Abstract
The epidermal growth factor receptor (EGFR) is a well-established target for cancer treatment. EGFR tyrosine kinase (TK) inhibitors, such as gefinitib and erlotinib, have been developed as anti-cancer drugs. Although non-small cell lung carcinoma with an activating EGFR mutation, L858R, responds well to gefinitib and erlotinib, tumors with a doubly mutated EGFR, T790M-L858R, acquire resistance to these drugs. The C. elegans EGFR homolog LET-23 and its downstream signaling pathway have been studied extensively to provide insight into regulatory mechanisms conserved from C. elegans to humans. To develop an in vivo screening system for potential cancer drugs targeting specific EGFR mutants, we expressed three LET-23 chimeras in which the TK domain was replaced with either the human wild-type TK domain (LET-23::hEGFR-TK), a TK domain with the L858R mutation (LET-23::hEGFR-TK[L858R]), or a TK domain with the T790M-L858R mutations (LET-23::hEGFR-TK[T790M-L858R]) in C. elegans vulval cells using the let-23 promoter. The wild-type hEGFR-TK chimeric protein rescued the let-23 mutant phenotype, and the activating mutant hEGFR-TK chimeras induced a multivulva (Muv) phenotype in a wild-type C. elegans background. The anti-cancer drugs gefitinib and erlotinib suppressed the Muv phenotype in LET-23::hEGFR-TK[L858R]-expressing transgenic animals, but not in LET-23::hEGFR-TK[T790M-L858R] transgenic animals. As a pilot screen, 8,960 small chemicals were tested for Muv suppression, and AG1478 (an EGFR-TK inhibitor) and U0126 (a MEK inhibitor) were identified as potential inhibitors of EGFR-mediated biological function. In conclusion, transgenic C. elegans expressing chimeric LET-23::hEGFR-TK proteins are a model system that can be used in mutation-specific screens for new anti-cancer drugs.
Introduction
Development of a high-throughput, low-cost in vivo screening system for small molecule anti-cancer reagents would ideally be able to overcome the major problems of conventional in vitro screening methods. Due to fast generation time, high progeny numbers, low cost, and well established genetic tools, the nematode Caenorhabditis elegans (C. elegans) is an attractive candidate for an animal model screening system, with many of the advantages of in vitro screening systems and animal models [1].
EGFR is overexpressed or aberrantly activated in various types of human cancer, such as breast, ovarian, and non-small-cell lung carcinoma (NSCLC) [2]. EGFR is involved in various steps of cancer development including tumorigenesis, invasion, metastasis, and angiogenesis [3], and thus provides an attractive target for cancer drug development. Gefitinib (Commercial name: Iressa) was the first EGFR-TK inhibitor drug developed for the treatment of epithelial cancers such as NSCLC [4]. Mutations in the EGFR-TK domain have been linked to gefitinib sensitivity in a subset of lung cancers, and have also been found to activate anti-apoptotic pathways [5], [6].
C. elegans vulval development is a well-established model system used to study the EGFR signaling pathway [7]–[9]. Among the six vulval precursor cells (VPCs), P5.p, P6.p, and P7.p adopt the 2°-1°-2° cell fates, respectively, and continue dividing to form the mature vulva. The 1° cell fate is determined as a result of EGFR-Ras-MAPK signaling in P6.p, whereas the 2° cell fate is determined by LIN-12/Notch signaling in P5.p and P7.p, which is activated as a result of EGFR-Ras-MAPK signaling in the neighboring cell. Components of the EGFR pathway, including EGFR, Ras, Raf, MEK, and MAPK, are highly conserved between humans and C. elegans [8]. A limited number of chemical compounds that target the EGFR pathway have been tested using C. elegans vulval development as a model. Farnesyltransferase inhibitors, which inhibit Ras activity, and MCP compounds, which disrupt Ras-Raf interactions were found to act specifically on the orthologous proteins in the C. elegans EGFR-Ras pathway [10]–[12]. The toxicity of the EGFR kinase inhibitors BIBU1361 and BIBX1382 was also evaluated in C. elegans [13]. These studies suggest the potential for using C. elegans as a tool for anti-EGFR pathway drug screening.
In this study, we developed and analyzed a human EGFR-driven C. elegans model, which exhibits the Muv phenotype. Using this model, a pilot screen of 8,960 chemicals was conducted, and an EGFR inhibitor and a MEK inhibitor were isolated as suppressors, suggesting that this C. elegans-based system can be used efficiently to screen for new EGFR-inhibitory drugs.
Materials and Methods
Worm culture and strains
Wild-type N2 and mutant strains were cultured as described by Brenner [14]. Mutant alleles used in this work are let-23(sy1), let-23(sa62), let-60(n1700) and lin-15(n765). Integration lines used in this work are jgIs6[let-23p::LET-23::hEGFR-TK[L858R], rol-6(su1006)], jgIs19[let-23p::LET-23::hEGFR, rol-6(su1006)], jgIs25[let-23p::LET-23::hEGFR-TK[T790M-L858R], rol-6(su1006), myo-2p::mCherry], jgIs14[egl-17p::EMR-1::RFP, dhs-31p::NLS::GFP, rol-6(su1006)], JJ1136 (HMP-1::GFP), PS4657 (AJM-1::GFP) and PS3352 (CDH-3::GFP).
Construction of LET-23::hEGFR chimeric plasmids
We used genomic DNA of the let-23 gene and cDNA encoding human EGFR. Each DNA fragment was amplified by PCR, cloned into the pGEM-T easy vector (Promega Inc., Madison, WI, USA), and confirmed by sequencing. We then assembled the DNA fragments using appropriate restriction enzymes and the corresponding sites of the pPD117.01 vector (Dr. Andrew Fire, Stanford Univ., CA, USA). QuikChange site-directed mutagenesis (Cat # 200523, Agilent Technologies Inc., Santa Clara, CA, USA) of EGFR-TK cDNA was performed to produce EGFR[L858R], EGFR[T790M] and EGFR[T790M-L858R]. The detailed procedure and primers used in this study are provided in the Supplementary Materials (Fig. S1B). To use as a secondary cell fate marker, pJG205 was constructed by combining a PCR fragment amplified from the genomic DNA sequence 4.0 kb upstream of egl-17 with emr-1 cDNA, DsRed (RFP, Clontech of TAKARA Bio Inc.) and pPD95.77 (A. Fire). The GFP encoding sequence of pPD95.77 was replaced with DsRed cDNA. Another cell fate marker, pJG207, was made by cloning the dhs-31 promoter region into pPD95.69 (A. Fire), which contains the SV40 nuclear localization signal (NLS) and GFP. All plasmid constructs were confirmed by sequencing.
Microinjection and integration
The injection concentration of DNA constructs and markers were 75 µg/ml pRF4[rol-6(su1006)], 50 or 25 µg/ml EGFR chimeric plasmids, 50 µg/ml ttx-3::gfp, 35 µg/ml pJG205[egl-17p::EMR-1::RFP], 40 µg/ml pJG207[dhs-31p::NLS::GFP], and 5 µg/ml pCFJ90[myo-2p::mCherry] (Addgene, Cambridge, MA, USA). The total DNA concentration of each injection mixture was 150 µg/ml, with pBluescript SK+ DNA added when necessary. All integrated lines were made using UV irradiation and out-crossed four times.
