Abstract
Exaggerated levels of VEGF (vascular endothelial growth factor) are present in persons with asthma, but the role(s) of VEGF in normal and asthmatic lungs has not been defined. We generated lung-targeted VEGF165 transgenic mice and evaluated the role of VEGF in T-helper type 2 cell (TH2)-mediated inflammation. In these mice, VEGF induced, through IL-13–dependent and –independent pathways, an asthma-like phenotype with inflammation, parenchymal and vascular remodeling, edema, mucus metaplasia, myocyte hyperplasia and airway hyper-responsiveness. VEGF also enhanced respiratory antigen sensitization and TH2 inflammation and increased the number of activated DC2 dendritic cells. In antigen-induced inflammation, VEGF was produced by epithelial cells and preferentially by TH2 versus TH1 cells. In this setting, it had a critical role in TH2 inflammation, cytokine production and physiologic dysregulation. Thus, VEGF is a mediator of vascular and extravascular remodeling and inflammation that enhances antigen sensitization and is crucial in adaptive TH2 inflammation. VEGF regulation may be therapeutic in asthma and other TH2 disorders.
Exaggerated TH2 inflammation and airway remodeling are cornerstones in the pathogenesis of asthma1–3. In keeping with the importance of neovascularization in inflammation and remodeling, a number of investigators have characterized vascular responses in asthmatic tissues. These studies demonstrated prominent increases in vessel number, vessel size, vascular surface area and vascular leakage, and important correlations between these alterations and disease severity4–12. As a result, it has been assumed that asthmatic inflammation stimulates the growth of new blood vessels9,11,12 and that these vascular alterations contribute to the airway obstruction or airway hyper-responsiveness (AHR), or both, in this disorder12–14. Yet the mechanisms that generate these vascular alterations have not been defined. In addition, the possibility that the processes inducing these alterations also contribute to the pathogenesis of the inflammatory and immune alterations that are central to asthma has not been investigated.
Vascular endothelial growth factor (VEGF) was originally described as vascular permeability factor (VPF) based on its ability to generate tissue edema15. It has subsequently been understood to be a multifunctional angiogenic regulator that stimulates epithelial cell proliferation, blood vessel formation and endothelial cell survival16,17. Exaggerated levels of VEGF have been detected in tissues and biologic samples from people with asthma10,11,18,19, where these levels correlate directly with disease activity7 and inversely with airway caliber and airway responsiveness10,11,18,19. VEGF has been postulated to contribute to asthmatic tissue edema through its effect on vascular permeability12–14. However, the role of VEGF in the pathogenesis of other aspects of the asthmatic phenotype and the effector functions of VEGF in the lung have not been defined. Even the contribution of VEGF to asthmatic vascular alterations is not clear because VEGF has been reported to lack angiogenic properties in the respiratory tract20,21.
The lung is unique amongst mucosal compartments in that it is constantly exposed to airborne particulates. In normal humans, the immune response differentiates between harmless agents that should not induce sensitization and potentially injurious pathogens against which an immune response is warranted. In contrast, the lungs of atopic asthmatics show an enhanced ability to sensitize and mount pathologic TH2 responses after exposure to largely innocuous allergens22–24. Infection-elicited innate immune responses are known to have an essential role in the development of adaptive TH2 immunity22,23. This is nicely illustrated by respiratory syncytial virus (RSV), which enhances aeroallergen sensitization and contributes to the development of asthma22,25. It has been proposed that antiviral innate immune responses contribute to the generation or maintenance, or both, of adaptive TH2 immunity by increasing mucosal permeability and altering local dendritic cells (DCs)22. The validity of these assumptions has not been tested and the mediators of these effects have not been defined. In addition, although earlier work from our laboratories demonstrated that RSV stimulates epithelial VEGF elaboration in vitro and in humans in vivo26, the ability of VEGF to facilitate immune sensitization has not been evaluated.
We hypothesized that VEGF is a critical mediator in asthma and TH2 inflammation. To test this hypothesis, we generated and characterized transgenic mice in which VEGF165, the 165-amino-acid isoform of VEGF, was overexpressed in the airway and evaluated the role of VEGF in antigen-induced TH2 inflammation. These studies demonstrate that VEGF is a potent stimulator of angiogenesis, edema, inflammation, vascular remodeling, parenchymal remodeling and physiologic dysregulation. They also link innate and adaptive TH2 immunity by demonstrating that VEGF enhances antigen sensitization and demonstrate that epithelial and TH2 cell–derived VEGF have central roles in TH2 inflammation.
RESULTS
Generation of inducible transgenic mice
VEGF overexpression during lung development causes fetal death27. To avoid this, we used a dual-construct transgenic system, developed in our laboratory28, that can be regulated externally (Fig. 1a). We identified four dual-transgenic CC10-rtTA-VEGF founder mice. At 1 month of age, they were randomized to receive normal water or doxycycline (dox)-containing water. In the wild-type mice given normal or dox water and the transgenic mice given normal water, VEGF levels in bronchoalveolar lavage fluids (BAL) were ≤15 pg/ml. Increased levels of BAL VEGF were noted within 24 h and steady-state levels between 0.05 and 12 ng/ml were seen after 1 week of dox administration (data not shown). These levels of VEGF are comparable with the levels in biologic fluids from normal humans and those with asthma or RSV infection18,19,26,29–31. In all cases, RT-PCR of multiple organs and tissues showed that VEGF was produced in a lung-specific fashion (data not shown). Qualitatively similar, dose-dependent alterations were seen in all mice with levels of BAL VEGF ≥0.1 ng/ml as summarized below.
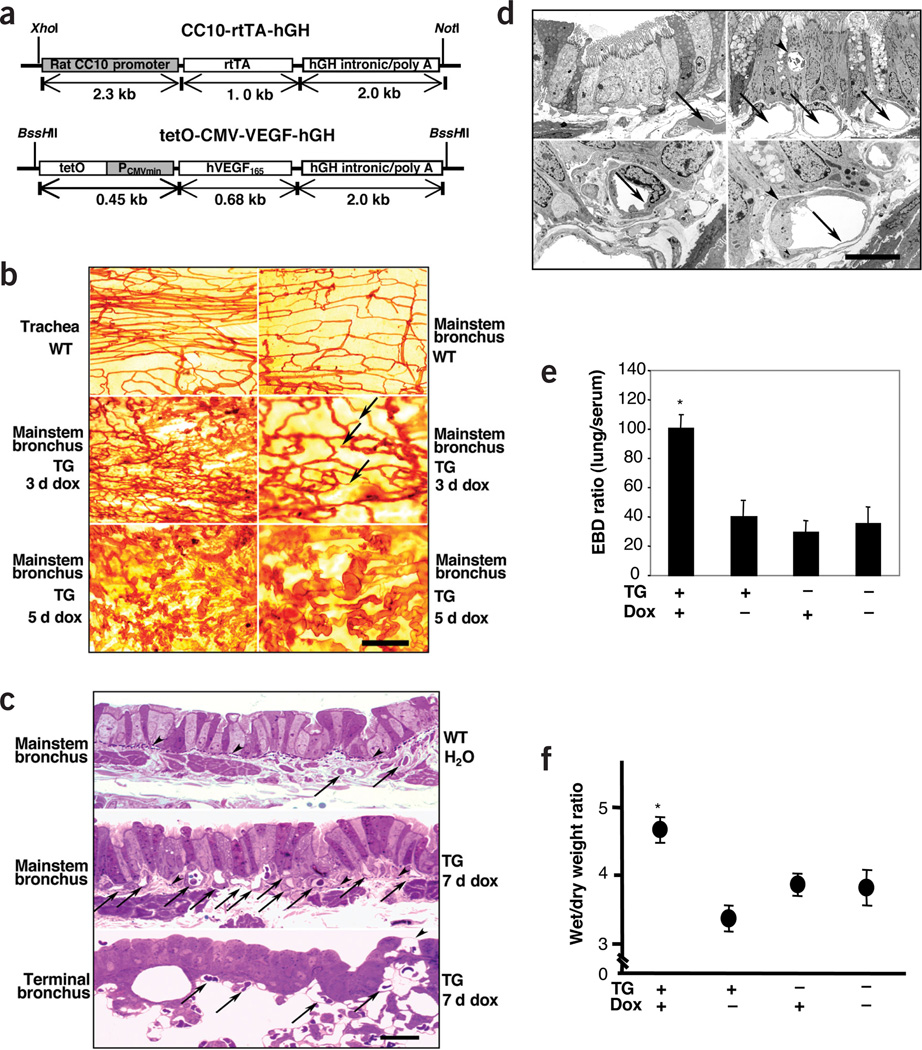
Figure 1.
