Abstract
Clonal growth allows plants to spread horizontally and to establish ramets in sites of contrasting resource status. If ramets remain physiologically integrated, clones in heterogeneous environments can act as cooperative systems – effects of stress on one ramet can be ameliorated by another connected ramet inhabiting benign conditions. But little is known about the effects of patch contrast on physiological integration of clonal plants and no study has addressed its effects on physiological traits like osmolytes, reactive oxygen intermediates and antioxidant enzymes. We examined the effect of physiological integration on survival, growth and stress indicators such as osmolytes, reactive oxygen intermediates (ROIs) and antioxidant enzymes in a clonal plant, Fragaria orientalis, growing in homogenous and heterogeneous environments differing in patch contrast of water availability (1 homogeneous (no contrast) group; 2 low contrast group; 3 high contrast group). Drought stress markedly reduced the survival and growth of the severed ramets of F. orientalis, especially in high contrast treatments. Support from a ramet growing in benign patch considerably reduced drought stress and enhanced growth of ramets in dry patches. The larger the contrast between water availability, the larger the amount of support the depending ramet received from the supporting one. This support strongly affected the growth of the supporting ramet, but not to an extent to cause increase in stress indicators. We also found indication of costs related to maintenance of physiological connection between ramets. Thus, the net benefit of physiological integration depends on the environment and integration between ramets of F. orientalis could be advantageous only in heterogeneous conditions with a high contrast.
Introduction
One of the features of clonal growth in plants is the capacity to exchange resources, such as water, photo-assimilates and nutrients [1]–[8] and non-resource agents, such as defence compounds, signalling molecules or pathogen agents [9]–[11] between interconnected ramets. Such physiological integration between potentially independent ramets is more important when different ramets within one clonal fragment experience heterogeneity of microhabitats [4], [12], [13]. Exchange of resources within a clone, i.e. support to one ramet from another one, may buffer the negative effects of individual microhabitats [3], [7], [13]–[16]. This buffer effect may also be accompanied with an improvement of physiological traits like osmolytes, reactive oxygen intermediates and antioxidant enzymes, but these effects have been little studied in clonal plants. Physiological integration may also have disadvantages such as energy cost of maintenance of inter-ramet connections and rapid spread of pathogens throughout the system of interconnected ramets [14], [17]–[20]. In fact, some studies have reported that under chronic or severe stress integration may result in lower fitness, and clonal plants may cease to support dependent ramets [21]–[24], whereas others have found that clones continue to support dependent ramets despite prolonged stress [3], [4], [15], [25], [26]. Intraclonal resource translocation may be mainly driven by the degree of difference (contrast, [4], [27]) between adjacent patches [28], [29], except in cases of developmental constraints (e.g., support to establishing ramets by an adult part of a clone [30], [31]. However, experimental evidence for this positive relationship between contrast and the strength of clonal integration is rather scarce [3], [4], [15], despite the fact that the level of contrast probably determines the balance between costs and benefits of physiological integration.
Water deficit is a major constraint for plant survival. In natural habitats, water often has a patchy distribution on the scale relevant to individual plants, so that neighbouring ramets of clonal plants may experience contrasting levels of water availability [32]–[34]. In clonal plants, physiological integration allows water to be transported from connected and well-watered ramets to ramets subjected to drought, thereby alleviating water stress in water-stressed ramets under heterogeneous water environments [15], [35]–[37]. Several studies have shown the effects of variable water availability on the morphology and gas exchange of clonal plants [3], [15], [36], [38]–[43]. However, no study has separated the effect of water stress on clonal plant from the effect of provision of support to a dependent ramet by a supporting one.
Osmolytes (osmoregulation substances) and reactive oxygen intermediates (ROIs) have been used as signals of drought stress in many studies of non-clonal plants [44]–[48]. Moreover, the level of stress can also be evaluated by estimation of the activity of antioxidant enzymes, which can clean out excessive active oxygen resulting from stress [47], [49]–[51]. Stress induced damage to plant cells can also be evaluated by measuring free radical induced peroxidation of lipid membranes [52]. The malondialdehyde (MDA) content is an indicator of membrane lipid peroxidation. This damage to membranes is also associated with accumulation of H2O2 [48], [53], [54]. Free proline content may serve as a means of osmotic adjustment, it could function as a hydroxyl radical scavenger to prevent membrane damage and protein de-naturation [55]. Plants also have evolved antioxidative enzymes, including peroxidase (POD), superoxide dismutase (SOD), ascorbate peroxidase (APX) and catalase (CAT), to protect plants against oxidative damage [47]. Above-mentioned stress-indicator traits have been extensively documented in non-clonal plants, but so far little study has been done in clonal plants.
In this study, we examine the effect of physiological integration between ramets and the level of contrast in water availability between patches on survival, growth, and level of stress on a clonal plant Fragaria orientalis. We specifically address the following questions: (1) Are the survival, growth, and level of stress of supporting ramets growing in well-watered soils affected by integration with dependent ramets growing in water shortage? I.e., how large is the cost of integration for a supporting ramet? (2) Is the level of support to a dependent ramet affected by the water availability contrast between patches inhabited by supporting and dependent ramet? We expect that the physiological integration ameliorates the negative effect of drought stress on dependent ramets, while the net benefit of integration on the whole clonal fragment increases with increasing contrast between patches inhabited by supporting and dependent ramets.
Materials and Methods
Plants and Experimental Design
Fragaria orientalis (Rosaceae) is a stoloniferous, perennial, rosette herb which is widely distributed in Korea, Mongolia, Eastern Russia and China. In China, it is common in North China and Eastern Qinghai-Tibetan Plateau, inhabiting forests and meadows on mountain slopes [56], [57]. The axillary buds on the vertical stems may grow out and form stolons. The stolons usually take root on stem nodes when reaching a moist substratum, and even single stem node can establish and grow as a ramet.
