Abstract
A foundational aspect of early social-emotional development is the ability to detect and respond to the actions of others who are coordinating their behavior with that of the self. Behavioral work in this area has found that infants show particular preferences for adults who are imitating them rather than adults who are carrying out noncontingent or mismatching actions. Here we explore the neural processes related to this tendency of infants to prefer others who act like the self. EEG was recorded from 14-month-olds while they were observing actions which either matched or mismatched the action the infant had just executed. Desynchronization of the EEG mu rhythm was greater when infants observed an action that matched their own most recently executed action. This effect was strongest immediately prior to the culmination of the goal of the observed action, which is consistent with recent ideas about the predictive nature of brain responses during action observation.
Keywords: EEG, mu rhythm, imitation, perception-action, infant
Imitation serves a number of important cognitive and social-emotional functions, and is a powerful learning tool for the developing individual. Imitation reduces the trial-and-error attempts inherent in learning in isolation; children can learn skilled action patterns and causal sequences by observing the actions of experts in the culture (Meltzoff, Kuhl, Movellan, & Sejnowski, 2009). In addition to supplying important information about the conventional use of objects and tools, imitation also provides infants with valuable information about the psychological attributes of other people. Through imitation, infants come to understand that they can act like others and that others can act like them. This recognition that others are “Like Me” has been argued to be a foundation for social-cognitive development (Meltzoff, 2007a, 2007b). This bidirectional process depends on infant’s abilities to detect and respond to a “match” between their own actions and the actions of socially interactive partners, particularly caregivers who are engaged in dyadic interactions with them. Related work in this area further suggests that infants’ responsivity to those who engage in reciprocal imitation goes beyond a surface-level matching process to engage emotional processes related to learning and affiliation.
Behavioral studies have demonstrated that preverbal infants show particular interest in watching an adult who acts like them (Agnetta & Rochat, 2004; Asendorpf, Warkentin, & Baudonniere, 1996; Hauf, Aschersleben, & Wolfgang, 2007; Meltzoff, 1990). In one sequence of studies, Meltzoff (1990, 2007a) sat 14-month-old infants across a table from two adult experimenters. Infants looked longer and smiled more often at the adult who imitated the actions of the infant, compared with the other adult who made contingent, but non-matching actions. Developmentally, infants’ responses to recognizing equivalence between self and other at the level of shared actions could be a stepping stone for their realization that others who act like them also have the capacity for feelings and intentions that are shared with their own (Meltzoff, Williamson, & Marshall, in press).
Interestingly, the power of mutual imitation is not limited to infancy. Adults often unconsciously copy the postures, expressions, and mannerisms of their social partners, and show increased affiliation and liking towards those who imitate them. In adults, this “chameleon effect” (Chartrand & Bargh, 1999) is thought to prompt prosocial feelings because it gives us the sense that the other is somehow synchronized with oneself. In adult work, there is increasing interest in identifying the neural correlates of such effects (Guionnet et al., 2012; Kuhn et al., 2010). In one early neuroimaging study in this area, Decety, Chaminade, Grèzes, and Meltzoff (2002) found activation of the superior temporal gyrus (STG) and inferior parietal lobe (IPL) during conditions of reciprocal imitation.
Adding to a substantive behavioral literature on how infants perceive and respond to the imitative actions of others, several recent studies have elucidated patterns of brain activity while infants observe other people’s actions. A focus of this work has been the application of electroencephalographic (EEG) methods to examine the neural linkages between action perception and action production in preverbal infants (for a review, see Marshall & Meltzoff, 2011). Investigators in this area have been particularly interested in the properties of the mu rhythm, an alpha-range EEG oscillation which is most salient over central electrode sites and which has also been useful in the study of perception-action linkages in adults (Hari, 2006).
Recent studies have suggested that the infant mu rhythm is desynchronized (reduced in amplitude) during infants’ execution of actions and also during the observation of similar actions performed by others (Marshall, Young, & Meltzoff, 2011; Southgate, Johnson, Osborne, & Csibra, 2009). Related work has focused on changes in the infant mu rhythm in relation to specific properties of observed actions (Nystrom, Ljunghammar, Rosander, & von Hofsten, 2011; Southgate, Johnson, El Karoui, & Csibra, 2010; Stapel, Hunnius, van Elk, & Bekkering, 2010; van Elk, van Schie, Hunnius, Vesper, & Bekkering, 2008). However, despite the interest in infant mu rhythm from developmental scientists investigating the neural correlates of early action processing, work in this area has tended to lack a genuinely social focus. To advance developmental social neuroscience, it seems important to develop new paradigms and to generate data related to more naturalistic social phenomena such as the reciprocal exchanges that infants have with caregivers in the form of mutual imitation.
Thus far, little work has examined the response of the infant mu rhythm to manipulations in the behavior of a social partner. In one recent study, Reid, Striano, and Iacoboni (2011) reported a reduction in alpha-range power at frontal and central sites when 14-month-old infants observed an experimenter who was attempting to imitate their movements, relative to a separate block of trials in which infants watched an experimenter carry out a series of unfamiliar, intransitive movements. This finding lends some support to the notion that changes in the infant mu rhythm during action observation may relate to changes in the behavior of a social partner. However, little is known about the nature of such effects, especially the fine-grained temporal relation of EEG changes to the observed behavior of adults who are engaged in imitative (or non-imitative) interactions with infants. In addition, the relations between EEG activity during infants’ own action production and EEG patterns during the observation of others’ actions which match or do not match the infants’ have not been explored. Yet, mutual imitation episodes are important aspects of everyday social interactions between infants and caretakers, and the experience of such matching has been argued to engender and support social-emotional and cognitive development (e.g., Meltzoff, 2007a; Meltzoff, 2007b)
The question of particular interest in the current study was whether infant EEG measures can shed further light on the nature of infants’ responses to being imitated. In addressing this question we developed an extension of an interactive turn-taking protocol that we had previously used during EEG collection with 14-month-old infants (Marshall et al., 2011). In that work we used only one type of action (a button press) which infants carried out themselves and also observed an experimenter performing. In the current protocol we also examined 14-month-old infants’ EEG responses to observing an experimenter’s button press action, but we systematically varied the action that the infants executed immediately before they observed the experimenter carry out the button press. This procedure enabled us to compare infants’ EEG responses during observation of the same action presented across two different psychological contexts: In one condition, the infant observed an experimenter who was imitating them, while in the other condition the experimenter’s action lacked the same connection to the infant’s prior act.