Consequences of transgenic VEGF expression. (a) Constructs in CC10-rtTA-VEGF mice. (b) Lycopersicon esculentum staining (arrows, endothelial sprouts; scale bar, 100 µm in upper, middle and lower left subpanels, and 50 µm in middle and lower right subpanels). (c) Toluidine blue–stained bronchi from wild-type (WT) and transgenic (TG) mice. (Arrows, blood vessels; arrowheads, subepithelial elastic lamina; scale bar, 20 µm.) (d) Electron micrographs of tissue from WT mouse on normal water (upper and lower left) and TG mouse given dox for 7 d (upper and lower right) (In upper panels, arrows, blood vessels; arrowhead, epithelial vacuole). Endothelial cell in lower left has a thick wall, whereas that in lower right has a thin wall and pericyte processes (arrowheads). Scale bar, 25 µm in upper and 10 µm in lower subpanels. (e,f) Evans Blue dye (EBD) and wet/dry ratios compare the permeability in WT and TG mice given normal or dox water for 7 d (*P < 0.01 versus the other three groups.)
Effect on neovascularization and edema
Lycopersicon esculentum lectin analysis demonstrated that blood vessels in the tracheas and intrapulmonary bronchi of wild-type mice given normal or dox water and transgenic mice given normal water were arranged in cascades with capillaries crossing between arterioles and venules (Fig. 1b,c). This bronchial circulation became less dense as it extended from the extrapulmonary to the intrapulmonary bronchi (Fig. 1b). In the transgenic mice, as little as 3 d of dox generated endothelial sprouts, mostly arising from the venules (Fig. 1b). Vascular density (the percent of the airway covered with vessels) was maximal after 7 d of dox (42.3 ± 5.4 versus 23.5 ± 1.3%; P < 0.01) (Fig. 1c) and remained elevated for at least a month thereafter. The new vessels were larger than the capillaries of the control airways (11.86 ± 0.37 versus 8.90 ± 0.14 µm, P < 0.001) and, as seen in asthma4, had migrated into the lamina reticularis and occasionally into the epithelium (Fig. 1c,d). The endothelial cells of these vessels were thin, had occasional fenestrations, and were enveloped by pericyte processes and basement membranes. In dox-treated transgenic mice, there was a significant increase in lung wet/dry ratios, Evans blue dye extravasation and edema compared to the wild-type and transgenic controls (Fig. 1e,f and data not shown) (P < 0.01 comparing dox-treated transgenic mice to all other groups). Thus, VEGF is a potent inducer of angiogenesis and edema in the mouse airway and lung.
Histologic and physiologic evaluation
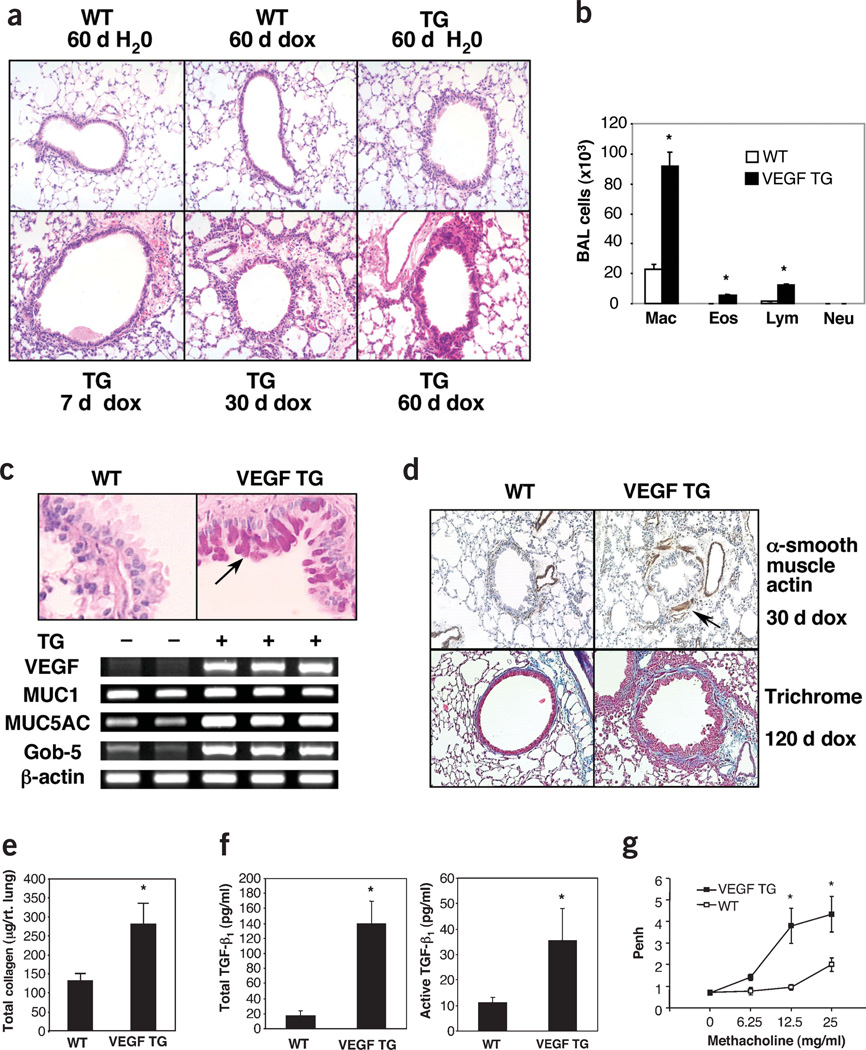
Lungs from wild-type mice given normal or dox water and transgenic mice given normal water could not be distinguished from lungs from control mice given normal water (Fig. 2a and data not shown). In contrast, VEGF overexpression caused conspicuous alterations that persisted throughout the 5-month study interval. Inflammation was seen after 2 d of dox. At early time points, increases in tissue mononuclear cells, B lymphocytes and occasional clusters of eosinophils were noted (Fig. 2a and data not shown). At later time points, CD4+ and CD8+ T cells were also more abundant (1.82% ± 0.3 to 9.92 ± 0.9% for CD4+ cells, 1.14 ± 0.4 to 8.64 ± 0.7% for CD8+ cells, P < 0.01 comparing wild-type to transgenic for both). Similarly, total cell recovery and the recovery of macrophages, lymphocytes and eosinophils were increased in BAL from dox-treated transgenic mice (Fig. 2b). Mucus metaplasia, characterized by D-PAS and Alcian blue staining of airway epithelial cells, increased Muc5ac and epithelial gob-5 (Clca3) gene expression (Fig. 2c and Supplementary Fig. 1 online) and myocyte hyperplasia with enlarged airway smooth muscle bundles (Fig. 2d) were seen after 7 d of dox. Histologic and biochemical increases in collagen were first seen after 2 months and were most prominent after 4 months of VEGF overexpression (Fig. 2d,e). At these time points, VEGF also stimulated the production and spontaneous activation of TGF-β1 (Fig. 2f). Thus, VEGF is a potent stimulator of airway inflammation and airway remodeling with mucus metaplasia, subepithelial fibrosis and smooth muscle hyperplasia.
