To the Editor
The mammalian epidermis is maintained by epithelial stem cells (SCs) capable of self-renewal and differentiation (Fuchs, 2009). While the transcription factor p63 is critical for the proliferative potential of epithelial SCs (Senoo et al., 2007), the molecular events underlying their transition to more differentiated progenitors remain largely unknown. Technical challenges in distinguishing SCs from transit-amplifying (TA) cells, early progeny of SCs (Watt, 2001), complicate such analyses and impedes development of new strategies to improve SC quality for therapeutic transplantation and SC-directed gene therapy.
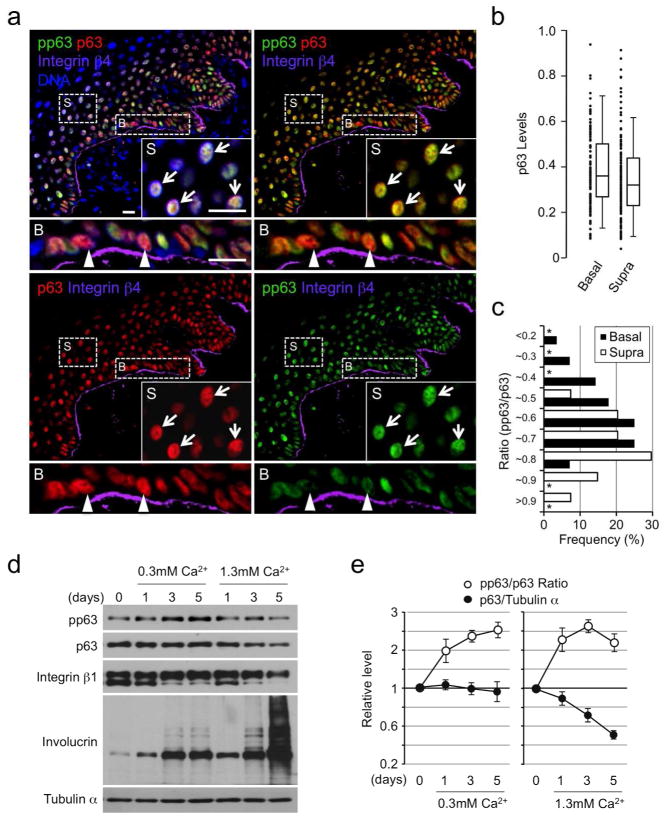
A current model dictates that SCs in the interfollicular epidermis are located in the basal layer while TA cells are found in both the basal and suprabasal layers (Watt, 2001; Fuchs, 2008). As epidermal SCs express relatively high levels of p63 (p63hi) in culture (Pellegrini et al., 2001; Senoo et al., 2007), we first investigated if p63hi cells were restricted to the epidermal basal layer. To identify the basal layer, we used integrin β4 (Radoja et al., 2006). Unexpectedly, we found p63hi cells in both basal and suprabasal layers in adult human epidermis (Figure 1a and 1b), suggesting that high p63 expression is not restricted to SCs. Because the decrease in p63 expression accompanying epidermal cell differentiation appears linked to phosphorylation and consequent targeting for proteasome-mediated degradation (Westfall et al., 2005), we next asked if initiation of SC differentiation is accompanied by an increase in p63 phosphorylation.
Figure 1. Expression of p63 and pp63 in human epidermis and cultured keratinocytes.
(a) Immunofluorescence staining of normal human epidermis for p63, pp63 and integrin β4. Insets and lower panels are enlarged views of representative suprabasal (S) and basal (B) cells, respectively. Arrowheads and arrows indicate representative p63hi cells with relatively low and high p63 phosphorylation, respectively; DNA, nuclear counterstaining; Bars=20μm.
(b) Comparison of the p63 levels normalized by nuclear counterstaining between the basal (integrin β4+, n=58) and the suprabasal (integrin β4−, n=192) cells from a single image by box-and-whisker plots. Shown are representative data from three independent experiments with similar results.
(c) Histogram of the pp63/p63 ratio among p63hi cells (top 50% in (b)). *, not detectable.
(d) Western blot analysis of Ca2+-induced differentiation of HPEKs.
(e) Quantification of (d) for the changes in p63 levels and the pp63/p63 ratio with time.
Toward this end, we first assessed total p63 and pp63 levels in individual adult human epidermal cells by quantitative immunofluorescence. The specificity of anti-pp63 antibodies was verified by immunohistochemistry and western blot analysis (Figure S1). Our data show that pp63 levels were variable within cells with similar p63 levels, including those with high p63 expression (Figure 1a, insets and lower panels). Notably, p63hi cells with extremely low p63 phosphorylation were found exclusively in the basal layer while those with increased p63 phosphorylation were observed in both the basal and suprabasal layers (Figure 1a and1c). These data suggest that epidermal SCs are included in the p63hipp63lo basal layer population and that p63hi cells with increased levels of p63 phosphorylation represent more differentiated epidermal progenitor cells.