At the start of the experiment, 15 plants of F. orientalis, each consisting of more than 12 newly produced ramets (on stolons), were excavated around Maoxian Ecological Station, Chinese Academy of Sciences (31°41′07″N, 103°53′58″E; 1,816 m asl.). The sampling site did not belong to the part of any farms or national parks. Fragaria orientalis is widespread in China and it is not an endangered or protected species, so we did not need any relevant permissions/permits for plant samples collection. The plants were collected at least 1000 m away from one another, and were thus considered as 15 distinct genotypes [58]. These original plants were dissected into clonal fragments, each fragment composed of two interconnected ramets of similar size. One ramet in each pair was referred to as the initial proximal part (called the “supporting ramet”; it is well-watered and intended to provide water to the distal ramet in the experiment), indicating its relative proximity to the mother rosette, while the other as the initial distal part (called the “dependent ramet”; it is intended to receive water from the supporting ramet in the experiment). With the stolon still intact between two ramets, these clonal fragments were planted in trays of sand for 20 days. Once well established (rooted), they were size-standardized by removing extra leaves so that only three youngest leaves remained, and transplanted into plastic pots (20 cm in diameter and 15 cm in height) filled with homogenized soil to a depth of 14 cm. Leaf removal could affect the source-sink relationship of individual ramets (e.g., the balance between above and belowground growth), but it would keep the source-sink relationship between the two interconnected ramets before the start of the experiment the same across all the treatments, which is crucial for the study of the effects of physiological integration [15], [58]–[60]. The supporting and dependent ramets of each clonal fragment were planted in separate pots (forming a pair of pots), and they were connected by an undamaged stolon. In half of the pots the stolon was cut off (severed treatment). Intact connection between paired ramets (intact stolon) allowed physiological integration between ramets, while in the severed treatments physiological integration was impeded. Plants were grown in a glasshouse under a semi-controlled environment, with the day temperature range of 12–31°C, night temperature range of 9–15°C, and the relative humidity range of 35–85%. After one week, all ramets were size-standardized again by removing all leaves except the youngest one so that foliage of similar area remained.
At the beginning of the experiment, all ramets were about 2 cm tall. Both the unsevered and severed pairs of ramets were divided into three groups: 1) homogeneous group, both ramets of each pair were well-watered [up to 90% of field capacity (FC)]; 2) low contrast group, the supporting ramet of each pair was well-watered [90% of FC] and the dependent ramet was subject to less-watered treatment [60% of FC]; 3) high contrast group, the supporting ramet was well-watered [90% of FC] and the dependent ramet was subject to severe drought [30% of FC] (Table 1). Thus, six different treatments were formed (two levels of physiological integration × three levels of water availability contrast). In each treatment, there were 15 replicates, each of which was derived from one of the 15 original rosettes.
Table 1. Experimental design.
| Treatment | Supporting ramet | Stolon connection | Dependent ramet |
| Homogeneity (Ho) | 90% FC | Intact | 90% FC |
| 90% FC | Severed | 90% FC | |
| Low contrast (L) | 90% FC | Intact | 60% FC |
| 90% FC | Severed | 60% FC | |
| High contrast (H) | 90% FC | Intact | 30% FC |
| 90% FC | Severed | 30% FC |
In each treatment, the pots were re-watered to their respective FC by replacing the amount of water transpired every second day. Evaporation from the soil surface was reduced by enclosing all pots in plastic bags sealed at the base of the stem of each ramet. The amount of water was determined by weighing the pots. An empirical relationship between plant fresh weight (g) and plant leaf area (cm2) was used to correct pot water for changes in plant biomass. In addition, 15 additional control pots were planted with dead F. orientalis and the pots enclosed in plastic bags in the same way as other treatments. These pots were also weighted every second day in order to estimate evaporation from the soil surface. A total 8 g of slow-release fertilizer (13% N, 10% P and 14% K) was added to each pot during the experiment. After 100 days, samples of the youngest fully expanded leaves were taken from the original ramets of each pot for determination of physiological indicator (osmolytes, ROIs and antioxidant enzyme activity). At the end of the experiment (the experiment lasted 110 days), all parts of each plant in each pot were marked and harvested.
Survival and Growth
At the end of the experiment, mortality rates were determined in supporting and dependent ramets, total number of new ramets produced by original ramet and leaves per pot were counted, total leaf area per pot was measured using a CI-203 Laser Area Meter (CID Inc.), and the biomass per pot was determined after drying the plants at 70°C for 48 h.
Osmolytes and ROIs
Osmolytes and ROIs were estimated by the contents of proline, malondialdehyde (MDA), H2O2 and total soluble protein.
The free proline content was determined according to the method described by Bates et al. [61]. The absorbance of the free proline concentration was measured at 520 nm. The MDA content was determined by the thiobarbituric acid (TBA) reaction as described by Heath and Packer [62]. The absorbance of MDA was measured at 532, 600 and 450 nm. The MDA content was calculated according to the formula: MDA [µM] = 6.45(A532–A600)–0.56A450. The H2O2 content was determined according to Prochazkova et al. [63]. The absorbance of the H2O2 concentration was measured at 415 nm. Total soluble protein content was determined using Coomassie brilliant blue followed the Bradford assay method [64]. Preparation of crude extract was based on the method of Pinto et al. [65].
Antioxidant Enzymes Activity
Antioxidant enzyme activities of ramets were estimated from the activities of peroxidase (POD), superoxide dismutase (SOD), ascorbate peroxidase (APX) and catalase (CAT).
Extracts for the determination of antioxidant enzyme activities were prepared from 1.0 g of fully developed new leaves homogenized under ice-cold conditions in 3 ml of extraction buffer, containing 50 mM phosphate buffer (pH 7.4), 1 mM EDTA, 1 g PVP and 0.5% (v/v) Triton X-100. The homogenates were centrifuged at 10,000×g for 30 min and the supernatant was used for the assays.
POD activity (EC 1.11.1.7) was based on the determination of guaiacol oxidation, as described by Ekmekci and Terzioglu [66]. Activity was determined by the increase in absorbance at 470 nm due to guaiacol oxidation. SOD activity (EC 1.15.1.1) was assayed by the inhibition of the photochemical reduction of nitroblue tetrazolium (NBT) [67]. The reduction in NBT was followed by reading absorbance at 560 nm. One unit of SOD was defined as the amount of enzyme inhibiting the photo-reduction of NBT by 50% [68]. APX activity (EC 1.11.1.11) was assayed followed Nakano and Asada [69]. The hydrogen peroxide-dependent oxidation of ascorbate was followed by decrease in the absorbance decrease at 290 nm. CAT activity (EC 1.11.1.6) was determined in the homogenates by measuring the decrease in absorption at 240 nm [70]. The CAT activity was calculated using the extinction coefficient for H2O2.