Figure 2.
Structural alterations in transgenic (TG) mice. (a) H&E staining (×10 original magnification) for wild-type (WT) and TG mice given dox for the noted intervals. (b) BAL from WT and TG mice given dox for 1 month (*P < 0.05 versus same cells in WT). (c) Levels of mucus (top) and mucin and gob-5 mRNA (bottom) were evaluated with D-PAS staining and RT-PCR evaluations (arrow, epithelial staining) (×20). (d) α-smooth muscle actin immunohistochemistry and tissue fibrosis (Mallory’s trichrome staining) are compared in lungs from WT and TG mice (×10). (e) Sircol evaluations were used to compare the collagen content of lungs from WT and TG mice given dox for 4 months (*P < 0.01). (f) ELISA evaluations of acid-activated (left) and untreated (right) BAL fluids from WT and TG mice given dox for 4 months (*P < 0.01). (g) Methacholine responsiveness of WT and TG mice given dox for 7 d is compared (*P < 0.01).
Responsiveness to methacholine was also assessed. When compared to wild-type mice given normal or dox water and transgenic mice given normal water, transgenic mice given dox water for as few as 7 d show impressive AHR (Fig. 2g and data not shown). Thus, VEGF induces AHR in the mouse lung.
Induction and role of IL-13
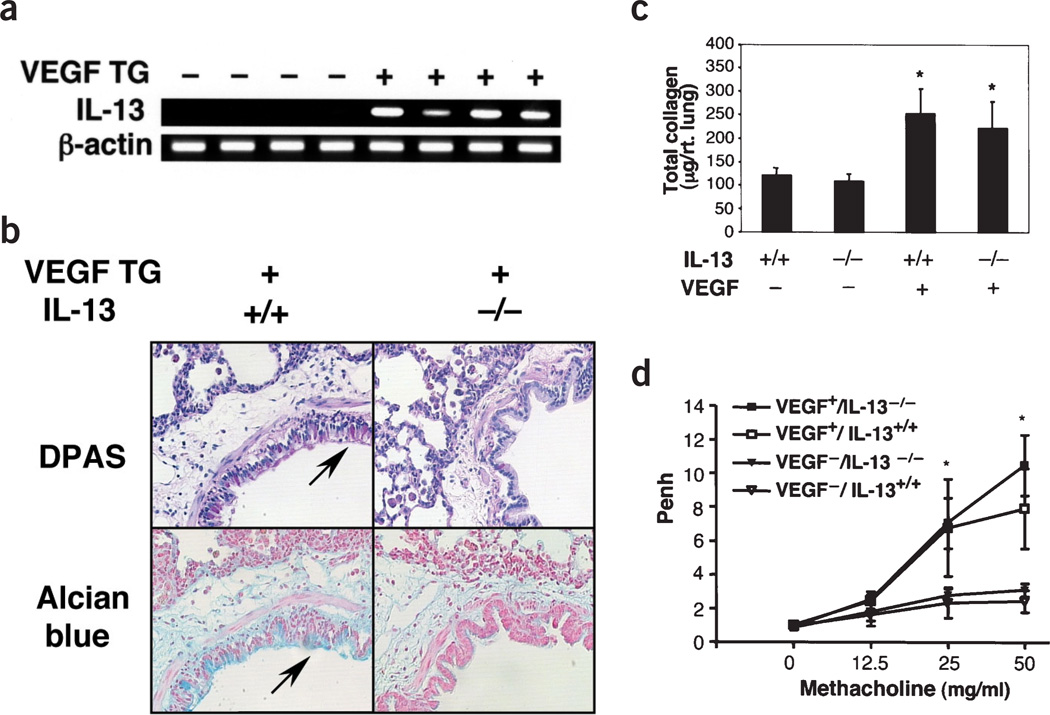
We also undertook studies to determine whether VEGF mediated its effects by stimulating TH2 cytokines. The levels of mRNA encoding IL-4, IL-5 and IL-9 in wild-type and transgenic mice given normal water or dox water were near or below the limits of detection of our assays (data not shown). Similarly, mRNA encoding IL-13 could not be detected in RNA from wild-type mice given normal or dox water or transgenic mice given normal water (Fig. 3a and data not shown). In contrast, the lungs of transgenic mice given dox showed increased expression of IL-13 mRNA (Fig. 3a).
Figure 3.
Role of IL-13 in the VEGF phenotype. (a) RT-PCR was used to evaluate the levels of IL-13 mRNA in lung RNA from wild-type (WT) and transgenic (TG) mice given dox for 1 month. (b–d) Ability of VEGF to induce mucus metaplasia (×20), pulmonary fibrosis and AHR, respectively, are compared in mice with wild-type (Il13+/+) and null mutant (Il13−/−) loci. The arrows in b highlight positive-staining cells. In c, *P < 0.01 versus VEGF− mice. In d, *P < 0.05 versus VEGF− controls.
To define the role of IL-13 in the VEGF phenotype, we compared the effects of VEGF in Il13+/+ and Il13–/– mice. In the absence of IL-13, the ability of VEGF to induce mucus metaplasia was completely abrogated (Fig. 3b). In contrast, VEGF induction of tissue and BAL inflammation, neovascularization, AHR, myocyte hyperplasia, subepithelial fibrosis, TGF-β1 elaboration and dendritic cell alterations (see below) were unaltered in Il13−/− mice (Fig. 3c,d and data not shown). These studies document the IL-13 dependence of VEGF-induced mucus metaplasia. Thus, the preponderance of the VEGF phenotype is mediated through an IL-13–independent mechanism.
Effect on sensitization, TH2 inflammation and DCs
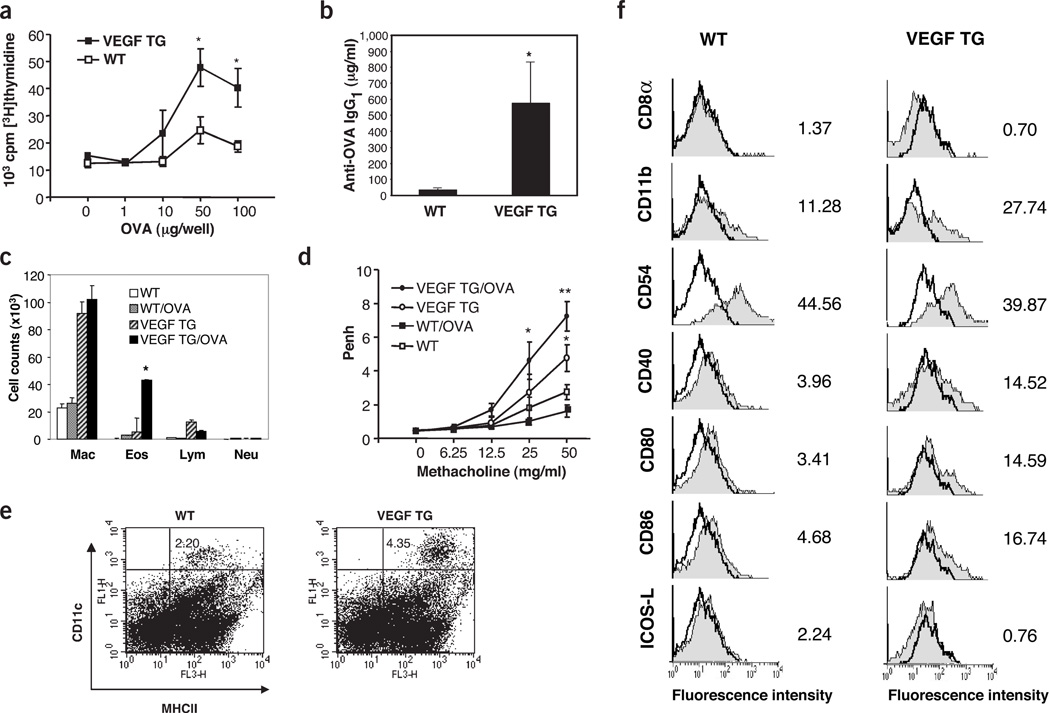
To determine whether VEGF altered respiratory antigen sensitization, wild-type and transgenic mice were exposed to ovalbumin (OVA) by aerosol and systemic sensitization was assessed by quantifying OVA-induced splenocyte proliferation and OVA-specific IgG1. OVA did not cause significant sensitization in wild-type mice given normal or dox water or in transgenic mice given normal water. It did, however, engender marked sensitivity in dox-treated transgenic mice (Fig. 4a,b). In agreement with this observation, transgenic mice showed increased BAL and tissue eosinophilic inflammation (Fig. 4c) and enhanced AHR (Fig. 4d) after aerosol antigen sensitization and challenge. Similar results were obtained when recombinant VEGF was administered with intranasal OVA to wild-type mice (data not shown). In both experimental systems, alterations in OVA-specific IgG2a and keyhole limpet hemocyanin (KLH)–induced splenocyte proliferation could not be observed (data not shown). In addition, enhanced respiratory antigen sensitization was not seen when transgenic CC10-IL-11 mice that overexpress IL-11 were similarly challenged (data not shown). Thus, VEGF selectively increases TH2 antigen sensitization and inflammation in the lung.