To investigate how pp63 levels change in relation to total p63 expression during epidermal cell differentiation, we analyzed Ca2+-induced differentiation of human primary epidermal keratinocytes (HPEKs). Consistent with an earlier report (Westfall et al., 2003), stimulation of HPEKs with high Ca2+ led to a rapid decrease in total p63 expression and an associated increase in the pp63/p63 ratio (Figure 1d and 1e). These changes were accompanied by a rapid decrease in integrin β1, a marker associated with epidermal SC function (Jones and Watt, 1993) and a significant increase in total and cross-linked involucrin, a marker for terminal differentiation (Banks-Schlegel and Green, 1981).
Notably, however, we found that stimulation of HPEKs with lower Ca2+ led to a gradual increase in p63 phosphorylation, with unchanged total p63 expression (Figure 1e), suggesting that phosphorylation of p63 precedes differentiation-dependent, proteasome-mediated degradation of p63. In support, integrin β1 expression was sustained in HPEKs cultured in lower Ca2+ and increased involucrin expression was restricted to lower molecular weight forms (Figure 1d). Although the latter effect appears as sensitive as p63 phosphorylation in vitro, basal and first suprabasal cells in the epidermis do not express detectable levels of these terminal differentiation markers (Banks-Schlegel and Green, 1981). Thus, we conclude that increased p63 phosphorylation is a useful marker that can be utilized to detect the initiation of epidermal cell differentiation both in vitro and in vivo before a significant decrease in p63 expression is observed.
Next, we sought to determine whether changes in pp63 levels reflect the proliferative potential of epidermal SCs in clonogenic cultures designed for generation of therapeutic skin grafts (Green, 2008). In these systems, epidermal cells form three types of clones; holoclones, meroclones and paraclones in decreasing order of proliferative potential and p63 expression, and increasing order of differentiation (Barrandon and Green, 1987; Pellegrini et al., 2001; Senoo et al., 2007). Holoclones consist primarily of SCs, are homogeneous for high p63 expression, and are morphologically immature (Figure S2). Long-term culture of holoclones generates meroclone-producing cells, which represent more differentiated epidermal progenitors. As such, these cultures are ideal for studying early stages of SC differentiation into TA cells.
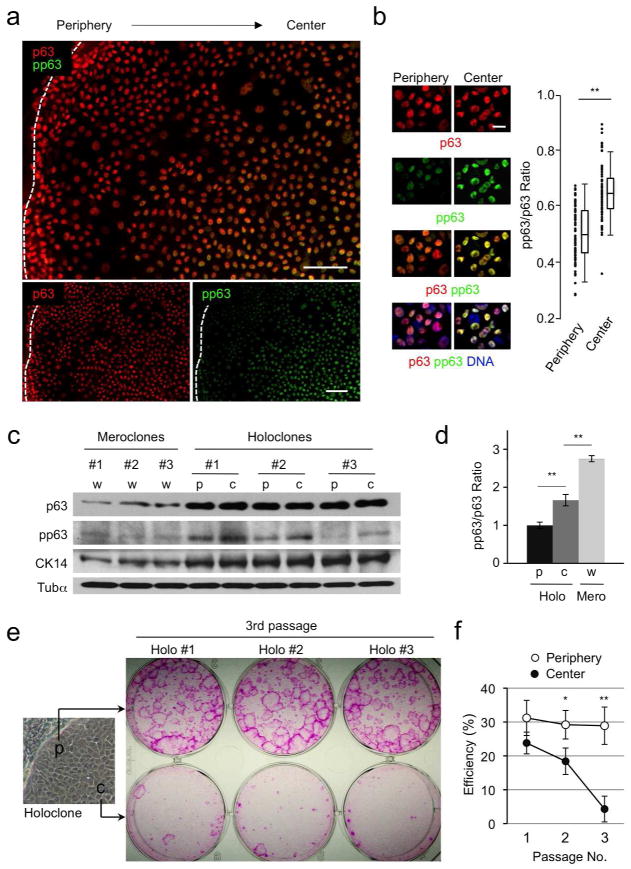
Notably, we found that although total p63 expression in holoclones was uniformly high, cells located within the center showed increased pp63 levels compared with those near the periphery (Figure 2a–2d). Staining with an anti-cytokeratin-14 (CK14) antibody, a marker for immature epidermal cells (Bragulla and Homberger, 2009), revealed that both peripheral and central cells in holoclones expressed similarly high levels of CK14 that differed significantly from those in meroclone-forming cells (Figure 2c and S2). These data indicate that single holoclone-forming SCs produce p63hi cells with variable levels of pp63 and that cells with relatively low and high levels of pp63 can be distinguished by their relative location within holoclones.
Figure 2. pp63 levels and the proliferative potential of epidermal SCs.
(a) Immunofluorescence staining of holoclones for p63 and pp63. Dotted lines indicate the clone border. Bars=50μm.