How might EEG activity be expected to vary during infant’s observation of actions which do or do not match their own? One key question we started with was whether mu rhythm desynchronization over central sites would differentiate between these conditions. A basis for expecting such differentiation comes from work in young children and adults showing that the extent of mu rhythm desynchronization is greater when an observed action is attended to in the context of joint action with another person (Kourtis, Sebanz, & Knoblich, 2010; Meyer, Hunnius, van Elk, van Ede, & Bekkering, 2011) or has direct relevance to task demands (Muthukumaraswamy & Singh, 2008; Schuch, Bayliss, Klein, & Tipper, 2010). Drawing on work on the neural manifestation of attention (Engel, Fries, & Singer, 2001) we further expected that a matching action may trigger enhanced activity in cortical systems associated with the motor requirements or sensory consequences of that action. Of particular relevance here is the finding that the mu rhythm appears to be mainly generated in primary somatosensory cortex (Hari & Salmelin, 1997; Ritter, Moosmann, & Villringer, 2009). This raises the possibility that mu desynchronization would be greater to matching than non-matching actions because the proprioceptive or kinesthetic concordance with one’s own immediate experience would result in enhanced engagement of cortical systems which process the sensorimotor consequences or requirements of observed actions (see Marshall & Meltzoff, 2011).
In the current study we also paid particular attention to the time course of EEG desynchronization. We used eight 125ms bins immediately prior to and after the culmination of a goal-directed action, both for infants’ action execution and during their observation of the experimenter’s action. The rationale for this approach comes from recent theoretical perspectives suggesting that brain responses during the early stages of action observation may have a predictive element (Csibra, 2007; Jacob, 2008; Kilner, 2011). Initial empirical support for this suggestion comes from the infant studies of Southgate et al. (2009; 2010) who reported desynchronization of the EEG mu rhythm in 9-month-olds during observation of reaching actions, prior to the fulfillment of the actor’s goal.
In the current study we examined the time course of EEG activity over infants’ execution of two different actions and during their observation of matching or non-matching actions. As in Marshall et al. (2011), we examined EEG activity at central sites and across a range of other scalp regions in order to further clarify the topography of electrophysiological responses during infants’ execution and observation of goal-directed actions.
Method
Participants
Forty-four 14-month-old infants participated in the study (M = 62 weeks, SD = 1.6; 24 male). Families were recruited from a diverse urban environment using commercially available mailing lists. Families were not invited to participate if their infant was born preterm, if both parents were left-handed, if the infant had experienced chronic developmental problems, or if the infant was on long-term medication. A number of infants did not have useable EEG data because of technical problems (n = 4) or because they became excessively fussy during cap preparation (n = 4). Four additional infants were excluded since they did not carry out the required button press action. Further exclusions (e.g., infants with insufficient numbers of artifact-free trials) are detailed in the results section.
Procedure
The procedure involved the use of a custom-made button box with a recessed button that produced a short melody when pressed. Prior to the start of the experiment, an experimenter demonstrated the button press to the infant.
Infants were fitted with an EEG cap (see below) and were seated at a table on their caregiver’s lap opposite an experimenter. Each trial consisted of a baseline epoch followed by an action execution epoch and an action observation epoch. During the baseline epoch, the experimenter held up a flashcard with an abstract visual pattern for approximately 5 s. Following the baseline, infants carried out one of two actions: pressing the button on the button box or grasping a small toy. For button press trials, the experimenter placed the button box in front of the infant. For grasp trials, the experimenter held out a small toy that was attached to a plastic handle. For both pressing and grasping, the interval between the offset of the baseline and the onset of the infant’s reach was around 5 s. After the infant had produced the pressing or grasping action, the object (toy or button box) was retrieved. The experimenter then verbally attracted the infant’s attention before reaching towards the button box and producing a button press with her right hand. The experimenter’s reach began 1–2 s after the infant had brought his or her hand back to a resting position. For analysis purposes, observation epochs which were preceded by the infant’s own button press action constituted the “observe-same” condition, while the “observe-different” condition comprised observation epochs that had been preceded by the infant’s grasping action. The observe-same and observe-different conditions were alternated on successive trials across the course of the experimental session.
The experimental protocol continued for as long as the infant produced the actions and remained attentive to the experimenter. The session was videotaped with a vertical interval time code (VITC) being placed on the video signal during recording. Calibration procedures had ensured that the VITC signal was synchronized with EEG collection, such that the video was aligned with the EEG data to the precision of one NTSC video frame (33 ms). Videos were coded offline for the time points marking the onset and offset of the baseline epochs as well as the frame in which the experimenter or infant pressed the button, and the frame in which the infant grasped the toy. Other frames that were marked included the onset and offset of reaches of the infant and experimenter towards the toy or button box. The videos were also coded for subtle infant behavioral movements, and baseline and observation epochs containing infant movements resembling reaching, grasping, pointing, or pressing were not included in subsequent EEG analyses.
EEG Collection and Processing
Collection and processing of the EEG signal was identical to the methods used in Marshall et al. (2011). EEG was recorded using a lycra stretch cap (Electro-Cap International, Inc.) from a range of sites across the scalp: Fp1, Fp2, F3, F4, Fz, F7, F8, C3, C4, T7, T8, P3, P4, Pz, P7, P8, O1, O2, and the left and right mastoids. Electro-Gel conducting gel was utilized and scalp electrode impedances were accepted if they were below 25 kilohms. The signal from each site was amplified using optically isolated, high input impedance (> 1 GΩ) custom bioamplifiers (SA Instrumentation) and was digitized using a 16-bit A/D converter (+/− 5 V input range). Bioamplifier gain was 4000 and the hardware filter (12 db/octave rolloff) settings were .1 Hz (high-pass) and 100 Hz (low-pass). The signal was collected referenced to the vertex (Cz) with an AFz ground, and EEG data were re-referenced offline to an average mastoids reference prior to further analysis. As in Marshall et al. (2011), a procedure involving independent component analysis (ICA) was used to clear the EEG data of ocular and muscle artifact. The ICA procedure was an automation of the method described by Jung et al. (2000). After this had been carried out, any epochs in which the EEG signal for any channel exceeded +/− 250 μV were excluded from further analysis.