Figure 4.
Immune effects of VEGF. Effect of intranasal OVA in wild-type (WT) and transgenic (TG) mice treated with dox for 2 weeks. (a,b) OVA-induced spleen cell proliferation (a) and OVA-specific IgG1 (b) were assessed (*P < 0.01). (c,d) Dox-treated WT and TG mice were challenged with intranasal OVA, re-challenged after 7 d and, 48 h later, BAL cellularity (c) and AHR (d) were evaluated. (In c, *P < 0.001 versus eosinophils in other groups; in d, *P < 0.01 versus WT and **P < 0.05 versus TG mouse challenged with vehicle.) (e,f) FACS analysis of lung cells isolated from WT mice and TG mice given dox for 7 d and stained with antibodies directed against CD11c, MHC II and with other antibodies as indicated. In f: Cells were sorted for CD11c. CD11c+ cells were incubated with test antibodies (gray) and control antibodies (transparent).
The number and state of activation of parenchymal DCs were similar in wild-type mice given normal or dox water and transgenic mice given normal water. In contrast, in transgenic mice, as little as 7 d of dox increased the number of CD11chigh major histocompatibility (MHC) IIhigh DCs in pulmonary tissues (Fig. 4e). These cells had increased CD11b and decreased CD8α expression (Fig. 4f). These DC2 cells had the characteristics of activated DCs because they expressed increased levels of MHC class II, CD80/B7.1, CD86/B7.2 and CD40 and decreased levels of ICOSL/B.7h (Fig. 4f). Thus, VEGF increases the number and state of activation of DC2 cells in pulmonary tissues.
Role(s) of Vegf in TH2 inflammation
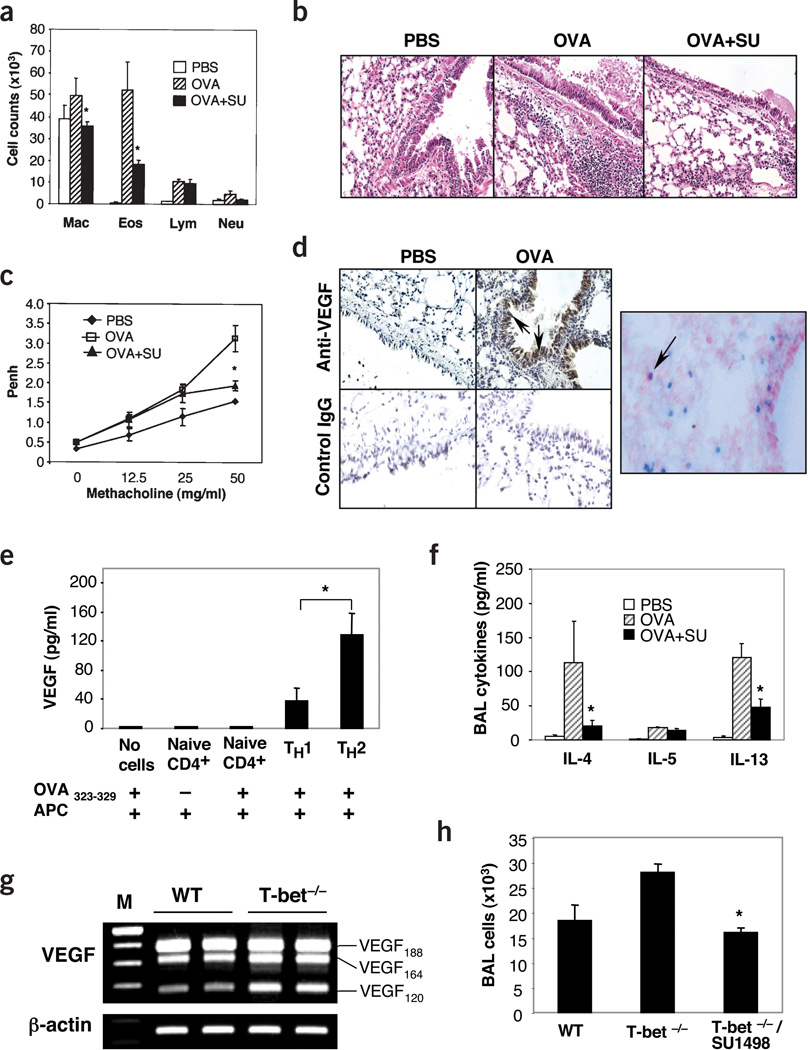
Next we undertook studies to evaluate the role(s) of Vegf in antigen-induced TH2 inflammation. Eosinophil-, lymphocyte- and macrophage-rich inflammation and AHR were seen in wild-type mice sensitized and challenged with OVA (Fig. 5a–c and data not shown). This response was associated with increased Vegf levels in BAL (5.9 ± 3.5 (range 5–15) pg/ml for controls versus 24.5 ± 2.2 (range 20–35) pg/ml for sensitized and challenged mice) (P < 0.01). This Vegf could be detected in CD3+ T cells and in a variety of airway epithelial cells including CC10-positive Clara cells (Fig. 5d and Supplementary Fig. 2 online). Studies using in vitro–polarized mouse T cells also showed that TH2 cells are potent producers of Vegf when compared to TH1 cells or naive CD4+ cells when stimulated with antigen in the presence of antigen-presenting cells (APCs) or with PMA and ionomycin in the absence of APC (Fig. 5e and Supplementary Fig. 3 online). Notably, the VEGF inhibitor SU1498 markedly decreased BAL and tissue inflammation and AHR (Fig. 5a–c). Similar results were seen with the VEGFR1R2 TRAP (Supplementary Fig. 4 online). SU1498 and the TRAP also decreased antigen-stimulated IL-13 and IL-4 production (Fig. 5f and data not shown). This response was not OVA-specific, because Vegf induction was readily observed and similar decreases in inflammation and AHR were seen in mice with a null mutation of the T-bet transcription factor that spontaneously develop an asthma-like phenotype32 treated in a similar fashion (Fig. 5g,h and data not shown). Thus, Vegf is produced by epithelial cells and TH2 cells and plays a critical role in TH2 inflammation, cytokine elaboration and AHR.
Figure 5.