Statistical Analysis
We used two-way ANOVA to assess the effects of water availability contrast (three levels) and severance of stolon connection (severed and intact) on all variables of supporting and dependent ramets. The variables include biomass, number of ramets, leaf area, number of leaves, proline content, MDA content, H2O2 content, protein content, POD, SOD, APX and CAT. In the two-way ANOVA models, water availability contrast and severance of stolon connection were treated as fixed factors. The data met the model assumptions of normality and homoscedasticity and thus were not transformed before analysis. The Duncan test was used to compare the means among the treatments. All statistical analyses were done with the SPSS 15 for Windows statistical software package.
Results
Survival and Growth
At the end of the experiment, 53% (8 ramets of 15 ramets) of severed dependent ramets in high water contrast died. All other ramets survived.
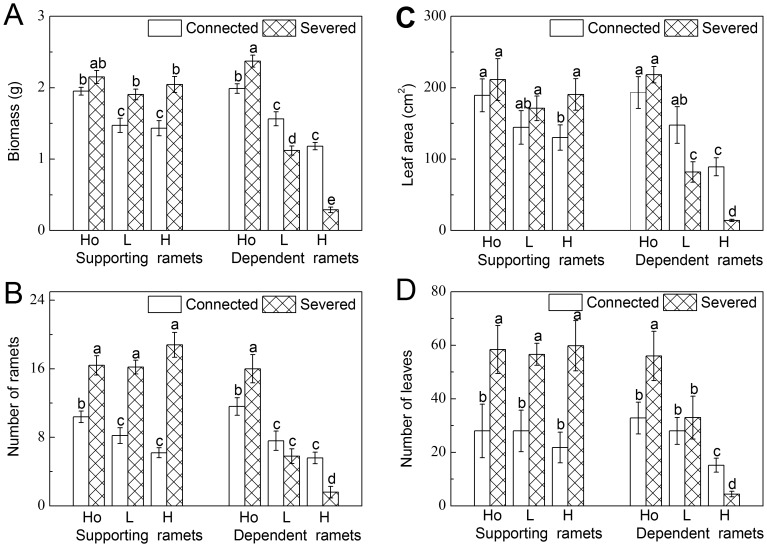
Supporting ramets with an intact stolon had, when compared to severed ramets, lower biomass, fewer ramets and leaves in heterogeneous environments (low contrast and high contrast) and smaller leaf area in high contrast treatments (Table 2 and Fig. 1A, B, C, D). There was no effect of severing on biomass of supporting ramets in the homogeneous treatments, and on leaf area in the homogeneous and low contrast treatments (Fig. 1A, C). Compared to intact ramets, severed dependent ramets had higher biomass, more ramets and more leaves in the homogeneous treatments but lower biomass and leaf area in the heterogeneous ones. Severed dependent ramets also had fewer ramets and leaves than intact dependent ramets in high contrast (Table 2 and Fig. 1A, B, C, D). There were no significant differences in leaf area in the homogeneous treatments as well as number of ramets and number of leaves in low contrast (Fig. 1B, C, D).
Table 2. F-values of two-way ANOVA which was used to test for the effects of contrast level of water availability (C), severance of stolon connection between the ramets (S) and their interaction (C*S) on the growth parameters and levels of stress indicators in supporting and dependent ramets.
| Characters | Supporting ramets | Dependent ramets | ||||
| C | S | C*S | C | S | C*S | |
| Biomass | 16.045** | 54.316** | 4.567* | 572.274** | 105.448** | 127.356** |
| No. of ramets | 1.511ns | 203.106** | 8.487** | 169.511** | 0.849ns | 29.545** |
| Leaf area | 6.682** | 12.035** | 1.281ns | 119.755** | 23.016** | 16.985** |
| No. of leaves | 4.196* | 63.412** | 7.467** | 43.832** | 4.037* | 10.924** |
| Proline | 0.299ns | 0.054ns | 2.072ns | 276.829** | 557.667** | 231.767** |
| MDA | 2.538ns | 0.938ns | 1.436ns | 80.027** | 179.396** | 67.987** |
| H2O2 | 0.388ns | 0.275ns | 1.321ns | 50.712** | 32.914** | 12.723** |
| Protein | 0.600ns | 0.891ns | 0.679ns | 17.631** | 20.605** | 15.253** |
| POD | 0.031ns | 1.039ns | 2.422ns | 87.597** | 212.281** | 56.125** |
| SOD | 0.989ns | 1.717ns | 2.347ns | 178.863** | 219.330** | 106.120** |
| APX | 1.577ns | 2.211ns | 3.024ns | 122.231** | 89.227** | 84.107** |
| CAT | 1.099ns | 0.370ns | 1.332ns | 353.226** | 815.577** | 308.031** |
For the supporting part, df (degrees of freedom) C, df S and df C*S are (2, 90), (1, 90), (2, 90), respectively. Because ramets of the severed dependent part died in high contrast, this treatment was not included in the ANOVA analysis for the dependent part. So df C, df S and df C*S are (2, 82), (1, 82), (2, 82), respectively. Significance level: ns P>0.05, *P<0.05, **P<0.01.
Figure 1. Growth parameters.
(A) Biomass, (B) number of ramets, (C) leaf area, and (D) number of leaves of the supporting and the dependent ramets. Data are means±SE (n = 15). Bars sharing the same lowercase letter are not different at p = 0.05. Treatments are coded as in Table 1.
Biomass, number of ramets and leaf area of connected supporting ramets in the heterogeneous treatments were smaller than those in the homogeneous ones. There was no effect of water availability contrast on any growth parameter of the severed ramets (Table 2 and Fig. 1A, B, C, D). Biomass, number of ramets, leaf area and number of leaves of both connected and severed dependent ramets decreased with the increasing contrast, while the degree of the decrease was higher in severed ramets than in connected ramets (Table 2 and Fig. 1A, B, C, D).