Event-related changes in band power between the baseline and the observation or execution epochs were derived using established methods for computing event-related desynchronization (ERD; Pfurtscheller, 2003). Based on previous work on the frequency of the mu rhythm in infants of this age (Marshall, Bar-Haim, & Fox, 2002), the primary frequency band of interest was taken to be 6–9 Hz. For the computation of ERD scores, the following sequence from Marshall et al. (2011) was used: (a) Bandpass filtering of the EEG signals between 6 and 9 Hz using a two-way least squares finite impulse response filter; (b) Squaring of the filtered signals to convert to a power metric; (c) Computation of event-related averages for each condition within each participant; (d) Computation of the mean power of the event-related signal in 125ms epochs. Desynchronization values were computed for each 125ms epoch as ([A−R]/R)*100, where A is band power during action observation or execution, and R is band power averaged over the baseline epochs (Pfurtscheller & Lopes da Silva, 1999). Negative ERD values reflect desynchronization (i.e., a decrease in band power relative to the baseline), while positive values reflect synchronization (i.e., an increase in band power relative to the baseline).
In prior work we analyzed mean ERD over a 1000-ms epoch that was centered on the first frame of the button press (Marshall et al., 2011). Here we introduced further temporal precision by carrying out separate analyses of mean ERD over the 500 ms prior to the infant or experimenter’s button press (the pre-button press epoch) and the 500 ms immediately following the first frame of the button press (the post-button press epoch). The analysis of the infant’s execution of the grasp was carried out in a similar fashion, with mean ERD being computed for 500ms epochs extending prior to and following the video frame depicting the onset of grasping. Inspection of the timing of events derived from the video coding showed that the 500 ms prior to the onset of the button press or grasp captured the latter part of the experimenter’s or infant’s reach toward the object. For example, the experimenter’s reach began an average of 800 ms prior to the first frame of the actual button press.
Results
Action Observation
Mean ERD scores for the two action observation conditions (observe-same vs. observe-different) were computed for mid-frontal (F3/F4), lateral frontal (F7/F8), central (C3/C4), mid-parietal (P3/P4), and lateral parietal (P7/P8) sites. This selection was based on the fact that prior studies of the infant mu rhythm have reported findings from a mixture of frontal, central, and parietal regions (Marshall et al., 2011; Reid et al., 2011; Southgate et al., 2010; Southgate et al., 2009; van Elk et al., 2008). This strategy also allowed examination of hemispheric asymmetries in the EEG, which to this point have not been well documented in infant studies of action processing.
A number of infants were excluded from further analyses based on the exclusion criteria used by Marshall et al. (2011). Specifically, 7 infants had fewer than 3 artifact-free trials in either or both of the observation conditions. In addition, initial data inspection showed that 9 of the remaining 25 participants had extreme ERD values (more than 1.5 times the interquartile range from the median) at one or more regions during action observation. For the remaining 16 infants there was an average of 6.8 (SD = 3.4) artifact-free observation trials per participant for the observe-same condition and an average of 9.1 trials (SD = 4.4) for the observe-different condition. Seven of the 16 infants that were included in the analysis of the observation condition were not included in the analysis of action execution (see below).
For the comparison of mean ERD between the two conditions, a repeated measures ANOVA was conducted with the following factors: Pre/Post (500 ms before/after the button press), Condition (observe-same, observe-different), Region (mid-frontal, lateral frontal, central, mid-parietal, lateral parietal), and Hemisphere (left, right). Probability values in all reported ANOVAs have been adjusted based on the Greenhouse-Geisser correction factor (epsilon).
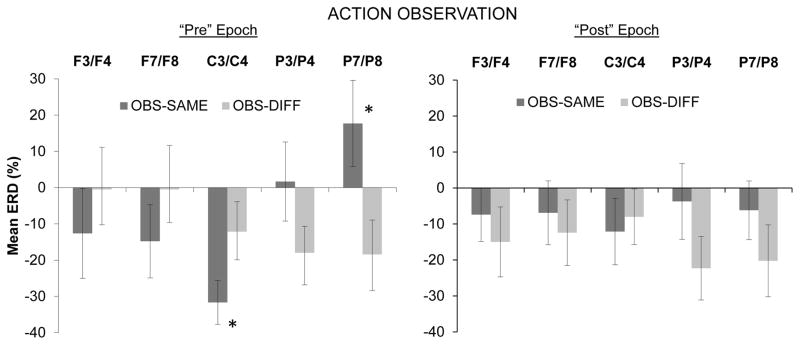
Results showed a significant interaction effect for Condition × Region, F(4,60) = 3.62, ∈ = .41, p < .05. This interaction was qualified by a trend towards significance for the Pre/Post × Condition × Region interaction, F(4,60) = 2.95, ∈ = .59, p = .06. No other main effects or interaction effects approached significance. The three-way interaction was followed up by individual repeated-measures ANOVAs for the pre- and post-button press epochs separately (see Figure 1).
Figure 1.
Mean event-related desynchronization (% ERD) during infants’ observation of the experimenter’s button press action for the observe-same and observe-different conditions separately. The left panel corresponds to the 500 ms epoch immediately preceding the button press by the experimenter, and the right panel shows results from the 500 ms epoch immediately following the button press. Means are shown for mid-frontal (F3/F4), lateral frontal (F7/F8), central (C3/C4), mid-parietal (P3/P8), and lateral parietal (P7/P8) regions. Significant differences between conditions are indicated (* indicates p < .05). Error bars indicate 1 S.E.
In the follow-up ANOVA for the pre-button press epoch there was a significant interaction between Condition and Region, F(4,60) = 5.17, ∈ = .44, p < .05 Additional repeated-measures ANOVAs within each region indicated a significant main effect of Condition at the central region, F(1,15) = 5.59, p < .05, with the observe-same condition being associated with greater ERD at central sites than the observe-different condition (see Figure 1). The reverse effect was noted at the lateral parietal region, with the observe-different condition being associated with a greater ERD than the observe-same condition, F(1,15) = 6.14, p < .05. The means for the observe-same condition over the lateral parietal region showed a positive ERD value, indicating the presence of EEG synchronization rather than desynchronization. For the analysis of the pre-button press epoch, no other main effects or interactions approached significance.