VEGF in TH2 inflammation. (a–c) Comparison of BAL cellularity (a), histology (×20; b) and AHR (c) of mice that were sensitized with OVA and then challenged in the presence or absence of SU1498 (SU) (*P < 0.01 versus the absence of inhibitor). (d) Staining with anti-VEGF and control antibodies in sensitized mice challenged with OVA or PBS (×20) (left). Right, staining with anti-VEGF (red) and anti-CD3 (blue) (×40). The arrow highlights a VEGF-positive T cell. (e) VEGF was quantified by ELISA in supernatants from naive CD4+ cells or polarized TH1 or TH2 cells cultured in the presence of APC and in the presence or absence of antigen (OVA323−329 peptide). (f) Levels of TH2 cytokines in BAL from OVA-sensitized mice challenged with OVA in the presence or absence of SU1498. (*P < 0.01 for presence versus absence of SU1498.) (g) Level of the mRNA encoding VEGF in lungs from wild-type (WT) and T-bet null (T-bet−/−) mice. (h) BAL cellularity of WT and T-bet−/− mice treated with SU1498 or vehicle control (*P < 0.01).
Reversibility of the VEGF phenotype
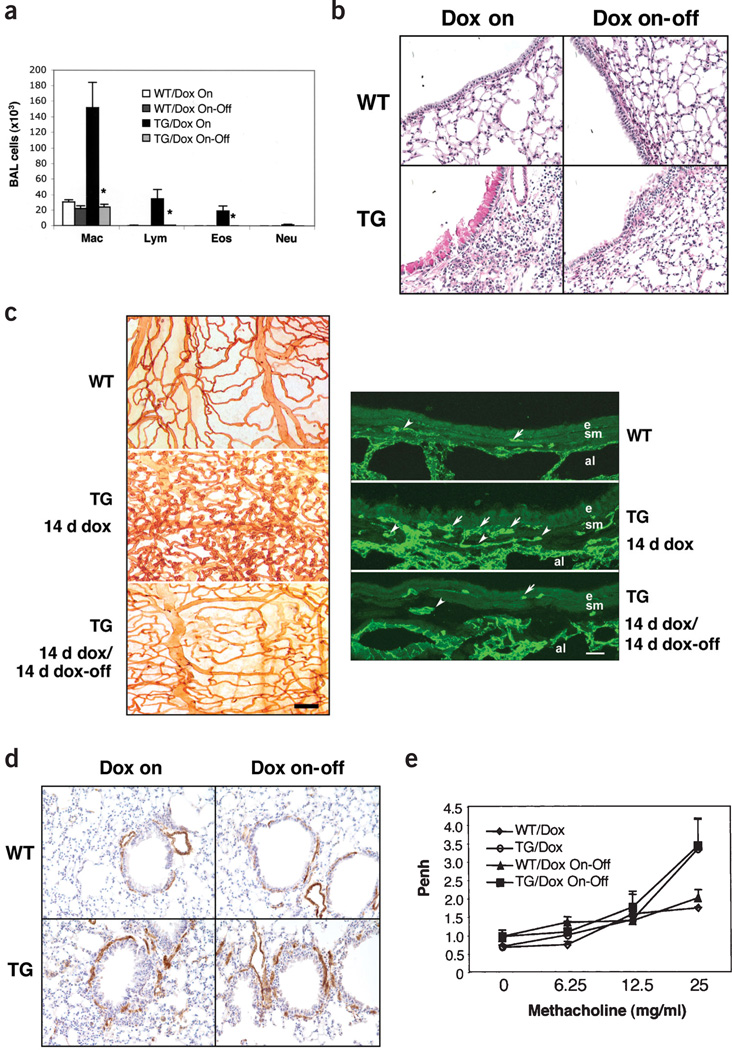
To define the reversibility of VEGF-induced alterations, wild-type and transgenic mice were treated with dox for 2 weeks. They were then switched to normal water and characterized at intervals thereafter. Transgenic VEGF was a potent stimulator of inflammation, vascular remodeling, mucus metaplasia, mucin gene expression, DC alterations, smooth muscle hyperplasia and AHR. Two weeks after cessation of transgenic VEGF elaboration, BAL and tissue inflammation (Fig. 6a and data not shown), DC numbers, mucus metaplasia, mucin gene expression (Fig. 6b and data not shown) and angiogenesis (Fig. 6c) had returned to basal levels. In contrast, 1 month after the cessation of transgene expression, smooth muscle hyperplasia and AHR were still readily apparent in lungs from transgenic mice (Fig. 6d,e). Thus, many VEGF-induced alterations are readily reversible after the cessation of Vegf elaboration; however, VEGF-induced smooth muscle and physiologic abnormalities have a lesser propensity toward normalization.
Figure 6.
Reversibility of VEGF effects. (a–e) Wild-type (WT) and transgenic (TG) mice received dox water for 2 weeks. They were then killed or placed on normal water and evaluated 2 (a–c) or 4 weeks (d,e) later. (a) Effects of transgene deactivation on BAL cell recovery (*P < 0.01 versus TG mice given dox for 2 weeks); (b) mucus metaplasia (×20); (c) vascular remodeling, comparing L. esculentum evaluations (left) and CD 31 IHC (right); (d) smooth muscle hyperplasia (×10); and (e) AHR are illustrated. Scale bar in c, 100 µm on the left and 20 µm on the right. Arrows, CD31-positive endothelial cells. e, epithelium; sm, smooth muscle cells; a, alveolar space.
DISCUSSION
Leonardo da Vinci is generally credited with the discovery that new blood vessels form at sites of pulmonary pathology12. It has since become clear that he was viewing bronchial vascular remodeling and angiogenesis at sites of infection, inflammation and neoplasia12. An increase in vessel size, number and surface area and the exaggerated expression of VEGF and VEGF receptors is well documented in the asthmatic airway4–8,10,11,18,19. Notably, the mechanisms of asthmatic vascular remodeling and the vascular and nonvascular contributions of VEGF to asthma pathogenesis have not been defined. To address this issue, we generated and characterized lung-targeted VEGF165 transgenic mice and evaluated the role of VEGF in pulmonary TH2 inflammation. These studies show that levels of VEGF that are comparable to those in human tissues and biologic fluids18,19,29–31 induce an asthma-like phenotype characterized by inflammation, edema, angiogenesis, vascular remodeling, mucus metaplasia, subepithelial fibrosis, smooth muscle hyperplasia and AHR. They also demonstrate that VEGF enhances respiratory antigen sensitization, augments antigen-induced TH2 inflammation, increases the accumulation and activation of pulmonary DCs and has a key role in antigen-induced TH2 inflammation and cytokine elaboration. Prior studies from our laboratories and others demonstrated that VEGF is produced during innate immune responses induced by RSV and endotoxin26,33. Taken together, these studies demonstrate that VEGF has a critical role in pulmonary TH2 inflammation, and provide important insights into a number of aspects of asthma pathogenesis.
First, these studies demonstrate that the VEGF produced during innate immune responses can generate asthma-like inflammation, airway and vascular remodeling and physiologic dysregulation. They also demonstrate that, in contrast to some earlier reports20,21, VEGF is a potent mediator of vascular remodeling and angiogenesis in the lung. It is presently believed that asthmatic airway remodeling is caused by chronic TH2 inflammation. The present studies demonstrate that remodeling can also be caused by innate immune responses and highlight the importance of VEGF in the genesis of these alterations. This provides a potential explanation for the observation that airway remodeling can be seen in childhood asthma well before the ravages of chronic TH2 inflammation would be expected34.
Second, these studies demonstrate that VEGF augments respiratory TH2 sensitization while simultaneously increasing tissue permeability and the number and activation of pulmonary DCs. These findings suggest that RSV and endotoxin may enhance antigen sensitization and TH2 inflammation by inducing VEGF and that this induction may explain how RSV infection early in life contributes to the development of asthma22,25. The exaggerated levels of VEGF in asthma may also contribute to the proclivity of asthmatics to become sensitized to respiratory antigens. The ability of cockroach antigen to directly stimulate epithelial VEGF elaboration14 may also account for the impressive levels of sensitization that are caused by even low-level exposure to this antigen35. Additional investigation will be required to determine whether VEGF-facilitated sensitization is mediated by the vascular leak, DC alterations and/or vascular alterations that it induces. It is clear, however, that VEGF can link innate and adaptive immunity by predisposing the lung (and possibly other organs) to antigen sensitization and, after antigen exposure, pathologic TH2 cytokine production and inflammation. Previous studies from our laboratories demonstrated that IL-13 also stimulates lung Vegf production36. When viewed together, these studies define a positive feedback loop with VEGF enhancing TH2 sensitization and inflammation and IL-13 subsequently enhancing VEGF production. This interaction may contribute to the severity and or chronicity of VEGF or IL-13–mediated disorders.
