Abstract
Gonadotrophin-releasing hormone (GnRH) antagonist rescue is performed by replacing a GnRH agonist with a GnRH antagonist in patients with rapidly rising serum oestradiol who are at risk of ovarian hyperstimulation syndrome (OHSS) during stimulation. It results in a rapid reduction in serum oestradiol, allowing for the avoidance of cycle cancellation and the continuation of exogenous gonadotrophin administration. A total of 387 patients who underwent GnRH antagonist rescue for ovarian hyperresponse were compared with 271 patients who did not receive GnRH antagonist rescue and had oestradiol concentrations >4000 pg/ml on the day of human chorionic gonadotrophin (HCG) administration. GnRH antagonist rescue decreased the mean oestradiol concentration by 35% on the first day of use. There was no difference in oocyte maturity (82% versus 83%) or fertilization rate (69% versus 67%) between the antagonist rescue and comparison groups, respectively. The percentage of high-grade embryos on day 3 and the blastocyst development rate were also similar between groups. The live-birth rate was 41.9% in the antagonist rescue group and 36.9% in the comparison group. GnRH antagonist rescue enabled cycle completion with high live-birth rates in patients at risk for OHSS. GnRH antagonist was associated with high oocyte quality, blastocyst development and pregnancy.
Keywords: assisted reproduction, cancellation, GnRH antagonist rescue, infertility, IVF, OHSS
Introduction
Ovarian hyperstimulation syndrome (OHSS) is one of the more severe risks associated with IVF. Complications of OHSS include deep venous thrombosis, pulmonary embolus, abdominal pain and hospitalization. Short of cycle cancellation, the suggested strategies for mitigating the risk of OHSS include coasting, lupron trigger in gonadotrophin-releasing hormone (GnRH) antagonist cycles, dopamine antagonist administration, post-retrieval GnRH antagonist administration, freezing all embryos, intracycle metformin, administering albumin or hespan and GnRH antagonist rescue.
High oestradiol concentrations have been associated with increasing likelihood of developing OHSS (Delvigne et al., 1993). GnRH antagonist rescue has been used to suppress rapidly rising oestradiol concentrations in patients requiring additional days of follicular growth. Gustofson et al. (2006a,b) first demonstrated that antagonist rescue could rapidly reduce serum oestradiol and provide good cycle outcomes in patients at high risk of OHSS. In a randomized controlled trial, Aboulghar et al. (2007) showed that antagonist rescue shortened the time to human chorionic gonadotrophin (HCG) administration and produced more oocytes and high-quality embryos when compared with coasting.
For the past 7 years, the study centre has utilized antagonist rescue as the primary intervention for patients undergoing ovarian stimulation who have rapidly rising oestradiol concentrations and are at risk for OHSS. Studies to date have been limited by small patient numbers, unknown effect on embryo development beyond the cleavage stage and difficulty ascertaining the risk of a rare complication like OHSS (Aboulghar et al., 2007, Gustofson et al., 2006a, Gustofson et al., 2006b). The primary objective of this study was to assess the effect of antagonist rescue on embryo development to the blastocyst stage and on assisted cycle outcomes. Additional objectives were to estimate the effect of antagonist rescue on serum oestradiol, cycle cancellation and factors associated with the development of OHSS.
Materials and methods
Design
The research protocol was submitted and approved by the Department of Clinical Investigation at Walter Reed Army Medical Center (WRAMC). Electronic medical records were reviewed for all patients who underwent a fresh autologous assisted cycle at WRAMC from January 2004 to May 2010. Electronic records were verified by cross-checking data points from paper charts. The primary outcomes analysed were oocyte yield, oocyte maturity and embryo development. Secondary outcomes included clinical pregnancy, live birth and rate of OHSS. The following definitions were used to measure cycle outcomes: pregnancy was a positive serum qualitative HCG; clinical pregnancy was the presence of a fetal pole on transvaginal ultrasound with positive fetal cardiac activity; and spontaneous abortion was a clinical pregnancy which proceeded to abort. Severe OHSS was defined by signs and symptoms of OHSS necessitating paracentesis (Grossman et al., 2010).
Patients
The study group (GnRH antagonist rescue group) included all patients who underwent a GnRH agonist (long LA or MDF) stimulation protocol and received at least 1 day of GnRH antagonist for ovarian hyperresponse. Hyperresponse was defined as a high or rapidly rising serum oestradiol concentration prior to HCG trigger for which cycle cancellation was considered due to the risk of OHSS. Antagonist rescue was utilized in patients when the serum oestradiol concentration was projected to be >5000 pg/ml if stimulation was continued and the size of the lead follicle was <18 mm, indicating the need for additional follicular growth. The estimated oestradiol concentration was projected by calculating the rate of oestradiol rise from the previous day and continuing the same rise on the following day. GnRH antagonist (250 g subcutaneously once daily; ganirelex acetate; Organon) was administered for 1–3 days to decrease the oestradiol concentration and permit further follicular growth prior to HCG administration for oocyte maturation. With the initiation of the GnRH antagonist, the GnRH agonist was discontinued while exogenous gonadotrophins were continued. The median FSH dose adjustment was 0 units (no change) with the start of antagonist rescue. No patients who had GnRH antagonist rescue were excluded. OHSS was classified as severe when patients exhibited physical symptoms and ultrasonographic evidence of ascites necessitating paracentesis. No patients underwent coasting to reduce oestradiol concentrations prior to HCG. Patients who underwent a traditional GnRH antagonist protocol as a means of ovulation suppression were not included. In addition, no donor oocyte or frozen embryo cycles were included.