Microscopy and Hoechst33342 staining
All microscopic images were captured and processed using an AxioCam HRc digital camera attached to an Imager M1 fluorescence microscope and Axiovision Rel. 4.6 software (Zeiss Inc., Germany). For Hoechst33342 (Molecular Probes of Life Technologies Co., Grand Island, NY, USA) staining, jgIs6 worms were harvested and washed several times with M9 buffer. The worms were then soaked in 1 µg/ml Hoechest33342 solution for 30 minutes. After washing, worms were prepared for observation.
Chemical treatment and statistics
Chemicals including gefitinib, erlotinib, U0126, AZD6244, PD0325901 and WZ4002 were purchased from Selleck Chemicals LLC. (Houston, TX, USA), and the 1,280 chemical library was purchased from Sigma-Aldrich Co. LLC. (St. Louis, MO, USA). The other 7,680 chemicals were obtained from the Korea Chemical Bank of KRICT. Chemicals were dissolved in 100% DMSO solution, and kept at −20°C as 1 or 10 mM stocks for screening. All chemical tests were executed in 96-well plates, and the final volume per well was 100 µl including worms, cholesterol, and dead E. coli. The DMSO concentration of the control group was kept at 0.5%, and DMSO concentrations of all experimental groups were below 0.5%. The final chemical concentration used for the screen was 5 µM and 20 to 50 L1 worms were cultured in each well of the 96-well plate. Each chemical test included at least three wells per chemical and was repeated at least twice. The error bars in all graphs represent SD (standard deviation), and P values relative to the control were calculated by unpaired Student's t-test.
Results
Chimeric LET-23::hEGFR-TK protein is functional in C. elegans
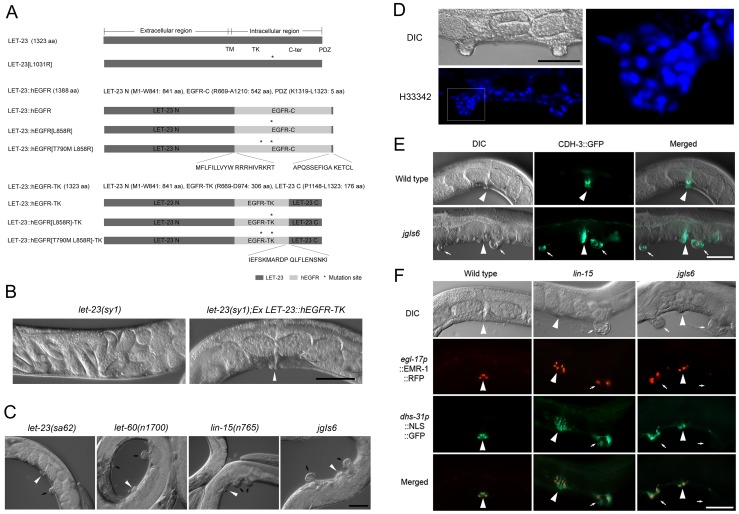
To develop a C. elegans model system to screen for chemicals that inhibit human EGFR (hEGFR) activity, we designed plasmid constructs that express the C. elegans EGFR ortholog, LET-23, and the hEGFR fusion protein, by swapping the cytoplasmic or TK domain of LET-23 with each hEGFR domain (Fig. 1A and Fig. S1). We aimed to facilitate functional expression of the human counterpart in C. elegans by retaining most of the C. elegans LET-23 coding and regulatory sequences, for example, by maintaining C-terminal residues important for proper trafficking [15]. The putative protein products of these transgenes have 1388 amino acids (LET-23::hEGFR) or 1323 amino acids (LET-23::hEGFR-TK). As shown in Figure 1A and Figure S2, LET-23::hEGFR includes 841 amino acids of the LET-23 N-terminal domain, 542 amino acids of the human EGFR C-terminal domain, and 5 amino acids of the LET-23 PDZ interacting motif. LET-23::hEGFR-TK includes 841 amino acids of the LET-23 N-terminal domain, 306 amino acids of the human EGFR-TK domain, and 176 amino acids of the LET-23 C-terminal domain. To test whether this chimeric LET-23::hEGFR-TK protein is functional, the construct was microinjected into the let-23(sy1) mutant. Most let-23 mutants are lethal, but let-23(sy1) is viable and vulvaless (Vul) due to aberrant trafficking of LET-23 [15]–[17]. The chimeric LET-23::hEGFR-TK protein rescued the Vul phenotype of let-23(sy1) (Fig. 1B), indicating that the chimeric protein is functional in C. elegans. When the let-23(sy1) mutant was rescued with jgIs19, an integrated strain containing the LET-23::hEGFR transgene, the let-23(sy1);jgIs19 strain showed a significantly reduced vulvaless population (9.1%) compared to the let-23(sy1) mutant (92%) (Fig. S1C).
Figure 1. The development of the human oncogenic EGFR induced Muv model.
(A) LET-23 and LET-23-based chimeric receptor constructs. All constructs were designed to express each chimeric receptor from the let-23 promoter. (B) The LET-23::hEGFR-TK chimera rescues the vulvaless phenotype of the let-23(sy1) mutant. (C) A comparison of several Muv mutants and the jgIs6 transgenic strain which expresses LET-23::hEGFR-TK[L858R]. C. elegans expressing LET-23::hEGFR-TK[L858R] exhibited a larger pseudovulva compared to let-23(sa62) and let-60(n1700) Muv mutants. (D) Hoechst33342 (H33342) staining of the pseudovulval region in jgIs6 revealed many nuclei. The boxed region of the lower panel is enlarged in the right panel. (E) Expression of a 1° cell marker, CDH-3::GFP in the wild-type worm and jgIs6. CDH-3::GFP is highly expressed both in the vulva and pseudovulva of jgIs6. (F) Expression of 2° vulval cell fate markers in the lin-15 Muv mutant and jgIs6. Reporter genes controlled by promoters of egl-17 and dhs-31 were expressed in the vulva and pseudovulva. Arrowheads indicate normal vulvae and small arrows indicate pseudovulvae. Scale bars, 50 µm.