Lastly, in accord with studies in an isocyanate model37, we demonstrated that VEGF signaling is required for antigen-induced TH2 inflammation and AHR. In the present studies, we have added to these findings by demonstrating that specific Vegf neutralization abrogates this antigen-induced response and by characterizing the role(s) of Vegf in classic aeroallergen-induced and genetic asthma models. Notably, we have also defined the mechanism of this inhibition by demonstrating that VEGF is induced in both models, that Vegf is produced by epithelial cells and T cells in the allergen-challenged lung, that Vegf is selectively elaborated by TH2 versus TH1 cells and that Vegf is required for antigen-induced TH2 cytokine elaboration. These effects of VEGF may relate to its ability to increase and activate DCs. In fact, our studies demonstrated that VEGF increases the number of DC2 cells and generates change in DC phenotype (B-7low ICOS-Lhigh to B-7high ICOS-Llow) similar to that seen in the allergic antigen-challenged lung38. Alternatively, VEGF may have previously unappreciated regulatory effects on T cells, which are known to express the VEGF receptors10. On superficial analysis, these findings would appear to conflict with studies that suggest that VEGF inhibits T cell development, is induced during TH1 inflammation and inhibits DC function39,40. It is important to point out, however, that these studies focused on the role of VEGF in antitumor and mycobacterial responses and that a TH1-to-TH2 or DC1-to-DC2 shift, such as is described here, would appear to be inhibitory in those settings.
Our studies demonstrate that VEGF-induced alterations differ in their degree of VEGF dependence with inflammation, mucus metaplasia, angiogenesis and DC alterations reversing rapidly, whereas smooth muscle hyperplasia and AHR did not reverse over similar intervals. These findings suggest that therapies that inhibit VEGF can ameliorate inflammation, angiogenesis and mucus responses, even in people with established disease. If VEGF-induced DC alterations have an essential role in pulmonary antigen sensitization and TH2 inflammation, it is reasonable to believe that anti-VEGF–based therapies will also ameliorate these responses. The demonstration that VEGF-induced smooth muscle and physiologic alterations are relatively less VEGF-dependent also has important implications. First, it suggests that these responses may not reverse or may require longer periods of time to reverse with anti-VEGF–based therapies. It also demonstrates that even short periods of VEGF expression can have long-lasting tissue remodeling and physiologic consequences. Lastly, it provides pathogenic mechanisms that can account for the dissociation of inflammation and AHR and the persistent AHR and remodeling that are seen in asthma and chronic antigen-driven experimental systems41.
In summary, these studies indicate that VEGF is a potent stimulator of inflammation, airway and vascular remodeling and physiologic dysregulation that augments antigen sensitization and TH2 inflammation and increases the number and activation of DCs. They also demonstrate that these effects are mediated by IL-13–dependent and –independent pathways and highlight the impressive reversibility of some, but not all, of these VEGF-induced responses. Lastly, they show that VEGF production is a critical event in TH2 inflammation and TH2 cytokine elaboration and that epithelial cells and TH2 cells are potent producers of VEGF in the antigen-challenged lung. These findings demonstrate how asthma-relevant responses can be induced by innate as well as adaptive inflammation and highlight mechanisms by which innate immune responses can predispose to antigen sensitization and TH2 inflammation. Thus, these findings provide a rationale for the use of VEGF regulators to prevent and or treat asthma and other TH2-dominated disorders.
METHODS
Transgenic mice
The CC10-rtTA-VEGF mice in these studies used the Clara cell 10 kDa protein (CC10) promoter and two transgenic constructs to target VEGF to the lung in a way that could be regulated externally. These mice were generated using approaches previously described by our laboratories28. Construct 1, CC10-rtTA-hGH, contains the CC10 promoter, the reverse tetracycline transactivator (rtTA) and human growth hormone (hGH) intronic and polyadenylation sequences (Fig. 1a). Construct 2, tet-O-CMV-VEGF-hGH, contains a polymeric tetracycline operator (tet-O), minimal cytomegalovirus (CMV) promoter, human VEGF165 cDNA and hGH (Fig. 1a).
All mice were evaluated for the presence of both rtTA and VEGF165 using PCR analysis. PCR for VEGF165 used the following primers: sense 5′-CCTCCGCGGCCATGAACTTT-3′ and antisense 5′-TCTTTCCGGATCCGAGATCTGG-3′. All founders were bred for at least eight generations onto a C57BL/6 background.
Dox water administration and phenotypic analysis
One-month-old transgenic and wild-type littermate controls were randomized to receive normal water or water containing doxycycline (dox) (0.5 mg/ml) as described28 and evaluated at intervals thereafter. BAL VEGF quantification, histologic analysis, mRNA analysis, in situ hybridization, immunohistochemistry (IHC), collagen quantification, and the quantification of total and bioactive TGF-β1 were done as previously described28,36,42,43. Airway methacholine responsiveness was assessed using noninvasive whole body phlethysmography as previously described44.
Breeding to Il13−/− mice
CC10-rtTA-VEGF mice were bred with wild-type and Il13−/− mice (from A.N.J. McKenzie) as previously described42
Staining of airway microvasculature
The vasculature was labeled by perfusion with L. esculentum, a lectin that binds uniformly to the luminal surface of endothelial cells, as described previously45,46.
Electron microscopy
After vascular perfusion with paraformaldehyde and glutaraldehyde, tissues were fixed, sectioned, treated with osmium tetroxide followed by aqueous uranyl acetate, dehydrated in acetone, and embedded in epoxy. We stained 0.5 µm sections with toluidine blue for light microscopy, and 50–100 nm sections with 0.8% lead citrate for electron microscopy.
Calculation of wet/dry ratios
Lungs were excised en bloc and extrapulmonary tissues were removed. They were then weighed, placed in a desiccating oven at 65 °C for 48 h and reweighed.
Evans blue dye extravasation
Evans blue dye (EBD) was used to assess plasma leakage as previously described20. The results are expressed as the ratio of the EBD absorbance at 620 nm of paired lung homogenates and serum.
Evaluation of antigen sensitization
The effects of transgenic VEGF, transgenic IL-11 (ref. 47) or recombinant VEGF165 were evaluated. In the case of transgenic VEGF, wild-type and transgenic mice were randomized to normal or dox water. Two weeks later, mice were exposed to aerosolized OVA (2%, grade V, Sigma) twice a day for 10 d. In the case of recombinant VEGF, wild-type mice received ten daily doses of intranasal recombinant VEGF (10 µg/mouse) or vehicle followed 20 min later by the same dose of OVA. After an additional week, representative mice were killed and serum IgG1 and IgG2a were quantified. The remaining mice received intranasal OVA (25 µg/mouse) and inflammation was assessed 48 h later.
Splenocyte proliferation assay
Splenocytes were harvested 2 d after the last OVA challenge and were incubated (5 × 105 cells/well) in 96-well flat-bottom plates at 37 °C for 48 h in medium alone or with 1, 5, 10, 50 or 100 µg OVA/well. KLH (Pierce) was the antigen control. After 18 h of culture with 1 µCi [3H]thymidine, cellular thymidine incorporation was assessed.