For the follow-up ANOVA involving only the post-button press epoch, no main effects or interactions approached significance.
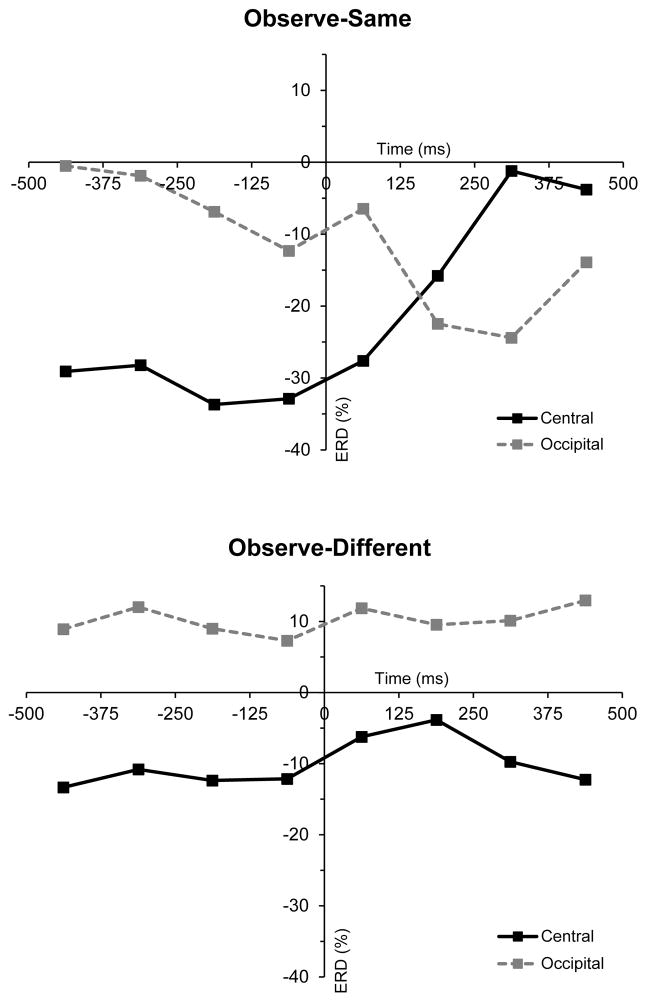
In order to further visualize the effects documented above, plots were constructed showing the mean values at central sites for the individual 125-ms bins in the action observation data (see Figure 2). These plots further illustrate the greater ERD in at central sites for the observe-same condition in the time period immediately preceding the button press, with the magnitude of this ERD decreasing rapidly immediately following the button press. Values from the occipital region were also included in these plots and confirmed that these results for the mu rhythm at central sites were independent of changes in the classical alpha rhythm which is typically recorded over visual cortex.
Figure 2.
The time course of event-related desynchronization (% ERD) at central (C3/C4) and occipital (O1/O2) sites during action observation. The upper and lower panels show mean ERD per 125ms bin across all included participants from the observe-same and observe-different conditions respectively. Each time series is centered on the first video frame of the button press action, which corresponds to 0ms.
Action Execution
Mean ERD scores were computed for the two action execution conditions (button press vs. grasp) for mid-frontal, lateral frontal, central, mid-parietal, and lateral parietal regions. Infants were excluded from further analyses based on criteria from Marshall et al. (2011). Specifically, 13 infants had fewer than 3 artifact-free trials in either or both of the execution conditions. Initial data inspection further showed that 6 of the remaining 19 participants had extreme ERD values (more than 1.5 times the interquartile range from the median) at one or more scalp regions. For the remaining 13 infants there were an average of 9.7 (SD = 3.5) artifact-free trials per participant for the button press condition and an average of 9.6 trials (SD = 3.5) for the grasp condition. Of these infants, all but one predominantly used his or her right hand to complete the executed actions. Nine of the 13 infants in the analysis of the execution data were also included in the observation condition.
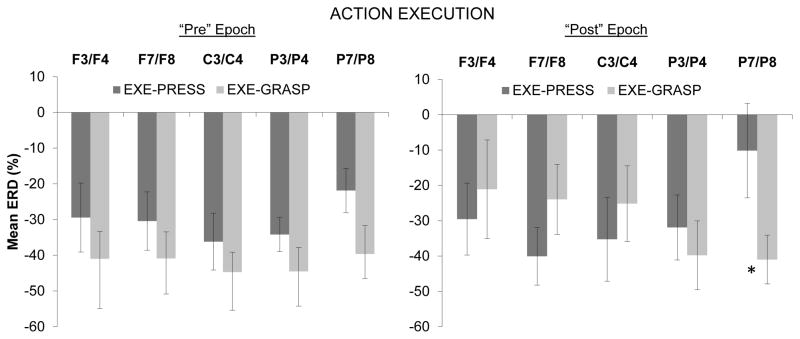
For the comparison of ERD between the two execution conditions, a repeated measures ANOVA was conducted with the following factors: Pre/Post (500 ms before/after the button press or grasp), Condition (execute button press, execute grasp), Region (mid-frontal, lateral frontal, central, mid-parietal, lateral parietal), and Hemisphere (left, right). There was a significant main effect of Hemisphere, F(1,12) = 8.50, p < .05, with greater overall ERD in the right hemisphere (M = −36.23, SD = 16.40) compared with the left hemisphere (M = −29.85, SD = 17.13). In addition, there was a significant interaction effect for Pre/Post × Condition × Region, F(4,48) = 3.16, ∈ = .66, p < .05.
The three-way interaction effect was followed up by examining Region × Condition interactions for the Pre- and Post- epochs separately (see Figure 3). For the Pre-epoch, no main effects or interactions approached significance. In the ANOVA for the Post-epoch there was a significant interaction for Condition × Region, F(4,48) = 3.41, ∈ = .56, p < .05. Follow-up repeated-measures ANOVAs within each region indicated a significant main effect of Condition over the lateral parietal region, F(1,12) = 7.08, p < .05, with the grasp condition being associated with a greater ERD over this region than the button press condition (see Figure 3). There were no significant differences between the two execution conditions over any other scalp regions.
Figure 3.
