Abstract
Objectives
The current study examines the awakening cortisol level in midlife mothers (M=51.4 years old, SD=8.4) of individuals (M=22.1 years old, SD=7.1) with autism spectrum disorders (ASD) under stressful conditions that are not specific to the son or daughter's ASD symptoms.
Methods
In addition to completing a set of questionnaires and in-home interviews, 82 mothers from the Adolescents and Adults with Autism Study (AAA) participated in a Daily Diary Study.
Results
Findings from the multilevel models indicated that mothers who previously were exposed to no negative life events in the previous period had an increased awakening cortisol level on days following a greater number and more severe stressors, a normative stress response. In contrast, we observed a flatter cortisol level of daily stressors in mothers who experienced a greater number of negative life events in the previous period.
Conclusion
These findings highlight the sustained toll that global and everyday stressors have on awakening cortisol level of midlife and aging mothers of individuals with ASD.
Keywords: Stress, caregivers, cortisol
Introduction
Autism is an increasingly prevalent diagnosis, and individuals who have autism spectrum disorders (ASD) are affected throughout the life course (Happe & Charlton, 2011). In general, the life expectancy of individuals with developmental disabilities (DD) has risen over the years (e.g., Janicki, Dalton, Henderson, & Davidson, 1999), and many remain dependent on family members and services throughout adulthood (Seltzer, Krauss, Orsmond, & Vestal, 2000). According to Braddock et al. (2008), approximately 1.7 million adults with DD are being cared for by middle-age and older family caregivers. Autism is the most prevalent DD condition, but until recently research on the health and well-being of aging parental caregivers has not specifically focused on this group (e.g., Pruchno & Meeks, 2004; Seltzer, Floyd, Song, Greenberg, & Hong, 2011; Yamaki, Hsieh, & Heller, 2009).
Parents of children and youth with ASD have elevated levels of stress and poorer well-being than parents of other types of DD conditions (Abbeduto et al., 2004). Studies consistently have shown that characteristics of ASD, particularly the son or daughter's behavior problems, are significant sources of stress for these parents (Davis & Carter, 2008), with high levels of behavior problems continuing into adulthood (Seltzer et al., 2010). Thus, midlife and aging parents of individuals with ASD experience elevated stress over decades, which highlights the need for more research on this group of caregivers.
Although factors directly related to the child's disability are stressful for parents of children with ASD, this parenting role is embedded in a larger social context. In addition to the unique challenges of parenting a son or daughter with ASD, these parents also are exposed to major negative life events (e.g., death of a family member) and daily stressors (e.g., arguments) that are experienced by everyone. In the current study, we examine how stressors not necessarily related to the child's disability are associated with maternal physiological functioning during midlife and older age. Specifically, we investigate how negative life events and daily stressors are associated with awakening cortisol level in a sample of middle-aged and older mothers of individuals with ASD.
Types of Stress: Life Events and Daily Stressors
Our study combines two distinct approaches to the study of stress and well-being. The first approach, the life events tradition (e.g., Holmes & Rahe, 1967), focuses on the global impact of life changes. Relatively infrequent in occurrence, major life events, such as death of a family member or marital separation, often result in long-term changes in lifestyle, residence, financial well-being, and social relations (Neugarten, 1979; Wheaton, 1990) and lead to distress, constraining other areas of life (Pearlin, Schieman, Fazio, & Meersman, 2005). A number of studies of families of individuals with ASD have examined the psychological effects of negative life events (Barker et al., 2010). However, little is known about the physiological effects of life events for parents of individuals with ASD, which is one focus of the present study.
The second approach focuses on everyday or ‘quotidian’ stressors (e.g., Almeida, 2005), which are defined as the routine challenges of day-to-day living, including arguments with a family member or unexpected deadlines. Life events and daily stressors differ in terms of frequency and may also have differential effects on well-being and health. Major life events occur less frequently and tend to have a more distal impact on well-being and physical health, whereas daily stressors tend to have a more proximal effect (Almeida, 2005). Furthermore, the two types of stressors may interact, such that the impact of daily stressors may be magnified in the context of exposure to negative life events. This is the central hypothesis of the present study.
