Abstract
Background
Cytochrome P450 2E1 (CYP2E1), an ethanol-inducible enzyme, has been shown to metabolically activate various carcinogens, which is critical for the development and progression of cancers. It has demonstrated that CYP2E1 polymorphisms alter the transcriptional activity of the gene. However, studies on the association between CYP2E1 polymorphisms (PstI/RsaI or DraI) and gastric cancer have reported conflicting results. Thus, the aim of the present study was to investigate whether CYP2E1 polymorphisms is associated with the development and progression of gastric cancer and its prognosis in Chinese patients.
Methods
A case-control study was conducted in which CYP2E1 PstI/RsaI and DraI polymorphisms were analyzed in 510 Chinese patients with gastric cancer and 510 age- and sex- matched healthy controls by PCR-RFLP. Odds ratios were estimated by multivariate logistic regression, and the lifetime was calculated by Kaplan-Meier survival curves. In addition, a meta-analysis was also conducted to verify the findings.
Results
For CYP2E1 PstI/RsaI polymorphism, C2C2 homozygotes (OR = 2.15; CI: 1.18–3.94) and C2 carriers (OR = 1.48; CI: 1.13–1.96) were associated with an increased risk of gastric cancer when compared with C1C1 homozygotes. Both C1C2 and C2C2 genotypes were associated with advanced stage, but not the grade of gastric cancer. Moreover, C2C2 genotype was identified as an independent marker of poor overall survival for gastric cancer. However, there was not any significant association between CYP2E1 DraI polymorphism and the risk of gastric cancer. In the meta-analysis, pooled data from 13 studies confirmed that the CYP2E1 PstI/RsaI polymorphism was associated with a significantly increased risk of gastric cancer.
Conclusion
CYP2E1 PstI/RsaI polymorphism is associated with increased risk of development, progression and poor prognosis of gastric cancer in Chinese patients. Pooled data from 13 studies, mainly in Asian countries, are in agreement with our findings.
Background
Gastric cancer, one of the most common cancers and the second leading cause of cancer death worldwide, remains an important public health problem. Studies have shown that 989,600 new gastric cancer cases occurred in 2008 and that 738,000 patients die annually of this disease [1]–[2]. There is a considerable geographic variation in the gastric cancer incidence, with higher rates in Asia and some parts of South America. Gastric cancer is one of the most prevalent malignant tumors in China, accounting for 38% of worldwide cases every year [3]–[4]. Although it is well known that environmental factors, dietary habits, and Helicobacter pylori infection are associated with the risk of gastric cancer, the host genetic factor is also believed to be important in gastric carcinogenesis [5]. Genetic susceptibilities could be explained, in part, by single nucleotide polymorphisms (SNPs) of susceptible genes [6]–[7]. Therefore, determination and understanding of genetic and molecular factors involved in gastric cancer development and prognosis may help identify novel genetic biomarkers and highlight potential avenues of investigation for targeted therapies.
Cytochrome P450 2E1 (CYP2E1), which is located on chromosome 10q26.3, is an 11.7 kb gene consisting of 9 exons and 8 introns, and encoding a 493 amino acid protein [8]. CYP2E1 is an ethanol-inducible enzyme that metabolically activates various carcinogens, such as benzene, vinyl chloride and N-nitrosamines [9]–[10]. N-nitrosamines are present in tobacco smoke, and activation of N-nitrosamines has been linked to the development of various cancers, including gastric cancer [11]–[12]. Functional CYP2E1 gene polymorphisms that alter the transcriptional activity of the gene and thus its substances such as N-nitrosamines would influence the susceptibility of cancers. Two genetic polymorphisms in the 5′-flank region (identified by RsaI is −1053C>T (rs2031920) and PstI is −1293G>C (rs3813867), respectively), which are in close linkage disequilibrium, have been reported to alter transcriptional activity of the gene [13]–[14]. The individuals with predominant homozygous allele (C1/C1), the heterozygous allele (C1/C2) and the rare homozygous allele (C2/C2) of PstI/RsaI polymorphism are named the wild-type homozygote, the heterozygote and the rare homozygote, respectively [15]–[17]. Another important polymorphism detectable with DraI in intron 6 is T7632A (rs6413432), a mutation of T to A, which is reported to may alter transcription of the CYP2E1 gene [13].
Over the last two decades, several studies have explored the association of the CYP2E1 polymorphism with the risk of lung cancer [18], oral cancer [19], and pancreatic cancer [20]. Recently, a few studies on the association between the CYP2E1 polymorphism and gastric cancer have also been published, but those studies have yielded contradictory results [21]–[33]. Moreover, there has been no report on the association between CYP2E1 polymorphism and survival of patients with gastric cancer. Therefore, the aim of this study was to investigate whether CYP2E1 polymorphism is associated with the development and progression of gastric cancer and its prognosis in Chinese patients. In addition, we also carried out a meta-analysis of selected high quality studies published between 1990 and 2011, in order to reveal more precise association between CYP2E1 polymorphism and gastric cancer.
Materials and Methods
Study Population
The study included 510 patients who were admitted for gastric cancer treatment to the First Affiliated Hospital of Nanjing Medical University between May 2006 and September 2008 and 510 age- and sex-matched healthy controls. All subjects were unrelated ethnic Han Chinese and residents in Jiangsu Province. All cases were newly diagnosed and histologically confirmed without previous chemotherapy or radiotherapy. The pathological stage of gastric cancer was classified according to the tumor-lymph node-metastasis (TNM) classification system into stage I (T1–T2N0M0), stage II (T1–T2N1M0 or T3N0M0), stage III (T3N1M0, T1–T3N2M0, TanyN3M0, or T4NanyM0), or stage IV (TanyNanyM1) [34]. Tumor grade was grouped into low (well differentiated), intermediate (moderately differentiated), or high grade (poor differentiated) according to the World Health Organization (WHO) grade classification [35]. The healthy controls were recruited from individuals living in the same residential areas who took part in routine medical examination at the same hospital withnormal findings during the examination and were age- (±5 years) and sex-matched to the cases.