A comparison group of high-responder patients was chosen to further assess outcomes. The comparison group included all patients during the study period who had a high response to ovarian stimulation but did not require the GnRH antagonist to reduce the oestradiol concentration. High-response patients were defined as those who had a serum oestradiol concentration of ≥4000 pg/ml on the day of HCG administration. This group did not receive antagonist rescue, despite high oestradiol concentrations, because the lead follicles were ≥18 mm and these patients received HCG rather than continuing stimulation. It has been the study centre’s clinical practice to give antagonist rescue to patients at risk of OHSS who need additional days of gonadotrophin stimulation but not to patients at risk of OHSS who are ready for oocyte retrieval without the need for additional stimulation.
For both groups, patients underwent either a GnRH microdose flare (MDF) or long luteal agonist (LA) protocol as previously described (Levens et al., 2009). Prior studies have demonstrated that, even in normal-responding patients under the age of 30, MDF and LA result in similar outcomes and OHSS rates (Levens et al., 2009). The study centre’s programme routinely utilizes MDF in normal-responding patients due to a significant reduction in the amount of GnRH agonist needed to achieve pituitary down-regulation, thus providing a protocol that maximizes resource allocation (Levens et al., 2009). The patients were monitored with serial serum oestradiol and transvaginal ultrasound follicle measurements. The clinic operated 7 days a week, so ultrasounds and serum testing could be performed on consecutive days of the week when indicated. When there were at least two follicles of ≥18 mm, a single intramuscular injection of either 5000 or 10,000 IU HCG was administered and ultrasound-guided transvaginal oocyte retrieval was performed 36 h later. Conventional IVF or intracytoplasmic sperm injection was performed as indicated. Luteal progesterone support (50 mg daily progesterone i.m.; AAP Parmaceuticals, Shaumburg, IL, USA; or 100 mg three times daily endometrin vaginal suppository; Ferring Pharmaceuticals, Parsippany, NY, USA) was provided on the day of retrieval and thereafter.
Embryo grading for cleavage-stage embryos was performed using the criteria developed by Veeck (1986). Day-5 embryos were assessed and assigned a developmental stage (expanded blastocyst, blastocyst, early blastocyst, morula or atretic). All embryo transfers were performed under ultrasound guidance either 3 or 5 days post retrieval. Serum quantitative HCG testing was performed 14 and 16 days after oocyte retrieval. Transvaginal ultrasound was performed at 6–7 weeks’ gestation to confirm an intrauterine pregnancy (clinical pregnancy). Live-birth outcomes were recorded. All patients were monitored for evidence of OHSS as previously described (Golan et al., 1989, Csokmay et al., 2010, Practice Committee of American Society for Reproductive, 2008). Specifically, severe OHSS was diagnosed when patients had ultrasound evidence of ascites with laboratory abnormalities and symptoms to include a tense abdomen, persistent abdominal pain, respiratory difficulty, rapid weight gain or oliguria.
The antagonist rescue cycles were further analysed based upon the type of GnRH agonist suppression (MDF versus LA), days of GnRH antagonist received, oestradiol concentration at time of GnRH antagonist initiation, oestradiol concentration on day of HCG, total number of follicles and amount of daily human menopausal gonadotrophin (HMG). A subgroup analysis was also performed comparing baseline and cycle characteristics between those patients who did or did not develop OHSS.
Statistical analysis
Statistical analyses were performed using Excel (Microsoft, 2007) and VassarStats online software (http://vassarstats.net). Student’s t-test was used to compare the mean values for normally distributed data. For data that were not normally distributed, a Mann–Whitney rank sum test was used to compare the mean values. Differences in outcome rates were analysed using a chi-squared test or Fisher’s Exact test, as appropriate. Bonferonni correction was applied for multiple comparisons. An alpha error of 0.05 was considered significant for all comparisons. All data were reported as mean ± standard deviation.
Results
A total of 387 patients underwent GnRH antagonist rescue between January 2004 and May 2010 and thus comprised the study group. During the same time period, 271 patients met criteria for the comparison group with a serum oestradiol concentration ≥4000 pg/ml on day of HCG administration. A total of 2620 assisted cycles were performed during the study period. Patients in the study and comparison groups were similar with respect to age, body mass index, gravidity, parity and diagnosis (Table 1). Because patients at highest risk for OHSS received antagonist rescue, the basal antral follicle count was significantly higher in the antagonist rescue group (22.2 versus 18.7; P < 0.001). Use of intracytoplasmic sperm injection and MDF protocol was similar between the two groups. The temporal distribution of the proportion of patients in each group was similar annually from 2004–2010. Of those receiving the GnRH antagonist, 323 patients received it for 1 day, 58 patients for 2 days and six patients for 3 days.