Cortisol
Cortisol is the main hormone product of the hypothalamic-pituitary-adrenocortical (HPA) axis and is considered to be a primary marker of biological stress reactivity (Adam & Gunnar, 2001). Under routine conditions, cortisol facilitates normal adaptation to the environment and the maintenance of homeostasis through various processes including glucose level stabilization, cell metabolism, and inflammatory responses (Heim, Ehlert, & Hellhammer, 2000). Under conditions of threat or distress, the HPA axis activates and secretes cortisol into the bloodstream (Dickerson & Kemeny, 2004). Dysregulated cortisol is implicated in a host of psychological, physiological, and physical health conditions, including depression, immune disorders, and cardiovascular disease (Bhattacharyya, Molloy, & Steptoe, 2008; McEwen et al., 1997).
While the cortisol literature has focused more on cortisol awakening response (referring to the increase in cortisol release observed over the first 30 minutes post awakening and often is assessed by the difference between cortisol levels at 30 minutes post-awakening and upon awakening) than other measurements of cortisol reactivity, research examining the magnitude of cortisol awakening response continues to be mixed (see Chida & Steptoe, 2009 and Fries, Dettenborn, & Kirschbaum, 2009 for review). This may be the case because a portion of the population does not exhibit the cortisol awakening response (see Wust et al. 2000 who observed cortisol awakening response in only 75% of their sample of healthy adults) masking the linkages between stressors and cortisol reactivity.
In addition, awakening cortisol levels are more responsive than cortisol levels assessed later in the day to subtle changes in the HPA axis associated with environmental stressors and psychiatric conditions such as chronic fatigue syndrome, depression, and sleep disturbance (e.g., Backhaus, Junghanns, & Hohagen, 2004; Dahlgren, Kecklund, Theorell, & Akerstedt, 2009). Prior research (e.g., Dahlgren et al., 2009) also has showed that low cortisol levels in the morning are associated with anxiety, symptoms of exhaustion, and poor health the day before. By focusing on awakening cortisol levels, we can better understand the time-order effects of stressors on next day's cortisol. Unlike cortisol level at awakening, cortisol values later in the day are likely to be influenced by same-day events, confounding the examination of the effect of prior-day events on next day cortisol values later in the day since these effects may be affected by ongoing stress during the second day. In this regards, the examination of cortisol levels at awakening also may provide us with clearer insights to cortisol reactivity to stressors for this sample of caregivers than the magnitude of cortisol awakening response.
Stressors and Cortisol
Stressors represent challenges and demands that trigger a response from the HPA axis. Whereas acute stressors (e.g., daily stressors) typically lead to a temporary increase in cortisol (see Miller, Chen, & Zhou, 2007, for a review), a reduced or blunted pattern of cortisol response often is observed in individuals with a history of chronic stressors (e.g., burnout, post-traumatic stress disorders; Neeck, Federlin, Graef, Rusch, & Schmidt, 1990; Yehuda, 2000). As a consequence, the literature on stress and cortisol responsiveness has been mixed, with some studies reporting increased cortisol levels (e.g., Nicolson, 2004) and others documenting blunted cortisol response (e.g., Bloch, Peleg, Koren, Aner, & Klein, 2007). In this study, we are interested in the effects of both negative life events and daily stressors on the awakening cortisol level of mothers of individuals with ASD.
The work of Smith and colleagues (2010) demonstrated that mothers of adolescents and adults with ASD are exposed to more daily stressors than mothers of similarly-aged children without disabilities. They found that, during an eight-day diary study, mothers of adolescents and adults with ASD reported more days with at least one stressful event (65% of days vs. 43%) than the comparison group. Recently, research has shown that the stress of parenting an adolescent or adult with disabilities is associated with a pattern of hypocortisolism. Comparing parents of individuals with disabilities and without disabilities, Seltzer and colleagues (2009) found that parents of grown children with disabilities exhibited a flatter cortisol decline across the day when they spent more time with their son or daughter, a pattern not evident in the comparison group. In another study, mothers of sons and daughters with ASD exhibited significantly lower levels of cortisol at all collection points (e.g., awakening, 30 minutes post awakening, bedtime) than mothers of typically developing sons and daughters after controlling for maternal age, prescription medications usage, and saliva collection time (Seltzer et al., 2010). The same study also found that mothers of sons and daughters with ASD had a less pronounced (more blunted) cortisol awakening response on the morning after their adult child with ASD had more behavior problems, provided that the son or daughter had a history of clinically significant behavior problems (Seltzer et al., 2010). These studies highlight the toll of child-related stressors on an important neuroendocrine process in parents of children with DD, resulting in lower levels and more blunted slopes of cortisol at various times throughout the day. However, less is known about the adrenal hormone activity of such parents under stressful conditions that are not specific to the child's symptoms. Only one study has examined the physiological effects of such life events (Seltzer, Barker et al., 2011) in mothers of children with fragile X, and found that mothers with a specific genotype had a blunted cortisol response when exposed to negative life events. However, that study did not examine the effects of daily stress.