Osmolytes and ROIs
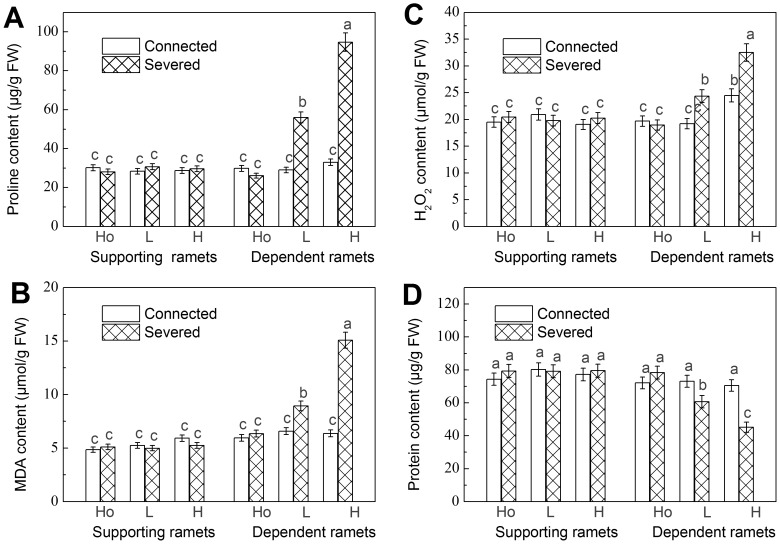
Proline, MDA, H2O2 and soluble protein content of both connected and severed supporting ramets were not significantly different between the three water availability contrasts. There were also no significant differences in these parameters between connected and severed supporting ramets. In dependent ramets, proline, MDA and H2O2 of severed ramets increased and soluble protein content decreased with the increase of the contrast level. Proline, MDA and H2O2 content of severed ramets was significantly higher, while soluble protein content was significantly lower than in connected ramets in low and high contrast, and there was no difference in the homogeneous treatments (Table 2 and Fig. 2).
Figure 2. Osmolytes and ROIs.
(A) Proline content, (B) MDA content, (C) H2O2 content and (D) protein content of the supporting and the dependent ramets. Data are means±SE (n = 15). Bars sharing the same lowercase letter are not different at p = 0.05. Treatments are coded as in Table 1.
Antioxidant Enzymes Activity
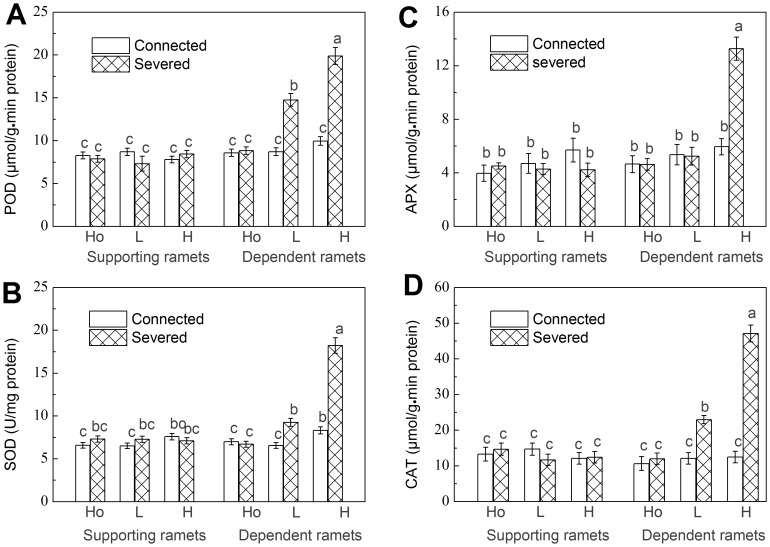
There were no significant differences between connected and severed ramets or among the contrasts in the levels of POD, SOD, APX and CAT of the supporting ramets. For dependent ramets, POD, SOD and CAT content in severed ramets was higher than in connected ramets in the heterogeneous treatments and APX content in high contrast. POD, SOD, APX and CAT levels in the homogeneous treatments and APX content in low contrast showed no significant differences between severing and non-severing treatments. There were no significant differences between different contrast levels in POD, APX and CAT content of connected ramets. But the SOD content of connected ramets in high contrast was higher than that in low contrast and homogeneous treatments. POD, SOD and CAT content in severed ramets increased with increasing contrast level. APX content in severed dependent ramets was higher than in connected dependent ramets in high contrast, but there was no difference in the homogeneous and low contrast treatments (Table 2 and Fig. 3).
Figure 3. Antioxidant enzymes activity.
(A) POD, (B) SOD, (C) APX and (D) CAT of the supporting and the dependent ramets. Data are means±SE (n = 15). Bars sharing the same lowercase letter are not different at p = 0.05. Treatments are coded as in Table 1.
Discussion
Drought Induced Stress
Our study clearly indicates an increased stress in plants with increasing shortage of water and this response is very consistent regardless of the size characteristic or physiological indicator studied. Severed plants subjected to stress without the support from a ramet grown in benign conditions were smaller and had fewer leaves as well as less clonal offspring ramets (Fig. 1). The effect was proportional to the level of induced stress.
To cope with an increased water shortage, plants may adjust their osmotic potential. Free proline content may serve as a means of osmotic adjustment, and improve water relations under drought conditions [71]. In addition, it could function as a hydroxyl radical scavenger to prevent membrane damage and protein denaturation [55]. Higher content of free proline has been reported in many drought-induced plants [71]–[73]. We found a greater proline accumulation in severed dependent ramets subjected to water shortage (Fig. 2A). Thus, plants may adjust to water shortage by increasing proline content, but reduced protein content in dry conditions (Fig. 2D) indicates that they could only adjust to a certain extent and damage could not be avoided.
In our study, both MDA and H2O2 increased with increasing water shortage in severed ramets, which indicates that plants sustained considerable damage and that damage was proportional to water shortage. Their increase during drought stress has been reported in non-clonal plants as indicators of drought stress [48], [71], [74]. We also found a clear increase in POD, SOD, APX, and CAT levels in stressful conditions, which is consistent with the findings in many non-clonal plants during drought stress [47], [75], [76]. POD, SOD, APX and CAT are important enzymes which protect plants against oxidative damage. Their increase in our study can be interpreted as a measure of effort plants put into repair. This repair will be costly to plants and is not entirely successful. Our results showed that when water availability is reduced to as low as 30% of soil water field capacity this has markedly negative effects even on the survival of F. orientalis. During the experiment 53% of severed dependent ramets growing in such conditions died, indicating that drought was a major stress factor in the model environment when ramets where denied support from a physiologically integrated ramet growing in benign conditions.
