Abstract
Side population (SP) is identified as cells capable of excluding the fluorescent Hoechst dye and anticancer drugs, and represents hematopoietic stem cells and chemoresistant cells from several solid tumors. In this study, we confirmed the presence of SP cells in tumors from melanoma patients. Melanoma SP cells overexpressed ATP-binding-cassette (ABC) transporters, ABCB1 and ABCB5. We generated a direct in vivo xenograft model, and demonstrated that SP cells were resistant to paclitaxel, a substrate of ABCB1, both in vitro and in vivo. However, melanoma SP cells were also resistant to temozolomide, which is not a substrate for ABC transporters, through IL-8 upregulation. In addition, gene profiling studies identified three signaling pathways (NF- κB, α6-β4-integrin and IL-1) as differentially upregulated in melanoma SP cells, and there was a significant increase of PCDHB11 and decrease of FUK and TBX2 in these cells. Therefore, we provide evidence that SP is an enriched source of chemoresistant cells in human melanomas, and suggest that the selected genes and signaling pathways of SP cells may be a potential target for effective melanoma therapies. To our knowledge, this is previously unreported study to isolate SP cells from melanoma patients and to investigate the gene expression profiling of these cells.
INTRODUCTION
Malignant melanoma is the fifth most common cancer in the USA (Siegel et al., 2011) and the deadliest form of skin cancer. Despite various types of treatments, advanced stages of melanoma cells have proven to be intrinsically resistant to many therapies. Multiple mechanisms contribute to the development of drug resistance in melanoma, including induction of enzyme systems, reduced apoptosis, increased DNA repair, and intra- and extra-cellular drug transport (Helmbach et al., 2001; La Porta, 2007).
One of the well-known mechanisms of chemoresistance in cancers is the enhanced efflux of a broad spectrum of hydrophobic cytotoxic drugs. Recently, the ability to exclude the fluorescent Hoechst 33342 dye has been employed to isolate a side population (SP) from hematopoietic cells (Goodell et al., 1996). This phenotype of low fluorescent staining pattern results from a family of transporter proteins known as ATP-binding cassette (ABC) transporters, which are responsible for the exclusion of Hoechst 33342 (Gottesman et al., 2002; Helmbach et al., 2001). These SP cells have stem cell-like properties and are capable of self-renewal and differentiation (Dean et al., 2005). These characteristics of SP cells have also been found in various types of tumors (Dean, 2009; Dou et al., 2009; Grichnik et al., 2006; Hirschmann-Jax et al., 2004; Zhong et al., 2010), and SP cells with a high drug efflux capacity are identified as the potential cause of the chemoresistant phenotypes of cancer stem cells in human tumors (Dean et al., 2005; Hirschmann-Jax et al., 2004).
SP cells have previously been isolated from human melanoma cell lines (Grichnik et al., 2006), but yet to be isolated directly from patient melanoma tumors. One major challenge of analyzing patient samples is that substantial quantities of fresh tumor tissues are not available for isolation and investigation of SP cells that comprise only a small percentage of the tumor cells. Another difficulty is that experiments to further characterize biology and function of these cells are limited by the technical difficulties in preserving and expanding tumor cells that faithfully represent patient tumor biology. To that end, a direct in vivo xenograft model provides cohorts of tumor-bearing mice suitable for further biological analyses and drug treatment (Rubio-Viqueira et al., 2006). Maintaining tumor xenografts exclusively in vivo retains fundamental genotypic features of the primary tumor of direct relevance to preclinical drug testing (Daniel et al., 2009; Rubio-Viqueira et al., 2006). Therefore, to isolate and characterize the molecular features of a small fraction of SP cells in patient melanoma samples, we generated a direct in vivo xenograft model from human melanoma tumors. Furthermore, our gene profiling analysis discovered a series of genes and pathways potentially involved in chemoresistance in melanoma SP cells.
RESULTS
Patient melanoma tumors contain a side population that excludes Hoechst dye
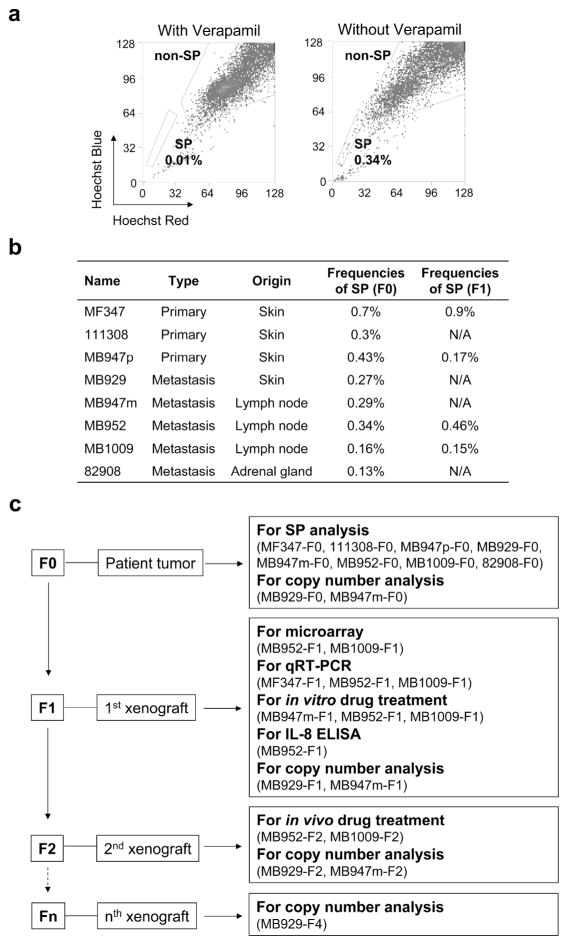
The presence of SP cells in patient melanoma tissues was first investigated by staining single cell suspensions with Hoechst 33342 dye. Flow cytometry plots depict Hoechst excitation at blue and red wavelengths. The cells with the least amount of Hoechst staining, which disappear in the presence of verapamil, an inhibitor of Hoechst 33342 transport, are gated as SP (Figure 1a). All of the patient melanoma tumors, including 3 skin primary melanomas and 5 metastatic melanomas, contained a small but clear SP fraction, ranging from 0.13% to 0.7% of all gated cells (Figure 1b). We then generated a direct in vivo xenograft model from human melanoma specimens to characterize SP cells from patient-derived tumors (Figure 1c). Original patient tumors were designated as F0 tumors whereas those grown from F0 tumors were designated as F1 tumors. F1 tumors were further implanted in F2 mice for in vivo drug treatment (Figure 1c). After characterizing SP cells using this model, cells from HS294T were utilized for further biological and mechanistic studies because the cell line contains a larger SP fraction (Supplementary Figure S1).