Next, we assessed the effects of over-expressing the activating mutant form of LET-23::hEGFR-TK in a let-23(+) background. Increased activity of the EGFR-Ras-MAPK pathway results in the hyper-induction of vulval cells, referred to as a Muv phenotype, as is seen with the semi-dominant let-23(sa62) mutation and the constitutively active let-60(n1700) mutation. lin-15 acts upstream of let-23 to negatively regulate the EGFR-Ras-MAPK pathway [18]. This negative regulation is disrupted in the lin-15(n765) mutant, resulting in a strong Muv phenotype [18], [19]. Thus, we expected that the activating mutations in the EGFR-TK region would also cause a Muv phenotype. Two activating EGFR mutations that confer gefitinib sensitivity to certain lung cancers were tested: EGFR[L858R] and EGFR[Δ747–752] [20], [21]. In-frame deletions in exon 19 including Δ747–749 (44%), and single point mutations in exon 21 including L858R (41%) are the most frequently found EGFR-TK activating mutations in NSCLC [20], . The gefitinib-resistant EGFR[T790M-L858R] mutation was also tested. T790M is a secondary mutation which endows gefitinib resistance to the L858R lesion [21]. Chimeric LET-23::hEGFR-TK containing any of these mutations induced the hyper-induction of vulval cells resulting in a Muv phenotype (Fig. 1C and Table 1). Transgenic animals expressing LET-23::hEGFR-TK[L858R] or LET-23::hEGFR-TK[T790M-L858R] had a phenotype similar to lin-15(n765), with 2-4 large pseudovulvae. In contrast, transgenic animals expressing LET-23::hEGFR-TK[T790M] did not exhibit a Muv phenotype. To facilitate further analysis, we selected a LET-23::hEGFR-TK[L858R] transgenic line to generate jgIs6, a strain with the transgenic array integrated into the genome. Integration of the transgene greatly enhanced the penetrance of the Muv phenotype; 49% Muv before integration versus 94.6% Muv after integration (Table 1). Staining of nuclei with Hoechst33342 revealed the presence of many nuclei in the pseudovulval region (Fig. 1D), and this means that cell numbers were increased in the pseudovulva of our transgenic strain similar to other Muv mutants such as let-60(n1700), and lin-15(n765). Transgenic animals showed a rolling phenotype because pRF4[rol-6(su1006)] was used as a transgenic marker. To facilitate Muv phenotype detection, we introduced the sqt-1(jg52) mutation into the transgenic lines. This sqt-1 mutant suppresses the rolling phenotype of rol-6 without any obvious defects [23]. Unexpectedly, we found that the sqt-1(jg52) mutation enhanced the Muv phenotype of the EGFR transgenic lines (Table 2).
Table 1. The multivulva phenotype of transgenic strains expressing LET-23::hEGFR-TK proteins.
| Name | % Muva | Integration line (% Muv, n) |
| LET-23::hEGFR | 0 | jgIs19 (0, 175) |
| LET-23::hEGFR[L858R] | 78 | |
| LET-23::hEGFR[T790M-L858R] | 75 | |
| LET-23::hEGFR-TK | 0 | |
| LET-23::hEGFR-TK[L858R] | 49 | jgIs6 (94.6±1.33, 1465) |
| LET-23::hEGFR-TK[T790M] | 0 | |
| LET-23::hEGFR-TK[Δ747–752] | 12 | |
| LET-23::hEGFR-TK[T790M-L858R] | 45 | jgIs25 (94.9±0.32, 410) |
| LET-23[L1031R] | 10 |
All transgenes were expressed by the let-23 promoter, and transgenic lines which show highest stability were selected out of 2 or 3 stable lines for each transgene. 100 worms were counted for each transgenic line. The amino acid L1031 in LET-23 is analogous to L858 in EGFR.
% Muv, the penetrance of Muv phenotype among transgenic animals.
Table 2. The sqt-1 mutation enhances the Muv phenotype of integrated strains.
| Strain | % Muv | Number |
| jgIs6 | 94.6±1.33 | 1465 |
| sqt-1(jg52);jgIs6 | 97.4±0.42 | 2012 |
| jgIs25 | 94.9±0.32 | 410 |
| sqt-1(jg52);jgIs25 | 95.1±0.19 | 593 |
| jgIs26 | 50.9±4.13 | 494 |
| sqt-1(jg52);jgIs26 | 94.5±1.03 | 445 |
The sqt-1(jg52) mutation was introduced to suppress the Rol phenotype of the integrated strains. sqt-1 effect is different in each integrated line. There was no difference between jgIs25 and sqt-1;jgIs25 (P = 0.834), but the Muv ratio of jgIs6 is changed in the sqt-1 mutant background (P = 0.00163). In particular, jgIs26 which is another integration line of LET-23::hEGFR-TK[T790M-L858R] exhibited the dramatic increase of Muv in the sqt-1 mutant background (P<0.001). This Rol suppression by sqt-1 allowed us to score the multivulva more clearly compared to the rolling strain.
The Muv phenotype of jgIs6 is due to ectopic activation of the LET-23/EGFR pathway
To confirm that pseudovulvae formation in jgIs6 is due to specific activation of the EGFR-Ras-MAPK pathway by the chimeric LET-23::hEGFR-TK[L858R] protein, we performed RNAi of genes in the EGFR-Ras-MAPK pathway. Consistent with ectopic activation of the EGFR-Ras-MAPK pathway, knock-down of genes downstream of let-23, including let-60/Ras, mek-2/MEK, and mpk-1/MAPK, suppressed the Muv phenotype of jgIs6. RNAi of the LET-23/EGFR upstream gene lin-3/EGF also suppressed the Muv phenotype. The Muv phenotype of another transgenic line, jgIs25, which expresses LET-23::hEGFR-TK[L858R-T790M], was also suppressed by RNAi of lin-3, let-60, mek-2, and mpk-1(Fig. S3A). Since the Wnt pathway acts in parallel to lin-3 to maintain VPC competence [24], we also performed Wnt pathway gene knockdown by RNAi to test whether Wnt activity affects Muv formation in jgIs25. Two Wnt pathway genes (bar-1 and cwn-2) were tested, because their knockdown phenotypes are associated with vulval development [25], [26]. Similar to lin-3 knockdown, RNAi of bar-1 or cwn-2 resulted in suppression of the Muv phenotype of jgIs25 (). We observed rare larval lethality from these RNAi experiments because synchronized L1 larvae were treated with RNAi, same as the same method we used for drug treatment described below. To confirm the lethal RNAi effect of EGFR downstream genes, we treated jgIs25 L4 larvae with let-60 or mpk-1 RNAi, and counted the numbers of F1 progeny. Larval lethality was significantly increased by let-60 or mpk-1 RNAi (Fig. S3C).
We compared vulval cell fate markers in jgIs6 and Muv mutants that have the EGFR-Ras-MAPK pathway activated. When the 1° cell fate marker was examined, jgIs6 pseudovulvae showed expression of the 1° cell fate marker CDH-3::GFP (Fig. 1E). CDH-3 is a cadherin expressed in the 1° vul C, D, E, and F cells [27]. To test for 2° fate marker expression, we constructed an integrated transgenic line expressing both egl-17p::EMR-1::RFP and dhs-31p::NLS::GFP. EGL-17 is expressed in the 2° vul C and D cells, and DHS-31 in the 2° vul B1, B2, and D cells at the adult stage [27], [28]. EMR-1 is a homolog of the human integral nuclear membrane protein emerin [29], and therefore, causes localization to the nuclear envelope. In a wild-type background, egl-17p::EMR-1::RFP and dhs-31p::NLS::GFP are expressed at the nuclear envelope and nucleus of the 2° vulval cells, respectively. Both lin-15(n765) and jgIs6 animals had similar patterns of expression of egl-17p::EMR-1::RFP and dhs-31p::NLS::GFP (Fig. 1F). For the further comparison of jgIs6 and Muv mutants, we examined the expression of AJM-1::GFP and HMP-1::GFP, which are both expressed at the adherens junction and mark the boundaries of proliferating and differentiating epithelial cells [30]. AJM-1::GFP and HMP-1::GFP expression was observed in ventral invaginations, including putative pseudovulval regions in jgIs6, let-60(n1700), and lin-15(n765) mutants at the L4 stage. These two junction markers were also observed in the pseudovulva of jgIs6 at the adult stage (Fig. S4). Taken together, the results described above suggest that the jgIs6 transgenic strain expressing the chimeric LET-23::hEGFR-TK[L858R] protein display characteristics that are consistent with over-activation of the EGFR pathway in the Muv mutants.