Benefits from Physiological Integration
Ramets of F. orientalis in favourable conditions provide support to connected drought stressed ramets. This result is consistent with several studies with other clonal species [37], [39], [40], [77]–[84] where it was found that drought had negative effects on severed ramets, and the negative effects were ameliorated in connected ramets. In our case with F. orientalis the support by a supporting ramet was so efficient that the level of most of the stress hormones did not increase in the dependent ramet. Only the levels of H2O2, protein content, and SOD in the high-contrast treatments increased in connected dependent ramets. However, the growth of connected ramets was reduced even in the low-contrast treatments, showing that water shortage affected them, even though stress was not detected in the chemical analyses of these plants.
In the case of extremely severe drought or in conditions of chronic water shortage clonal plants may cease to support dependent ramets [21], [23], [24]. We can conclude that in our experiment the support to dependent ramets of F. orientalis never stopped. The higher the contrast between paired patches, the stronger was the support to a dependent ramet (as indicated by the increasing difference between severed and connected ramets). Thus, water sharing between connected ramets was very efficient [25], [38], [77], [78], [83] and it increased with increasing demand.
Cost of Support
Apart from benefits, integration may also have a cost for ramets exporting resources, especially when the supported ramets are located in more stressful conditions [85]. In our study, supporting ramets severed from a dependent ramet were always bigger than supporting ramets with intact stolon connection (Fig. 1), and this difference is most obvious in the number of leaves and ramets. To a lesser extent this also holds true for severed dependent ramets. The fact that connected ramets were smaller even when both of them received the same amount of water indicates the metabolic costs of maintaining the connection between ramets [15], [19], [85]. This cost was also shared between both proximal and distal ramets even though the former seemed to bear the greater share. The cost was surprisingly large, if the increase in number of modules (ramets or leaves) in severed ramets was an indication of maintenance costs of a connection. However, the increase in number of modules in severed ramets may be also an indication of a release from apical dominance.
When clonal fragments of F. orientalis were partially exposed to drought, and stolon connections between the droughty and wet ramets remained intact, clonal integration showed great benefits to the ramets in the dry patches (which imported resources) and significant costs to the connected wet ramets (which exported resources). There was a clear increase in growth reduction in connected supporting ramets with increasing water deficiency in dependent ramets, but there were no differences in stress indicators between connected and severed supporting ramets. This indicates that translocation of substances to resource-deficient ramets clearly reduces performance of the supplying part of a clonal fragment [15], [19], [28], [85]. The effect is primarily associated with growth reduction, but supporting ramets do not export water at a level which would cause the increase in production of stress-related chemicals. Thus, we conclude that the support is not given at a level that would directly harm the supporting ramet. A possible explanation for this might be that vascular translocation of water may require expenditure of energy [86] and therefore is expected to affect the growth of supporting ramets. Removal of stem and shoot apex usually results in the growth of one or more of the lateral buds and production of more new shoots [87]. Thus another possible explanation is that the loss of apical dominance caused by stolon severance promotes the growth of supporting ramets. Significant costs of water export in clonal plants also have previously been reported in C. flacca and Ficus tikoua spanning a gradient in water availability [39], [43]. However, this contrasts with the results in H. bonariensis [37], C. hirta [39], P. anserina [80] and Buchloe dactyloides [88], which showed that clonal integration confers benefits on drought stressed ramets but no costs on the connected supporting ramets. The support from well-watered ramets to drought ramets is not given at a level that would directly harm the supporting ramets, even though we did not know whether the growth difference of supporting ramets connected to and severed from dependent ramets were caused by maintenance costs of a connection or a release from apical dominance.
Conclusions
Our results, together with previous studies on the effect of clonal integration on morphology and photosynthesis of F. orientalis [3], [15], suggest that clonal integration can help F. orientalis to deal with drought stress, especially in conditions where contrast between patches is large. In such conditions the benefits from connection could outweigh the considerable costs of maintaining the clonal connection between ramets which make persistence of connections in homogeneous conditions disadvantageous. Clonal integration can therefore be understood as part of a stress tolerance strategy that enhances the survival and growth of clonal plants growing in patchy environments [4], [19], [89]–[91]. However, excessive integration can be adaptive only when its benefits outweigh the costs and not in every environment.
Funding Statement
This research was financially supported by ERMOS Postdoctoral Research Grants (ERMOS 11), grant no 8745 from the Estonian Science Foundation and the National Natural Science Foundation of China (31170502). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Alpert P (1991) Nitrogen sharing among ramets increases clonal growth in Fragaria Chiloensis . Ecology 72: 69–80. [Google Scholar]
- 2. D'Hertefeldt T, Jónsdóttir IS (1994) Effects of resource availability on integration and clonal growth in Maianthemum bifolium . Folia Geobotanica and Phytotaxonomica 29: 167–179. [Google Scholar]
- 3. Zhang YC, Zhang QY, Yirdaw E, Luo P, Wu N (2008) Clonal integration of Fragaria orientalis driven by contrasting water availability between adjacent patches. Botanical Studies 49: 373–383. [Google Scholar]
- 4. Guo W, Song YB, Yu FH (2011) Heterogeneous light supply affects growth and biomass allocation of the understory fern Diplopterygium glaucum at high patch contrast. Plos One 6: e27998 doi:27910.21371/journal.pone.0027998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Witte LC, Stöcklin J (2010) Longevity of clonal plants: why it matters and how to measure it. Annals of Botany 106: 859–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yu FH, Dong M, Krusi B (2004) Clonal integration helps Psammochloa villosa survive sand burial in an inland dune. New Phytologist 162: 697–704. [DOI] [PubMed] [Google Scholar]
- 7. Yu FH, Wang N, He WM, Chu Y, Dong M (2008) Adaptation of rhizome connections in drylands: increasing tolerance of clones to wind erosion. Annals of Botany 102: 571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xu L, Yu FH, van Drunen E, Schieving F, Dong M, et al. (2012) Trampling, defoliation and physiological integration affect growth, morphological and mechanical properties of a root-suckering clonal tree. Annals of Botany 109: 1001–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gómez S, Stuefer JF (2006) Members only: induced systemic resistance to herbivory in a clonal plant network. Oecologia 147: 461–468. [DOI] [PubMed] [Google Scholar]
- 10. Chen JS, Lei NF, Liu Q (2011) Defense signaling among interconnected ramets of a rhizomatous clonal plant, induced by jasmonic-acid application. Acta Oecologica 37: 355–360. [Google Scholar]
- 11. Gómez S, Onoda Y, Ossipov V, Stuefer JF (2008) Systemic induced resistance: a risk-spreading strategy in clonal plant networks? New Phytologist 179: 1142–1153. [DOI] [PubMed] [Google Scholar]
- 12. Stuefer JF (1996) Potential and limitations of current concepts regarding the response of clonal plants to environmental heterogeneity. Vegetatio 127: 55–70. [Google Scholar]
- 13. Zhou J, Dong BC, Alpert P, Li HL, Zhang MX, et al. (2012) Effects of soil nutrient heterogeneity on intraspecific competition in the invasive, clonal plant Alternanthera philoxeroides . Annals of Botany 109: 813–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jónsdóttir IS, Watson MA (1997) Extensive physiological integration: an adaptive trait in resource-poor environments? In: de Kroon H, Groenendael JM, editors. The ecology and evolution of clonal plants. Leiden: Backhuys Publishers. 109–136.