Figure 1. SP is a rare population in patient melanoma tissues.
(a) A representative SP flow cytometry profile from a patient tumor tissue (MB952-F0). Cells were stained with Hoechst 33342 dye in the presence (left panel) or absence (right panel) of verapamil. SP cells are shown in the lower left gated area. (b) Frequency of SP fractions in eight patient melanoma specimens (F0 tumors) and selected F1 tumors. (c) Study schema. Clinical melanoma specimens (F0) were used for SP analysis and copy number analysis. Tumor specimens used in each analysis are listed in parentheses. Tumors were serially passaged into mice (F1, F2, ---, Fn) for further analyses.
Melanoma SP cells are resistant to paclitaxel and temozolomide IN VITRO and IN VIVO
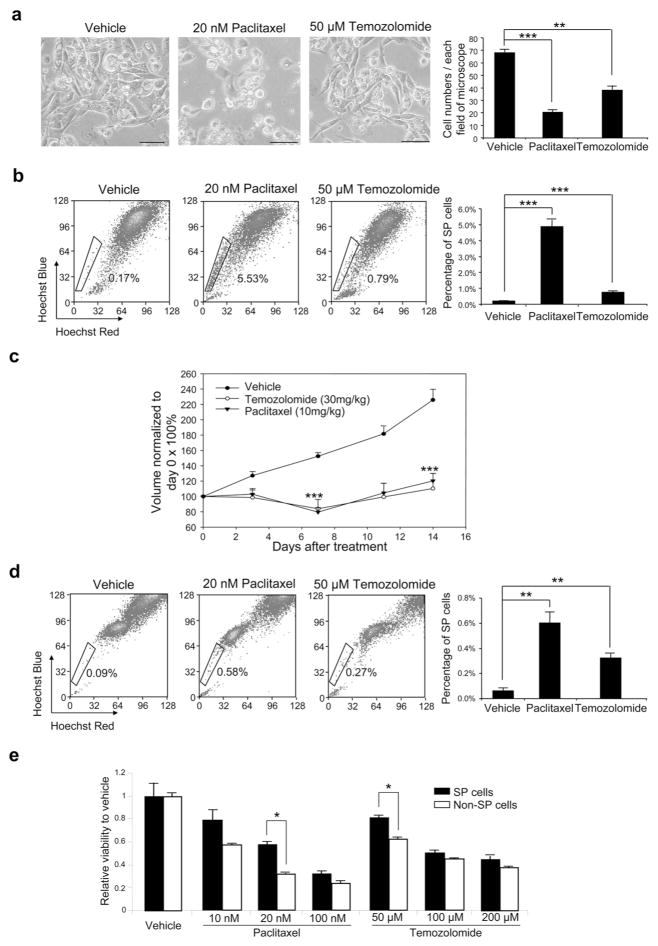
SP cells expressing ABC transporters have been shown to be resistant to multiple drugs (Dean, 2009; Duan et al., 2004). Paclitaxel, a substrate of ABCB1 transporter (Duan et al., 2004), has been widely evaluated and shown to have positive outcomes in phase II/III clinical trials of malignant melanoma (Bedikian et al., 2004; Hauschild et al., 2009). Therefore, we treated human melanomas with paclitaxel to examine the drug sensitivity of SP cells. Temozolomide, an alkylating agent for melanoma treatment (Bleehen et al., 1995), is not a substrate of ABC transporters and thus was used as a control. As shown in Figure 2a, melanoma cells from MB1009-F1 tumor were sensitive to 20 nM paclitaxel treatment in vitro. This treatment resulted in a 32.5-fold increase in SP cells compared with vehicle control (Figure 2b). On the other hand, temozolomide treatment induced less cell death, and induced moderate increase in SP cells. Increase in SP cells after drug treatment despite absence of overall cell increase indicates that SP cells are more resistant to the drugs than non-SP cells.
Figure 2. Drug resistance of SP cells.
(a) Left panels, microscopic images of MB1009-F1 cells following 48-hour treatment with vehicle, paclitaxel or temozolomide in vitro. Bar = 100 μm. Right panel, average number of cells in each microscopic field (n=6). (b) Left panels, SP cells from MB952-F1 tumors after in vitro treatment. Right panel, percentage of SP cells (n=3). (c) Growth curve of MB1009-F2 tumors after treatment with vehicle, paclitaxel or temozolomide in vivo (n=6). ***P<0.001 compared to vehicle. (d) Left panels, SP cells from MB952-F2 tumors after in vivo treatment. Right panel, percentage of SP cells (n=3). (e) Viability of SP and non-SP cells from HS294T after 72 hours of treatment with vehicle, paclitaxel, or temozolomide (n=3). All data represent mean ± S.E. *P<0.05, **P<0.01, ***P<0.001.
To further analyze drug resistance in vivo, mice with MB1009-F2 tumors were treated with paclitaxel or temozolomide. Following paclitaxel treatment, a significant decrease in tumor size (79.4 ± 5.9% of original size) was observed after 7 days, but the tumors grew thereafter (125.1 ± 13.2% of original size on day 14) (Figure 2c). Temozolomide showed inhibitory effects similar to paclitaxel in vivo. Tumor analysis on day 14 revealed an increase in SP cells after paclitaxel treatment and a modest increase after temozolomide treatment when compared with vehicle (Figure 2d), indicating that SP cells are resistant to paclitaxel and temozolomide in vivo. This relative resistance of SP cells to drugs was further investigated by treating SP and non-SP cells with drugs separately. When SP cells and non-SP cells from HS294T were treated with paclitaxel or temozolomide, SP cells showed less sensitivity to both drugs than non-SP cells, confirming the drug resistance of SP cells compared to non-SP cells (Figure 2e).