The Current Study
In the current study, we examine how general life stress may influence awakening cortisol level of midlife mothers -- above and beyond the stressors directly related to the son or daughter with ASD. Specifically, we extend past work by examining how both exposure to negative life events and the experience of daily stressors are associated with awakening cortisol level in mothers of individuals with ASD. We hypothesize that negative life events and daily stressors will work in combination, rather than independently, to predict maternal awakening cortisol level. We base this prediction on the prior work of Seltzer and colleagues (2010) who found that the association between daily behavior problems and cortisol secretion in mothers of adolescents and adults with ASD was moderated by the child's history of behavior problems. By extension, we hypothesized that the association between daily stressors and awakening cortisol level in these mothers would be moderated by previous exposure to negative life events. Specifically, those mothers who previously were exposed to fewer negative life events would have higher awakening cortisol levels on days following more stressors. In contrast, we predict a pattern of blunted cortisol level on days following more daily stressors for mothers who previously were exposed to more negative life events.
To further the understanding of the different aspects of the daily stressor characteristics on awakening cortisol level, we also examine the interactive effect of appraisal of stressor severity and negative life events on awakening cortisol level. Similar to the pattern expected for the interactive effect of number of daily stressors and negative life events, we predict that mothers who previously were exposed to fewer negative life events would have higher awakening cortisol levels on days following more severe daily stress. We also predict that mothers who previously were exposed to greater numbers of negative life events would exhibit lower awakening cortisol levels on days following more severe daily stress.
Methods
Participants
Participants were selected from the ongoing longitudinal study of Adolescents and Adults with Autism (the AAA study; Barker et al., 2011; Seltzer et al., 2003). Mothers were recruited through agencies, schools, clinics, and the media. To qualify for the study, families met the following three criteria: (a) included a child 10 years of age or older; (b) child received an independent diagnosis of ASD from a professional, as reported by parents; and (c) scores on the Autism Diagnostic Interview-Revised (ADI-R; Lord, Rutter, & Le Couteur, 1994; Rutter, Le Couteur, & Lord, 2003), administered by research staff, were consistent with the parental report of an ASD.
At the beginning of the study in 1998, families of 406 individuals with ASD participated. Data were collected primarily from mothers in their homes eight times between 1998 and 2011 (see Barker et al., 2011; Seltzer, Greenberg et al., 2011, for study methods description). During 2006 and 2007, the AAA Daily Dairy Study was conducted with mothers whose son or daughter with ASD still lived at home with her. By the time of the Daily Dairy Study, 136 individuals with ASD remained co-resident with their parents. Six mothers were unreachable and 34 declined participation. Of the 96 mothers in the Daily Diary Study, three declined saliva collection and seven did not provide complete data on medication use, which was needed for the present analysis. Because of the associations between sleep-related factors and cortisol responsiveness (Fries et al., 2009), one mother who reported staying awake for more than 20 hours and waking up after 12 noon on all saliva collection days was excluded. Three mothers did not provide information on the Life Events Checklist, thereby resulting in a final sample of 82 mothers with adolescents or adults with ASD for the present analyses. Analyses of mothers included in this study and those who did not participate showed that the two groups did not differ significantly by age, education, martial status, or household income, or the age, gender, or behavior problems of the individual with ASD. Mothers who were not included in this study were significantly more likely to be working than those included.