Table 1.
Baseline demographics and IVF protocol differences between the GnRH antagonist rescue and comparison groups.
| Antagonist rescue (n = 387) | Comparison (n = 271) | P- value | |
|---|---|---|---|
| Age (years) | 33.0 ± 4.2 | 33.6 ± 4.2 | NS |
| BMI (kg/m2) | 24.9 ± 4.7 | 25.0 ± 4.9 | NS |
| Gravidity | 1.1 ± 1.3 | 1.0 ± 1.3 | NS |
| Parity | 0.4 ± 1.0 | 0.4 ± 1.0 | NS |
| AFC | 22.2 ± 12 | 18.7 ± 10 | <0.001 |
| Diagnosis | NS | ||
| Tubal factor | 37.2 | 28.8 | |
| Male factor | 30.0 | 34.3 | |
| Unexplained | 12.4 | 14.8 | |
| Anovulation | 11.6 | 10.3 | |
| Endometriosis | 5.4 | 7.4 | |
| Other | 3.4 | 4.4 | |
| ICSI | 317 (81.9) | 215 (79.3) | NS |
| MDF | 316 (81.6) | 222 (81.9) | NS |
| Days of stimulation | 10.5 ± 1.4 | 10.6 ± 1.4 | NS |
| Ampoules of gonadotrophins | 32.6 ± 18 | 39.9 ± 1.7 | <0.001 |
| Serum oestradiol (pg/ml) | |||
| On HCG day –1 | 4315 ± 1186 | 3052 ± 526 | <0.001 |
| On HCG day | 2969 ± 1121 | 4632 ± 594 | <0.001 |
| On HCG day +1 | 5773 ± 1837 | 5940 ± 1067 | NS |
| Follicles 16 mm | 10.7 ± 4.8 | 8.8 ± 3.5 | <0.001 |
| Severe OHSS | 31 (8.0) | 1 (0.4) | <0.01 |
| Follicles aspirated | 28.2 ± 9.7 | 21.4 ± 7.3 | <0.001 |
| Oocytes retrieved | 24.5 ± 10 | 18.8 ± 8.0 | <0.001 |
| Oocyte yield (%) | 93 ± 30 | 90 ±29 | NS |
| Mature oocytes (MII) | 18.9 ± 8.3 | 14.5 ± 6.5 | <0.001 |
| Oocyte maturity (%) | 82 ± 67 | 83 ± 63 | NS |
| 2PN | 13.2 ± 6.9 | 9.9 ± 5.6 | <0.001 |
| Fertilization rate (%) | 69 ± 19 | 67 ± 20 | NS |
| Pregnancy | 244 (63.0) | 162 (59.8) | NS |
| Clinical pregnancy | 201 (51.9) | 133 (49.1) | NS |
| Spontaneous abortion | 36 (9.3) | 31 (11.4) | NS |
| Live birth | 162 (41.9) | 100 (36.9) | NS |
Values are mean ± SD,% or n (%).
AFC = antral follicle count; BMI = body mass index; HCG = human chorionic gonadotrophin; ICSI = intracytoplasmic sperm injection; MDF = gonadotrophin-releasing hormone microdose flare; OHSS = ovarian hyperstimulation syndrome.
The peak oestradiol concentrations on the day after HCG administration were similar between the two groups (antagonist rescue 5773 pg/ml versus comparison 5940 pg/ml). Patients in the antagonist rescue group had a higher number of follicles aspirated, oocytes retrieved, mature oocytes and fertilized oocytes (2PN) (Table 1). However, this was primarily a function of more follicles, as the percentage oocyte yield, oocyte maturity and fertilization rate were similar between the two groups.