Mean event-related desynchronization (% ERD) during infants’ execution of the button press action and grasping action. The left panel corresponds to the 500 ms epoch immediately preceding the infant’s button press or grasp onset, and the right panel shows results from the 500 ms epoch immediately following the press or grasp onset. Means are shown for mid-frontal (F3/F4), lateral frontal (F7/F8), central (C3/C4), mid-parietal (P3/P8), and lateral parietal (P7/P8) regions. Significant differences between conditions are indicated (* indicates p < .05). Error bars indicate 1 S.E.
Discussion
In the current study we employed a socially interactive protocol in which 14-month-olds took turns with an experimenter in executing and observing specific goal-directed actions. In a prior study we had documented patterns of EEG responses while infants carried out a punctate action and observed an experimenter carry out the same action (Marshall et al., 2011). That particular study utilized only one type of action throughout. In the current protocol we extended our investigations to examine the effect of infant’s own immediate action-production experience on the EEG patterns during action observation. In addition, we analyzed infants’ EEG responses with greater temporal precision.
Our primary motivation was to compare EEG responses while infants observed a topographically identical action (the button press) presented across two different psychological contexts. In one context, the experimenter was imitating the infant, while in the other the experimenter’s action – while still being temporally contingent on the infant’s act – lacked the same imitative connection to the infant’s own action. In order to generate these contexts, in the preceding part of each trial infants had either executed a button press (which led to the “observe-same” condition) or they had grasped a small toy (which led to the “observe-different” condition). One limitation of our protocol is that the experimenter always carried out the button press action and so did not appear to flexibly adjust her actions based on the infants’ behavior. However, the alternative would be a more complex 2×2 experimental design which would also vary across trials whether the experimenter carried out the grasp or the button press. Given the distinct problems with obtaining an adequate number of trials in infant EEG studies involving multiple conditions, this more complex design was essentially untenable with infants this age.
While the EEG signal does not allow a great deal of spatial resolution, here we were able to leverage its high level of temporal resolution to provide details on the time course of EEG desynchronization during infants’ observation and execution of actions. An examination of temporal considerations would seem to be particularly important for social neuroscience given that interpersonal timing is a key aspect of reciprocal social interactions. In comparing the EEG responses across the two action observation conditions, we therefore examined the time course of the EEG response as the experimenter’s button-press action unfolded. The rationale for this approach derives from ideas that temporal patterns of brain activity during action observation may partly reflect the activation and updating of predictive processes as the child observes goal-directed actions (Csibra, 2007; Jacob, 2008; Kilner, 2011). In this respect it is notable that for the action observation epochs, significant effects of our experimental manipulation were found in the time period prior to the culmination of the observed action and not in the one immediately after. Specifically, desynchronization in the 6–9 Hz band during the 500-ms epoch preceding the button press – while the experimenter was reaching for the button – was greater at central electrode sites when the infant had just carried out the (same) button press action than when he or she had just carried out the (different) grasping action. Importantly, the desynchronization in the 6–9 Hz band at central sites was independent of activity in the same frequency range over occipital sites, which suggests that our findings are not simply a product of correlated changes in the classical visual alpha rhythm.
The presence of an effect at central sites is noteworthy given recent interest the functional correlates of the infant mu rhythm (Marshall & Meltzoff, 2011). Reid et al. (2011) reported greater desynchronization of the infant mu rhythm averaged over 3-minute epochs while 14-month-olds observed an experimenter who was attempting to imitate infants’ body movements and posture, compared with a condition in which the experimenter performed a sequence of unfamiliar body movements in a non-interactive fashion. These results are suggestive, although the study broadly contrasted “interactive” and “non-interactive” conditions using primarily intransitive body movements and did not attempt a more fine-grained manipulation of “matching actions” or a detailed temporal analysis involving goal-directed actions.
The results reported here are also related to other recent work demonstrating that patterns of EEG activity during action observation can be modulated by social factors. One study with adults found that desynchronization over frontal and central sites during action observation was stronger when participants were engaged in a joint-action task compared when they were observing actions of a third person who acted alone (Kourtis et al., 2010). With young children, Meyer et al. (2011) found that EEG desynchronization at central sites during action observation was enhanced when 3-year-olds were engaged in a joint-action task with an experimenter. Although our study did not involve a context of joint action toward a common goal, our findings suggest that mu rhythm desynchronization during infants’ observation of action is enhanced when there is there is an imitative connection between the infant’s prior action and the observed action of the other. From a developmental perspective, such reciprocal imitative actions may be the earliest ontogenetic building blocks for later-emerging shared and collaborative activities aimed at a common goal (Meltzoff et al., in press).
In terms of understanding the ways in which “being imitated” relates to changes in the mu rhythm, it is notable that EEG work with adults has shown greater desynchronization of sensorimotor rhythms when the observer’s task requires particular attention to the action. For example, Muthukumaraswamy and Singh (2008) found that the observation of finger movements led to greater beta desynchronization when the action was relevant to other task requirements, compared with passive viewing of the same movements. Along similar lines, Schuch, Bayliss, Klein, and Tipper (2010) found greater mu rhythm desynchronization during action observation when the action itself, rather than other aspects of the visual display, pertained to the participant’s task. In this sense it is possible that the differential patterns of mu rhythm activity during the two conditions in our study partly reflect differences in allocation of attention to the observed actions. Such differences in attention could arise through the differential connection of the observed actions to the infant’s prior self-experience.
A related process through which the observation of others who are imitating one’s actions may affect the EEG mu rhythm comes from neuroimaging studies of adults suggesting that the mu rhythm reflects activity in primary somatosensory cortex (Arnstein, Cui, Keysers, Maurits, & Gazzola, 2011; Formaggio et al., 2008; Hari & Salmelin, 1997; Ritter et al., 2009). Neuroimaging studies with adults suggest consistent activation of somatosensory cortex during action observation (Gazzola & Keysers, 2009; Keysers, Kaas, & Gazzola, 2010) and prior work using EEG and MEG in adults has also suggested a link between changes in alpha-range oscillations and somatosensory processing during action observation (Babiloni et al., 2010; Cheng, Yang, Lin, Lee, & Decety, 2008). In addition, studies with adults have also shown greater activation of somatosensory cortex when an observed action is seen from the first-person perspective compared to the third-person perspective (Jackson, Meltzoff, & Decety, 2006; Ruby & Decety, 2001, 2004). It is possible that the first-person perspective more strongly activates the somatosensory system because the observed action is seen as being concordant with the proprioceptive or kinesthetic information gained by doing the action oneself, which may facilitate imitation (Jackson et al., 2006). Given these findings, elucidating possible links between our EEG findings from central sites and somatosensory processes involved in action observation are an interesting target for further investigation. We further note that attentional and sensory processes may be closely linked, with attention being manifested in increased activation of cortical networks related to the processing of task-relevant sensory information (Engel et al., 2001). This suggests a potentially promising avenue for future EEG/MEG studies in which the sensory consequences of observed actions could be manipulated, perhaps in combination with manipulations in participants’ experience with those consequences.