The study was approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University, and the number of the document was 2008(1101). Written informed consent was obtained from all subjects.
DNA Extraction and Genotyping of CYP2E1
Whole blood was collected into EDTA-coated tubes and centrifuged for 15 min, and the buffy coat layer was isolated. Genomic DNA was extracted from 200 mL of buffy coat using a Qiagen QIAamp DNA Blood Mini kit (Qiagen Inc.,Valencia, CA). Polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) was used for gene analysis. The primer structure and restriction enzyme are shown in Table 1. Genotyping was performed without knowledge of the case/control status. The gel images were read independently by two research assistants. If a consensus was not reached on the tested genotypes, then the genotyping was repeated independently until a consensus was reached. To validate the RFLP method, 100 (50 from cases and 50 from controls) samples were selected randomly for direct sequencing with an ABI PRISM Dye Terminator sequencing Kit (Applied Biosystems, Foster City, Calif) with the samples loaded onto an ABI 3700 sequencer. The concurrence rate of these two methods was 99%.
Table 1. Gene loci, restriction enzymes and primer sequences.
| Gene | Location | Restriction enzyme | primer sequence |
| CYP2E1 | 5′-flanking | PstI | 5′-CCA GTC GAG TCT ACA TTG TCA-3′ |
| 5′-TTC ATT CTG TCT TCT AAC TGG-3′ | |||
| CYP2E1 | Intron 6 | DraI | 5′-TCG TCA GTT CCT GAA AG C AGG-3′ |
| 5′-GAG CTC TGA TGC AAG TAT CGC-3′ |
Statistical Analysis
Hardy-Weinberg equilibrium of alleles was assessed by chi-square test. Comparison of age between cases and controls was assessed by the Mann-Whitney U test. The difference in the distribution of genotypes between cases and controls was determined using chi-square test, and the association between the CYP2E1 polymorphisms and gastric cancer risk was estimated by odds ratio (OR) with the 95% confidence interval (CI). Logistic regression was used to control for selected potential confounders (sex, age, and smoking habit) and to estimate crude and adjusted OR and 95% CI. Cumulative overall survival curves were constructed using the method of Kaplan-Meier and the difference was evaluated by the log-rank test. All data analyses were done using SPSS software (version 11.0, Chicago, IL, USA). A P value of <0.05 was considered statistically significant.
Meta-analysis
The electronic databases PubMed, Embase and Web of Science, were searched for studies eligible for inclusion in the present meta-analysis, using the terms: “CYP2E1”, “P4502E1”, “polymorphism(s)”, “gastric” and “cancer or carcinoma or tumor or neoplasm”. An upper date limit of December 5, 2011 was applied, while a lower date limit was 1990. All published English language papers with full text matching the eligible criteria were retrieved. The citations in identified articles and in review articles were also examined. When the same patient population was included in more than one publication, only the most recent or most complete one was included in the meta-analysis. Inclusion criteria included: (a), case-control study on the association between the CYP2E1 RsaI/PstI or DraI polymorphism and gastric cancer; (b), English publication; (c), sufficient published data available for estimating an OR with 95% CI; and (d), the genotypes of the controls consistent with the Hardy-Weinberg equilibrium distribution. Information was carefully and independently extracted from all eligible publications by two of the authors according to the inclusion criteria listed above. Disagreement was resolved by discussion between the two authors. If the two authors did not reach a consensus, a third author was consulted and a final decision was made by the majority of the votes. The following data were collected from each study: first author’s name, publication date, ethnicity, study design, pathological types of gastric cancer, the total numbers of cases and controls, and information on CYP2E1 (PstI/RsaI and/or DraI) polymorphisms. Then we used the Review Manager 4.2 software (The Cochrane Collaboration, Oxford, United Kingdom) for meta-analysis.
Results
Characteristics of Study Population
The demographic and pathological characteristics of patients and controls are listed in Table 2. There were no significant differences in demographic data between cases and controls. However, 51.6% of cases were smokers whereas the rate was 44.1% for the controls (OR = 1.35, 95% CI: 1.05–1.73, P = 0.017). Of the patients with gastric cancer, 41 (8.0%), 181 (35.5%), 160 (31.8%) and 25 (4.9%) had stages I, II, III, and IV tumors, respectively, and 56 (11.0%), 116 (22.7%) and 231 (45.3%) had low-, intermediate-, and high-grade tumors, respectively.
Table 2. Demographic and pathological characteristics of patients and controls.
| Cases (n = 510) | Control (n = 510) | ||||
| Variable | No. | % | No. | % | P a value |
| Sex | 0.402 | ||||
| Men | 321 | 62.9 | 308 | 60.4 | |
| Women | 189 | 37.1 | 202 | 39.6 | |
| Age (y) | 0.133 | ||||
| <58 | 240 | 47.1 | 264 | 51.8 | |
| ≥58 | 270 | 52.9 | 246 | 48.2 | |
| Smoking status | 0.017 | ||||
| Smoker | 263 | 51.6 | 225 | 44.1 | |
| Nonsmoker | 247 | 48.4 | 285 | 55.9 | |
| Stage at diagnosis | |||||
| I | 41 | 8.0 | |||
| II | 181 | 35.5 | |||
| III | 162 | 31.8 | |||
| IV | 25 | 4.9 | |||
| Unknow | 101 | 19.8 | |||
| Grade at diagnose | |||||
| Low | 56 | 11.0 | |||
| Intermediate | 116 | 22.7 | |||
| High | 231 | 45.3 | |||
| Unknow | 107 | 21.0 | |||
Two-side chi-square test.
