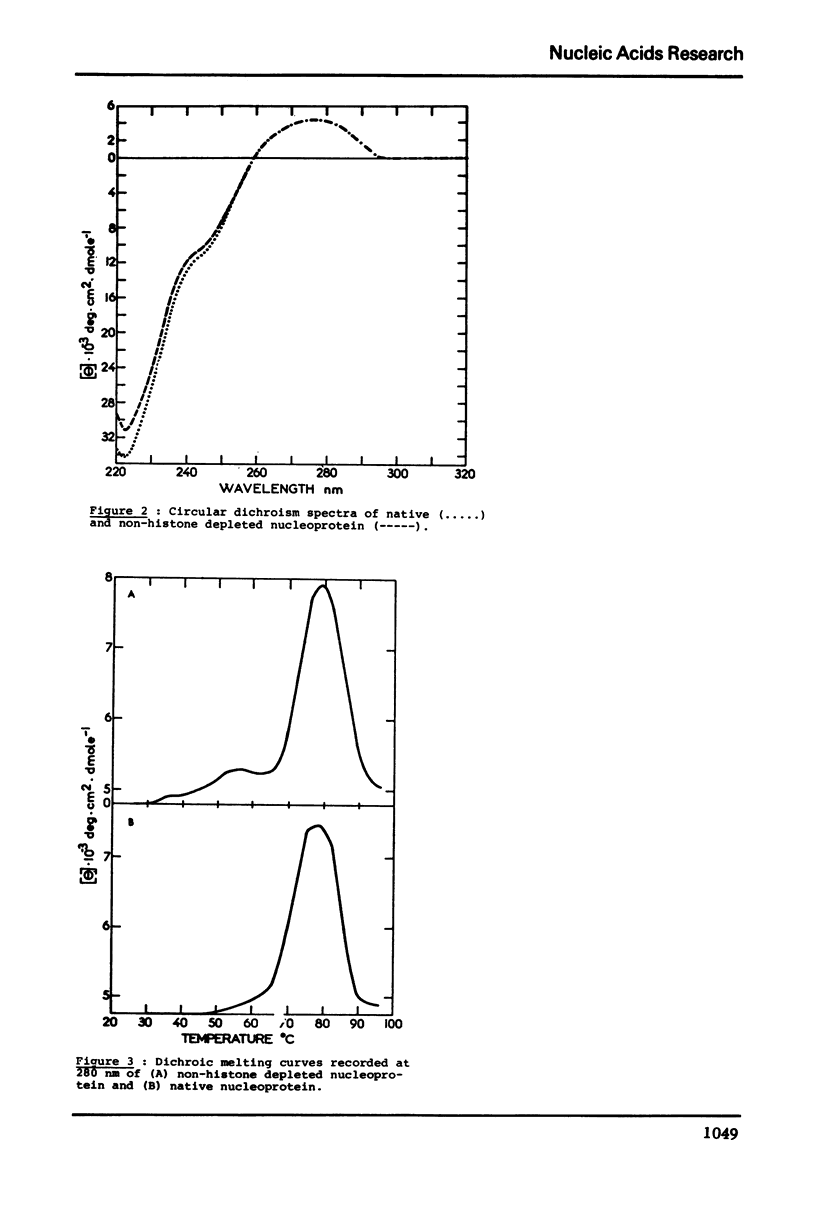
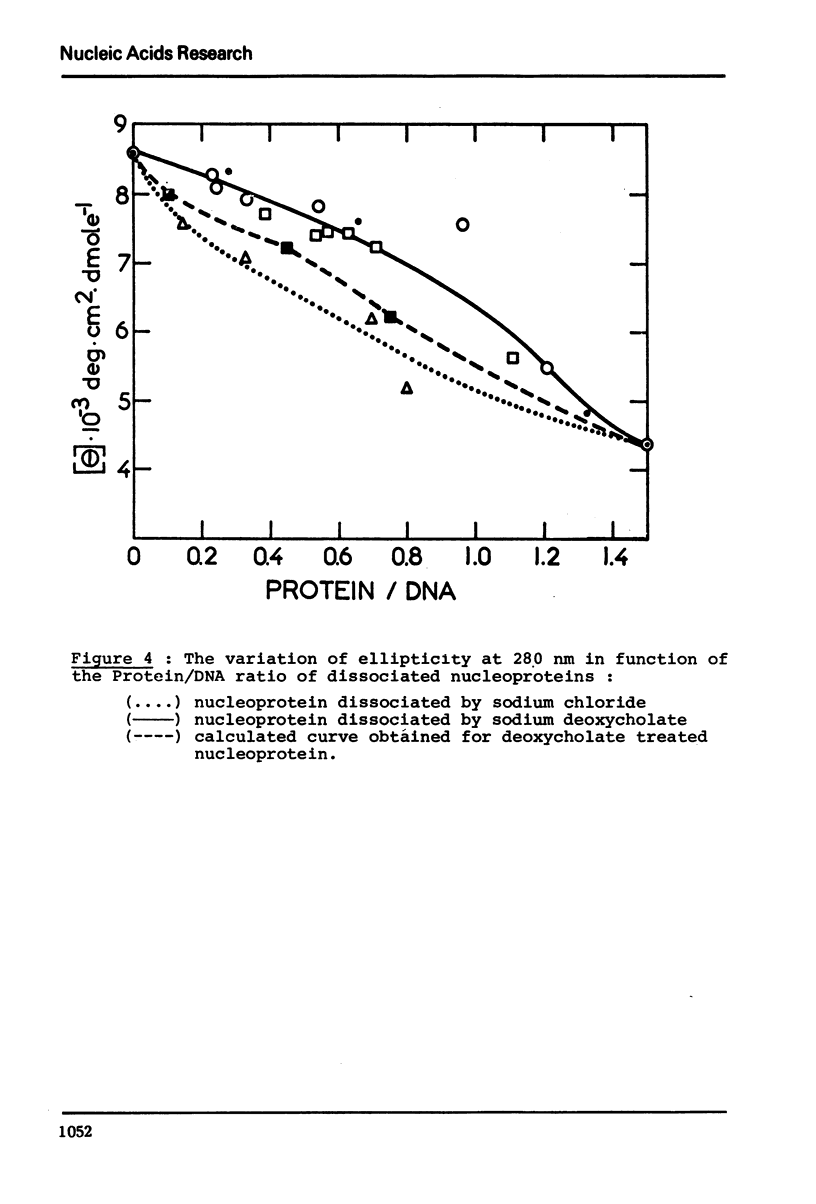
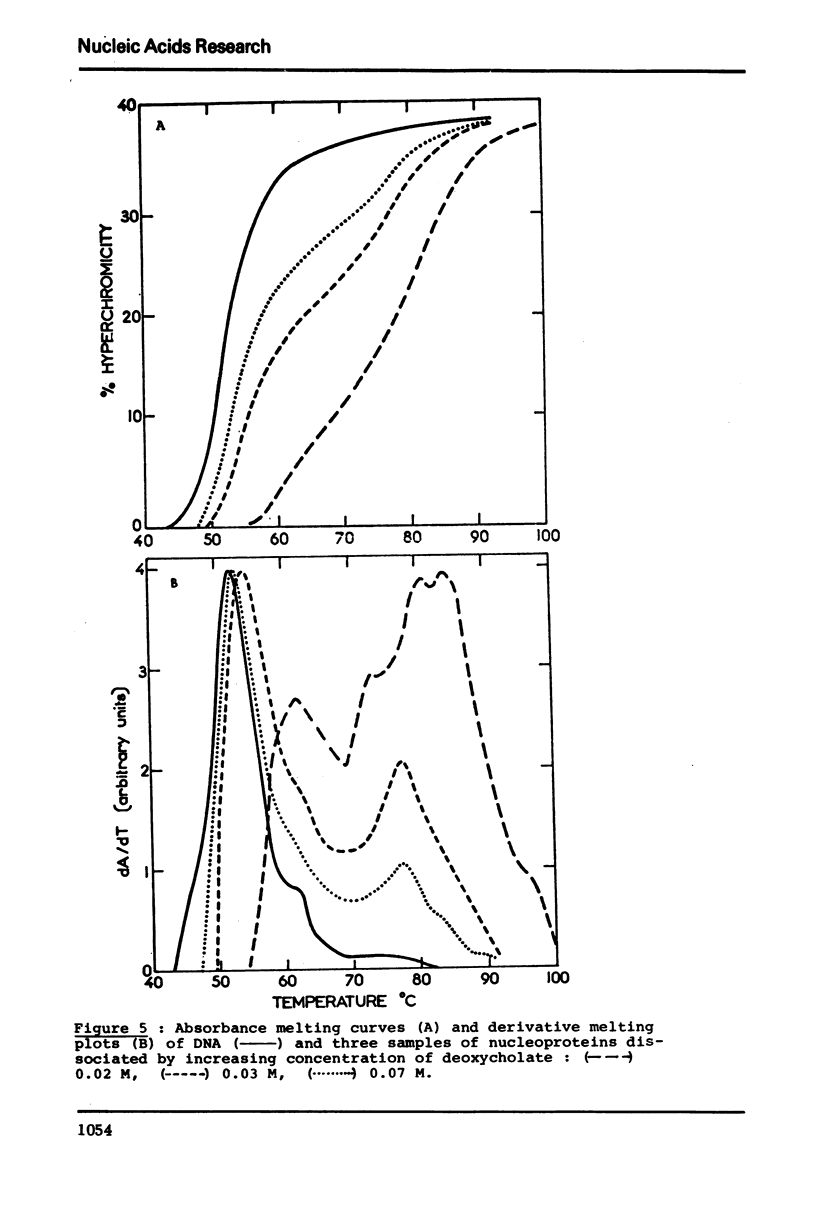
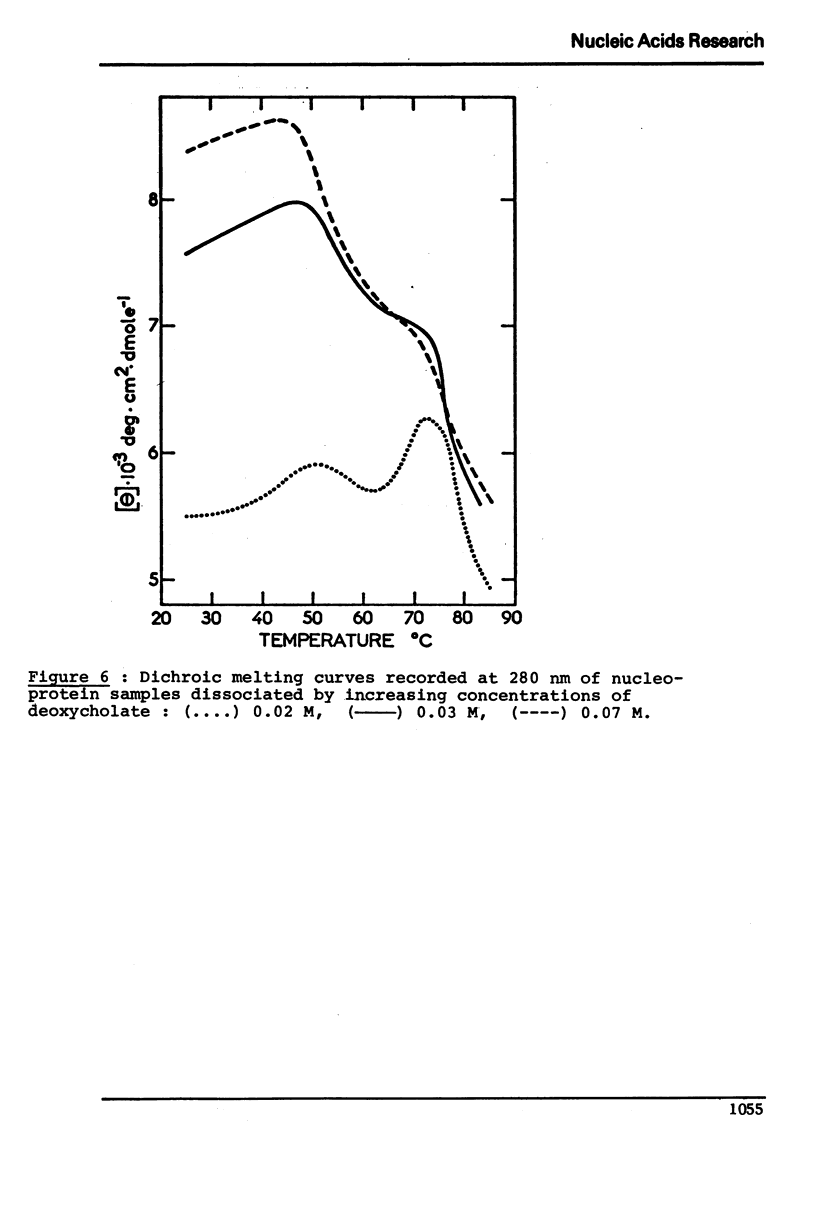
In native nucleoprotein, the premelting structural changes of DNA are not observed by circular dichroism measurements. In order to determine which protein fraction of chromatin is responsible for the absence of premelting we have examined a series of nucleoproteins depleted of different protein fractions by treatment with sodium chloride or sodium deoxycholate.