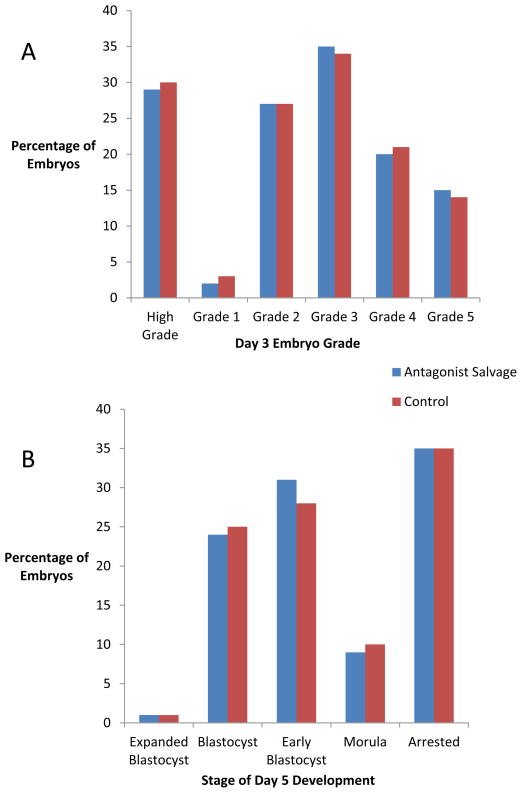
Patients in the GnRH antagonist rescue group had more high-grade cleavage-stage embryos (grade 1 or 2) (4.0 versus 2.9; P < 0.001) because they had more fertilized oocytes. However, the distribution of embryos at each grade was not significantly different (Figure 1A), supporting similar embryo quality between the groups. Patients in the GnRH antagonist rescue and comparison groups had similar numbers of expanded blastocysts, blastocysts and morulas. The GnRH antagonist rescue group had higher overall numbers of early blastocysts per patient (5.4 versus 3.7; P < 0.01). The distribution of embryos at each stage of blastocyst development was not different between the two groups (Figure 1B). Patients in the GnRH antagonist rescue group were further stratified by age groups (<35, 35–37, 38–40 and 41–42 years) and embryo quality at the cleavage and blastocyst stage was compared between the four age groups. No statistically significant differences were noted in embryo quality between any of the age groups receiving GnRH antagonist rescue (Supplementary Figure 1, available online only). However, this analysis may be limited by the relatively small number of patients in the oldest age group.
Figure 1.
Day-3 embryo grading and blastocyst development in the antagonist rescue and comparison groups. (A) High-grade embryos were classified as embryos grade I and II combined. (B) Blastocyst development. There were no statistical differences.
There was no difference in clinical pregnancy rates between the two groups (antagonist rescue 51.9% versus comparison 49.1%) (Table 1). Live-birth rates were also similar between the two groups (antagonist rescue 41.4% versus comparison 36.9%). Rates of biochemical pregnancy and spontaneous abortion were not different between the two groups. Within the antagonist rescue group, the live-birth rates were 42%, 34% and 67% in patients receiving 1, 2 and 3 days of antagonist rescue, respectively.
There was no difference in the rate of cycle cancellation prior to HCG administration due to severe risk of OHSS between the two groups (antagonist rescue 1.5% versus comparison 1.1%). Four patients in the antagonist rescue group (1%) and one patient in the comparison group (0.4%) developed signs of severe OHSS after oocyte retrieval but prior to embryo transfer and therefore had all embryos cryopreserved to mitigate the risk of worsening OHSS. The overall rate of severe OHSS was 1.2% (32/2620 cycles) during the study period. Consistent with the fact that all patients at high risk for OHSS were treated with antagonist rescue, the rate of OHSS was higher in the antagonist rescue cohort (8.0% versus 0.4%; P < 0.01). No adjunct treatments for mitigating OHSS risk (i.e. dopamine agonist, albumin) were given to any patient.
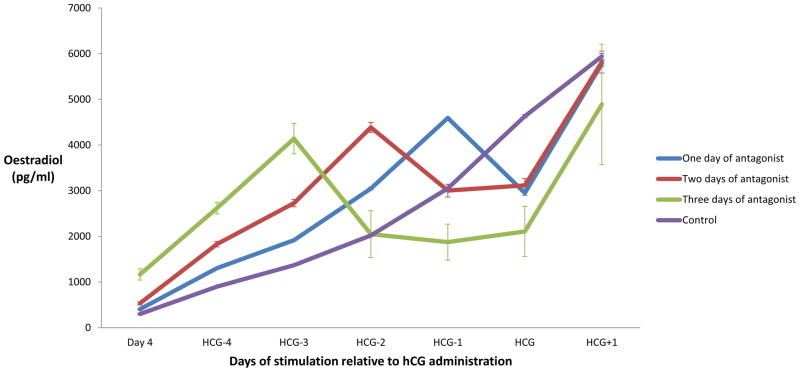
Figure 2 displays the mean serum oestradiol trends in the comparison group and in patients receiving 1, 2 and 3 days of GnRH antagonist rescue. The mean serum oestradiol for all antagonist rescue patients fell by 35% on the first day of GnRH antagonist treatment. Subsequently, the mean oestradiol rose by 96% in response to HCG administration. In patients receiving 2 days of antagonist rescue, oestradiol concentrations remained relatively stable on day 2 of antagonist treatment (mean 4% rise). In patients receiving 3 days of antagonist rescue, serum oestradiol concentrations also remained relatively stable on days 2 and 3 of antagonist (means –9% and 12%, respectively). The peak oestradiol concentrations on the day after HCG administration were not significantly different between the three groups, regardless of how many days of antagonist.
Figure 2.
Mean serum oestradiol concentrations (pg/ml) as measured at each point during stimulation. Day 4 refers to day 4 of stimulation; 1 day of gonadotrophin-releasing hormone antagonist: n = 323; 2 days of antagonist: n = 58; 3 days of antagonist: n = 6; control: n = 271. Error bars represent standard error for each group. HCG = human chorionic gonadotrophin.