Gefitinib and erlotinib inhibit the Muv phenotype of jgIs6
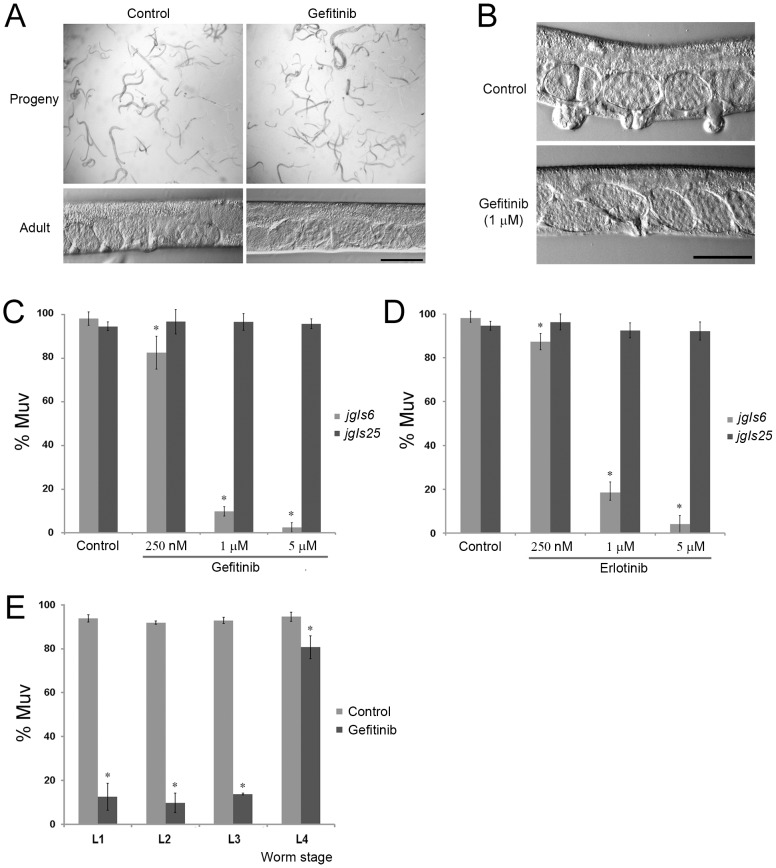
To determine whether the jgIs6 transgenic strain expressing the chimeric LET-23::hEGFR-TK[L858R] protein could be used in a large-scale screen for human EGFR-TK inhibitors, we tested the effects of the drugs gefitinib and erlotinib. When wild-type C. elegans were treated with gefitinib, even high doses (40 µM) did not affect embryogenesis or larval development (Fig. 2A). This indicated that gefitinib is not toxic to wild-type C. elegans, and appears to have little to no effect on LET-23 function, which is not surprising given the low sequence identity with the human EGFR protein or correlation of gefitinib response and activating EGFR mutations [31]. In contrast, the Muv phenotype of jgIs6 was inhibited up to 90% by 1 µM gefitinib and completely inhibited by 5 µM gefitinib (Fig. 2B and C). Considering that 250 or 500 mg of gefitinib orally once daily is recommended for cancer patients (approximately 10 µM) [4], [32], the inhibitory effect of gefitinib appears to be similar in C. elegans and humans. To verify the mechanism of action of reversible EGFR-TK inhibitors (TKIs) for our LET-23::hEGFR-TK-expressing transgenic C. elegans, we treated jgIs25 with gefitinib. The T790M mutation may result in an alteration of EGFR topology that precludes the binding of reversible EGFR-TKIs through steric hindrance, or T790M may increase the affinity of the kinase domain for ATP [21], [33]–[35]. Gefitinib treatment did not inhibit the Muv phenotype of jgIs25 (Fig. 2C). Erlotinib, another EGFR-TKI anti-cancer drug, produced similar inhibitory effects to gefitinib (Fig. 2D). We tried to compare the expression level of two chimeric proteins from jgIs6 and jgIs25, but we were unable to quantify the chimeric protein. Assuming similar levels of transgenic expression, the observed difference in activity is likely due to a decrease in drug sensitivity conferred by the L858R mutation.
Figure 2. The Muv phenotype of jgIs6 and jgIs25 reflects similar responses as human cancers have against the anti-cancer drugs, gefitinib and elrotinib.
(A) A high dose of gefitinib (40 µM) did not affect normal embryogenesis, larval growth, or vulval formation of the wild-type C. elegans. (B) Gefitinib inhibited the Muv phenotype of jgIs6 which expresses LET-23::hEGFR-TK[L858R]. (C) Gefitinib inhibited the Muv phenotype of jgIs6 in a dose dependent manner, but did not inhibit that of jgIs25, which expresses LET-23::hEGFR-TK[T790M-L858R]. Numbers of worms counted (n) were 159, 96, 130, 85, 87, 125, 108 and 88 from left along the X-axis. (D) Erlotinib produced a similar effect as gefitinib in both jgIs6 and jgIs25 models. (n = 159, 96, 67, 110, 80, 106, 95 and 176). (E) Determination of the developmental stage of jgIs6 that is most responsive to gefitinib. As in normal vulval development, early larvae (L1–L3) responded well to gefitinib. (n = 128, 224, 134, 116, 139, 167, 99 and 66). Scale bars, 50 µm (A, B). X-axis, concentration of gefitinib (C), erlotinib (D) and worm stage of gefitinib treatment (E). Y-axis, % of worms showing the Muv phenotype (C–E). * P<0.001.
To determine the stage of worm development during which gefitinib treatment is most effective, we treated jgIs6 of each larval stage with 1 µM gefitinib, and scored the Muv phenotype at the adult stage. Animals exposed to gefitinib from the L1, L2, or L3 stages showed a marked reduction in the Muv phenotype, and the degree of response was similar between the three stages (Fig. 2E). L4 animals were not as susceptible to gefitinib, indicating that gefitinib treatment before the L3/L4 molt, when vulval development initiates, is critical for effective inhibition of the LET-23::hEGFR-TK[L858R] protein. From this result, we concluded that early larvae, from L1 to L3, can be used for screening new inhibitors against the activated EGFR-TK signaling pathway.