Procedure
The present analyses utilized data from two waves (Times 4 and 5) of the AAA study. At Time 4 (2004), mothers completed a set of self-administered questionnaires before participating in an in-home interview. The present analyses specifically focused on measures from the self-administered questionnaires (i.e., the Life Events Checklist, maternal education, child's behavior problems).
At Time 5 (2006-2007), mothers participated in the Daily Diary Study, which consisted of telephone interviews on eight consecutive evenings. Lasting 15 to 25 minutes, the daily telephone interview included questions about experiences in the previous 24 hours. Questions focused on time use, daily stressors, positive events, mood, and physical symptoms (Almeida, Wethington, & Kessler, 2002). The Daily Diary interviews were conducted by The Pennsylvania State University's Survey Research Center.
On Days 2 through 5 of the Dairy Diary Study, mothers provided four saliva samples each day. A week prior to the telephone interview, participants received a Home Saliva Collection Kit. Each kit included 16 numbered and color-coded salivettes (Sarstedt, Nümbrecht, Germany), which contained a small absorbent wad about ¾ of an inch long, an instruction sheet, and a sample collection time log. Respondents were instructed to record the exact time they provided each saliva sample on the log. Respondents were instructed to collect their first saliva sample before eating, drinking, or brushing their teeth, not to consume any caffeinated products before taking their subsequent samples throughout the day, and to store all samples in the refrigerator (Almeida, Piazza, & Stawski, 2009).
Measures
Negative Life Events
At Time 4 of the AAA study, mothers reported whether they or their spouse or a child had experienced a range of life events in the past 12 months. Derived from Abidin's (1986) Life Stress Scale of the Parenting Stress Index, the 21-item Life Events Checklist consisted of positive and negative events. We focused only on negative events because of our interest in examining the impact of stress on maternal cortisol levels. The 11 negative items used in this study pertained to marital dissolution, finances (e.g., went deeply into debt), substance abuse, deaths, health problems, and caring for an aging parent. The final item (caring for an aging parent) was added to the life events list in Abidin's Checklist by the AAA study investigators. The most commonly reported events were health problems (42.7%), caring for an aging parent (26.8%), and death of a family member (24.4%). The items were summed to create a total negative life events score. Only 4.8% of the sample reported experiencing more than 4 negative life events. To reduce skew, the total negative life events score was capped at 4, with 4 representing 4 or more negative life events. Due to the design of the AAA study, the Life Events Checklist was administered prior to the Daily Diary Study. Because of the time lapse between the assessments of life events and daily stressors, the life events assessed in this study reflect events that occurred two to three years prior to the measurement of daily stress and cortisol level. Therefore, we controlled for the duration of time that elapsed between the experience of life events and the measurement of daily stressors (see below).
Salivary Cortisol
Saliva samples at awakening, 30 minutes post awakening, before lunch, and before bedtime were assayed for cortisol at Time 5. Cortisol concentrations were quantified with a commercially available luminescence immunoassay (IBL, Hamburg, Germany), with intra-assay coefficients of variation below 5% (Dressendörfer, Kirschbaum, Rohde, Stahl, & Strasburger, 1992). In this study, we focused on the cortisol level at the awakening point because we were interested in the lagged effect of stress experienced the day before. Cortisol data was log transformed to correct for positively skewed distributions. Before the log transformation, salivary cortisol values higher than 60 nmol/l were recoded as 61 to minimize the influence of extreme outliers (Dixon & Yuen, 1974). In our sample, only one observation of awakening cortisol level was greater than 60 nmol/l.
For our sample of 82 mothers, we started with an initial data set of 318 days of cortisol data at awakening. Days when mothers woke unusually late or remained awake for more than 20 hours were dropped. We also dropped days when mothers had missing data for the time-varying predictors/covariates. Our final sample consisted of 82 mothers with 279 days of saliva samples.