On the day prior to antagonist rescue, patients treated with MDF had higher mean oestradiol concentrations than patients treated with LA (3095 versus 2875 pg/ml, respectively; P = 0.05). However, serum oestradiol concentrations were not statistically different between the two protocols on the day after antagonist administration or the day after HCG. GnRH antagonist rescue reduced the mean serum oestradiol concentrations by 38% in patients receiving MDF and by 34% in patients receiving LA (Supplementary Figure 2).
The mean oestradiol change in patients receiving less than one, one, two or three vials of HMG on the day of GnRH antagonist rescue was analysed (Supplementary Figure 3). Oestradiol changes in response to antagonist were –45% in patients receiving less than one vial of HMG, –37% for one vial of HMG, –10% for two vials of HMG and 2% for three vials of HMG. The fall in oestradiol concentrations for patients receiving less than one or one vial of HMG was significantly greater than those receiving two or three vials (P < 0.0001).
Compared with patients who did not develop OHSS, antagonist rescue patients who went on to develop severe OHSS had higher basal antral follicle counts (29.1 versus 21.6; P < 0.001) and more follicles >10 mm on the day of HCG (35.1 versus 27.5; P < 0.001). While patients who developed OHSS had higher oestradiol concentrations on the day of GnRH antagonist rescue (4752 versus 4287 pg/ml; P = 0.03), the two groups had similar oestradiol concentrations on the day after antagonist administration (3068 versus 2953 pg/ml). Despite having similar oestradiol concentrations on the day of HCG administration, patients who developed OHSS had higher peak oestradiol concentrations 1 day after HCG than patients who did not develop OHSS (6806 versus 5684 pg/ml; P < 0.001).
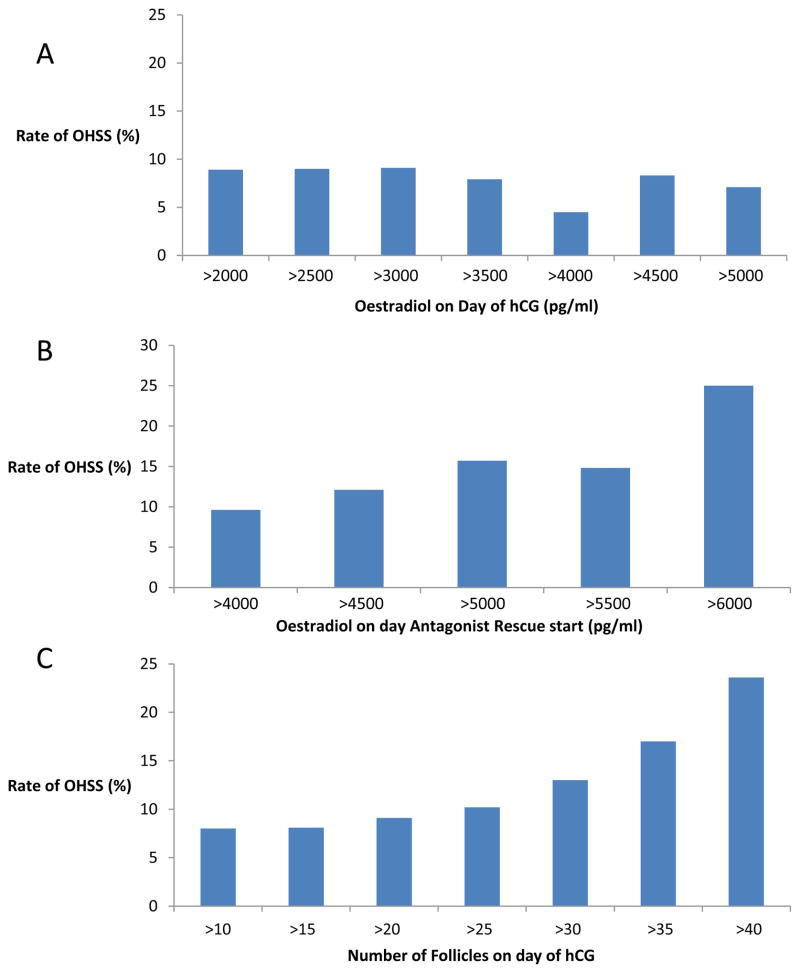
There was no significant difference in the rate of severe OHSS in GnRH antagonist rescue patients based upon threshold values of oestradiol on the day of HCG administration (Figure 3A). However, the rate of severe OHSS was found to increase in patients with higher oestradiol concentrations on the day that GnRH antagonist was initiated (Figure 3B). Rates of severe OHSS were 16% in patients with oestradiol concentrations 5000 pg/ml (5000–8390 pg/ml) on the day of antagonist start and 25% in patients with oestradiol concentrations 6000 pg/ml (6000–8390 pg/ml). While oestradiol concentrations on the day of HCG were not associated with severe OHSS because of antagonist suppression of oestradiol, the number of follicles ≥10 mm measured by transvaginal ultrasound were correlated to severe OHSS risk (Figure 3C). The percentage of patients with OHSS was 13%, 17% and 24% in patients with 10–30, 10–35 and 10–40 follicles, respectively. The percentage of patients with OHSS was not statistically different in patients receiving 1, 2 and 3 days of antagonist (8.0%, 8.6% and 0%, respectively).