- 15. Zhang YC, Zhang QY, Luo P, Wu N (2009) Photosynthetic response of Fragaria orientalis in different water contrast clonal integration. Ecological Research 24: 617–625. [Google Scholar]
- 16.Liu RH, Dong BC, Li HL, Zhang Q, Yu FH (2012) Patchy distributions of Spirogyra arcta do not affect growth of the submerged macrophyte Ceratophyllum demersum. Plant Species Biology doi: 10.1111/j.1442–1984.2011.00346.x.
- 17. Kelly CK (1995) Thoughts on clonal integration: facing the evolutionary context. Evolutionary Ecology 9: 575–585. [Google Scholar]
- 18. Stuefer JF, Gomez S, Van Molken T (2004) Clonal integration beyond resource sharing: implications for defence signalling and disease transmission in clonal plant networks. Evolutionary Ecology 18: 647–667. [Google Scholar]
- 19.Pitelka LF, Ashmun JW (1985) Physiology and integration of ramets in clonal plants. In: Jackson JBC, Buss LW, Cook RE, editors. Population Biology and Evolution of Clonal Organisms. New Haven: Yale University Press. 399–437.
- 20. Koubek T, Herben T (2008) Effect of systemic diseases on clonal integration: modelling approach. Evolutionary Ecology 22: 449–460. [Google Scholar]
- 21. Hartnett DC, Bazzaz FA (1983) Physiological integration among intraclonal ramets in Solidago Canadensis . Ecology 64: 779–788. [Google Scholar]
- 22. Jónsdóttir IS, Callaghan TV (1989) Localized defoliation stress and the movement of 14C-photoassimilates between tillers of Carex bigelowii . Oikos 54: 211–219. [Google Scholar]
- 23. Ong CK, Marshall C (1979) The growth and survival of severely-shaded tillers in Lolium perenne L. Annals of Botany. 43: 147–155. [Google Scholar]
- 24. Matlaga DP, Sternberg LDL (2009) Ephemeral Clonal Integration in Calathea Marantifolia (Marantaceae): Evidence of Diminished Integration over Time. American Journal of Botany 96: 431–438. [DOI] [PubMed] [Google Scholar]
- 25. Salzman AG, Parker MA (1985) Neighbors ameliorate local salinity stress for a rhizomatous plant in a heterogeneous environment. Oecologia 65: 273–277. [DOI] [PubMed] [Google Scholar]
- 26. Slade AJ, Hutchings MJ (1987) An analysis of the costs and benefits of physiological integration between ramets in the clonal perennial herb Glechoma hederacea . Oecologia 73: 425–431. [DOI] [PubMed] [Google Scholar]
- 27. Kotliar NB, Wiens JA (1990) Multiple scales of patchiness and patch structure: a hierarchical framework for the study of heterogeneity. Oikos 59: 253–260. [Google Scholar]
- 28. Caraco T, Kelly CK (1991) On the adaptive value of physiological integration in clonal plants. Ecology 72: 81–93. [Google Scholar]
- 29.Eriksson O, Jerling L (1990) Hierarchical selection and risk spreading in clonal plants. In: van Groenendael J, de Kroon H, editors. Clonal Growth in Plants: Regulation and Function. The Hague: SPB Academic Publishing. 79–94.
- 30.Marshall C (1990) Source-sink relations of interconnected ramets. In: van Groenendael J, de Kroon H, editors. Clonal Growth in Plants: Regulation and Function. The Hague: SPB Academic Publishing. 23–41.
- 31. Jònsdòttir IS, Callaghan TV (1990) Intraclonal translocation of ammonium and nitrate nitrogen in Carex bigelowii Torr. ex Schwein. using 15N and nitrate reductase assays. New Phytologist 114: 419–428. [DOI] [PubMed] [Google Scholar]
- 32.Kolasa J, Pickett ST (1991) Ecological heterogeneity. New York: Springer-Verlag. 332p.