DC analysis
Single cell suspensions from lungs from wild-type and transgenic mice on normal or dox water were prepared as described previously38. FACS analysis was performed with monoclonal antibodies from BD Biosciences Pharmingen: anti-I-Abb-biotin, anti-CD3-FITC, anti-CD4-PE (GK1.5), anti-CD8α-PE (53-6.7), anti-CD11b-APC-Cy7 (M1/70), anti-CD11c-FITC, anti-CD40-PE, anti-CD80-PE, anti-CD86-PE and anti-B220-PE. PE-labeled anti-B7h/ICOS-L monoclonal antibodies were obtained from eBioscience. PE-conjugated rat IgG2a and rat IgG2b were isotype controls. SAV-PerCP was used as a second-step reagent. When necessary, cells were preincubated with anti-CD16/CD32 monoclonal antibodies to block cell surface Fc receptors. Cells gated by forward- and side-scatter parameters were analyzed on a FACScalibur flow cytometer (Becton Dickinson) using CELLQuest software.
Role of VEGF in OVA- and T-bet null–stimulated inflammation
OVA sensitization and challenge were undertaken in the presence and absence of the VEGF receptor inhibitor SU1498 (ref. 48) (10 mg/kg/d) as previously described49. In selected experiments the VEGF TrapR1R2 (Regeneron) (25 mg/kg), a fusion protein containing the extracellular domains of VEGFR1 and VEGFR2 coupled to the Fc-portion of IgG50, was used. T-bet null mutant mice32 (Jackson Laboratories) were randomized to SU1498 or control vehicle, and 2 weeks later phenotypic characterization was undertaken as described above.
TH1 and TH2 cell Vegf production
As previously described by our laboratories51, CD4 T cells from OT-II mice that are transgenic for OVA-specific TCR were generated and polarized in vitro into TH1 or TH2 cells. They were then washed and incubated either in the presence and absence of APCs (syngeneic T-depleted splenocytes) and antigen (pOVA323–339, 15 µg/ml) for 24 h, or with phorbol-12 myristate 13-acetate (10 ng/ml, Sigma) and ionomycin (500 ng/ml, Sigma). Cell supernatant VEGF was quantified by ELISA as described above.
Assessment of reversibility
VEGF transgenic mice were randomized to dox or normal water for 2–4 weeks and then placed on normal water. VEGF-induced alterations were assessed at the end of the period of dox administration and after 2–4 weeks of normal water.
Statistics
Data were assessed using the Student’s t-test, ANOVA or Wilcoxon rank sum test as appropriate. Data are expressed as mean ± s.e.m. and are representative of evaluations in a minimum of six mice.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank K. Bertier and S. Ardito for their excellent secretarial assistance, A. Haskell for performing the electron microscopy and E. Ator for help with lectin staining. The authors also thank the other investigators and institutions that provided the reagents that were used. This research was supported in part by NIH grants HL-64642, HL-61904, HL-56389 and HL-78744 (J.A.E.) and HL-24136 and HL-59157 (D.M.) from the NHLBI of the National Institutes of Health USA.
Footnotes
Note: Supplementary information is available on the Nature Medicine website.
COMPETING INTERESTS STATEMENT
The authors declare that they have no competing financial interests.
References
- 1.Wills-Karp M, Chiaramonte M. Interleukin-13 in asthma. Curr. Opin. Pulm. Med. 2003;9:21–27. doi: 10.1097/00063198-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Elias JA, Zhu Z, Chupp G, Homer RJ. Airway remodeling in asthma. J. Clin. Invest. 1999;104:1001–1006. doi: 10.1172/JCI8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elias JA, et al. New insights into the pathogenesis of asthma. J. Clin. Invest. 2003;111:291–297. doi: 10.1172/JCI17748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vrugt B, et al. Bronchial angiogenesis in severe glucocorticoid-dependent asthma. Eur. Respir. J. 2000;15:1014–1021. doi: 10.1034/j.1399-3003.2000.01507.x. [DOI] [PubMed] [Google Scholar]
- 5.Salvato G. Quantitative and morphological analysis of the vascular bed in bronchial biopsy specimens from asthmatic and non-asthmatic subjects. Thorax. 2001;56:902–906. doi: 10.1136/thorax.56.12.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X, Wilson JW. Increased vascularity of the bronchial mucosa in mild asthma. Am. J. Respir. Crit. Care Med. 1997;156:229–233. doi: 10.1164/ajrccm.156.1.9607066. [DOI] [PubMed] [Google Scholar]
- 7.Lee YC, Lee HK. Vascular endothelial growth factor in patients with acute asthma. J. Allergy Clin. Immunol. 2001;107:1106–1108. doi: 10.1067/mai.2001.115628. [DOI] [PubMed] [Google Scholar]
- 8.Orsida BE, et al. Effect of a long-acting beta2-agonist over three months on airway wall vascular remodeling in asthma. Am. J. Respir. Crit. Care Med. 2001;164:117–121. doi: 10.1164/ajrccm.164.1.2006003. [DOI] [PubMed] [Google Scholar]
- 9.Hogg JC. Vascularity in asthmatic airways: Relation to inhaled steroid dose. Thorax. 1999;54:283. doi: 10.1136/thx.54.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoshino M, Nakamura Y, Hamid QA. Gene expression of vascular endothelial growth factor and its receptors and angiogenesis in bronchial asthma. J. Allergy Clin. Immunol. 2001;107:1034–1038. doi: 10.1067/mai.2001.115626. [DOI] [PubMed] [Google Scholar]
- 11.Hoshino M, Takahashi M, Aoike N. Expression of vascular endothelial growth factor, basic fibroblast growth factor, and angiogenin immunoreactivity in asthmatic airways and its relationship to angiogenesis. J. Allergy Clin. Immunol. 2001;107:295–301. doi: 10.1067/mai.2001.111928. [DOI] [PubMed] [Google Scholar]
- 12.Charan NB, Baile EM, Pare PD. Bronchial vascular congestion and angiogenesis. Eur. Respir. J. 1997;10:1173–1180. doi: 10.1183/09031936.97.10051173. [DOI] [PubMed] [Google Scholar]
- 13.Thurston G, et al. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat. Med. 2000;6:460–463. doi: 10.1038/74725. [DOI] [PubMed] [Google Scholar]
- 14.Antony AB, Tepper RS, Mohammed KA. Cockroach extract antigen increases bronchial airway epithelial permeability. J. Allergy Clin. Immunol. 2002;110:589–595. doi: 10.1067/mai.2002.127798. [DOI] [PubMed] [Google Scholar]
- 15.Senger DR, et al. Vascular permeability factor (VPF, VEGF) in tumor biology. Cancer Metastasis Rev. 1993;12:303–324. doi: 10.1007/BF00665960. [DOI] [PubMed] [Google Scholar]
- 16.Gerber HP, Dixit V, Ferrara N. Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cells. J. Biol. Chem. 1998;273:13313–13316. doi: 10.1074/jbc.273.21.13313. [DOI] [PubMed] [Google Scholar]
- 17.Clauss M. Molecular biology of the VEGF and the VEGF receptor family. Semin. Thromb. Hemost. 2000;26:561–569. doi: 10.1055/s-2000-13213. [DOI] [PubMed] [Google Scholar]
- 18.Kanazawa H, Hirata K, Yoshikawa J. Involvement of vascular endothelial growth factor in exercise induced bronchoconstriction in asthmatic patients. Thorax. 2002;57:885–888. doi: 10.1136/thorax.57.10.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asai K, et al. Imbalance between vascular endothelial growth factor and endostatin levels in induced sputum from asthmatic subjects. J. Allergy Clin. Immunol. 2002;110:571–575. doi: 10.1067/mai.2002.127797. [DOI] [PubMed] [Google Scholar]
- 20.Kaner RJ, et al. Lung overexpression of the vascular endothelial growth factor gene induces pulmonary edema. Am. J. Respir. Cell Mol. Biol. 2000;22:657–664. doi: 10.1165/ajrcmb.22.6.3779. [DOI] [PubMed] [Google Scholar]