(b) Left, Expression of p63 and pp63 in the peripheral and central cells isolated from holoclones. Right, Comparison of the pp63/p63 ratios between the peripheral (n=83) and the central (n=74) cells by box-and-whisker plots. DNA, nuclear counterstaining; Bar=20μm; **, p<0.01.
(c) Western blot analysis of p63, pp63 and CK14 levels in the peripheral (p) and central (c) cells from holoclones and whole (w) meroclone-forming cells.
(d) Quantification of the pp63/p63 ratio from the data shown in (c). **, p<0.01.
(e) Representative data for the proliferative potential of cells isolated from the periphery and the center of holoclones at passage 3.
(f) Comparison of holoclone-forming efficiency between peripheral and central cells at different passages (n=3). *, p<0.05; **, p<0.01.
To determine if p63hi cells with increased pp63 levels had reduced proliferative potential, we performed clonogenic analysis of isolated cells from the center and periphery of holoclones. Isolated cells were cultured at clonal density and equal percentages of cells were serially passaged at two-week intervals. Our data show that while peripheral cells with relatively low levels of pp63 continuously formed holoclones at high efficiency through multiple passages, the proliferative potential of central cells with higher levels of pp63 declined rapidly (Figure 2e and 2f). These data clearly indicate that phosphorylation of p63 inversely correlates with the proliferative potential of epidermal cells and that pp63 levels distinguish self-renewing SCs from TA cells with more limited proliferative potential.
Our current work demonstrates that high p63 expression alone is not sufficient to identify epidermal SCs and that increased p63 phosphorylation serves as a biomarker that delineates exit from the SC state into more differentiated progenitors. Thus, our studies highlight the significance of post-translational modification of stem cell self-renewal factors in adult stem cells. Investigation of the signals responsible for p63 phosphorylation will likely provide essential insight into the signaling pathways that regulate self-renewal and differentiation of epidermal SCs.
Supplementary Material
Acknowledgments
We are grateful to Dr. H. Green and Dr. J. Seykora for 3T3-J2 cells and human epidermal sections, respectively. The authors thank Drs. S. Ryeom, J. Holcombe and L. King for critical comments and proof reading of the manuscript. This work was supported in part by grants from the Pennsylvania Department of Health and the Skin Disease Research Center (5-P30-AR-057217-02) to M.S.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- Banks-Schlegel S, Green H. Involucrin synthesis and tissue assembly by keratinocytes in natural and cultured human epithelia. J Cell Biol. 1981;90:732–37. doi: 10.1083/jcb.90.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrandon Y, Green H. Three clonal types of keratinocyte with different capacities for multiplication. Proc Natl Acad Sci USA. 1987;84:2302–6. doi: 10.1073/pnas.84.8.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragulla HH, Homberger DG. Structure and functions of keratin proteins in simple, stratified, keratinized and cornified epithelia. J Anat. 2009;214:516–59. doi: 10.1111/j.1469-7580.2009.01066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E. Skin stem cells: rising to the surface. J Cell Biol. 2008;180:273–84. doi: 10.1083/jcb.200708185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E. The tortoise and the hair: slow-cycling cells in the stem cell race. Cell. 2009;137:811–19. doi: 10.1016/j.cell.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green H. The birth of therapy with cultured cells. Bioessays. 2008;30:897–903. doi: 10.1002/bies.20797. [DOI] [PubMed] [Google Scholar]
- Jones PH, Watt FM. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell. 1993;73:713–24. doi: 10.1016/0092-8674(93)90251-k. [DOI] [PubMed] [Google Scholar]
- Pellegrini G, Dellambra E, Golisano O, et al. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci USA. 2001;98:3156–61. doi: 10.1073/pnas.061032098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radoja N, Gazel A, Banno T, et al. Transcriptional profiling of epidermal differentiation. Physiol Genomics. 2006;27:65–78. doi: 10.1152/physiolgenomics.00031.2006. [DOI] [PubMed] [Google Scholar]
- Senoo M, Pinto F, Crum CP, et al. p63 is essential for the proliferative potential of stem cells in stratified epithelia. Cell. 2007;129:523–36. doi: 10.1016/j.cell.2007.02.045. [DOI] [PubMed] [Google Scholar]
- Watt FM. Stem cell fate and patterning in mammalian epidermis. Curr Opin Genet Dev. 2001;11:410–17. doi: 10.1016/s0959-437x(00)00211-2. [DOI] [PubMed] [Google Scholar]
- Westfall MD, Mays DJ, Sniezek JC, et al. The ΔNp63α phosphoprotein binds the p21 and 14-3-3 sigma promoters in vivo and has transcriptional repressor activity that is reduced by Hay-Wells syndrome-derived mutations. Mol Cell Biol. 2003;23:2264–76. doi: 10.1128/MCB.23.7.2264-2276.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall MD, Joyner AS, Barbieri CE, et al. Ultraviolet radiation induces phosphorylation and ubiquitin-mediated degradation of ΔNp63α. Cell Cycle. 2005;4:710–16. doi: 10.4161/cc.4.5.1685. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.