ABCB1 and ABCB5 are selectively upregulated in human melanoma SP cells
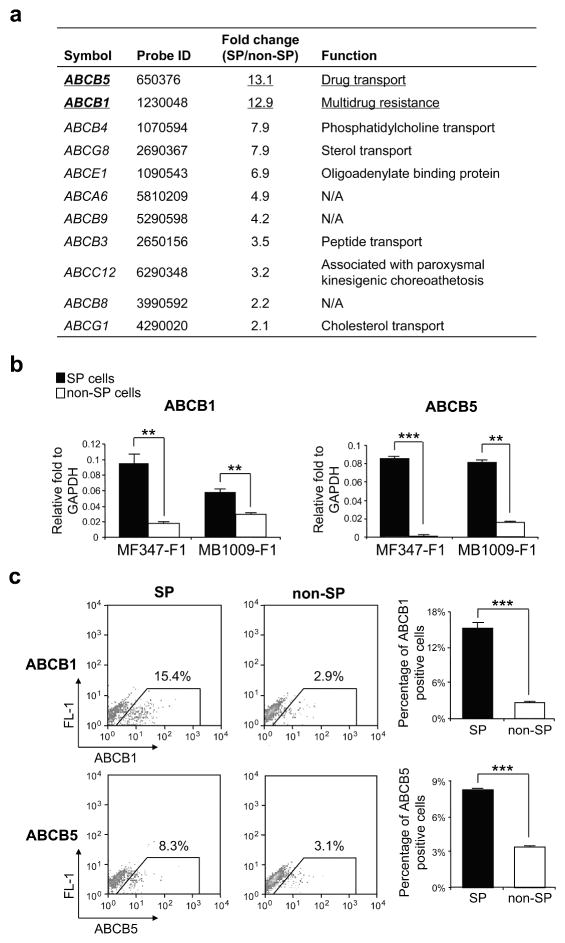
ABC transporters, particularly ABCB1 and ABCG2, have been associated with resistance to multiple drugs (Dean, 2009; Dean et al., 2001; Duan et al., 2004; Frank et al., 2005). To identify the ABC transporters responsible for the exclusion of Hoechst 33342 in melanoma SP cells, we first compared the expression levels of all 48 ABC transporters between SP and non-SP cells (from MB952-F1 and MB1009-F1 tumors) via microarray analysis. Results demonstrated that SP cells expressed 13-fold more ABCB1 and ABCB5 than non-SP cells (Figure 3a), verifying the accuracy of the SP isolation method and suggesting that these ABC transporters but not ABCG2 are likely responsible for the exclusion of Hoechst 33342. Upregulation of ABCB1 and ABCB5 in SP cells was confirmed by real-time quantitative reverse transcription-PCR (qRT-PCR) using MF347-F1 and MB1009-F1 tumors (Figure 3b), and flow cytometric analysis using HS294T (Figure 3c).
Figure 3. Expression of ABC transporters in SP cells.
(a) List of ABC transporters upregulated by more than 2-fold in SP cells from the microarray analysis, their fold changes and functions. The genes with a fold change of more than 10.0 were underlined. N/A indicates that gene function is not characterized. (b) qRT-PCR validation of ABCB5 and ABCB1 expression (mean ± S.E.) in SP cells from MF347-F1 and MB1009-F1 tumors (n=3). (c) Left panel, representative flow cytometry analysis of ABCB1 and ABCB5 in SP and non-SP cells from HS294T. Right panel, percentage of positive cells in SP and non-SP cells. **P<0.01, ***P<0.001.
Resistance to paclitaxel in SP cells is associated with ABC transporters
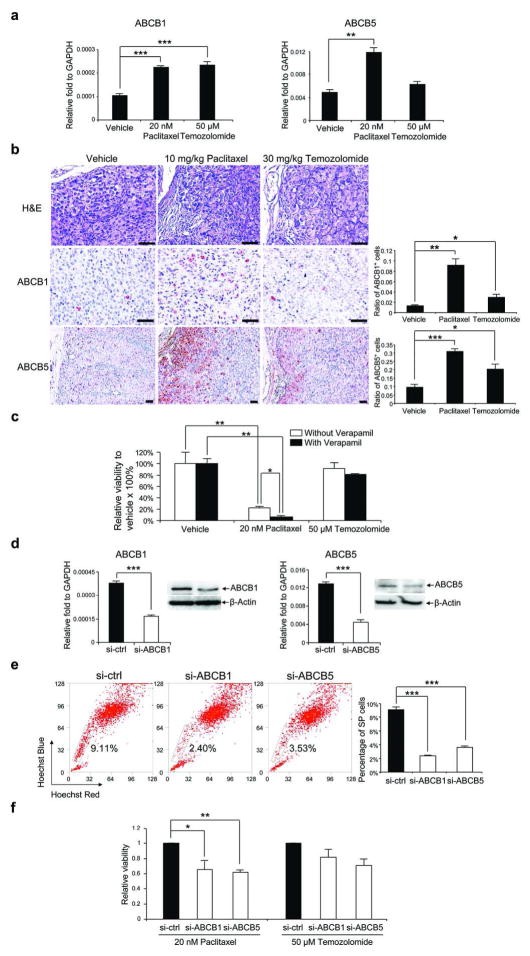
Because of the upregulation of ABCB1 and ABCB5 in SP cells, we hypothesized that the drug resistance is associated with these transporters. Consistent with the hypothesis, the expression of ABCB1 and ABCB5 was upregulated after treatment with paclitaxel or temozolomide in vitro (in HS294T) (Figure 4a) and in vivo (in MB1009-F2 tumors) (Figure 4b).
Figure 4. Mechanisms for paclitaxel resistance in SP cells.
(a) qRT-PCR of ABCB1 and ABCB5 in HS294T after in vitro drug treatment (n=3). (b) Left panels, H&E, ABCB1 and ABCB5 staining of M1009-F2 tumors after in vivo treatment. Scale bar = 100 μm. Right panel, percentage of positive cells (n=3). (c) Viability of MB952-F1 melanoma cells following treatment, with or without verapamil in vitro (n=3). (d) qRT-PCR and Western blot analysis of ABCB1 and ABCB5 in HS294T after siRNA transfection of scrambled (si-ctrl), ABCB1 (si-ABCB1) or ABCB5 (si-ABCB5) (n=3). (e) Left panels, SP cells in HS294T after siRNA transfection. Right panel, percentage of SP cells (n=3). (f) Viability of siRNA transfected cells after 72 hours of treatment with paclitaxel or temozolomide (n=9). All data represent mean ± S.E. *P<0.05, **P<0.01, ***P<0.001.