Figure 3.
Threshold graphs showing the rates of severe ovarian hyperstimulation syndrome (OHSS) above various cut-off values of oestradiol and follicle number in the GnRH antagonist rescue group according to threshold values of: (A) oestradiol measured on day of human chorionic gonadotrophin (HCG) administration; (B) oestradiol measured on the day of gonadotrophin-releasing hormone antagonist start ; and (C) follicles ≥10 mm on transvaginal ultrasound measured on day of HCG.
Discussion
GnRH antagonist rescue decreased serum oestradiol concentrations and avoided cycle cancellation in patients at high risk for OHSS while achieving excellent outcomes. Despite the rapid reduction in serum oestradiol concentrations with antagonist rescue, there was no adverse impact on oocyte maturity, fertilization rate, day-3 embryo grading, blastocyst development or live birth.
While coasting has been shown to be effective at avoiding cycle cancellation in this same patient population, it has been associated with poorer cycle outcome. Coasting patients for more than 3 days results in fewer oocytes retrieved, lower implantation rates and lower pregnancy rates in fresh and oocyte donor cycles (Ulug et al., 2002, Mansour et al., 2005, Isaza et al., 2002). In contrast, the current study found no decrease in live-birth rate regardless of the number of days of antagonist rescue. Antagonist rescue resulted in a rapid reduction in oestradiol concentrations, allowing 1–3 days of continued follicle growth and maturation while avoiding cycle cancellation. While antagonist rescue produces a significant decrease in oestradiol concentrations 1 day after administration, oestradiol concentrations fall more slowly when coasting is implemented and so the interval between coasting and HCG administration is necessarily prolonged. The negative impact seen on implantation with coasting may be due to this prolonged period between intervention and HCG administration. However, when Aboulghar et al. (2007) compared 1–2 days of coasting versus 1–2 days of antagonist rescue, coasting still resulted in fewer oocytes and fewer high-quality embryos. Thus, antagonist rescue may be superior to coasting even in patients requiring short intervals of intervention. Mansour et al. (2005) showed that prolonged coasting resulted in fewer granulosa cells surrounding oocytes, potentially due to the detrimental effect of complete withdrawal of exogenous gonadotrophin stimulation and prolonged coasting was associated with a decrease in implantation and clinical pregnancy rates.
This study differs from that of Aboulghar et al. (2007) in several respects. The investigators utilized antagonist rescue in patients with peak oestradiol concentrations >3000 pg/ml (Aboulghar et al., 2007), whereas the mean oestradiol in this study was 4500 pg/ml at antagonist rescue initiation. Specifically, this study aimed to initiate antagonist rescue in patients for whom the projected oestradiol was ≥5000 pg/ml on day of HCG administration. Therefore, the antagonist rescue patient population represented a group at significantly greater risk of OHSS. Additionally, all embryos in Aboulghar et al. (2007) were transferred on day 2 or day 3, but in this study, 109 patients (28%) underwent blastocyst transfer, allowing the impact on embryo development to be evaluated up to day 6. All patients in Aboulghar et al. (2007) were on a long luteal protocol, whereas the majority of this study’s patients were on a MDF protocol. Despite these differences, both studies support antagonist rescue as an effective method for avoiding cycle cancellation in high OHSS risk patients without diminishing embryo quality or outcome.
These data demonstrate that GnRH antagonist rescue enabled cycle completion with high live-birth rates in patients at risk for OHSS. The reduction seen in oestradiol concentrations allowed continuation of ovarian stimulation for 1–3 additional days, allowing for follicular growth and oocyte maturation when OHSS was a clinical concern. The mean reduction in serum oestradiol was 35% on the first day of antagonist administration and is consistent with the 36% drop reported in prior studies (Aboulghar et al., 2007, Gustofson et al., 2006b). In patients receiving 2 or 3 days of antagonist, there was a similar drop on the initial day of antagonist administration and then oestradiol concentrations plateaued until HCG administration.