Association between CYP2E1 Polymorphisms and Gastric Cancer Risk
The genotype frequencies of the polymorphisms in the controls were consistent with the Hardy-Weinberg equilibrium distribution (P = 0.055 for PstI, and P = 0.056 for DraI). Table 3 shows the frequency distribution of CYP2E1 PstI or DraI genotypes and the estimated ORs (95% CI) for gastric cancer. In CYP2E1 PstI restriction analysis, there was a significant difference in the distribution of genotypes between the case and control groups. Individuals with the C1C2 or C2C2 genotype had a significantly elevated risk of developing gastric cancer compared with the C1C1 homozygotes, with an adjusted OR (95% CI) of 1.35 (1.01–1.80) and 2.15 (1.18–3.94), respectively. Moreover, C2 carriers (C1C2 or C2C2) had an adjusted OR (95% CI) of 1.48 (1.13–1.96), compared with the C1C1 homozygotes. However, in CYP2E1 DraI restriction analysis, there was no significant difference between the case and control group in the distribution of genotypes. The adjusted OR (95% CI) for TA, AA, and A carriers were 0.76 (0.58–1.01), 1.34 (0.83–2.17), and 0.85 (0.65–1.10), respectively, compared with the TT homozygotes. When the CYP2E1 PstI and DraI genotypes were analyzed together, the individuals with C2C2/AA, had a significantly elevated risk of gastric cancer, with an adjusted OR (95% CI) of 2.66 (1.27–5.57), compared with the C1C1/TT genotype (Table 3).
Table 3. Associations between CYP2E1 polymorphisms and gastric cancer risk.
| Polymorphism of CYP2E1 | Case | Control | Crude OR (95% CI) | Adjusteda OR (95% CI) | ||
| No. | % | No. | % | |||
| PstI genotypes | ||||||
| C1C1 | 348 | 68.2 | 374 | 73.3 | 1.00 | 1.00 |
| C1C2 | 128 | 25.1 | 119 | 23.3 | 1.31 (0.98–1.74) | 1.35 (1.01–1.80) |
| C2C2 | 34 | 6.7 | 17 | 3.3 | 2.21 (1.21–4.03) | 2.15 (1.18–3.94) |
| C1C2+C2C2 | 162 | 31.8 | 136 | 26.7 | 1.42 (1.09–1.86) | 1.49 (1.13–1.96) |
| DraI genotypes | ||||||
| TT | 334 | 65.5 | 318 | 62.3 | 1.00 | 1.00 |
| TA | 131 | 25.7 | 160 | 31.4 | 0.78 (0.59–1.03) | 0.76 (0.58–1.01) |
| AA | 45 | 8.8 | 32 | 6.3 | 1.33 (0.83–2.16) | 1.34 (0.83–2.17) |
| TA+AA | 176 | 34.5 | 192 | 37.6 | 0.86 (0.66–1.11) | 0.85 (0.65–1.10) |
| PstI and DraI genotypes | ||||||
| C1C1/TT | 212 | 41.6 | 233 | 45.7 | 1.00 | 1.00 |
| C1C2/TA | 83 | 16.3 | 77 | 15.1 | 1.20 (0.83–1.72) | 1.13 (0.79–1.63) |
| C2C2/AA | 26 | 5.1 | 11 | 2.2 | 2.62 (1.26–5.44) | 2.66 (1.27–5.57) |
Adjusted for sex, age and smoking habit.
Association between CYP2E1 Polymorphisms and Gastric Cancer Disease Status
In CYP2E1 PstI restriction analysis, both C1C2 and C2C2 genotypes were associated with advanced stage, but not the grade of gastric cancer (Table 4). The frequencies of C1C2 genotype were 21.9%, 24.9%, 31.5%, and 44.0% in stages I, II, II and IV, respectively, whereas the frequencies of C2C2 genotype were 4.9%, 4.4%, 11.1%, and 24.0%, respectively. C2C2 genotype was associated with the advanced stage of gastric cancer, among all of the three subgroup analyses (i.e. III vs. I; III+ IV vs. I; III+ IV vs. I+ II), and the adjusted ORs (95% CI) were 5.17 (1.05–25.54), 4.80 (1.03–22.45), and 4.38 (1.92–9.97), respectively, compared with the C1C1 homozygotes. In addition, C1C2 genotype was associated with the advanced stage of gastric cancer only in the subgroup analysis comparing stages III+ IV with stages I+ II) (adjusted OR = 1.89; CI: 1.18–3.03), compared with the C1C1 homozygotes. However, no association between CYP2E1 RsaI polymorphism and gastric cancer grade was detected (Table 4).
Table 4. Association with CYP2E1 RsaI/PstI polymorphism and progression of gastric cancer.
| Total | C1C1 | C1C2 | C2C2 | ||||
| No. | No. | % | No. | % | No. | % | |
| Tumor stage | |||||||
| I | 41 | 30 | 73.2 | 9 | 21.9 | 2 | 4.9 |
| II | 181 | 128 | 70.7 | 45 | 24.9 | 8 | 4.4 |
| III | 162 | 93 | 57.4 | 51 | 31.5 | 18 | 11.1 |
| IV | 25 | 8 | 32.0 | 11 | 44.0 | 6 | 24.0 |
| Adjusteda OR (95% CI) | |||||||
| III vs. I | 1.00 | 2.29 (0.94–5.58) | 5.17 (1.05–25.54) | ||||
| III+ IV vs. I | 1.00 | 2.30 (0.98–5.38) | 4.80 (1.03–22.45) | ||||
| III+ IV vs. I+ II | 1.00 | 1.89 (1.18–3.03) | 4.38 (1.92–9.97) | ||||
| Tumor grade | |||||||
| Low | 56 | 39 | 69.6 | 13 | 23.2 | 4 | 7.1 |
| Intermediate | 116 | 79 | 68.1 | 20 | 17.2 | 17 | 14.7 |
| High | 231 | 152 | 65.8 | 67 | 20.3 | 12 | 5.2 |
| Adjusteda OR (95% CI) | |||||||
| Intermediate vs. Low | 1.00 | 0.59 (0.26–1.35) | 1.88 (0.58–6.14) | ||||
| High vs. Low | 1.00 | 0.50 (0.20–1.26) | 0.65 (0.19–2.24) | ||||
Adjusted for sex, age and smoking habit.