In the current study, the direction of the ERD effect seen at central sites was reversed at parietal sites, with the observe-same condition being associated with synchronization (rather than desynchronization) in the alpha band over this region during the observation of the experimenter’s reach. This may be related to findings that alpha desynchronization over sensorimotor cortex is often paired with synchronization (an increase in power) over adjacent cortical areas, with the latter possibly reflecting inhibition of task-irrelevant cortical systems (Pfurtscheller & Neuper, 1994; Suffczynski, Kalitzin, Pfurtscheller, & Lopes da Silva, 2001). It is also notable that a growing literature in adults has connected alpha synchronization at posterior sites to the deployment of selective attention (Capotosto, Babiloni, Romani, & Corbetta, 2009; Foxe, Simpson, & Ahlfors, 1998), an effect which seems particularly salient during anticipation (Snyder & Foxe, 2010). Although further investigation is needed, related evidence from developmental work has noted alpha synchronization over parietal cortex during periods of anticipatory visual attention in infants (Orekhova, Stroganova, & Posikera, 2001) and older children (Fernandez et al., 1998).
Concerning EEG patterns during action execution, one clear finding was that alpha-range desynchronization was greater in the right than the left hemisphere while infants carried out either the button press or grasping actions. This effect was ipsilateral to the hand predominantly used by the infants (right), was not specific to one particular scalp region within the right hemisphere, and was present in the epochs immediately preceding and following the onset of the button press or grasp. This finding of increased right-sided ERD during action execution is consistent with some work in adults (Stancak & Pfurtscheller, 1996) although results from infant EEG studies of action execution and action observation have thus far yielded inconsistent findings concerning hemispheric asymmetries (see Marshall & Meltzoff, 2011).
In terms of the overall pattern of ERD during infant’s own action production, there appear to be some differences relative to our prior findings (Marshall et al., 2011). In our original study, ERD during infant’s execution of the button press was strongest at central sites, while in the current study a similar pattern was not clearly observed. One contributing factor to this difference is that in our original work, the ERD measures for frontal and parietal regions combined a number of disparate electrodes (e.g., mean ERD from F3/F4, F7/F8 and Fz was averaged to create a frontal ERD measure). Supplementary inspection of the current data for button-press execution epochs suggested that such an analysis strategy – which reduces topographic specificity – moved the pattern of means more towards the pattern reported in our original study (Marshall et al., 2011). One additional difference between the current study and our prior work is that the magnitude of ERD across the scalp during infants’ action execution was somewhat greater in the current results. It is possible that known differences in the experimental protocols between the studies contributed to this effect. For instance, the current protocol differed from our original work in the use of two kinds of executed action versus only one in the original work, which may introduce issues of differing accuracy or effort required and also of habituation effects. In addition, the current study design necessitated a fixed order of execution and observation epochs rather than the alternation used in Marshall et al. (2011), and it employed only one baseline epoch per trial versus two in the original study.
As well as generating the two observation conditions, the inclusion of two action execution conditions in the current study allowed us to undertake a novel comparison of the pattern of EEG responses associated with the two different goal-direct actions (button pressing vs. grasping). In the 500 ms preceding the button press or grasp, during which the infants were reaching for the objects, there were no significant differences between the two kinds of action in their effect on the EEG response. However, a relatively subtle difference did emerge in the 500 ms following contact with the button or the toy. Specifically, the button press action was associated with significantly less desynchronization over the lateral parietal region, compared with the grasping action. Interestingly, this regional pattern of ERD following the execution of the infant’s button press bears at a least a superficial resemblance to that elicited during the observation of the experimenter’s reach toward the button. While formal statistical tests were not conducted to examine this resemblance more closely, this again raises the possibility of a link between cortical activity during the observation of the early stages of an unfolding action and the anticipated goals or consequences of that action.
In summary, the current study suggests utility in employing EEG methods to advance our understanding of the ways in which infants develop within an interactive, social world. In an analysis of the extant developmental work using EEG to investigate infants’ action processing, Marshall and Meltzoff (2011) framed several key theoretical questions pertaining to how prior self-experience in performing an action affects infants’ EEG responses to perceiving the actions. We developed a novel approach to addressing this question by manipulating the actions that infants carried out immediately before they observed an experimenter carry out a particular goal-directed action. Although our sample size was relatively small we were able to identify some noteworthy patterns that warrant further investigation. The finding that EEG responses during infants’ action observation varied as a function of their immediately preceding action experience is particularly provocative. Moreover, it is particularly interesting to begin to fit these emerging neuroscience results within a larger framework of established theory and behavioral data from developmental psychology showing infants’ preferential visual, action, and emotional responses to adults who act “Like Me” (e.g., Meltzoff 2007b; Meltzoff et al., in press; Meltzoff & Decety, 2003). Further work on this connection will help to elucidate more specifics of the neural processes linking action production and action perception in a social context.
Acknowledgments
This work was supported by NIH (HD-68734) and NSF (BCS-0642404) awards to PJM and NSF (OMA-0835854) and ONR (N00014-0910097) awards to ANM. We are grateful to Christina Comalli, Hayley Haaf, Jamie Matthews, Kate Ridge, and Sarah Sanders for assistance with data collection and coding.