Pilot screen for inhibitors that suppress the Muv phenotype of jgIs6
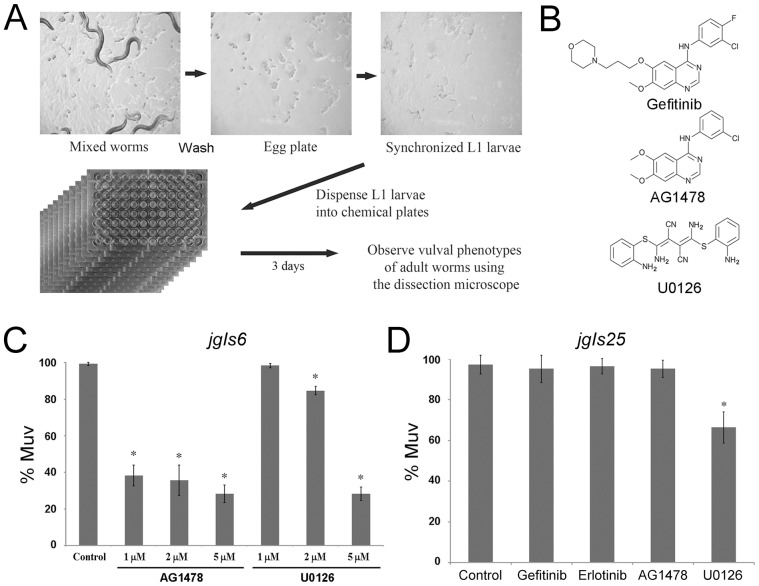
Based on our early results, we designed a protocol for large-scale high-throughput screening of EGFR inhibitors using jgIs6. To prepare a large number of synchronized larvae, C. elegans were grown until the plates were filled with eggs, and larvae and adults were removed by simple washing with M9 buffer. After 12 hours, hatched larvae were harvested and washed three times with M9 buffer. We dispensed L1 larvae into 96-well plates that contained a mix of dead E. coli and the chemicals being tested. After 3–4 days, the Muv phenotype was observed on a dissecting microscope (Fig. 3A). Using this protocol, we conducted a screen of 1,280 small molecules with known molecular targets and efficacy. Among the 1,280 chemicals, AG1478 and U0126 inhibited the Muv phenotype of jgIs6. AG1478 is an EGFR inhibitor frequently used in in vitro experiments, with a structure similar to gefitinib (Fig. 3B). U0126, which is known as an inhibitor of MEK, inhibited the jgIs6 Muv phenotype at 5 µM, but not at lower concentrations (Fig. 3C). When the effects of AG1478 and U0126 on the gefitinib-resistant LET-23::hEGFR-TK[T790M-L858R] model jgIs25 were tested, only U0126 had an inhibitory effect, whereas AG1478 was ineffective (Fig. 3D).
Figure 3. A pilot screen of 1,280 chemicals for EGFR-TK inhibitors using jgIs6.
(A) Screening method, including synchronization of C. elegans and liquid culture using the 96-well plate for inhibitor screen. (B) Chemical structures of gefitinib, AG1478 and U0126. (C) Both AG1478 and U0126 inhibit the Muv phenotype of jgIs6 in a dose dependent manner. (n = 295, 193, 381, 133, 254, 304 and 415 from left along the X-axis). (D) Effect of gefitinib, erlotinib, AG1478 and U0126 on jgIs25. Gefitinib, erlotinib, and AG1478 did not inhibit the Muv phenotype of jgIs25, but U0126 inhibited. (n = 81, 99, 106, 90 and 81). Chemical concentration, 5 µM. * P<0.001.
MEK inhibitors may potentially treat gefitinib-resistant cancers
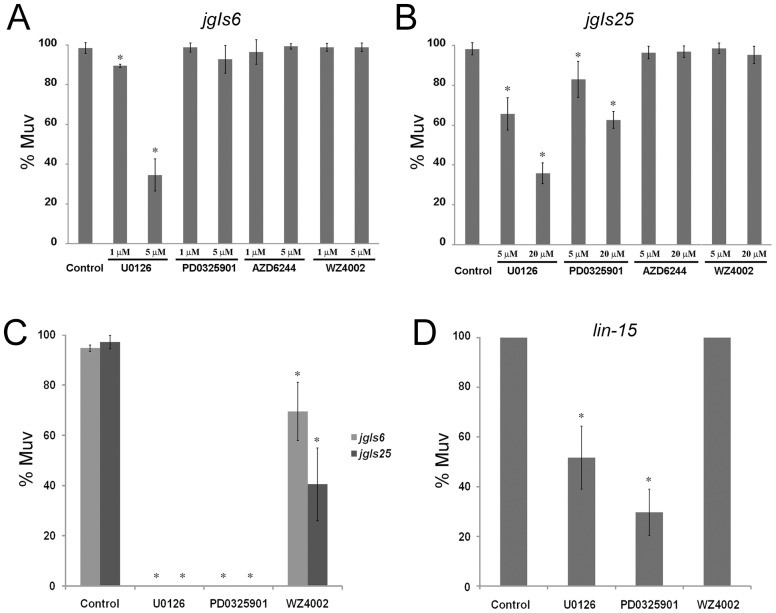
The observation that U0126 inhibited the Muv phenotype of jgIs25 suggested that some MEK inhibitors may have the potential to inhibit gefitinib-resistant forms of EGFR mutations. Therefore, we tested the effects of another MEK inhibitor, PD0325901, as well as a Raf[V600E] inhibitor (AZD6233), and an EGFR[T790M] inhibitor (WZ4002) in our model system. None of these chemicals inhibited the Muv phenotype of jgIs6 animals at doses effective for U0126 (1 µM or 5 µM) (Fig. 4A). However, at higher doses (5 µM or 20 µM), the MEK inhibitor PD0325901 slightly inhibited the Muv phenotype of jgIs25 (Fig. 4B). These results were confirmed in a side-by-side test, where jgIs6 and jgIs25 animals were treated with 100 µM of each chemical to observe the effects on the Muv phenotype. WZ4002, which is an inhibitor against the EGFR[T790M] gatekeeper mutation [36], inhibited the Muv phenotype of jgIs25 better than that of jgIs6. Similar to U0126, PD0325901 perfectly inhibited the Muv phenotype of both jgIs6 and jgIs25 (Fig. 4C). We also tested whether these chemicals target C. elegans genes by treating the lin-15 Muv mutant with U0126 and PD0325901. Both U0126 and PD0325901 suppressed the Muv phenotype of lin-15 at an excessive concentration (Fig. 4D). This result suggests that mek-2, one of MEKs in C. elegans that is related to vulval development, is one of the possible target candidates of U0126 and PD0325901.
Figure 4. MEK inhibitors rescue the gefitinib-resistant Muv phenotype of jgIs25.