In CYP2E1 DraI restriction analysis, no significant association was observed with either gastric cancer stage or grade (Table 5).
Table 5. Association with CYP2E1 DraI polymorphism and progression of gastric cancer.
| Total | TT | TA | AA | ||||
| No. | No. | % | No. | % | No. | % | |
| Tumor stage | |||||||
| I | 41 | 25 | 61.0 | 12 | 29.3 | 4 | 9.7 |
| II | 181 | 122 | 67.4 | 38 | 21.0 | 21 | 11.6 |
| III | 162 | 98 | 60.5 | 52 | 32.1 | 12 | 7.4 |
| IV | 25 | 15 | 60.0 | 7 | 28 | 3 | 12.0 |
| Adjusteda OR (95% CI) | |||||||
| III vs. I | 1.00 | 1.00 (0.45–2.20) | 0.90 (0.25–3.17) | ||||
| III+ IV vs. I | 1.00 | 0.98 (0.45–2.14) | 0.93 (0.28–3.11) | ||||
| III+ IV vs. I+ II | 1.00 | 1.37 (0.86–2.19) | 0.75 (0.37–1.52) | ||||
| Tumor grade | |||||||
| Low | 56 | 39 | 69.6 | 11 | 19.7 | 6 | 10.7 |
| Intermediate | 116 | 75 | 64.7 | 31 | 26.7 | 10 | 8.6 |
| High | 231 | 171 | 74.0 | 34 | 14.7 | 26 | 11.3 |
| Adjusteda OR (95% CI) | |||||||
| Intermediate vs. Low | 1.00 | 1.39 (0.62–3.09) | 1.02 (0.33–3.12) | ||||
| High vs. Low | 1.00 | 0.71 (0.33–1.54) | 1.08 (0.41–2.81) | ||||
Adjusted for sex, age and smoking habit.
Association between CYP2E1 Polymorphisms and Gastric Cancer Survival
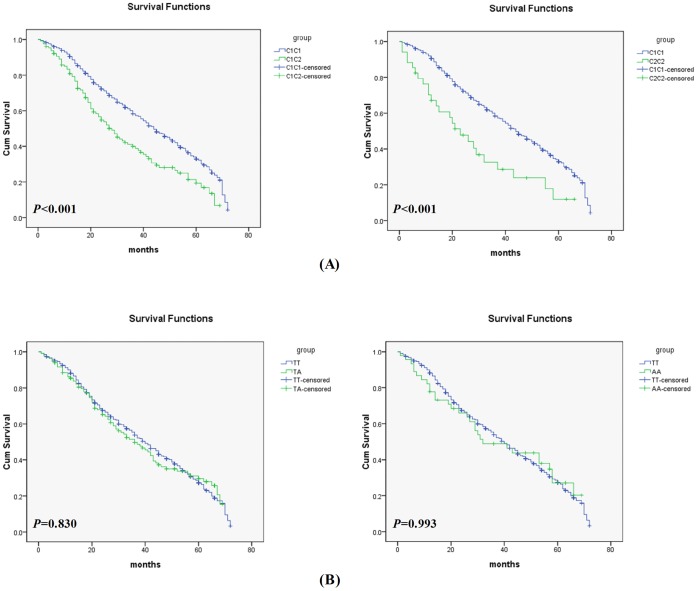
Overall, patients with gastric cancer were followed up for a median (range) of 39 (3–72) months. Kaplan-Meier survival curves (Figure 1A) and log-rank test show that CYP2E1 PstI polymorphism was associated with the poor overall survival of gastric cancer. C1C2 or C2C2 genotype had a markedly poor overall survival, compared with the C1C1 genotype (P<0.001). The median estimated cumulative survival was significantly lower in C1C2 or C2C2 carriers (28 months; 95% CI: 22.1–33.9 months, or 23months; 95% CI: 14.0–32.0 months, respectively), compared with C1C1 carriers (44 months; 95% CI, 38.6–49.4 months). However, the survival was not significantly associated with the CYP2E1 DraI polymorphism (Figure 1B).
Figure 1. Kaplan-Meier survival curves for gastric cancer patients with CYP2E1 PstI/RsaI (A) and DraI (B) polymorphisms.
(A), C1C2 or C2C2 genotype had a markedly poor overall survival, compared with C1C1 genotype (P<0.001); (B), The survival was not significantly associated with the CYP2E1 DraI polymorphism.