Contributor Information
Joni N. Saby, Temple University
Peter J. Marshall, Temple University
Andrew N. Meltzoff, University of Washington
References
- Agnetta B, Rochat P. Imitative games by 9-, 14-, and 18-month-old infants. Infancy. 2004;6:1–36. [Google Scholar]
- Arnstein D, Cui F, Keysers C, Maurits NM, Gazzola V. Mu-suppression during action observation and execution correlates with BOLD in dorsal premotor, inferior parietal, and SI cortices. Journal of Neuroscience. 2011;31:14243–14249. doi: 10.1523/JNEUROSCI.0963-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asendorpf JB, Warkentin V, Baudonniere P. Self-awareness and other-awareness. II: Mirror self-recognition, social contingency awareness, and synchronic imitation. Developmental Psychology. 1996;32:313–321. [Google Scholar]
- Babiloni C, Capotosto P, Del Percio C, Babiloni F, Petrini L, Buttiglione M, et al. Sensorimotor interaction between somatosensory painful stimuli and motor sequences affects both anticipatory alpha rhythms and behavior as a function of the event side. Brain Research Bulletin. 2010;81:398–405. doi: 10.1016/j.brainresbull.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Capotosto P, Babiloni C, Romani GL, Corbetta M. Frontoparietal cortex controls spatial attention through modulation of anticipatory alpha rhythms. Journal of Neuroscience. 2009;29:5863–5872. doi: 10.1523/JNEUROSCI.0539-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartrand TL, Bargh JA. The chameleon effect: The perception-behavior link and social interaction. Journal of Personality and Social Psychology. 1999;76:893–910. doi: 10.1037//0022-3514.76.6.893. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Yang CY, Lin CP, Lee PL, Decety J. The perception of pain in others suppresses somatosensory oscillations: A magnetoencephalography study. NeuroImage. 2008;40:1833–1840. doi: 10.1016/j.neuroimage.2008.01.064. [DOI] [PubMed] [Google Scholar]
- Csibra G. Action mirroring and action understanding: An alternative account. In: Haggard P, Rosetti Y, Kawato M, editors. Sensorimotor foundations of higher cognition: Attention and performance. XXII. Oxford: Oxford University Press; 2007. pp. 435–459. [Google Scholar]
- Decety J, Chaminade T, Grèzes J, Meltzoff AN. A PET exploration of the neural mechanisms involved in reciprocal imitation. NeuroImage. 2002;15:265–272. doi: 10.1006/nimg.2001.0938. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P, Singer W. Dynamic predictions: Oscillations and synchrony in top-down processing. Nature Reviews Neuroscience. 2001;2:704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- Fernandez T, Harmony T, Silva J, Galan L, Diaz-Comas L, Bosch J, et al. Relationship of specific EEG frequencies at specific brain areas with performance. Neuroreport. 1998;9:3681–3687. doi: 10.1097/00001756-199811160-00021. [DOI] [PubMed] [Google Scholar]
- Formaggio E, Storti SF, Avesani M, Cerini R, Milanese F, Gasparini A, et al. EEG and fMRI coregistration to investigate the cortical oscillatory activities during finger movement. Brain Topography. 2008;21:100–111. doi: 10.1007/s10548-008-0058-1. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Simpson GV, Ahlfors SP. Parieto-occipital approximately 10 Hz activity reflects anticipatory state of visual attention mechanisms. Neuroreport. 1998;9:3929–3933. doi: 10.1097/00001756-199812010-00030. [DOI] [PubMed] [Google Scholar]
- Gazzola V, Keysers C. The observation and execution of actions share motor and somatosensory voxels in all tested subjects: Single-subject analyses of unsmoothed fMRI data. Cerebral Cortex. 2009;19:1239–1255. doi: 10.1093/cercor/bhn181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guionnet S, Nadel J, Bertasi E, Sperduti M, Delaveau P, Fossati P. Reciprocal Imitation: Toward a Neural Basis of Social Interaction. Cerebral Cortex. 2012;22:971–978. doi: 10.1093/cercor/bhr177. [DOI] [PubMed] [Google Scholar]
- Hari R. Action-perception connection and the cortical mu rhythm. Progress in Brain Research. 2006;159:253–260. doi: 10.1016/S0079-6123(06)59017-X. [DOI] [PubMed] [Google Scholar]
- Hari R, Salmelin R. Human cortical oscillations: A neuromagnetic view through the skull. Trends in Neurosciences. 1997;20:44–49. doi: 10.1016/S0166-2236(96)10065-5. [DOI] [PubMed] [Google Scholar]
- Hauf P, Aschersleben G, Wolfgang P. Baby do-baby see! How action production influences action perception in infants. Cognitive Development. 2007;22:16–32. [Google Scholar]
- Jackson PL, Meltzoff AN, Decety J. Neural circuits involved in imitation and perspective-taking. NeuroImage. 2006;31:429–439. doi: 10.1016/j.neuroimage.2005.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob P. What do mirror neurons contribute to human social cognition? Mind and Language. 2008;23:190–223. [Google Scholar]
- Jung TP, Makeig S, Humphries C, Lee TW, McKeown MJ, Iragui V, et al. Removing electroencephalographic artifacts by blind source separation. Psychophysiology. 2000;37:163–178. [PubMed] [Google Scholar]
- Keysers C, Kaas JH, Gazzola V. Somatosensation in social perception. Nature Reviews Neuroscience. 2010;11:417–428. doi: 10.1038/nrn2833. [DOI] [PubMed] [Google Scholar]
- Kilner JM. More than one pathway to action understanding. Trends in Cognitive Sciences. 2011;15:352–357. doi: 10.1016/j.tics.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourtis D, Sebanz N, Knoblich G. Favouritism in the motor system: Social interaction modulates action simulation. Biology Letters. 2010;6:758–761. doi: 10.1098/rsbl.2010.0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn S, Muller BC, van Baaren RB, Wietzker A, Dijksterhuis A, Brass M. Why do I like you when you behave like me? Neural mechanisms mediating positive consequences of observing someone being imitated. Social Neuroscience. 2010;5:384–392. doi: 10.1080/17470911003633750. [DOI] [PubMed] [Google Scholar]
- Marshall PJ, Bar-Haim Y, Fox NA. Development of the EEG from 5 months to 4 years of age. Clinical Neurophysiology. 2002;113:1199–1208. doi: 10.1016/s1388-2457(02)00163-3. [DOI] [PubMed] [Google Scholar]