- 33. Hutchings MJ, Wijesinghe DK (1997) Patchy habitats, division of labour and growth dividends in clonal plants. Trends in Ecology and Evolution 12: 390–394. [DOI] [PubMed] [Google Scholar]
- 34. Wang YH, Dong M, Yu FH, Jiang H, Yu SQ, et al. (2011) Mechanical shaking and soil water affect the growth of Psammochloa villosa in the Mu Us Sandland. Journal of Arid Environments 75: 974–977. [Google Scholar]
- 35. Alpert P (1999) Clonal integration in Fragaria chiloensis differs between populations: ramets from grassland are selfish. Oecologia 120: 69–76. [DOI] [PubMed] [Google Scholar]
- 36. Zhang CY, Yu FH, Chen YF, Dong M (2003) Phenotypic plasticity in response to the heterogeneous water supply in the rhizomatous grass species, Calamagrostis epigejos in the Mu Us sandy land of China. Acta Botanica Sinica 45: 1210–1217. [Google Scholar]
- 37. Evans JP (1991) The effect of resource integration on fitness related traits in a clonal dune perennial, Hydrocotyle Bonariensis . Oecologia 86: 268–275. [DOI] [PubMed] [Google Scholar]
- 38. Alpert P, Mooney HA (1986) Resource sharing among ramets in the clonal herb, Fragaria chiloensis . Oecologia 70: 227–233. [DOI] [PubMed] [Google Scholar]
- 39. de Kroon H, Fransen B, van Rheenen JWA, van Dijk A, Kreulen R (1996) High levels of inter-ramet water translocation in two rhizomatous Carex species, as quantified by deuterium labelling. Oecologia 106: 73–84. [DOI] [PubMed] [Google Scholar]
- 40. de Kroon H, van der Zalm E, van Rheenen JWA, van Dijk A, Kreulen R (1998) The interaction between water and nitrogen translocation in a rhizomatous sedge (Carex flacca). Oecologia 116: 38–49. [DOI] [PubMed] [Google Scholar]
- 41. Liu FH, Liu J, Yu FH, Dong M (2007) Water integration patterns in two rhizomatous dune perennials of different clonal fragment size. Flora 202: 106–110. [Google Scholar]
- 42. Savini G, Giorgi V, Scarano E, Neri D (2008) Strawberry plant relationship through the stolon. Physiologia Plantarum 134: 421–429. [DOI] [PubMed] [Google Scholar]
- 43. Liu CC, Liu YG, Guo K, Fan DY, Yu LF, et al. (2011) Exploitation of patchy soil water resources by the clonal vine Ficus tikoua in karst habitats of southwestern China. Acta Physiologiae Plantarum 33: 93–102. [Google Scholar]
- 44. Prakash J, Bhattacharyya S, Chattopadhyay K, Roy S, Das SP, et al. (2009) PQM-1: a newly developed superior clone of pineapple for northeastern India as evident through phenotype, fruit quality and DNA polymorphism. Scientia Horticulturae 120: 288–291. [Google Scholar]
- 45. Mizuki I, Takahashi A (2009) Secondary dispersal of Dioscorea japonica (Dioscoreaceae) bulbils by rodents. Journal of Forest Research 14: 95–100. [Google Scholar]
- 46. Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science 7: 405–410. [DOI] [PubMed] [Google Scholar]
- 47. Uzilday B, Turkan I, Sekmen AH, Ozgur R, Karakaya HC (2012) Comparison of ROS formation and antioxidant enzymes in Cleome gynandra (C4) and Cleome spinosa (C3) under drought stress. Plant Science 182: 59–70. [DOI] [PubMed] [Google Scholar]
- 48. Sohrabi Y, Heidari G, Weisany W, Golezani KG, Mohammadi K (2012) Changes of antioxidative enzymes, lipid peroxidation and chlorophyll content in chickpea types colonized by different Glomus species under drought stress. Symbiosis 56: 5–18. [Google Scholar]
- 49. Cavalcanti FR, Oliveira JTA, Martins-Miranda AS, Viégas RA, Silveira JAG (2004) Superoxide dismutase, catalase and peroxidase activities do not confer protection against oxidative damage in salt-stressed cowpea leaves. New Phytologist 163: 563–571. [DOI] [PubMed] [Google Scholar]
- 50. Ederli L, Reale L, Ferranti F, Pasqualini S (2004) Responses induced by high concentration of cadmium in Phragmites australis roots. Physiologia Plantarum 121: 66–74. [DOI] [PubMed] [Google Scholar]
- 51. Zhuang L, Chen YN (2006) Physiological responses of three contrasting plant species to groundwater level changes in an arid environment. Journal of Integrative Plant Biology 48: 520–526. [Google Scholar]
- 52. Jain M, Mathur G, Koul S, Sarin N (2001) Ameliorative effects of proline on salt stress-induced lipid peroxidation in cell lines of groundnut (Arachis hypogaea L.). Plant Cell Reports 20: 463–468. [Google Scholar]
- 53. Chowdhury SR, Choudhuri MA (1985) Hydrogen peroxide metabolism as an index of water stress tolerance in jute. Physiologia Plantarum 65: 476–480. [Google Scholar]
- 54. Li L, van Staden J (1998) Effects of plant growth regulators on the antioxidant system in callus of two maize cultivars subjected to water stress. Plant Growth Regulation 24: 55–66. [Google Scholar]
- 55. Ain-Lhout F, Zunzunegui M, Diaz Barradas MC, Tirado R, Clavijo A, et al. (2001) Comparison of proline accumulation in two mediterranean shrubs subjected to natural and experimental water deficit. Plant and Soil 230: 175–183. [Google Scholar]
- 56.Guan WB, Wu JA, Liang GL, Wang B, Ma KM, et al. (2004) Vegetation classification and the main types in the headwater area of the Minjiang River. In: Chen YY, editor. Proceedings of the fifth national symposium on the conservation and sustainable use of biodiversity in China. Beijing: China Meteorological Press. 288–300.
- 57.Li CL, Ikeda H, Ohba H (2003) Fragaria Linnaeus. In: Wu ZY, Raven PH, Hong DY, editors. Flora of China. Beijing: Science Press, St. Louis: Missouri Botanical Garden Press. 337–340.