Meta-Analysis on the Association between CYP2E1 Polymorphisms and Gastric Cancer Risk
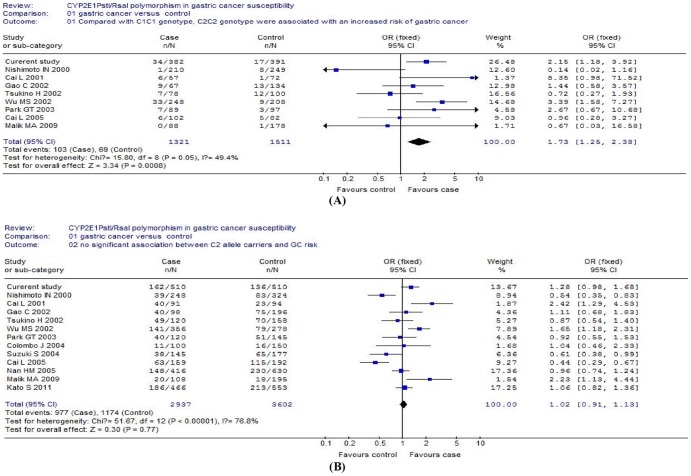
A total of 13 publications met the inclusion criteria [21]–[33]. Of these studies, one study [33] was excluded because the same data was available in a later publication [27]. With the pooled data from those previous studies and our current investigation, this meta-analysis included 2937 cases and 3602 controls. The characteristics of these studies are provided in Table 6. There was a statistically significant association between C2C2 genotype (OR = 1.73; 95% CI: 1.26–2.38; P = 0.0008) and gastric cancer risk (Figure 2A). However, there was no significant association between C2 carriers and gastric cancer risk. The pooled ORs for C2 carriers were 1.01 (95% CI: 0.80–1.28; P = 0.93), compared with the homozygous wild-type genotype (C1/C1) (Figure 2B). Prior to the present study, there were only two previous studies evaluating the association between CYP2E1 DraI polymorphism and gastric cancer risk [21], [29]. Because the samples of these studies were too small to generate a sufficient power, we did not conduct the meta-analysis on this polymorphism. Nevertheless, all the three studies reported that there was no significant association between DraI polymorphism and gastric cancer risk.
Table 6. Characteristics of gastric cancer case-control studies included in meta-analysis on the association between CYP2E1 polymorphisms and gastric cancer.
| Study | Year | Population | Case | Control | ||||||
| PstI restriction analysis | C1C1 | C1C2 | C2C2 | C1C1 | C1C2 | C2C2 | ||||
| Park et al | 2003 | Koreans | 80 | 33 | 7 | 94 | 48 | 3 | ||
| Cai et al | 2001 | Chinese | 58 | 27 | 6 | 71 | 22 | 1 | ||
| Nishimoto et al | 2000 | Brazilian | 209 | 38 | 1 | 241 | 75 | 8 | ||
| Tsukino et al | 2002 | Japanese | 71 | 42 | 7 | 88 | 58 | 12 | ||
| Gao et al | 2002 | Chinese | 58 | 31 | 9 | 121 | 62 | 13 | ||
| Colombo et al | 2004 | Brazilian | 89 | 11 | 0 | 134 | 16 | 0 | ||
| Nan et al | 2005 | Korean | 268 | 148a | 400 | 230a | ||||
| Suzuki et al | 2004 | Japanese | 107 | 38a | 112 | 65a | ||||
| Wu et al | 2002 | Chinese | 215 | 108 | 33 | 199 | 70 | 9 | ||
| Cai et al | 2005 | Chinese | 96 | 57 | 6 | 77 | 110 | 5 | ||
| Malik et al | 2009 | Indian | 88 | 20 | 0 | 177 | 17 | 1 | ||
| Kato et al | 2011 | Japanese | 280 | 186a | 340 | 213a | ||||
| Current study | Chinese | 348 | 128 | 34 | 374 | 119 | 17 | |||
| DraI restriction analysis | TT | TA | AA | TT | TA | AA | ||||
| Park et al | 2003 | Koreans | 35 | 7 | 78 | 45 | 8 | 85 | ||
| Wu et al | 2002 | Chinese | 195 | 120 | 41 | 158 | 100 | 20 | ||
| Current study | Chinese | 334 | 131 | 45 | 348 | 121 | 41 | |||
C1C2+C2C2.
Figure 2. Forest plots on the association of CYP2E1 PstI/RsaI polymorphism with gastric cancer risk.
(A), CYP2E1 PstI/RsaI C2C2 genotype and gastric cancer risk (fixed-effect model); (B), CYP2E1 PstI/RsaI C2 allele carriers and gastric cancer risk (random-effect model).
Discussion
In the present case-control study, for CYP2E1 PstI polymorphism, we observed that both C2 carriers and C2C2 genotypes were significantly associated with gastric cancer risk and poor clinical prognosis. However, we did not found any significant association between CYP2E1 DraI polymorphism and both gastric cancer risk and clinical prognosis. In addition, our meta-analysis also confirmed that the CYP2E1 PstI polymorphism, but not DraI, was associated significantly with the risk of gastric cancer, which provided further evidence indicating an association between this functional polymorphism and gastric cancer susceptibility.
Gastric cancer is a multistep process in which genetic and environmental factors interact in the development of cancer. Interindividual genetic differences in susceptibility to chemical carcinogens are among the most important host factors in human cancer [36]–[38]. It has been proposed that various host factors affect susceptibility to cancer, even following the same exposure to environmental carcinogenic factors [39]–[41]. Of special interest is CYP2E1 whose polymorphisms are related to substantial interindividual variation in metabolizing carcinogens and cancer risks [42]–[44]. Our results indicating the association between the CYP2E1 polymorphism and the risk of gastric cancer are biologically plausible. CYP2E1 catalyzes oxidation and DNA adduct formation of some low-molecular carcinogens of gastric cancer [45]–[46]. It has been revealed that the PstI and RsaI restriction sites are in the transcription-regulation region of CYP2E1 [14]. A tenfold increase in gene expression of the homozygous C2/C2 genotype of CYP2E1 using PstI or RsaI digestion has been reported [14]. In contrast, the polymorphism detected by DraI digestion of CYP2E1 is located in intron 6, and no functional significance of this polymorphism exists. This may explain why the PstI/RsaI, rather than DraI, polymorphism of CYP2E1 conferred a greater risk of gastric cancer in this study. Our meta-analysis provided further evidence indicating an association between CYP2E1 PstI/RsaI polymorphism and gastric cancer susceptibility. It is agreed with the previous meta-analysis in 2007 [47], which reported that CYP2E1 PstI/RsaI polymorphism may be a risk factor for gastric cancer in Asians.