(A) U0126, PD0325901 (MEK inhibitor), AZD6244 (RAF[V600E] inhibitor) and WZ4002 (EGFR[T790M] inhibitor) were added to jgIs6. (n = 86, 94, 116, 64, 88, 66, 79, 110 and 98 from left along the X-axis). (B) Chemicals were added to gefitinib resistant jgIs25. MEK inhibitors, U0126 and PD0325901, inhibited the Muv phenotype of jgIs25, but the other chemicals did not. (n = 64, 100, 128, 113, 89, 64, 63, 79 and 98). (C) Excessive doses (100 µM) of chemicals were added to jgIs6 and jgIs25. Two MEK inhibitors perfectly inhibited the Muv phenotype of jgIs6 and jgIs25. WZ4002 inhibited the Muv phenotype of jgIs25 better than that of jgIs6. (n = 160, 213, 186, 312, 195, 323, 192 and 237). (D) The Muv phenotype of lin-15 was suppressed by U0126 and PD0325901; chemical concentration, 100 µM (X-axis). (n = 119, 89, 90 and 143). * P<0.001.
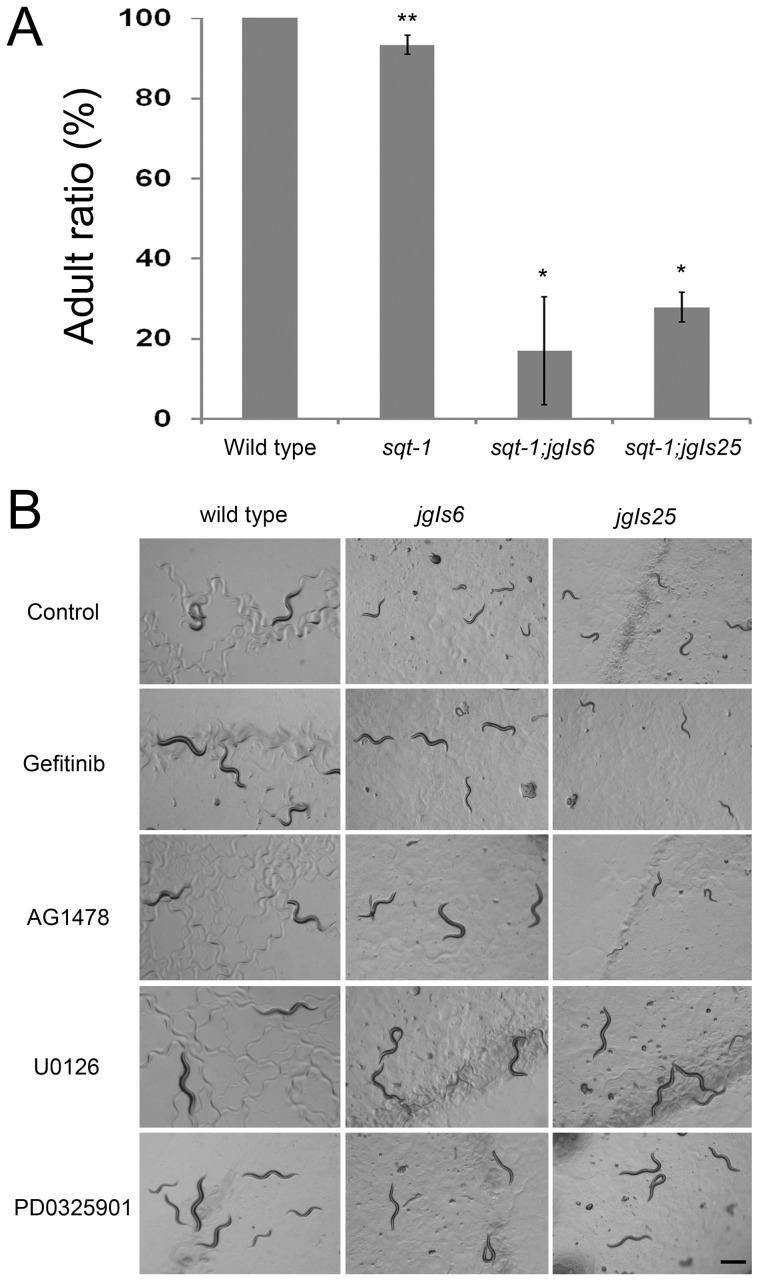
All transgenic strains expressing the activated EGFR chimeras, including jgIs6 and jgIs25, grow slowly (Fig. 5A and Fig. S5) similar to transgenic strains ectopically expressing LIN-3/EGF [37]. Interestingly, EGFR-TK inhibitors repaired the growth rate of jgIs6 to the level of wild type, and U0126 and PD0325901 rescued the growth rate of both jgIs6 and jgIs25 (Fig. 5B).
Figure 5. The Muv inhibitors suppressed the slow growth phenotype of jgIs6 and jgIs25.
(A) Ratios of adults 3 days after placing embryos on plate. All wild-type strains were adults, but most jgIs6 and jgIs25 transgenic strains were younger than the L4 stage. Five adults were transferred to each plate and were removed after 6 hours of egg-laying. Three days after removing P0 worms, F1 worms were observed. Four plates were counted for each strain. (n = 488, 313, 104 and 94 from left along the X-axis). * P<0.001 and ** P<0.05. (B) U0126 and PD0325901 suppressed the slow growth phenotype of jgIs6 and jgIs25. Gefitinib and AG1478 suppressed the slow growth phenotype of jgIs6, but not jgIs25. L1 stage larvae were incubated with each chemical for 4 days in 96-well plates, and they were recovered on a fresh plate for 6 hours before taking pictures. C. elegans grew slowly when cultured in 96-well plates. Control (0.5% DMSO) and chemical concentration (50 µM). Scale bar, 500 µm.
Discussion
We constructed transgenic C. elegans containing several different EGFR constructs. Transgenic lines expressing LET-23::hEGFR chimeric receptors exhibited a much stronger phenotype than those expressing LET-23::hEGFR-TK chimeric receptors (Table 1). Unfortunately, we failed to get integration lines for those constructs and only have data produced from the integration lines of LET-23::hEGFR-TK transgenic lines. The cytoplasmic tail of hEGFR may have evolved to transmit the activated signals more efficiently than LET-23, C. elegans EGFR, even in VPCs.
To establish our model system, the chimeric LET-23::hEGFR-TK transgene was expressed in a let-23(+) background. The potential formation of heterodimers of endogenous LET-23 with the chimeric LET-23::hEGFR-TK protein may explain some observations that were made over the course of our study. We occasionally observed that high doses of gefitinib inhibited normal vulval development in jgIs6. Also, RNAi of the EGFR upstream gene, lin-3/EGF, suppressed the Muv phenotype of jgIs6 and jgIs25 (Fig. S3A), which may be explained by heterodimerization of the endogenous and transgenic chimeric protein; although, the early role of lin-3 in establishing and maintaining vulval cell competence may contribute to this phenotype [24]. In addition, Wnt pathway gene knockdown by RNAi affected the Muv formation in jgIs25 (Fig. S3B). The Wnt pathway acts in parallel to lin-3 during VPC competence [24]. As in many previous studies, vulval development in jgIs6 and jgIs25 appears to involve several signaling pathways rather than simple EGFR activation. Nevertheless, expressing the chimeric transgene in a let-23(+) background produces an advantage for screening purposes compared to a let-23 null background. If the LET-23::EGFR-TK transgene were expressed in a let-23 null mutant, inhibitor treatment would cause lethality or slow growth, making it difficult to distinguish true inhibitors of human EGFR-TK from chemicals that inhibit other endogenous essential genes or chemicals that display toxicity.