- 21.Partovian C, et al. Adenovirus-mediated lung vascular endothelial growth factor overexpression protects against hypoxic pulmonary hypertension in rats. Am. J. Respir. Cell Mol. Biol. 2000;23:762–771. doi: 10.1165/ajrcmb.23.6.4106. [DOI] [PubMed] [Google Scholar]
- 22.Schwarze J, Gelfand EW. Respiratory viral infections as promoters of allergic sensitization and asthma in animal models. Eur. Respir. J. 2002;19:341–349. doi: 10.1183/09031936.02.00254302. [DOI] [PubMed] [Google Scholar]
- 23.Eisenbarth SC, et al. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J. Exp. Med. 2002;196:1645–1651. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Umetsu DT, McIntire JJ, Akbari O, Macaubas C, DeKruyff RH. Asthma: an epidemic of dysregulated immunity. Nat. Immunol. 2002;3:715–720. doi: 10.1038/ni0802-715. [DOI] [PubMed] [Google Scholar]
- 25.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am. J. Respir. Crit. Care Med. 2000;161:1501–1507. doi: 10.1164/ajrccm.161.5.9906076. [DOI] [PubMed] [Google Scholar]
- 26.Lee CG, et al. Respiratory syncytial virus stimulation of vascular endothelial cell growth Factor/Vascular permeability factor. Am. J. Respir. Cell Mol. Biol. 2000;23:662–669. doi: 10.1165/ajrcmb.23.5.4188. [DOI] [PubMed] [Google Scholar]
- 27.Zeng X, Wert SE, Federici R, Peters KG, Whitsett JA. VEGF enhances pulmonary vasculogenesis and disrupts lung morphogenesis in vivo. Dev. Dyn. 1998;211:215–227. doi: 10.1002/(SICI)1097-0177(199803)211:3<215::AID-AJA3>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 28.Ray P, et al. Regulated overexpression of interleukin 11 in the lung. Use to dissociate development-dependent and -independent phenotypes. J. Clin. Invest. 1997;100:2501–2511. doi: 10.1172/JCI119792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohta Y, et al. Vascular endothelial growth factor expression in airways of patients with lung cancer: a possible diagnostic tool of responsive angiogenic status on the host side. Chest. 2002;121:1624–1627. doi: 10.1378/chest.121.5.1624. [DOI] [PubMed] [Google Scholar]
- 30.Meyer KC, Cardoni A, Xiang ZZ. Vascular endothelial growth factor in bronchoalveolar lavage from normal subjects and patients with diffuse parenchymal lung disease. J. Lab. Clin. Med. 2000;135:332–338. doi: 10.1067/mlc.2000.105618. [DOI] [PubMed] [Google Scholar]
- 31.Nishigaki Y, et al. Increased vascular endothelial growth factor in acute eosinophilic pneumonia. Eur. Respir. J. 2003;21:774–778. doi: 10.1183/09031936.03.00085903. [DOI] [PubMed] [Google Scholar]
- 32.Finotto S, et al. Development of spontaneous airway changes consistent with human asthma in mice lacking T-bet. Science. 2002;295:336–338. doi: 10.1126/science.1065544. [DOI] [PubMed] [Google Scholar]
- 33.Hahn RG. Endotoxin boosts the vascular endothelial growth factor (VEGF) in rabbits. J. Endotoxin. Res. 2003;9:97–100. doi: 10.1179/096805103125001478. [DOI] [PubMed] [Google Scholar]
- 34.Payne DN, et al. Early thickening of the reticular basement membrane in children with difficult asthma. Am. J. Respir. Crit. Care Med. 2003;167:78–82. doi: 10.1164/rccm.200205-414OC. [DOI] [PubMed] [Google Scholar]
- 35.Matsui EC, et al. Cockroach allergen exposure and sensitization in suburban middle-class children with asthma. J. Allergy Clin. Immunol. 2003;112:87–92. doi: 10.1067/mai.2003.1588. [DOI] [PubMed] [Google Scholar]
- 36.Corne J, et al. IL-13 stimulates vascular endothelial cell growth factor and protects against hyperoxic acute lung injury. J. Clin. Invest. 2000;106:783–791. doi: 10.1172/JCI9674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee YC, Kwak Y-G, Song CH. Contribution of vascular endothelial growth factor to airway hyperresponsiveness and inflammation in a murine model of toluene diisocyanate-induced asthma. J. Immunol. 2002;168:3595–3600. doi: 10.4049/jimmunol.168.7.3595. [DOI] [PubMed] [Google Scholar]
- 38.Vermaelen KY, Pauwels RA. Accelerated airway dendritic cell maturation, trafficking and elimination in a mouse model of asthma. Am. J. Respir. Cell Mol. Biol. 2003 doi: 10.1165/rcmb.2003-0008OC. [DOI] [PubMed] [Google Scholar]
- 39.Gabrilovich DI, Ishida T, Nadaf S, Ohm JE, Carbone DP. Antibodies to vascular endothelial growth factor enhance the efficacy of cancer immunotherapy by improving endogenous dendritic cell function. Clin. Cancer. Res. 1999;5:2963–2970. [PubMed] [Google Scholar]
- 40.Matsuyama W, et al. Purified protein derivative of tuberculin upregulates the expression of vascular endothelial growth factor in T lymphocytes in vitro. Immunology. 2002;106:96–101. doi: 10.1046/j.1365-2567.2002.01395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leigh R, et al. Type 2 cytokines in the pathogenesis of sustained airway dysfunction and airway remodeling in mice. Am. J. Respir. Crit. Care Med. 2004;169:860–867. doi: 10.1164/rccm.200305-706OC. [DOI] [PubMed] [Google Scholar]
- 42.Lee CG, et al. Transgenic overexpression of interleukin (IL)-10 in the lung causes mucus metaplasia, tissue inflammation, and airway remodeling via IL-13-dependent and -independent pathways. J. Biol. Chem. 2002;277:35466–35474. doi: 10.1074/jbc.M206395200. [DOI] [PubMed] [Google Scholar]
- 43.Lee CG, et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating TGF-β1. J. Exp. Med. 2001;194:809–821. doi: 10.1084/jem.194.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu Z, et al. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities and eotaxin production. J. Clin. Invest. 1999;103:779–788. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thurston G, Baluk P, Hirata A, McDonald DM. Permeability related changes revealed at endothelial cell borders in inflamed vessels by lectin staining. Am. J. Physiol. 1996;271:H2547–H2562. doi: 10.1152/ajpheart.1996.271.6.H2547. [DOI] [PubMed] [Google Scholar]
- 46.Baluk P, et al. Regulated angiogenesis and vascular regression in mice overexpressing vascular endothelial growth factor in airways. Am. J. Pathol. doi: 10.1016/S0002-9440(10)63369-X. (in the press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang W, et al. Targeted expression of IL-11 in the murine airway causes lymphocytic inflammation, bronchial remodeling, and airways obstruction. J. Clin. Invest. 1996;98:2845–2853. doi: 10.1172/JCI119113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor stimulates neurogenesis in vitro and in vivo. Proc. Natl. Acad. Sci. USA. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang J, et al. IL-11 selectively inhibits aeroallergen-induced pulmonary eosinophilia and Th2 cytokine production. J. Immunol. 2000;165:2222–2231. doi: 10.4049/jimmunol.165.4.2222. [DOI] [PubMed] [Google Scholar]
- 50.Cursiefen C, et al. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J. Clin. Invest. 2004;113:1040–1050. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cohn L, Homer RJ, Marinov A, Rankin J, Bottomly K. Induction of airway mucus production by T helper 2 (Th2) cells: a critical role for interleukin 4 in cell recruitment but not mucus production. J. Exp. Med. 1997;186:1737–1747. doi: 10.1084/jem.186.10.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.