Another interesting finding in the present study was the association of the CYP2E1 PstI/RsaI polymorphism with gastric cancer stage, which may mirror the substantial role of this polymorphism in the progression. We also reported, for the first time, that this polymorphism affected gastric cancer survival. Recently, studies have demonstrated that CYP2E1 plays an important role in tumor progression, and may be used as a prognostic indicator. For instance, Vaclavikova et al. observed that increased CYP2E1 expression was associated with an invasive breast cancer, and suggested its potential role as a breast cancer prognosis marker [48]. Tsunedomi et al. also found that the expression of CYP2E1 was associated with the progression of hepatitis C virus-associated hepatocellular carcinoma [49]. In addition, CYP2E1 positivity is closely correlated with a poor survival of patients with non-small cell lung carcinoma, and the expression of CYP2E1 in bronchial epithelium has a prognostic potential [50]. Conversely, an animal study demonstrated that blockade of cytochrome P450 significantly reduced capillary formation and tumor size in glial tumors formed by injection of rat glioma 2 (RG2) cells, and also resulted in an increased animal survival time [51]. Furthermore, epidemiologic studies have also indicated that the CYP2E1 PstI/RsaI polymorphism is associated with cancer progression and prognosis [52], [53]. CYP2E1 C2C2 genotype is significantly associated with advanced clinical stages, and also associated with tumor recurrence, since it is important for determining the parameters associated with tumor progression and poor outcomes in patients with head and neck squamous cell carcinoma [52]. Haque AK et al. observed that CYP2E1 wild-type allele was significantly associated with better survival of non-small cell lung carcinoma and the expression of p53 [53]. However, it remains unclear whether CYP2E1 PstI/RsaI polymorphism is associated with the differentiation of cancer. In our study, we found there was no significant association with the differentiation of gastric cancer.
There were a few limitations in the present study. First, data on the cancer stage and differentiation status were unknown for a few patients, which may bring some bias to the results indicating the association between the polymorphism and cancer status. Second, although we carried out a meta-analysis, in order to further confirm our results, only published studies were included in it. This may have limited the power of the pooled results.
In conclusion, CYP2E1 PstI/RsaI polymorphism is associated with development and progression of gastric cancer and poor prognosis of patients with gastric cancer. However, there was no significant association between CYP2E1 DraI polymorphism and the risk of gastric cancer. Future investigation in this area should aim to elucidate the underlying mechanisms between CYP2E1 PstI/RsaI polymorphism and gastric cancer.
Acknowledgments
The authors thank Medjaden Bioscience Limited for assisting in the preparation of this manuscript.
Funding Statement
This work was supported by the National Natural Science Foundation of China [NO. 81072031(BA10)]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, et al. (2011) Global cancer statistics. CA Cancer J Clin 61(2): 69–90. [DOI] [PubMed] [Google Scholar]
- 2. Brenner H, Rothenbacher D, Arndt V (2009) Epidemiology of stomach cancer. Methods Mol Biol 472: 467–77. [DOI] [PubMed] [Google Scholar]
- 3. Parkin DM, Bray F, Ferlay J, Pisani P (2001) Estimating the world cancer burden: Globocan 2000. Int J Cancer 94(2): 153–6. [DOI] [PubMed] [Google Scholar]
- 4. Yang L (2006) Incidence and mortality of gastric cancer in China. World J Gastroenterol 12(1): 17–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kelley JR, Duggan JM (2003) Gastric cancer epidemiology and risk factors. J Clin Epidemiol 56: 1–9. [DOI] [PubMed] [Google Scholar]
- 6. Hwang IR, Kodama T, Kikuchi S, Sakai K, Peterson LE, et al. (2002) Effect of interleukin 1 polymorphisms on gastric mucosal interleukin 1beta production in Helicobacter pylori infection. Gastroenterology 123: 1793–803. [DOI] [PubMed] [Google Scholar]
- 7. Xue H, Lin B, Ni P, Xu H, Huang G (2010) Interleukin-1B and interleukin-1 RN polymorphisms and gastric carcinoma risk: a meta-analysis. J Gastroenterol Hepatol 25(10): 1604–17. [DOI] [PubMed] [Google Scholar]
- 8. Umeno M, McBride OW, Yang CS, Gelboin HV, Gonzalez FJ (1988) Human ethanol-inducible P450IIE1: complete gene sequence, promoter characterization, chromosome mapping, and cDNA-directed expression. Biochemistry 27(25): 9006–13. [DOI] [PubMed] [Google Scholar]
- 9. Yamazaki H, Inui Y, Yun CH, Guengerich FP, Shimada T (1992) Cytochrome P450 2E1 and 2A6 enzymes as major catalysts for metabolic activation of N-nitrosodialkylamines and tobacco-related nitrosamines in human liver microsomes. Carcinogenesis 13(10): 1789–94. [DOI] [PubMed] [Google Scholar]