The chimeric LET-23::hEGFR-TK model system was designed for screening inhibitors that target the tyrosine kinase domain of human EGFR. As expected, well-known EGFR inhibitors, such as gefitinib or erlotinib which target the TK domain, were effective in suppressing the Muv phenotype in our model, but have little to no effect on the wild-type C. elegans. In addition, the chemicals that inhibit the Muv phenotype of jgIs6 in another screen of 7,680 chemicals were similar in structure to gefitinib (Fig. S6). Thus, this approach has an advantage in screening for chemicals targeting specific protein domains.
This model system can be easily modified to screen for new chemicals that target drug-resistant cancers. Gefitinib resistance is caused by secondary mutations in EGFR [21], over-expression of c-Met [38] or IGFR [39], or mutations in EGFR downstream genes, such as Ras [40]. Here, we identified potential inhibitors of gefitinib-resistant secondary EGFR mutations. Gefitinib effectively suppressed the Muv phenotype of transgenic strains expressing EGFR mutations such as EGFR[L858R] (jgIs6), and was ineffective on gefitinib-resistant mutations, such as EGFR[T790M-L858R] (jgIs25). Using this model system, a MEK inhibitor was identified as a potential inhibitor of gefitinib-resistant EGFR mutations. In our models, MEK inhibitors produced a much stronger effect than WZ4002, which targets the EGFR[T790M] mutation [36] (Fig. 4C). Similarly, one could use transgenic C. elegans lines simultaneously expressing oncogenic EGFR and c-Met for drug screening, or use transgenic lines expressing chimeras of C. elegans and human EGFR downstream genes such as Raf or MEK [20], [41].
Because we screened inhibitors by observing the Muv phenotype using the dissection microscope, high-throughput screening (HTS) is difficult at a small laboratory level. However, C. elegans is a versatile model system, easily adapted for HTS. Chemical screening methods using C. elegans in automated bio-sorting machines, such as COPAS (Complex Object Parametric Analyzer and Sorter) have been reported [42], [43]. The use of fluorescence markers in conjunction with COPAS, such as AJM-1::GFP or egl-17p::EMR-1::RFP to mark the pseudovulvae, could enable high-throughput screening methods for new anti-cancer drugs in our model system. We propose another screening method that uses jgIs6 or jgIs25 would perform better than the one used in this study. These transgenic strains grow slowly and EGFR pathway inhibitors suppress the growth phenotype (Fig. 5). With these strains, we will be able to screen inhibitors first by selecting fast growing C. elegans and then confirm the Muv phenotype suppression.
By expressing a chimera of the C. elegans and human EGFR proteins in C. elegans, we have developed and characterized an animal model system that can be used to screen EGFR inhibitor anti-cancer drugs. The transgenic C. elegans have a multivulva phenotype, which is consistent with the activated EGFR pathway. In a pilot screen of 8,960 molecules, chemicals such as AG1478, which share a common backbone structure with gefitinib and erlotinib, as well as a MEK inhibitor, U0126, inhibited the Muv phenotype of jgIs6. We also provide evidence that MEK inhibitors may be effective in treating cancers that are resistant to known EGFR-TKIs. The humanized C. elegans makes a highly specific in vivo animal screening model system.
Supporting Information
Plasmid constructs for expressing LET-23::hEGFR chimeric receptors.
(PDF)
Amino acid sequences of the LET-23::hEGFR-TK and LET-23::hEGFR transgene products.
(PDF)
Knock-down of Ras-MAPK and Wnt pathway genes in jgIs6 and jgIs25 by feeding RNAi.
(PDF)
Expression of epithelial junction proteins in the jgIs6 transgenic worm which expresses LET-23::hEGFR-TK[L858R].
(PDF)
Adult ratios of wild type and two integrated strains over time.
(PDF)
Seven chemicals found to inhibit the Muv phenotype of jgIs6 in another screen are similar in structure to gefitinib.
(PDF)
Acknowledgments
We thank the Korea Chemical Bank of KRICT for providing 7,680 chemicals. Some nematode strains including let-23, let-60, lin-15, JJ1136, PS4657 and PS3352 used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (NCRR).
Funding Statement
This work was solely supported by a research grant from the National Cancer Center (NCC-1110060) of South Korea (www.ncc.re.kr). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Artal-Sanz M, de Jong L, Tavernarakis N (2006) Caenorhabditis elegans: a versatile platform for drug discovery. Biotechnol J 1: 1405–1418. [DOI] [PubMed] [Google Scholar]
- 2. Hynes NE, Lane HA (2005) ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer 5: 341–354. [DOI] [PubMed] [Google Scholar]
- 3. Gschwind A, Fischer OM, Ullrich A (2004) The discovery of receptor tyrosine kinases: targets for cancer therapy. Nat Rev Cancer 4: 361–370. [DOI] [PubMed] [Google Scholar]
- 4. Muhsin M, Graham J, Kirkpatrick P (2003) Gefitinib. Nat Rev Drug Discov 2: 515–516. [DOI] [PubMed] [Google Scholar]
- 5. Pao W, Miller V, Zakowski M, Doherty J, Politi K, et al. (2004) EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A 101: 13306–13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sordella R, Bell DW, Haber DA, Settleman J (2004) Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science 305: 1163–1167. [DOI] [PubMed] [Google Scholar]
- 7. Sternberg PW (2005) Vulval development. WormBook 1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chang C, Sternberg PW (1999) C. elegans vulval development as a model system to study the cancer biology of EGFR signaling. Cancer Metastasis Rev 18: 203–213. [DOI] [PubMed] [Google Scholar]
- 9. Moghal N, Sternberg PW (2003) The epidermal growth factor system in Caenorhabditis elegans . Exp Cell Res 284: 150–159. [DOI] [PubMed] [Google Scholar]
- 10. Hara M, Han M (1995) Ras farnesyltransferase inhibitors suppress the phenotype resulting from an activated ras mutation in Caenorhabditis elegans . Proc Natl Acad Sci U S A 92: 3333–3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gonzalez-Perez V, Reiner DJ, Alan JK, Mitchell C, Edwards LJ, et al. (2010) Genetic and functional characterization of putative Ras/Raf interaction inhibitors in C. elegans and mammalian cells. J Mol Signal 5: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lackner MR, Kindt RM, Carroll PM, Brown K, Cancilla MR, et al. (2005) Chemical genetics identifies Rab geranylgeranyl transferase as an apoptotic target of farnesyl transferase inhibitors. Cancer Cell 7: 325–336. [DOI] [PubMed] [Google Scholar]
- 13. Dengg M, van Meel JC (2004) Caenorhabditis elegans as model system for rapid toxicity assessment of pharmaceutical compounds. J Pharmacol Toxicol Methods 50: 209–214. [DOI] [PubMed] [Google Scholar]