Daily Stressors
Exposure to (number) and appraisal of (severity) daily stressors were assessed at Time 5 using the Daily Inventory of Stressful Events (DISE; Almeida et al., 2002). The DISE is comprised of a series of seven stem questions that identify whether certain types of stressful events occurred in the past 24 hours (arguments, avoided arguments; home, work, or network stressors; discrimination; and other stressors). Responses to the seven items were summed to create a total number of stressors score per day. Scores ranged from 0 (experienced no stressful event) to 7 (experienced all seven stressful events). The most commonly reported daily stressors across the study period were home-related stressors (29.4% of the study days), avoided arguments (24.4%), and arguments (20.7%). When a stressful event was reported on a given day, follow-up question asked the mother to rate the severity of stress (1=not stressful at all to 4=very stressful). To measure stress severity, we followed similar stressor severity scoring procedures outlined by Mroczek and Almeida (2004). On days when a stressor was reported, the severity scores for all seven items were summed. Stressor severity scores ranged from 1 (experienced one stressor at a not stressful level) to 28 (experienced all seven events at very stressful levels).
To better able to interpret the directionality of the association of stressors and cortisol levels, we examined the number and severity of stressors from the previous day predicting awakening cortisol level the next morning.
Control Variables
Characteristics of the mother that were controlled included age, education, marital status, medication usage, total number of children in the family, saliva collection time (Keenan, Licinio, & Veldhuis 2001), and length of time between the date of the mother's interview at Time 4 and the date of the beginning of the mother's participation in the Daily Diary Study at Time 5, which ranged from 21 to 39 months (M=31.7, SD=3.9). Education level was coded on a 1 to 4 scale (1=less than high school to 4=college graduate). Marital status was coded as 0=not married and 1=married. Studies have shown that certain types of medications may influence cortisol response (Granger, Hibel, Fortunato, & Kapelewski, 2009). To control for potential medication effects, the analyses included a variable that accounted for whether respondents reported taking any medication from a 7 item list (e.g., steroids, anti-depressants or anti-anxiety; 0=no medication, 1=at least one medication). Saliva collection time also was controlled. A saliva collection time log was included in the home saliva collection kit, and respondents were asked to record the exact time that they provided each saliva sample on the log. Mothers, on average, collected their awakening saliva sample at 6:44 AM (SD=61 minutes). Based on prior research which showed the effects of behavior problems on maternal well-being and cortisol level (Seltzer et al., 2010), the behavior problems of the individual with ASD at Time 4 also was controlled. The measure of behavior problems was based on the Scales of Independent Behavior-Revised (SIB-R; Bruininks, Woodcock, Weatherman, & Hill, 1996). Developed for the broad population with developmental disabilities, the SIB-R scales assess the frequency and severity of eight types of behavior problems (e.g., hurtful to self, unusual or repetitive, disruptive). Using standardized algorithm, frequency and severity scores were translated into a summary score.
The average age of the mothers at Time 4 was 51.4 years (SD=8.4). Average maternal education level was “some college” (M=3.1, SD=0.8). Approximately 83% of the mothers were married at Time 4 and nearly 65% reported taking at least one medication during the Daily Diary Study period. The average number of children in the family, including the target child with ASD, was 2.8 (SD=1.3). At Time 4, the average age of the individuals with ASD was 22.1 (SD=7.1). Fully 79% of the individuals with ASD were males. The average behavior problem score at Time 4 was 110.8 (SD=10.1), which fell between the upper end of the cutoff for ‘not clinically significant’ and the lower end of ‘marginally significant’ behavior problems (Bruininks et al., 1996).
Data Analysis Plan
A set of two-level multilevel models (SAS Proc Mixed), where days were nested within persons, assessed the extent to which mothers’ negative life events affect the associations between daily stress and cortisol level on the following morning. For number of daily stressors from the previous day, analyses were carried out in two models—main effects only (model 1) and interaction effect (model 2). The same structure of analyses was conducted for the examination of severity of stressors from the previous day, but only for days when respondents reported experiencing at least one daily stressor. For our analyses of number of daily stressors, the sample consisted of 82 mothers with 279 days of saliva samples. Constrained to days when a stressor was reported, our stressor severity analyses comprised of 80 mothers and 200 days of saliva samples.