The incidence of OHSS in this antagonist rescue cohort may be explained by several factors. First, the highest OHSS risk patients received antagonist rescue. The overall incidence of severe OHSS in this programme during the study period was 1.2%, which is comparable to rates of 0.1–5% reported by other authors (Aboulghar et al., 2007, Csokmay et al., 2010, Maxwell et al., 2008, Delvigne and Rozenberg, 2002). Additionally, it is the centre’s clinical practice to perform outpatient paracentesis early and often in OHSS patients to reduce the pathophysiological effects of abdominal compartment syndrome (Grossman et al., 2010, Csokmay et al., 2010) and this aggressive approach may have led to an overestimation of the rate of severe OHSS as compared with other programmes. The present study was not designed to assess whether GnRH antagonist rescue decreases the likelihood of severe OHSS. The prevalence of OHSS in the study group is interpreted as reflecting the fact that they are high-risk patients. The cycle cancellation rate in this group of patients at high risk of cycle cancellation was 1.5% and is consistent with the literature (Aboulghar et al., 2007, Gustofson et al., 2006b). These data support the conclusion that GnRH antagonist rescue is effective at avoiding cycle cancellation in patients who are at high risk of OHSS. GnRH antagonist rescue has been shown to be superior to coasting by producing more oocytes and more high-quality embryos and decreasing cycle cancellation (Aboulghar et al., 2007).
These data also identified factors in the subgroup of patients undergoing GnRH antagonist rescue that developed OHSS. There was a strong relationship between increasing follicle number and the incidence of OHSS, as has been reported by others (Whelan and Vlahos, 2000, Amer Soc Reproductive, 2008, Enskog et al., 1999). On this basis, the study centre now weights follicle number more heavily in the clinical management of these patients. The incidence of OHSS was similar at all serum oestradiol thresholds on the day of HCG. The suppressed concentration of oestradiol in response to antagonist rescue did not appear to be a good marker for OHSS risk. However, higher oestradiol concentrations prior to the initiation of antagonist were associated with OHSS risk. These data may help identify patients most at risk for severe OHSS prior to utilizing GnRH antagonist rescue and may help identify a subgroup of patients whose elevated risk may warrant cycle cancellation rather than GnRH antagonist rescue. For example, cancelling all patients with an oestradiol concentration >6000 pg/ml rather than utilizing GnRH antagonist rescue would have resulted in 28 cycle cancellations and prevented seven cases of severe OHSS at the expense of the 10 live births that occurred in those patients.
This study is limited by its retrospective design and the selection of the comparison group. It would be unethical and unsafe to randomize patients with such rapidly rising oestradiol concentrations to antagonist rescue versus no treatment. The comparison group was chosen from patients with the highest oestradiol concentrations amongst those in whom GnRH antagonist rescue was not performed. This comparison group was chosen to provide a similar cohort of high-responder patients for comparison of blastocyst development and treatment outcomes. The study centre does not have an adequate control group with which to compare OHSS risk to the antagonist rescue cohort. A potential weakness of the study is that the use of MDF in young, good-responding patients may not be applicable to other programmes. The fact that patients receiving a MDF or LA protocol had similar response to antagonist rescue suggests that these findings would still be relevant to programmes who routinely utilize a LA protocol in this patient population.
These data and clinical experience demonstrate that GnRH antagonist rescue can result in excellent embryo development to the blastocyst stage. The GnRH antagonist rescue protocol enabled patients at high risk of developing OHSS to avoid cycle cancellation and successfully complete their assisted cycle, with excellent oocyte quality, embryo development and live birth.
Supplementary Material
Acknowledgments
This research was supported, in part, by intramural research programme of the Program in Reproductive and Adult Endocrinology (NICHD, NIH).
Biography

Dr Hill is a clinical fellow in reproductive endocrinology and infertility in the Program in Reproductive and Adult Endocrinology at the National Institutes of Health in Bethesda, Maryland. He received his medical degree from the Oklahoma State College of Osteopathic Medicine and completed his residency training in obstetrics and gynaecology at the Tripler Army Medical Center in Honolulu, Hawaii. Dr. Hill has published over 25 journal articles and book chapters. He is a Major in the US Army and an assistant professor at the Uniformed Services University of the Health Sciences.
Footnotes
The views expressed in this manuscript are those of the authors and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the US Government.