In order to determine if drug resistance is associated with a drug efflux capacity, tumor cells from MB952-F1 were treated with paclitaxel or temozolomide in vitro in the presence or absence of verapamil, an efflux pump inhibitor (Figure 4c). Verapamil treatment decreased sensitivity to paclitaxel but not temozolomide, suggesting that the resistance to paclitaxel in SP cells is at least partially dependent on drug efflux.
Next, to determine if the drug resistance of SP cells is associated with drug efflux by ABC transporters, we knocked down ABCB1 and ABCB5 using siRNA. Successful transfection of siRNA was confirmed by reduction in mRNA at 18 hours (60% and 65% reduction of ABCB1 and ABCB5, respectively) and protein levels at 72 hours in HS294T cells (Figure 4d). Compared with control siRNA, transfection of ABCB1 and ABCB5 siRNAs led to 74% and 62% reduction in SP percentage at 72 hours, respectively (Figure 4e), suggesting that SP phenotype of melanoma cells is associated with ABCB1 and ABCB5. Cell viability assay revealed that transient transfection of ABCB1 and ABCB5 siRNA in HS294T decreased sensitivity to paclitaxel (38% and 39% decrease with ABCB1 and ABCB5 siRNA, respectively) but not temozolomide (Figure 4f), suggesting that the resistance to paclitaxel in SP cells is dependent on ABCB1 and ABCB5, whereas other mechanisms are responsible for temozolomide resistance in these cells.
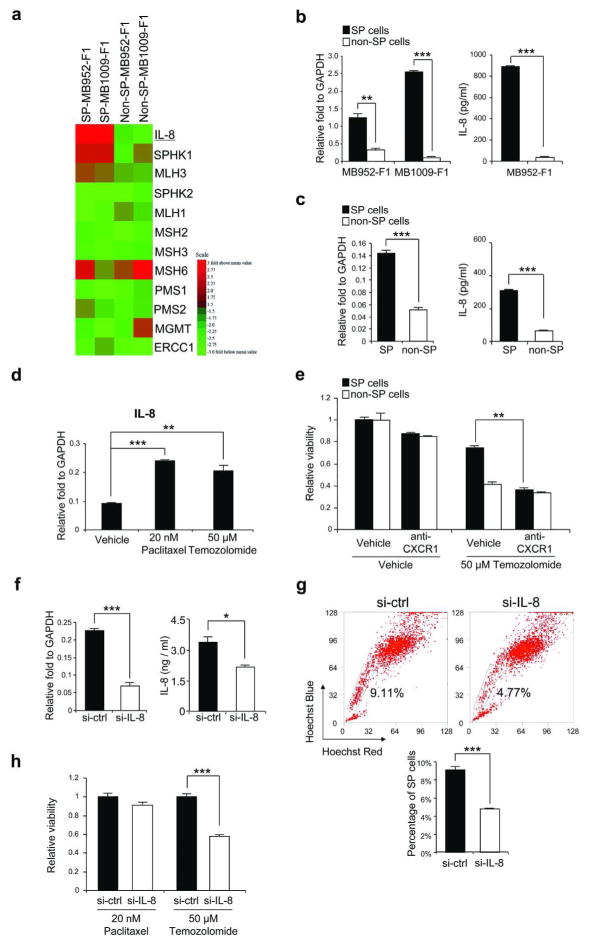
Resistance to temozolomide in SP cells is at least partly due to IL-8 upregulation
Previous studies by other researchers have shown that IL-8, sphingosine kinase (SPHK), mutL homolog (MLH), mutS homolog (MSH), postmeiotic segregation (PMS), O-6-methylguanine-DNA methyltransferase (MGMT), and excision repair cross-complementing rodent repair deficiency, complementation group (ERCC) 1 were related to the resistance to temozolomide (Boeckmann et al., 2009; Chelouche-Lev et al., 2004; Gerson, 2002; Narayan and Roy, 2003; Pilorget et al., 2007). The expression levels of these genes in SP and non-SP cells were therefore compared in our microarray data using patient-derived tumors, and we found that IL-8, SPHK1 and MLH3 were indeed selectively expressed in SP cells (Figure 5a). Since IL-8 was the most differentially expressed gene (22-fold in MB952-F1 and 55-fold in MB1009-F1), we focused on this molecule in our study. The upregulation of IL-8 in SP cells was verified by qRT-PCR in melanoma tissues (3.8-fold and 23.2-fold in MB952-F1 and MB1009-F1 tumors, respectively) (Figure 5b) and HS294T (2.8-fold) (Figure 5c). This was further confirmed by ELISA analysis in melanoma tumor cells (16.1-fold in MB952-F1) (Figure 5b) and HS294T (5.2-fold) (Figure 5c). When HS294T cells were treated with paclitaxel or temozolomide, IL-8 expression was enhanced 2.7-fold or 2.2-fold, respectively (Figure 5d). To determine whether this increased IL-8 expression plays a role in temozolomide resistance, we blocked the IL-8 signaling pathway using anti-CXCR1, a neutralizing antibody for IL-8R, in HS294T cells, and found this blockade significantly increased the sensitivity of SP cells to temozolomide, an effect not observed in non-SP cells (Figure 5e). We then knocked down IL-8 using siRNA. Successful transfection of siRNA was confirmed by reduction in mRNA at 18 hours (69%) and secreted cytokine level at 72 hours (37%) in HS294T cells (Figure 5f). Compared with control siRNA, transfection of IL-8 led to 48% reduction of SP percentage at 72 hours (Figure 5g), suggesting that SP phenotype of melanoma cells is associated with IL-8. Furthermore, cell viability assay revealed that transient transfection of IL-8 siRNA in HS294T decreased sensitivity to temozolomide (42%) but not paclitaxel (Figure 5h), suggesting that the resistance to temozolomide in SP cells is at least partially dependent on IL-8.