- 58. Yu FH, Chen YF, Dong M (2002) Clonal integration enhances survival and performance of Potentilla anserina, suffering from partial sand burial on Ordos plateau, China. Evolutionary Ecology 15: 303–318. [Google Scholar]
- 59. Chu Y, Yu FH, Dong M (2006) Clonal plasticity in response to reciprocal patchiness of light and nutrients in the stoloniferous herb Glechoma longituba L. Journal of Integrative Plant Biology. 48: 400–408. [Google Scholar]
- 60. Chen JS, Lei NF, Dong M (2010) Clonal integration improves the tolerance of Carex praeclara to sand burial by compensatory response. Acta Oecologica 36: 23–28. [Google Scholar]
- 61. Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant and Soil 39: 205–207. [Google Scholar]
- 62. Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Archives of Biochemistry and Biophysics 125: 189–198. [DOI] [PubMed] [Google Scholar]
- 63. Prochazkova D, Sairam RK, Srivastava GC, Singh DV (2001) Oxidative stress and antioxidant activity as the basis of senescence in maize leaves. Plant Science 161: 765–771. [Google Scholar]
- 64. Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72: 248–254. [DOI] [PubMed] [Google Scholar]
- 65. Pinto ME, Casati P, Hsu TP, Ku MS, Edwards GE (1999) Effects of UV-B radiation on growth, photosynthesis, UV-Babsorbing compounds and NADP-malic enzyme in bean (Phaseolus vulgaris L.) grown under different nitrogen conditions. Journal of Photochemistry and Photobiology B: Biology 48: 200–209. [DOI] [PubMed] [Google Scholar]
- 66. Ekmekci Y, Terzioglu S (2005) Effects of oxidative stress induced by paraquat on wild and cultivated wheats. Pesticide Biochemistry and Physiology 83: 69–81. [Google Scholar]
- 67. Becana M, Aparicio-Tejo P, Irigoyen JJ, Sanchez-Dıaz M (1986) Some enzymes of hydrogen peroxide metabolism in leaves and root nodules of Medicago sativa . Plant Physiology 82: 1169–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Costa H, Gallego SM, Tomaro ML (2002) Effects of UV-B radiation on antioxidant defense system in sunflower cotyledons. Plant Science 162: 939–945. [Google Scholar]
- 69. Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplast. Plant and Cell Physiology 22: 867–880. [Google Scholar]
- 70. Kato M, Shimizu S (1987) Chlorophyll metabolism in higher plants. VII. Chlorophyll degradation in senescing tobacco leaves; phenolic-dependent peroxidative degradation. Canadian Journal of Botany 65: 729–735. [Google Scholar]
- 71. Yin GH, Shen YJ, Tong N, Gu J, Hao L, et al. (2012) Drought induced changes of physio-biochemical parameters in maize. Journal of Food Agriculture & Environment 10: 853–858. [Google Scholar]
- 72. Yang Y, Liu Q, Han C, Qiao YZ, Yao XQ, et al. (2007) Influence of water stress and low irradiance on morphological and physiological characteristics of Picea asperata seedlings. Photosynthetica 45: 613–619. [Google Scholar]
- 73. Ashraf M, Iram A (2005) Drought stress induced changes in some organic substances in nodules and other plant parts of two potential legumes differing in salt tolerance. Flora 200: 535–546. [Google Scholar]
- 74. Yang F, Xu X, Xiao X, Li C (2009) Responses to drought stress in two poplar species originating from different altitudes. Biologia Plantarum 53: 511–516. [DOI] [PubMed] [Google Scholar]
- 75. Sankar B, Jaleel CA, Manivannan P, Kishorekumar A, Somasundaram R, et al. (2007) Effect of paclobutrazol on water stress amelioration through antioxidants and free radical scavenging enzymes in Arachis hypogaea L. Colloids and Surfaces B-biointerfaces. 60: 229–235. [DOI] [PubMed] [Google Scholar]
- 76. Fazeli F, Ghorbanli M, Niknam V (2007) Effect of drought on biomass, protein content, lipid peroxidation and antioxidant enzymes in two sesame cultivars. Biologia Plantarum 51: 98–103. [Google Scholar]
- 77. Lau RR, Young DR (1988) Influence of physiological integration on survivorship and water relations in a clonal herb. Ecology 69: 215–219. [Google Scholar]
- 78. Alpert P (1990) Water sharing among ramets in a desert population of Distichlis spicata (Poaceae). American Journal of Botany 77: 1648–1651. [Google Scholar]
- 79. Ren AZ, Gao YB, Liang Y, Chen SP, Liu S, et al. (1999) Effect of drought stress on clonal growth of Pennisetum Centrasiaticum and Leymus Secalinus . Journal of Desert Research 19: 30–34. [Google Scholar]
- 80. van Kleunen M, Stuefer JF (1999) Quantifying the effects of reciprocal assimilate and water translocation in a clonal plant by the use of steam-girdling. Oikos 85: 135–145. [Google Scholar]
- 81. Dong M, Alaten B (1999) Clonal plasticity in response to rhizome severing and heterogeneous resource supply in the rhizomatous grass Psammochloa villosa in an Inner Mongolian dune, China. Plant Ecology 141: 53–58. [Google Scholar]
- 82. Noda H, Muraoka H, Washitani I (2004) Morphological and physiological acclimation responses to contrasting light and water regimes in Primula sieboldii . Ecological Research 19: 331–340. [Google Scholar]
- 83. Mao SY, Jiang CD, Zhang WH, Shi L, Zhang JZ, et al. (2009) Water translocation between ramets of strawberry during soil drying and its effects on photosynthetic performance. Physiologia Plantarum 137: 225–234. [DOI] [PubMed] [Google Scholar]
- 84. Wang JC, Shi X, Yin LK, Zhang DY (2011) Role of Clonal Integration in Life Strategy of Sandy Dune Plant, Eremosparton Songoricum (Litv.) Vass (Fabaceae): Experimental Approach. Polish Journal of Ecology 59: 455–461. [Google Scholar]
- 85. Roiloa SR, Retuerto R (2012) Clonal integration in Fragaria vesca growing in metal-polluted soils: Parents face penalties for establishing their offspring in unsuitable environments. Ecological Research 27: 95–106. [Google Scholar]
- 86.Epstein E (1972) Mineral nutrition of plants: principles and perspectives. New York: John Wiey and Sons Inc. 415p.
- 87.Öpik H, Rolfe SA, Willis AJ (2005) The Physiology Of Flowering Plants. Cambridge: Cambridge University Press. 392p.
- 88. Sun XL, Niu JZ, Xu YF, Zhou H (2010) Long term water integration in interconnected ramets of stoloniferous grass, buffalograss. African Journal of Biotechnology 9: 5503–5510. [Google Scholar]
- 89.Grime JP (1979) Plant strategies and vegetation processes. New York: John Wiley & Sons. 222p.
- 90. He WM, Yu FH, Zhang LL (2010) Physiological integration impacts nutrient use and stoichiometry in three clonal plants under heterogeneous habitats. Ecological Research 25: 967–972. [Google Scholar]
- 91. Du J, Wang N, Alpert P, Yu MJ, Yu FH, et al. (2010) Clonal integration increases performance of ramets of the fern Diplopterygium glaucum in an evergreen forest in southeastern China. Flora 205: 399–403. [Google Scholar]