The premelting reappears as soon as non-histone proteins are removed or in residual complexes from which the two slightly lysine-rich histone fractions (F2a2+F2b) have been removed. On the other hand, it is shown that histone F1 alone is not able to suppress the premelting phenomenon. It is thus concluded that the absence of premelting is a property of native nucleoprotein where interactions between the different proteins complexed with DNA can occur and especially between the non-histone proteins and the two slightly lysine-rich histone fractions.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartley J., Chalkley R. An approach to the structure of native nucleohistone. Biochemistry. 1973 Jan 30;12(3):468–474. doi: 10.1021/bi00727a017. [DOI] [PubMed] [Google Scholar]
- Gennis R. B., Cantor C. R. Optical studies of a conformational change in DNA before melting. J Mol Biol. 1972 Apr 14;65(3):381–399. doi: 10.1016/0022-2836(72)90196-9. [DOI] [PubMed] [Google Scholar]
- Goodwin G. H., Johns E. W. The non-histone proteins of chromatin. FEBS Lett. 1972 Mar;21(1):103–104. doi: 10.1016/0014-5793(72)80174-1. [DOI] [PubMed] [Google Scholar]
- Henson P., Walker I. O. The partial dissociation of nucleohistone by salts. Circular dichroism and denaturation studies. Eur J Biochem. 1970 Nov;16(3):524–531. doi: 10.1111/j.1432-1033.1970.tb01112.x. [DOI] [PubMed] [Google Scholar]
- Johnson R. S., Chan A., Hanlon S. Mixed conformations of deoxyribonucleic acid in intact chromatin isolated by various preparative methods. Biochemistry. 1972 Nov 7;11(23):4347–4358. doi: 10.1021/bi00773a023. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MacGillivray A. J., Carroll Dana, Paul J. The heterogeneity of the non-histone chromatin proteins from mouse tissues. FEBS Lett. 1971 Mar 16;13(4):204–208. doi: 10.1016/0014-5793(71)80536-7. [DOI] [PubMed] [Google Scholar]
- Melchior W. B., Jr, Von Hippel P. H. Alteration of the relative stability of dA-dT and dG-dC base pairs in DNA. Proc Natl Acad Sci U S A. 1973 Feb;70(2):298–302. doi: 10.1073/pnas.70.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Permogorov V. I., Debabov V. G., Sladkova I. A., Rebentish B. A. Structure of DNA and histones in the nucleohistone. Biochim Biophys Acta. 1970 Feb 18;199(2):556–558. doi: 10.1016/0005-2787(70)90107-3. [DOI] [PubMed] [Google Scholar]
- Ramm E. I., Vorob'ev V. I., Birshtein T. M., Bolotina I. A., Volkenshtein M. V. Circular dichroism of DNA and histones in the free state and in deoxyribonucleoprotein. Eur J Biochem. 1972 Feb 15;25(2):245–253. doi: 10.1111/j.1432-1033.1972.tb01690.x. [DOI] [PubMed] [Google Scholar]
- Shapiro J. T., Stannard B. S., Felsenfeld G. The binding of small cations to deoxyribonucleic acid. Nucleotide specificity. Biochemistry. 1969 Aug;8(8):3233–3241. doi: 10.1021/bi00836a015. [DOI] [PubMed] [Google Scholar]
- Shih T. Y., Fasman G. D. Conformation of deoxyribonucleic acid in chromatin: a circular dichroism study. J Mol Biol. 1970 Aug 28;52(1):125–129. doi: 10.1016/0022-2836(70)90182-8. [DOI] [PubMed] [Google Scholar]
- Simpson R. T., Sober H. A. Circular dichroism of calf liver nucleohistone. Biochemistry. 1970 Aug 4;9(16):3103–3109. doi: 10.1021/bi00818a001. [DOI] [PubMed] [Google Scholar]
- Smart J. E., Bonner J. Selective dissociation of histones from chromatin by sodium deoxycholate. J Mol Biol. 1971 Jun 28;58(3):651–659. doi: 10.1016/0022-2836(71)90030-1. [DOI] [PubMed] [Google Scholar]
- Von Hippel P. H., Wong K. Y. Dynamic aspects of native DNA structure: kinetics of the formaldehyde reaction with calf thymus DNA. J Mol Biol. 1971 Nov 14;61(3):587–613. doi: 10.1016/0022-2836(71)90066-0. [DOI] [PubMed] [Google Scholar]
- Wagner T., Spelsberg T. C. Aspects of chromosomal structure. I. Circular dichroism studies. Biochemistry. 1971 Jun 22;10(13):2599–2605. doi: 10.1021/bi00789a029. [DOI] [PubMed] [Google Scholar]
- Wilhelm F. X., Champagne M. H., Daune M. P. Conformation du DNA dans la nucléoprotéine. Eur J Biochem. 1970 Aug;15(2):321–330. doi: 10.1111/j.1432-1033.1970.tb01010.x. [DOI] [PubMed] [Google Scholar]