Figure 5. Mechanisms for temozolomide resistance in SP cells.
(a) Heatmap of temozolomide resistance genes. Red/green colors represent high/low expressions. (b, c) qRT-PCR (left panels) and ELISA (right panels) of IL-8 in patient-derived tumors (b) and HS294T (c). (d) qRT-PCR of IL-8 in HS294T after treatment. (e) Viability of SP and non-SP from HS294T cultured with or without temozolomide, and treated with vehicle or anti-CXCR1. (f) qRT-PCR (left panel) and ELISA (right panel) of IL-8 after siRNA transfection of scrambled (si-ctrl) or IL-8 (si-IL-8) in HS294T. (g) Upper panel, representative SP cells after IL-8-siRNA transfection in HS294T. Lower panel, percentage of SP cells after transfection. (h) Viability of IL-8-siRNA-transfected HS294T after treatment. All data represent mean ± S.E. (n=3 except h where n=9). *P<0.05, **P<0.01, ***P<0.001.
Because ABCB1, ABCB5 and IL-8 are associated with SP phenotypes, we investigated if they are related each other. We found that transfection of IL-8 siRNA did not affect the expression levels of ABCB1 and ABCB5 at 18 hours (Supplementary Figure S2a), and that transfection of ABCB1 or ABCB5 siRNA did not affect IL-8 expression levels at 18 hours, either (Supplementary Figure S2b), suggesting that these 2 mechanisms (ABC transporters and IL-8) are not directly associated with each other.
Gene profiling analyses via microarray and qRT-PCR
To further delineate chemoresistant mechanisms, we conducted microarray analysis to compare differentially expressed genes between SP and non-SP cells. 1729 transcripts were upregulated and 1888 transcripts were downregulated in SP cells (P<0.05, fold change > 2.0) (Supplementary Figure S3a). The most common biological processes of these transcripts defined by gene ontology annotations included cellular process (28.54%), metabolic process (17.51%) and biological regulation (17.38%) (Supplementary Figure S3b). Significant pathway analysis using the 3617 differentially expressed transcripts identified NF- κB, α6-β4-integrin and IL-1 pathways (P<0.05) (Supplementary Figure S4).
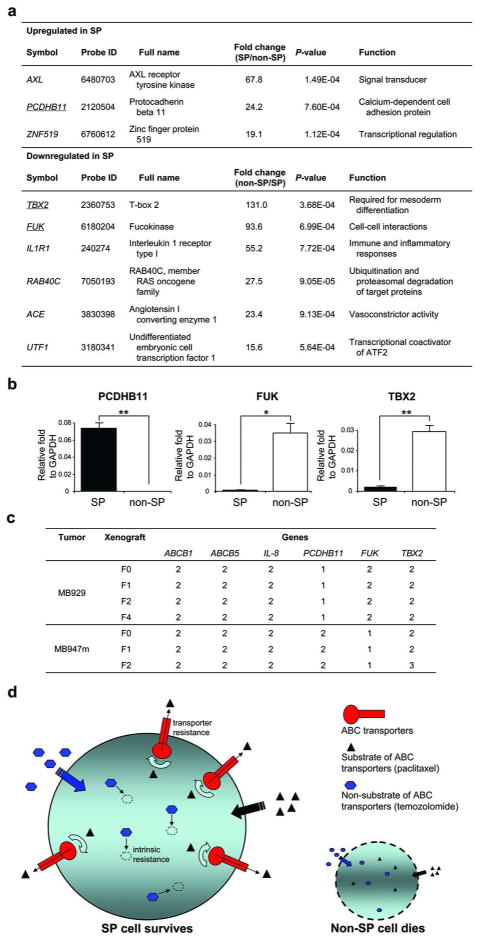
More stringent analysis (P<0.001 and fold change > 15) revealed three unregulated genes (AXL, PCDHB11 and ZNF519) and six downregulated genes (TBX2, FUK, IL1R1, RAB40C, ACE and UTF1) in SP cells (Figure 6a). Due to the limited amount of SP cDNA template, only one unregulated gene (PCDHB11) and two downregulated genes (TBX2 and FUK) were validated by qRT-PCR. Expression of PCDHB11 in SP cells from the MB1009-F1 tumor was 0.073-fold relative to that of GAPDH but its expression in non-SP cell was below the detection level (< 1 × 10−7 fold) (Figure 6b). Also, the non-SP/SP ratio was 91.7-fold for FUK measured by qRT-PCR, and 16.0-fold for TBX2. These results were further confirmed using the MB952-F1 tumor.
Figure 6. Differentially expressed genes in SP cells.
(a) A list of microarray genes that most discriminated SP from non-SP cells (P<0.001, fold change > 15). (b) qRT-PCR expression levels (mean ± S.E.) of PCDHB11, FUK and TBX2 in SP and non-SP cells (n=3). *P<0.05, **P<0.01. (c) Copy number analysis of genes analyzed in this study from F0-Fn tumors from 2 patient-derived tumors. (d) Two different drug resistance systems exist in SP cells. ABC transporters contribute to transporter resistance by pumping out drugs which are their substrates. Intrinsic resistance represents inherent survival systems in SP cells, such as induction of certain signaling pathways, enzyme systems, reduced apoptosis and increased DNA repair. Non-SP cells are sensitive to drug exposure because they lack the resistant systems.
Previous studies using a direct in vivo xenograft model of pancreatic cancer have shown that the status of key genes in pancreatic cancer is stable and drug susceptibility is a stable trait after passaging tumors into mice (Rubio-Viqueira et al., 2006). To address whether passaging melanoma tumors in mice alters genes in this study, we determined the copy number of 6 genes (ABCB1, ABCB5, IL8, PCDHB11, FUK and TBX2) in F0, F1, F2 and Fn tumors from 2 patient-derived samples. As shown in Figure 6c, we observed high concordance of gene copy number between original tumors and xenografted tumors.