Declaration: The authors report no financial or commercial conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ABOULGHAR MA, MANSOUR RT, AMIN YM, AL-INANY HG, ABOULGHAR MM, SEROUR GI. A prospective randomized study comparing coasting with GnRH antagonist administration in patients at risk for severe OHSS. Reproductive Biomedicine Online. 2007;15:271–279. doi: 10.1016/s1472-6483(10)60339-2. [DOI] [PubMed] [Google Scholar]
- AMER SOC REPRODUCTIVE M. Ovarian hyperstimulation syndrome. Fertility and Sterility. 2008;90:S188–S193. doi: 10.1016/j.fertnstert.2008.08.034. [DOI] [PubMed] [Google Scholar]
- CSOKMAY JM, YAUGER BJ, HENNE MB, ARMSTRONG AY, QUEENAN JT, SEGARS JH. Cost analysis model of outpatient management of ovarian hyperstimulation syndrome with paracentesis: ‘Tap early and often’ versus hospitalization. Fertility and Sterility. 2010;93:167–173. doi: 10.1016/j.fertnstert.2008.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELVIGNE A, DEMOULIN A, SMITZ J, DONNEZ J, KONINCKX P, DHONT M, ENGLERT Y, DELBEKE L, DARCIS L, GORDTS S, PUTTEMANS P, GERRIS J, SCHOYSMAN R, LEROY F. THE OVARIAN HYPERSTIMULATION SYNDROME IN IN-VITRO FERTILIZATION–A BELGIAN MULTICENTRIC STUDY.1. CLINICAL and BIOLOGICAL FEATURES. Human Reproduction. 1993;8:1353–1360. doi: 10.1093/oxfordjournals.humrep.a138260. [DOI] [PubMed] [Google Scholar]
- DELVIGNE A, ROZENBERG S. Epidemiology and prevention of ovarian hyperstimulation syndrome (OHSS): a review. Human Reproduction Update. 2002;8:559–577. doi: 10.1093/humupd/8.6.559. [DOI] [PubMed] [Google Scholar]
- ENSKOG A, HENRIKSSON M, UNANDER M, NILSSON L, BRANNSTROM M. Prospective study of the clinical and laboratory parameters of patients in whom ovarian hyperstimulation syndrome developed during controlled ovarian hyperstimulation for in vitro fertilization. Fertility and Sterility. 1999;71:808–814. doi: 10.1016/s0015-0282(99)00090-4. [DOI] [PubMed] [Google Scholar]
- GOLAN A, RON-EL R, HERMAN A, SOFFER Y, WEINRAUB Z, CASPI E. Ovarian hyperstimulation syndrome: an update review. Obstetrical and gynecological survey. 1989;44:430–40. doi: 10.1097/00006254-198906000-00004. [DOI] [PubMed] [Google Scholar]
- GROSSMAN LC, MICHALAKIS KG, BROWNE H, PAYSON MD, SEGARS JH. The pathophysiology of ovarian hyperstimulation syndrome: an unrecognized compartment syndrome. Fertility and Sterility. 2010;94:1392–1398. doi: 10.1016/j.fertnstert.2009.07.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUSTOFSON RL, LARSEN FW, BUSH MR, SEGARS JH. Treatment with gonadotropin-releasing hormone (GnRH) antagonists in women suppressed with GnRH agonist may avoid cycle cancellation in patients at risk for ovarian hyperstimulation syndrome. Fertility and Sterility. 2006a;85:251–254. doi: 10.1016/j.fertnstert.2005.07.1291. [DOI] [PubMed] [Google Scholar]
- GUSTOFSON RL, SEGARS JH, LARSEN FW. Ganirelix acetate causes a rapid reduction in estradiol levels without adversely affecting oocyte maturation in women pretreated with leuprolide acetate who are at risk of ovarian hyperstimulation syndrome. Human Reproduction. 2006b;21:2830–2837. doi: 10.1093/humrep/del059. [DOI] [PubMed] [Google Scholar]
- ISAZA V, GARCIA-VELASCO JA, ARAGONES M, REMOHI J, SIMON C, PELLICER A. Oocyte and embryo quality after coasting: the experience from oocyte donation. Human Reproduction. 2002;17:1777–1782. doi: 10.1093/humrep/17.7.1777. [DOI] [PubMed] [Google Scholar]
- LEVENS ED, WHITCOMB BW, KORT JD, MATERIA-HOOVER D, LARSEN FW. Microdose follicular flare: a viable alternative for normal-responding patients undergoing in vitro fertilization? Fertility and Sterility. 2009;91:110–114. doi: 10.1016/j.fertnstert.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANSOUR R, ABOULGHAR M, SEROUR G, AMIN Y, ABOU-SETTA AM. Criteria of a successful coasting protocol for the prevention of severe ovarian hyperstimulation syndrome. Human Reproduction. 2005;20:3167–3172. doi: 10.1093/humrep/dei180. [DOI] [PubMed] [Google Scholar]
- MAXWELL KN, CHOLST IN, ROSENWAKS Z. The incidence of both serious and minor complications in young women undergoing oocyte donation. Fertility and Sterility. 2008;90:2165–2171. doi: 10.1016/j.fertnstert.2007.10.065. [DOI] [PubMed] [Google Scholar]
- PRACTICE COMMITTEE OF AMERICAN SOCIETY FOR REPRODUCTIVE M. Ovarian hyperstimulation syndrome. Fertility and sterility. 2008;90:S188–93. doi: 10.1016/j.fertnstert.2008.08.034. [DOI] [PubMed] [Google Scholar]
- ULUG U, BAHCECI M, ERDEN HF, SHALEV E, BEN-SHLOMO I. The significance of coasting duration during ovarian stimulation for conception in assisted fertilization cycles. Human Reproduction. 2002;17:310–313. doi: 10.1093/humrep/17.2.310. [DOI] [PubMed] [Google Scholar]
- VEECK LL. Atlas of the Human Oocyte and Early Conceptus. 1. Baltimore, MD: Williams and Wilkins; 1986. [Google Scholar]
- WHELAN JG, VLAHOS NF. The ovarian hyperstimulation syndrome. Fertility and Sterility. 2000;73:883–896. doi: 10.1016/s0015-0282(00)00491-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.