- 14. Brenner S (1974) The genetics of Caenorhabditis elegans . Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kaech SM, Whitfield CW, Kim SK (1998) The LIN-2/LIN-7/LIN-10 complex mediates basolateral membrane localization of the C. elegans EGF receptor LET-23 in vulval epithelial cells. Cell 94: 761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aroian RV, Lesa GM, Sternberg PW (1994) Mutations in the Caenorhabditis elegans let-23 EGFR-like gene define elements important for cell-type specificity and function. EMBO J 13: 360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aroian RV, Koga M, Mendel JE, Ohshima Y, Sternberg PW (1990) The let-23 gene necessary for Caenorhabditis elegans vulval induction encodes a tyrosine kinase of the EGF receptor subfamily. Nature 348: 693–699. [DOI] [PubMed] [Google Scholar]
- 18. Huang LS, Tzou P, Sternberg PW (1994) The lin-15 locus encodes two negative regulators of Caenorhabditis elegans vulval development. Mol Biol Cell 5: 395–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Clark SG, Lu X, Horvitz HR (1994) The Caenorhabditis elegans locus lin-15, a negative regulator of a tyrosine kinase signaling pathway, encodes two different proteins. Genetics 137: 987–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sharma SV, Bell DW, Settleman J, Haber DA (2007) Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer 7: 169–181. [DOI] [PubMed] [Google Scholar]
- 21. Kobayashi S, Boggon TJ, Dayaram T, Janne PA, Kocher O, et al. (2005) EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med 352: 786–792. [DOI] [PubMed] [Google Scholar]
- 22. Gazdar AF (2009) Activating and resistance mutations of EGFR in non-small-cell lung cancer: role in clinical response to EGFR tyrosine kinase inhibitors. Oncogene 28 Suppl 1: S24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim TH, Kim YJ, Cho JW, Shim J (2011) A novel zinc-carboxypeptidase SURO-1 regulates cuticle formation and body morphogenesis in Caenorhabditis elegans . FEBS Lett 585: 121–127. [DOI] [PubMed] [Google Scholar]
- 24. Myers TR, Greenwald I (2007) Wnt signal from multiple tissues and lin-3/EGF signal from the gonad maintain vulval precursor cell competence in Caenorhabditis elegans . Proc Natl Acad Sci U S A 104: 20368–20373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Green JL, Inoue T, Sternberg PW (2007) The C. elegans ROR receptor tyrosine kinase, CAM-1, non-autonomously inhibits the Wnt pathway. Development 134: 4053–4062. [DOI] [PubMed] [Google Scholar]
- 26. Inoue T, Oz HS, Wiland D, Gharib S, Deshpande R, et al. (2004) C. elegans LIN-18 is a Ryk ortholog and functions in parallel to LIN-17/Frizzled in Wnt signaling. Cell 118: 795–806. [DOI] [PubMed] [Google Scholar]
- 27. Inoue T, Wang M, Ririe TO, Fernandes JS, Sternberg PW (2005) Transcriptional network underlying Caenorhabditis elegans vulval development. Proc Natl Acad Sci U S A 102: 4972–4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Burdine RD, Chen EB, Kwok SF, Stern MJ (1997) egl-17 encodes an invertebrate fibroblast growth factor family member required specifically for sex myoblast migration in Caenorhabditis elegans . Proc Natl Acad Sci U S A 94: 2433–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee KK, Gruenbaum Y, Spann P, Liu J, Wilson KL (2000) C. elegans nuclear envelope proteins emerin, MAN1, lamin, and nucleoporins reveal unique timing of nuclear envelope breakdown during mitosis. Mol Biol Cell 11: 3089–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Labouesse M (2006) Epithelial junctions and attachments. WormBook 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, et al. (2004) EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304: 1497–1500. [DOI] [PubMed] [Google Scholar]
- 32. Herbst RS, Fukuoka M, Baselga J (2004) Gefitinib- a novel targeted approach to treating cancer. Nat Rev Cancer 4: 956–965. [DOI] [PubMed] [Google Scholar]
- 33. Kwak EL, Sordella R, Bell DW, Godin-Heymann N, Okimoto RA, et al. (2005) Irreversible inhibitors of the EGF receptor may circumvent acquired resistance to gefitinib. Proc Natl Acad Sci U S A 102: 7665–7670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, et al. (2005) Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med 2: e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yun CH, Mengwasser KE, Toms AV, Woo MS, Greulich H, et al. (2008) The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci U S A 105: 2070–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhou W, Ercan D, Chen L, Yun CH, Li D, et al. (2009) Novel mutant-selective EGFR kinase inhibitors against EGFR T790M. Nature 462: 1070–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Van Buskirk C, Sternberg PW (2007) Epidermal growth factor signaling induces behavioral quiescence in Caenorhabditis elegans . Nat Neurosci 10: 1300–1307. [DOI] [PubMed] [Google Scholar]
- 38. Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, et al. (2007) MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 316: 1039–1043. [DOI] [PubMed] [Google Scholar]
- 39. Cappuzzo F, Toschi L, Tallini G, Ceresoli GL, Domenichini I, et al. (2006) Insulin-like growth factor receptor 1 (IGFR-1) is significantly associated with longer survival in non-small-cell lung cancer patients treated with gefitinib. Ann Oncol 17: 1120–1127. [DOI] [PubMed] [Google Scholar]
- 40. Uchida A, Hirano S, Kitao H, Ogino A, Rai K, et al. (2007) Activation of downstream epidermal growth factor receptor (EGFR) signaling provides gefitinib-resistance in cells carrying EGFR mutation. Cancer Sci 98: 357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wong KK (2009) Recent developments in anti-cancer agents targeting the Ras/Raf/MEK/ERK pathway. Recent Pat Anticancer Drug Discov 4: 28–35. [DOI] [PubMed] [Google Scholar]
- 42. Sprando RL, Olejnik N, Cinar HN, Ferguson M (2009) A method to rank order water soluble compounds according to their toxicity using Caenorhabditis elegans, a Complex Object Parametric Analyzer and Sorter, and axenic liquid media. Food Chem Toxicol 47: 722–728. [DOI] [PubMed] [Google Scholar]
- 43. Burns AR, Kwok TC, Howard A, Houston E, Johanson K, et al. (2006) High-throughput screening of small molecules for bioactivity and target identification in Caenorhabditis elegans . Nat Protoc 1: 1906–1914. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Plasmid constructs for expressing LET-23::hEGFR chimeric receptors.
(PDF)
Amino acid sequences of the LET-23::hEGFR-TK and LET-23::hEGFR transgene products.
(PDF)
Knock-down of Ras-MAPK and Wnt pathway genes in jgIs6 and jgIs25 by feeding RNAi.
(PDF)
Expression of epithelial junction proteins in the jgIs6 transgenic worm which expresses LET-23::hEGFR-TK[L858R].
(PDF)
Adult ratios of wild type and two integrated strains over time.
(PDF)
Seven chemicals found to inhibit the Muv phenotype of jgIs6 in another screen are similar in structure to gefitinib.
(PDF)