- 10. Bellec G, Dréano Y, Lozach P, Ménez JF, Berthou F (1996) Cytochrome P450 metabolic dealkylation of nine N-nitrosodialkylamines by human liver microsomes. Carcinogenesis 17(9): 2029–34. [DOI] [PubMed] [Google Scholar]
- 11. Hoffmann D, Hecht SS (1985) Nicotine-derived N-nitrosamines and tobacco-related cancer: current status and future directions. Cancer Res 45(3): 935–44. [PubMed] [Google Scholar]
- 12. Hecht SS, Hoffmann D (1988) Tobacco-specific nitrosamines, an important group of carcinogens in tobacco and tobacco smoke. Carcinogenesis 9(6): 875–84. [DOI] [PubMed] [Google Scholar]
- 13. Tang K, Li Y, Zhang Z, Gu Y, Xiong Y, et al. (2010) The PstI/RsaI and DraI polymorphisms of CYP2E1 and head and neck cancer risk: a meta-analysis based on 21 case-control studies. BMC Cancer 10: 575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hayashi S, Watanabe J, Kawajiri K (1991) Genetic polymorphisms in the 5-flanking region change transcriptional regulation of the human cytochrome p450IIE1 gene. J Biochem 110: 559–65. [DOI] [PubMed] [Google Scholar]
- 15. Hu Y, Hakkola J, Oscarson M, Ingelman-Sundberg M (1999) Structural and functional characterization of the 5′-flanking region of the rat and human cytochrome P450 2E1 genes: identification of a polymorphic repeat in the human gene. Biochem Biophys Res Commun 263(2): 286–93. [DOI] [PubMed] [Google Scholar]
- 16. Hayashi S, Watanabe J, Kawajiri K (1991) Genetic polymorphisms in the 5′-flanking region change transcriptional regulation of the human cytochrome P450IIE1 gene. J Biochem 110(4): 559–65. [DOI] [PubMed] [Google Scholar]
- 17. Watanabe J, Hayashi S, Kawajiri K (1994) Different regulation and expression of the human CYP2E1 gene due to the RsaI polymorphism in the 5′-flanking region. J Biochem 116(2): 321–6. [DOI] [PubMed] [Google Scholar]
- 18. Zhan P, Wang J, Zhang Y, Qiu LX, Zhao SF, et al. (2010) CYP2E1 Rsa I/Pst I polymorphism is associated with lung cancer risk among Asians. Lung Cancer 69(1): 19–25. [DOI] [PubMed] [Google Scholar]
- 19. Balaji L, Singh KB, Bhaskar LV (2011) Genetic Polymorphisms of the CYP2E1 Gene do not Contribute to Oral Cancer Susceptibility in South Indians. Asian Pac J Cancer Prev 12(6): 1523–7. [PubMed] [Google Scholar]
- 20. Lee HC, Yoon YB, Kim CY (1997) Association between genetic polymorphisms of the cytochromes P-450 (1A1, 2D6, and 2E1) and the susceptibility to pancreatic cancer. Korean J Intern Med 12(2): 128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Park GT, Lee OY, Kwon SJ, Lee CG, Yoon BC, et al. (2003) Analysis of CYP2E1 polymorphism for the determination of genetic susceptibility to gastric cancer in Koreans. J Gastroenterol Hepatol 18(11): 1257–63. [DOI] [PubMed] [Google Scholar]
- 22. Nishimoto IN, Hanaoka T, Sugimura H, Nagura K, Ihara M, et al. (2000) Cytochrome P450 2E1 polymorphism in gastric cancer in Brazil: case-control studies of Japanese Brazilians and non-Japanese Brazilians. Cancer Epidemiol Biomarkers Prev 9(7): 675–80. [PubMed] [Google Scholar]
- 23. Tsukino H, Kuroda Y, Qiu D, Nakao H, Imai H, et al. (2002) Effects of cytochrome P450 (CYP) 2A6 gene deletion and CYP2E1 genotypes on gastric adenocarcinoma. Int J Cancer 100(4): 425–8. [DOI] [PubMed] [Google Scholar]
- 24. Colombo J, Rossit AR, Caetano A, Borim AA, Wornrath D, et al. (2004) GSTT1, GSTM1 and CYP2E1 genetic polymorphisms in gastric cancer and chronic gastritis in a Brazilian population. World J Gastroenterol 10(9): 1240–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gao C, Takezaki T, Wu J, Li Z, Wang J, et al. (2002) Interaction between cytochrome P-450 2E1 polymorphisms and environmental factors with risk of esophageal and stomach cancers in Chinese. Cancer Epidemiol Biomarkers Prev 11(1): 29–34. [PubMed] [Google Scholar]
- 26. Cai L, Yu SZ, Zhan ZF (2001) Cytochrome P450 2E1 genetic polymorphism and gastric cancer in Changle, Fujian Province. World J Gastroenterol 7(6): 792–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nan HM, Park JW, Song YJ, Yun HY, Park JS, et al. (2005) Kimchi and soybean pastes are risk factors of gastric cancer. World J Gastroenterol 11(21): 3175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Suzuki S, Muroishi Y, Nakanishi I, Oda Y (2004) Relationship between genetic polymorphisms of drug-metabolizing enzymes (CYP1A1, CYP2E1, GSTM1, and NAT2), drinking habits, histological subtypes, and p53 gene point mutations in Japanese patients with gastric cancer. J Gastroenterol 39(3): 220–30. [DOI] [PubMed] [Google Scholar]
- 29. Wu MS, Chen CJ, Lin MT, Wang HP, Shun CT, et al. (2002) Genetic polymorphisms of cytochrome p450 2E1, glutathione S-transferase M1 and T1, and susceptibility to gastric carcinoma in Taiwan. Int J Colorectal Dis 17(5): 338–43. [DOI] [PubMed] [Google Scholar]
- 30. Cai L, Zheng ZL, Zhang ZF (2005) Cytochrome p450 2E1 polymorphisms and the risk of gastric cardia cancer. World J Gastroenterol 11(12): 1867–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Malik MA, Upadhyay R, Mittal RD, Zargar SA, Modi DR, et al. (2009) Role of xenobiotic-metabolizing enzyme gene polymorphisms and interactions with environmental factors in susceptibility to gastric cancer in Kashmir Valley. J Gastrointest Cancer 40(1–2): 26–32. [DOI] [PubMed] [Google Scholar]