- Marshall PJ, Meltzoff AN. Neural mirroring systems: Exploring the EEG mu rhythm in human infancy. Developmental Cognitive Neuroscience. 2011;1:110–123. doi: 10.1016/j.dcn.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall PJ, Young T, Meltzoff AN. Neural correlates of action observation and execution in 14-month-old infants: An event-related EEG desynchronization study. Developmental Science. 2011;14:474–480. doi: 10.1111/j.1467-7687.2010.00991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff AN. Foundations for developing a concept of self: The role of imitation in relating self to other, and the value of social mirroring, social modeling, and self-practice in infancy. In: Cicchetti D, Beeghly M, editors. The self in transition: Infancy to childhood. Chicago: The University of Chicago Press; 1990. pp. 139–164. [Google Scholar]
- Meltzoff AN. ‘Like me’: A foundation for social cognition. Developmental Science. 2007a;10:126–134. doi: 10.1111/j.1467-7687.2007.00574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff AN. The ‘like me’ framework for recognizing and becoming an intentional agent. Acta Psychologica. 2007b;124:26–43. doi: 10.1016/j.actpsy.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff AN, Decety J. What imitation tells us about social cognition: A rapprochement between developmental psychology and cognitive neuroscience. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences. 2003;358:491–500. doi: 10.1098/rstb.2002.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff AN, Kuhl PK, Movellan J, Sejnowski TJ. Foundations for a new science of learning. Science. 2009;325:284–288. doi: 10.1126/science.1175626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff AN, Williamson RA, Marshall PJ. Developmental perspectives on action science: Lessons from infant imitation and cognitive neuroscience. In: Prinz W, Beisert M, Herwig A, editors. Tutorials in action science. Cambridge, MA: MIT Press; (in press) [Google Scholar]
- Meyer M, Hunnius S, van Elk M, van Ede F, Bekkering H. Joint action modulates motor system involvement during action observation in 3-year-olds. Experimental Brain Research. 2011;211:581–592. doi: 10.1007/s00221-011-2658-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Singh KD. Modulation of the human mirror neuron system during cognitive activity. Psychophysiology. 2008;45:896–905. doi: 10.1111/j.1469-8986.2008.00711.x. [DOI] [PubMed] [Google Scholar]
- Nystrom P, Ljunghammar T, Rosander K, von Hofsten C. Using mu rhythm desynchronization to measure mirror neuron activity in infants. Developmental Science. 2011;14:327–335. doi: 10.1111/j.1467-7687.2010.00979.x. [DOI] [PubMed] [Google Scholar]
- Orekhova EV, Stroganova TA, Posikera IN. Alpha activity as an index of cortical inhibition during sustained internally controlled attention in infants. Clinical Neurophysiology. 2001;112:740–749. doi: 10.1016/s1388-2457(01)00502-8. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G. Induced oscillations in the alpha band: Functional meaning. Epilepsia. 2003;44(Suppl 12):2–8. doi: 10.1111/j.0013-9580.2003.12001.x. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clinical Neurophysiology. 1999;110:1842–1857. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Neuper C. Event-related synchronization of mu rhythm in the EEG over the cortical hand area in man. Neuroscience Letters. 1994;174:93–96. doi: 10.1016/0304-3940(94)90127-9. [DOI] [PubMed] [Google Scholar]
- Reid VM, Striano T, Iacoboni M. Neural correlates of dyadic interaction during infancy. Developmental Cognitive Neuroscience. 2011;1:124–130. doi: 10.1016/j.dcn.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter P, Moosmann M, Villringer A. Rolandic alpha and beta EEG rhythms’ strengths are inversely related to fMRI-BOLD signal in primary somatosensory and motor cortex. Human Brain Mapping. 2009;30:1168–1187. doi: 10.1002/hbm.20585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby P, Decety J. Effect of subjective perspective taking during simulation of action: A PET investigation of agency. Nature Neuroscience. 2001;4:546–550. doi: 10.1038/87510. [DOI] [PubMed] [Google Scholar]
- Ruby P, Decety J. How would you feel versus how do you think she would feel? A neuroimaging study of perspective-taking with social emotions. Journal of Cognitive Neuroscience. 2004;16:988–999. doi: 10.1162/0898929041502661. [DOI] [PubMed] [Google Scholar]
- Schuch S, Bayliss AP, Klein C, Tipper SP. Attention modulates motor system activation during action observation: Evidence for inhibitory rebound. Experimental Brain Research. 2010;205:235–249. doi: 10.1007/s00221-010-2358-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder AC, Foxe JJ. Anticipatory attentional suppression of visual features indexed by oscillatory alpha-band power increases: A high-density electrical mapping study. Journal of Neuroscience. 2010;30:4024–4032. doi: 10.1523/JNEUROSCI.5684-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southgate V, Johnson MH, El Karoui I, Csibra G. Motor system activation reveals infants’ on-line prediction of others’ goals. Psychological Science. 2010;21:355–359. doi: 10.1177/0956797610362058. [DOI] [PubMed] [Google Scholar]
- Southgate V, Johnson MH, Osborne T, Csibra G. Predictive motor activation during action observation in human infants. Biology Letters. 2009;5:769–772. doi: 10.1098/rsbl.2009.0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stancak A, Jr, Pfurtscheller G. Mu-rhythm changes in brisk and slow self-paced finger movements. Neuroreport. 1996;7:1161–1164. doi: 10.1097/00001756-199604260-00013. [DOI] [PubMed] [Google Scholar]
- Stapel JC, Hunnius S, van Elk M, Bekkering H. Motor activation during observation of unusual versus ordinary actions in infancy. Social Neuroscience. 2010;5:451–460. doi: 10.1080/17470919.2010.490667. [DOI] [PubMed] [Google Scholar]
- Suffczynski P, Kalitzin S, Pfurtscheller G, Lopes da Silva FH. Computational model of thalamo-cortical networks: Dynamical control of alpha rhythms in relation to focal attention. International Journal of Psychophysiology. 2001;43:25–40. doi: 10.1016/s0167-8760(01)00177-5. [DOI] [PubMed] [Google Scholar]
- van Elk M, van Schie HT, Hunnius S, Vesper C, Bekkering H. You’ll never crawl alone: Neurophysiological evidence for experience-dependent motor resonance in infancy. NeuroImage. 2008;43:808–814. doi: 10.1016/j.neuroimage.2008.07.057. [DOI] [PubMed] [Google Scholar]