DISCUSSION
This study isolated SP cells not only from the original patient melanoma tissues but also from in vivo xenograft mouse models. Melanoma SP cells were shown to be resistant to two melanoma drugs: paclitaxel and temozolomide, both in vitro and in vivo. Melanoma SP cells overexpressed ABCB1 and ABCB5, and their resistance to paclitaxel was at least partly attributed to ABC transporters. Furthermore, IL-8 plays a role in chemoresistance to temozolomide in SP cells. Microarray and qRT-PCR studies demonstrated upregulation of PCDHB11 and downregulation of FUK and TBX2 in SP cells, and identified NF- κB, α6-β4-integrin and IL-1 signaling networks differentially expressed between SP and non-SP cells. ABCB1, previously known as MDR1 or P-glycoprotein, has been demonstrated to confer resistance to multiple hydrophobic drugs (Gottesman et al., 2002). Primary cultures of human melanoma contain ABCB1-positive cells that co-express ABCB5 and ABCC2 (Keshet et al., 2008). ABCB5 was recently shown to mediate chemoresistance in human melanoma (Frank et al., 2005) and identify melanoma initiating cells (Schatton et al., 2008).
ABCB1, ABCB4 and ABCB5 are closely homologous (Tusnady et al., 2006), and ABCB5 and ABCB1 have overlapping substrate specificities (Frank et al., 2005), implying their cooperative functions in mediating multidrug resistance in SP cells. Furthermore, recent studies indicate that in addition to serving as drug efflux pumps, ABC transporters also play fundamental roles in melanogenesis (Chen et al., 2009), tumor biology (Fletcher et al., 2010) and immunomodulation (Schatton et al., 2010). Our results are consistent with the role that ABC transporters play in melanomas and the chemoresistance signature that characterizes SP cells.
The resistance of SP cells to temozolomide was observed in a glioma study (Bleau et al., 2009), but the specific mechanisms were not previously studied. Interestingly, we found that IL-8 was upregulated in melanoma SP cells, which is consistent with data from ovarian cancer and endometrial SP cells (Cervello et al., 2010; Moserle et al., 2008). In addition, we found that IL-8 upregulation is responsible for chemoresistance to temozolomide in SP cells. IL-8 signaling activates a set of cell survival and proliferation pathways including MAPK, PI3-K, JAK2-STAT3 and β-catenin signalings (Waugh and Wilson, 2008); thus, it comes as no surprise that this molecule plays an important role in the intrinsic chemoresistance of SP cells.
In addition to ABC transporters and IL-8, melanoma SP cells expressed genes and pathways associated with development, stemness and metabolic process. For example, PCDHB11 belongs to the protocadherin family, and the lack of protocadherins causes massive apoptosis of spinal interneurons in a mouse model (Lefebvre et al., 2008). TBX2 is necessary for cell proliferation and suppression of senescence in melanoma (Vance et al., 2005). FUK is associated with fucosylation, and reduced fucosylation in cancer induces immunoevasion and metastasis (Moriwaki et al., 2009).
Three pathways identified in melanoma SP cells may be closely related to chemoresistance. For example, NF- κB protects cells from apoptosis following DNA damage, and is a principal mechanism for chemoresistance (Yamamoto and Gaynor, 2001). Notably, NF- κB was shown to induce ABCB1 expression (Bentires-Alj et al., 2003; Zhou and Kuo, 1997). In addition, both α6-β4-integrin and IL-1 signaling pathways induce resistance to apoptosis by activation of NF- κB in tumors (Arlt et al., 2002; Weaver et al., 2002).
Therefore, it appears that chemoresistance of SP cells derives from at least two different types of mechanisms (Figure 6d). One is the ABC-transporter-conferred resistance due to efflux of drugs. The other is the intrinsic resistance to cytotoxic drugs possibly by induction of certain signaling pathways and enzyme systems, changes in cytoskeleton, reduced apoptosis and increased DNA repair.
In conclusion, we provide evidence that SP is an enriched source of chemoresistant cells in human melanoma tumors. These data will pave the road for further mechanistic studies of melanoma SP cells, and provide potential targets for future melanoma therapies.
MATERIALS AND METHODS
Clinical melanoma specimens
Human melanoma tissues were obtained from surgical specimens with written patient consent under institutional review board-approved protocols at the University of Colorado Hospital, adhering to the Helsinki Guidelines. Human melanoma tissues were cut into 3-mm3 pieces, and processed for cell isolation and direct in vivo xenograft.
Direct IN VIVO xenograft
Animal experiments were performed under the institutional guidelines for the use of laboratory animals. Under isofluorane anesthesia, patient tumors were implanted into 6-week-old female athymic (nu/nu) mice (NCI) by a small incision and subcutaneous pocket made in each side of the lower back (Rubio-Viqueira et al., 2006). These mice were designated as F1 mice. F1 tumors were harvested when reaching a size of 1,500 mm3 and implanted into F2 mice. Tumors were serially passaged into mice.
Tumor cell isolation and sorting of SP cells from tumor cells
Mechanically minced tumor tissues were digested with 235 U/ml collagenase (Sigma-Aldrich) and 850 U/ml hyaluronidase (Sigma-Aldrich) for 2 hours at 37 °C with intermittent vortex, followed by sequentially passing through 70- and 40-μm filters to obtain single cell suspension. Red blood cells were lysed using 1 X Red Blood Cell Lysis Buffer (eBioscience). Cells were washed and re-suspended in DMEM with 2% FBS and 1% HEPES at a concentration of 1 × 106 cells/ml, and stained with Hoechst 33342 dye (5 μg/ml, Sigma-Aldrich) in the presence or absence of Verapamil (50 μM, Sigma-Aldrich) at 37 °C for 90 minutes with intermittent vortex. Propidium iodide (1 μg/ml) was used to remove dead cells. Human stromal cells were eliminated from human melanoma tumors using phycoerythrin-cy7-labeled anti-human CD45 (eBioscience) and anti-human CD31 (eBioscience) antibodies. Mouse stromal cells were eliminated from xenografted tumors using phycoerythrin-cy7-labeled anti-mouse CD45 (eBioscience) and anti-mouse CD31 (eBioscience) antibodies. Human melanoma cells were then positively selected by allophycocyanin-labeled anti-human CD147 (eBioscience). SP and non-SP cells were sorted by MoFlo (DakoCytomation) and the results were analyzed with Summit software (DakoCytomation).