- 32. Kato S, Naito Z, Matsuda N, Onodera H, Sakurazawa N, et al. (2011) Localization of cytochrome P4502E1 enzyme in normal and cancerous gastric mucosa and association with its genetic polymorphism in unoperated and remnant stomach. J Nihon Med Sch 78(4): 224–34. [DOI] [PubMed] [Google Scholar]
- 33. Nan HM, Song YJ, Yun HY, Park JS, Kim H (2005) Effects of dietary intake and genetic factors on hypermethylation of the hMLH1 gene promoter in gastric cancer. World J Gastroenterol 11(25): 3834–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shparyk IaV (1998) [New classification of malignant tumors TNM (5th edition)]. Klin Khir (6): 36–8. [PubMed] [Google Scholar]
- 35. Sobin LH (1981) The international histological classification of tumours. Bull World Health Organ 59(6): 813–9. [PMC free article] [PubMed] [Google Scholar]
- 36. Glatt HR (2000) An overview of bioactivation of chemical carcinogens. Biochem Soc Trans 28: 1–6. [DOI] [PubMed] [Google Scholar]
- 37. Indulski JA, Lutz W (2000) Metabolic genotype in relation to individual susceptibility to environmental carcinogens. Int Arch Occup Environ Health 73: 71–85. [DOI] [PubMed] [Google Scholar]
- 38. Cai L, Yu SZ, Zhang ZF (2001) Glutathione S-transferases M1, T1 genotypes and the risk of gastric cancer: A case-control study. World J Gastroenterol 7: 506–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Augenlicht LH, Heerdt BG, Mariadason JM, Yang W, Wilson AJ, et al. (2002) Environment-gene interactions in intestinal cancer. Eur J Cancer Prev (Suppl 2): S12–7. [PubMed]
- 40. Malats N (2001) Gene-environment interactions in pancreatic cancer. Pancreatology 1: 472–6. [DOI] [PubMed] [Google Scholar]
- 41. Kiyohara C (2000) Genetic polymorphism of enzymes involved in xenobiotic metabolism and the risk of colorectal cancer. J Epidemiol 10: 349–60. [DOI] [PubMed] [Google Scholar]
- 42. Agundez JA (2004) Cytochrome p450 gene polymorphism and cancer. Curr Drug Metab 5: 211–24. [DOI] [PubMed] [Google Scholar]
- 43. Neuhaus T, Ko YD, Lorenzen K, Fronhoffs S, Harth V, et al. (2004) Association of cytochrome P450 2E1 polymorphisms and head and neck squamous cell cancer. Toxicol Lett 151: 273–82. [DOI] [PubMed] [Google Scholar]
- 44. Munaka M, Kohshi K, Kawamoto T, Takasawa S, Nagata N, et al. (2003) Genetic polymorphisms of tobacco- and alcohol-related metabolizing enzymes and the risk of hepatocellular carcinoma. J Cancer Res Clin Oncol 129: 355–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kuno S, Hirohata T (1996) Nutrition and cancer. Cancer Causes Control 7: 41–55. [DOI] [PubMed] [Google Scholar]
- 46. Camus AM, Geneste O, Honkakoshi P, Béréziat JC, Henderson CJ, et al. (1993) High variability of nitrosamine metabolism among individuals: role of cytochrome p450 2A6 and 2E1 in the dealkylation of N-nitrosodimethylamine and N-nitrosodiethylamine in mice and humans. Mol Carcinog 7: 268–75. [DOI] [PubMed] [Google Scholar]
- 47. Boccia S, De Lauretis A, Gianfagna F, van Duijn CM, Ricciardi G (2007) CYP2E1PstI/RsaI polymorphism and interaction with tobacco, alcohol and GSTs in gastric cancer susceptibility: a meta-analysis of the literature. Carcinogenesis 28(1): 101–6. [DOI] [PubMed] [Google Scholar]
- 48. Vaclavikova R, Hubackova M, Stribrna-Sarmanova J, Kodet R, Mrhalova M, et al. (2007) RNA expression of cytochrome P450 in breast cancer patients. Anticancer Res 27(6C): 4443–50. [PubMed] [Google Scholar]
- 49. Tsunedomi R, Iizuka N, Hamamoto Y, Uchimura S, Miyamoto T, et al. (2005) Patterns of expression of cytochrome P450 genes in progression of hepatitis C virus-associated hepatocellular carcinoma. Int J Oncol 27(3): 661–7. [PubMed] [Google Scholar]
- 50. Oyama T, Sugio K, Uramoto H, Iwata T, Onitsuka T, et al. (2007) Increased cytochrome P450 and aryl hydrocarbon receptor in bronchial epithelium of heavy smokers with non-small cell lung carcinoma carries a poor prognosis. Front Biosci 12: 4497–503. [DOI] [PubMed] [Google Scholar]
- 51. Zagorac D, Jakovcevic D, Gebremedhin D, Harder DR (2008) Antiangiogenic effect of inhibitors of cytochrome P450 on rats with glioblastoma multiforme. J Cereb Blood Flow Metab 28(8): 1431–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Olivieri EH, da Silva SD, Mendonça FF, Urata YN, Vidal DO, et al. (2009) CYP1A2*1C, CYP2E1*5B, and GSTM1 polymorphisms are predictors of risk and poor outcome in head and neck squamous cell carcinoma patients. Oral Oncol 45(9): e73–9. [DOI] [PubMed] [Google Scholar]
- 53. Haque AK, Au W, Cajas-Salazar N, Khan S, Ginzel AW, et al. (2004) CYP2E1 polymorphism, cigarette smoking, p53 expression, and survival in non-small cell lung cancer: a long term follow-up study. Appl Immunohistochem Mol Morphol 12(4): 315–22. [DOI] [PubMed] [Google Scholar]