Sorting of SP cells from cell lines
Human metastatic melanoma cell line, HS294T, was obtained from the American Type Culture Collection. Cells were maintained in RPMI 1640 medium (Mediatech, Inc.) supplemented with 10% fetal bovine serum (Mediatech, Inc.), 100 units/ml penicillin, 0.1 mg/ml streptomycin and 2 mM L-glutamine. Single cells were prepared using 1 mM EDTA (Sigma-Aldrich) for 15 minutes at 37 °C and stained with Hoechst 33342 dye for SP analysis and sorting. Single cells were also used for biological analysis.
Copy number analysis of melanoma cells
DNA from human melanoma cells was isolated using QIAamp DNA Mini Kit (Qiagen) and quantified by PicoGreen® dsDNA assay (Invitrogen). Melanoma DNA (250 ng) was hybridized to SNP probes (Illumina) at the University of Colorado Denver microarray core. Probes consisted of 595 markers: 293 for ABCB1 (chr7:87132948-87342611); 249 for ABCB5 (chr7:20654830-20816658); 6 for IL-8 (chr4:74606223-74609433); 6 for PCDHB11 (chr5:140579183-140582618); 31 for FUK (chr16:70488498-70514177); and 10 for TBX2 (chr17:59477257-59486827). Probe copy number was analyzed using CNApartition V3.1.6 (Illumina).
Other methods
Further information about the materials and methods used in this work are provided in the Supplementary Materials and Methods online section.
Supplementary Material
Acknowledgments
The authors thank the University of Colorado Denver (UCD) melanoma tissue bank for providing human melanoma samples, the Flow Core of the University of Colorado Cancer Center (UCCC) and the Skin Disease Research Center (SDRC) (Alistaire S. Acosta and Karen Helm) for helping with FACS sorting, the Molecular Genetics Core from the SDRC and microarray core from UCD for helping with copy number analysis, and the Mind Research Network from New Mexico (Marilee Morgan and Kent Hutchison) for helping with microarray analysis. We thank Garrick Talmage, Tracey Ferrara and Katherine Gowan for assisting experiments. This work was supported by US National Institutes of Health grants P30CA046934 (N.G.A. and M.F. and the Flow Core of the UCCC), R03CA125833 (M.F.), P30AR057212 (Flow Core and the Molecular Genetics Core of the SDRC), Wendy Will Case Cancer Fund (M.F.) and the Tadamitsu Cancer Research Fund (M.F.).
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
References
- Arlt A, Vorndamm J, Muerkoster S, Yu H, Schmidt WE, Folsch UR, et al. Autocrine production of interleukin 1beta confers constitutive nuclear factor kappaB activity and chemoresistance in pancreatic carcinoma cell lines. Cancer Res. 2002;62:910–6. [PubMed] [Google Scholar]
- Bedikian AY, Plager C, Papadopoulos N, Eton O, Ellerhorst J, Smith T. Phase II evaluation of paclitaxel by short intravenous infusion in metastatic melanoma. Melanoma Res. 2004;14:63–6. doi: 10.1097/00008390-200402000-00010. [DOI] [PubMed] [Google Scholar]
- Bentires-Alj M, Barbu V, Fillet M, Chariot A, Relic B, Jacobs N, et al. NF-kappaB transcription factor induces drug resistance through MDR1 expression in cancer cells. Oncogene. 2003;22:90–7. doi: 10.1038/sj.onc.1206056. [DOI] [PubMed] [Google Scholar]
- Bleau AM, Hambardzumyan D, Ozawa T, Fomchenko EI, Huse JT, Brennan CW, et al. PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell. 2009;4:226–35. doi: 10.1016/j.stem.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleehen NM, Newlands ES, Lee SM, Thatcher N, Selby P, Calvert AH, et al. Cancer Research Campaign phase II trial of temozolomide in metastatic melanoma. J Clin Oncol. 1995;13:910–3. doi: 10.1200/JCO.1995.13.4.910. [DOI] [PubMed] [Google Scholar]
- Boeckmann L, Schirmer M, Rosenberger A, Struever D, Thoms KM, Gutzmer R, et al. Effect of DNA repair host factors on temozolomide or dacarbazine melanoma treatment in Caucasians. Pharmacogenet Genomics. 2009;19:760–9. doi: 10.1097/FPC.0b013e3283307cd9. [DOI] [PubMed] [Google Scholar]
- Cervello I, Gil-Sanchis C, Mas A, Delgado-Rosas F, Martinez-Conejero JA, Galan A, et al. Human endometrial side population cells exhibit genotypic, phenotypic and functional features of somatic stem cells. PLoS One. 2010;5:e10964. doi: 10.1371/journal.pone.0010964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelouche-Lev D, Miller CP, Tellez C, Ruiz M, Bar-Eli M, Price JE. Different signalling pathways regulate VEGF and IL-8 expression in breast cancer: implications for therapy. Eur J Cancer. 2004;40:2509–18. doi: 10.1016/j.ejca.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Chen KG, Valencia JC, Gillet JP, Hearing VJ, Gottesman MM. Involvement of ABC transporters in melanogenesis and the development of multidrug resistance of melanoma. Pigment Cell Melanoma Res. 2009;22:740–9. doi: 10.1111/j.1755-148X.2009.00630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel VC, Marchionni L, Hierman JS, Rhodes JT, Devereux WL, Rudin CM, et al. A primary xenograft model of small-cell lung cancer reveals irreversible changes in gene expression imposed by culture in vitro. Cancer Res. 2009;69:3364–73. doi: 10.1158/0008-5472.CAN-08-4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean M. ABC transporters, drug resistance, and cancer stem cells. J Mammary Gland Biol Neoplasia. 2009;14:3–9. doi: 10.1007/s10911-009-9109-9. [DOI] [PubMed] [Google Scholar]
- Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–84. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- Dean M, Hamon Y, Chimini G. The human ATP-binding cassette (ABC) transporter superfamily. J Lipid Res. 2001;42:1007–17. [PubMed] [Google Scholar]
- Dou J, Wen P, Hu W, Li Y, Wu Y, Liu C, et al. Identifying tumor stem-like cells in mouse melanoma cell lines by analyzing the characteristics of side population cells. Cell Biol Int. 2009;33:807–15. doi: 10.1016/j.cellbi.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Duan Z, Brakora KA, Seiden MV. Inhibition of ABCB1 (MDR1) and ABCB4 (MDR3) expression by small interfering RNA and reversal of paclitaxel resistance in human ovarian cancer cells. Mol Cancer Ther. 2004;3:833–8. [PubMed] [Google Scholar]
- Fletcher JI, Haber M, Henderson MJ, Norris MD. ABC transporters in cancer: more than just drug efflux pumps. Nat Rev Cancer. 2010;10:147–56. doi: 10.1038/nrc2789. [DOI] [PubMed] [Google Scholar]
- Frank NY, Margaryan A, Huang Y, Schatton T, Waaga-Gasser AM, Gasser M, et al. ABCB5-mediated doxorubicin transport and chemoresistance in human malignant melanoma. Cancer Res. 2005;65:4320–33. doi: 10.1158/0008-5472.CAN-04-3327. [DOI] [PubMed] [Google Scholar]
- Gerson SL. Clinical relevance of MGMT in the treatment of cancer. J Clin Oncol. 2002;20:2388–99. doi: 10.1200/JCO.2002.06.110. [DOI] [PubMed] [Google Scholar]
- Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- Grichnik JM, Burch JA, Schulteis RD, Shan S, Liu J, Darrow TL, et al. Melanoma, a tumor based on a mutant stem cell? J Invest Dermatol. 2006;126:142–53. doi: 10.1038/sj.jid.5700017. [DOI] [PubMed] [Google Scholar]
- Hauschild A, Agarwala SS, Trefzer U, Hogg D, Robert C, Hersey P, et al. Results of a phase III, randomized, placebo-controlled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. J Clin Oncol. 2009;27:2823–30. doi: 10.1200/JCO.2007.15.7636. [DOI] [PubMed] [Google Scholar]
- Helmbach H, Rossmann E, Kern MA, Schadendorf D. Drug-resistance in human melanoma. Int J Cancer. 2001;93:617–22. doi: 10.1002/ijc.1378. [DOI] [PubMed] [Google Scholar]
- Hirschmann-Jax C, Foster AE, Wulf GG, Nuchtern JG, Jax TW, Gobel U, et al. A distinct "side population" of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci U S A. 2004;101:14228–33. doi: 10.1073/pnas.0400067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshet GI, Goldstein I, Itzhaki O, Cesarkas K, Shenhav L, Yakirevitch A, et al. MDR1 expression identifies human melanoma stem cells. Biochem Biophys Res Commun. 2008;368:930–6. doi: 10.1016/j.bbrc.2008.02.022. [DOI] [PubMed] [Google Scholar]
- La Porta CA. Drug resistance in melanoma: new perspectives. Curr Med Chem. 2007;14:387–91. doi: 10.2174/092986707779941078. [DOI] [PubMed] [Google Scholar]
- Lefebvre JL, Zhang Y, Meister M, Wang X, Sanes JR. gamma-Protocadherins regulate neuronal survival but are dispensable for circuit formation in retina. Development. 2008;135:4141–51. doi: 10.1242/dev.027912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriwaki K, Noda K, Furukawa Y, Ohshima K, Uchiyama A, Nakagawa T, et al. Deficiency of GMDS leads to escape from NK cell-mediated tumor surveillance through modulation of TRAIL signaling. Gastroenterology. 2009;137:188–98. 98, e1–2. doi: 10.1053/j.gastro.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Moserle L, Indraccolo S, Ghisi M, Frasson C, Fortunato E, Canevari S, et al. The side population of ovarian cancer cells is a primary target of IFN-alpha antitumor effects. Cancer Res. 2008;68:5658–68. doi: 10.1158/0008-5472.CAN-07-6341. [DOI] [PubMed] [Google Scholar]
- Narayan S, Roy D. Role of APC and DNA mismatch repair genes in the development of colorectal cancers. Mol Cancer. 2003;2:41. doi: 10.1186/1476-4598-2-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilorget A, Demeule M, Barakat S, Marvaldi J, Luis J, Beliveau R. Modulation of P-glycoprotein function by sphingosine kinase-1 in brain endothelial cells. J Neurochem. 2007;100:1203–10. doi: 10.1111/j.1471-4159.2006.04295.x. [DOI] [PubMed] [Google Scholar]
- Rubio-Viqueira B, Jimeno A, Cusatis G, Zhang X, Iacobuzio-Donahue C, Karikari C, et al. An in vivo platform for translational drug development in pancreatic cancer. Clin Cancer Res. 2006;12:4652–61. doi: 10.1158/1078-0432.CCR-06-0113. [DOI] [PubMed] [Google Scholar]
- Schatton T, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M, et al. Identification of cells initiating human melanomas. Nature. 2008;451:345–9. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatton T, Schutte U, Frank NY, Zhan Q, Hoerning A, Robles SC, et al. Modulation of T-cell activation by malignant melanoma initiating cells. Cancer Res. 2010;70:697–708. doi: 10.1158/0008-5472.CAN-09-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- Tusnady GE, Sarkadi B, Simon I, Varadi A. Membrane topology of human ABC proteins. FEBS Lett. 2006;580:1017–22. doi: 10.1016/j.febslet.2005.11.040. [DOI] [PubMed] [Google Scholar]
- Vance KW, Carreira S, Brosch G, Goding CR. Tbx2 is overexpressed and plays an important role in maintaining proliferation and suppression of senescence in melanomas. Cancer Res. 2005;65:2260–8. doi: 10.1158/0008-5472.CAN-04-3045. [DOI] [PubMed] [Google Scholar]
- Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735–41. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- Weaver VM, Lelievre S, Lakins JN, Chrenek MA, Jones JC, Giancotti F, et al. beta4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell. 2002;2:205–16. doi: 10.1016/s1535-6108(02)00125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J Clin Invest. 2001;107:135–42. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Guan K, Zhou C, Ma W, Wang D, Zhang Y, et al. Cancer stem cells sustaining the growth of mouse melanoma are not rare. Cancer Lett. 2010;292:17–23. doi: 10.1016/j.canlet.2009.10.021. [DOI] [PubMed] [Google Scholar]
- Zhou G, Kuo MT. NF-kappaB-mediated induction of mdr1b expression by insulin in rat hepatoma cells. J Biol Chem. 1997;272:15174–83. doi: 10.1074/jbc.272.24.15174. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.