Awakening cortisol level was the primary dependent variable. Preliminary analyses were conducted, and the control variables of marital status and number of children in the family were dropped in the final models because they had no significant effects on awakening cortisol level and did not change any of the estimates. The final set of control variables included maternal age, education, medication usage, saliva collection time, the length of time between Times 4 and 5, and the behavior problems of the individual with ASD. Continuous time-invariant predictors/covariates were centered at the sample mean. Because daily stressors (number and severity) and awakening saliva collection time were assessed repeatedly, we included both within- and between-person effects using the person-mean center approach outlined by Hoffman and Stawski (2009). Preliminary analyses indicated that a random intercept only model had acceptable fit, and that the random effect of daily stress (number as well as severity) did not improve the model fit. Similarly, we examined whether to include a random effect of awakening saliva collection time, and maximum likelihood deviance comparison showed that the random effect of awakening saliva collection time did not improve the model fit.
Results
Table 1 presents the descriptive statistics of negative life events, stressors, and cortisol. The average number of negative life events for this sample was 1.5 (SD=1.2). The average number and severity of daily stressors from the previous day were 1.3 (SD=1.2) and 5.4 (SD=3.3), respectively. The average log cortisol level for awakening was 2.4 nmol/l (SD=0.5).
Table 1.
Descriptive Statistics of Life Events, Stressors, and Cortisol
| M | SD | |
|---|---|---|
| Number of negative life events | 1.5 | 1.2 |
| Number of daily stressors-previous daya | 1.3 | 1.2 |
| Severity of stressors-previous daya | 5.4 | 3.3 |
| Log awakening cortisol levela | 2.4 | 0.5 |
Mean value reflects average taken across study days.
Number of Daily Stressors, Number of Negative Life Events, and Cortisol Level
The first set of analyses examined the effects of number of daily stressors, number of negative life events, and the interaction between number of daily stressors and number of negative life events on cortisol level at awakening the following day. Pearson correlation of life events and number of daily stressors from the previous day (r=0.02, p=0.72) indicated that these are different types of stressors.
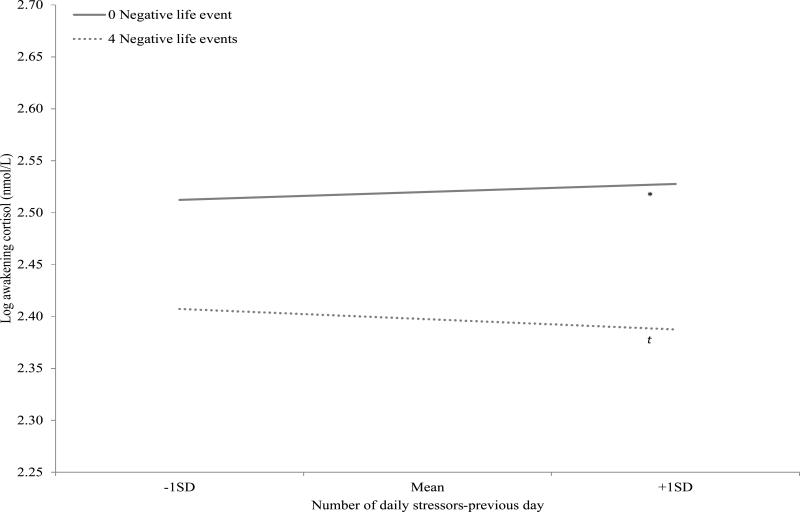
Results from the main effect model (Table 2, Model 1) showed that neither the number of stressors from the previous day nor the number of negative life events in the previous period was a significant predictor of maternal awakening cortisol level. However, there was a significant interaction effect of within-person number of daily stressors and between-person number of negative life events for awakening cortisol level the next day, suggesting that the effects of daily stressors on awakening cortisol level vary with exposure to negative life events (Table 2, Model 2 and Figure 1). To explore the interaction effect, the slopes of daily stressors on the awakening cortisol level the next day were estimated at different levels of negative life event. Results showed that for mothers who experienced no negative life events, there was a positive association between the number of daily stressors-previous day and awakening cortisol level (stressor slope estimated at zero negative life events: b = 0.097, SE = 0.048, p < 0.05). In contrast, there was a negative association at a trend level towards significance between the number of daily stressors-previous day and awakening cortisol level for mothers who experienced a greater number of negative life events (stressor slope estimated at four negative life events: b = -0.122, SE = 0.065, p = 0.06). These results are consistent with our hypotheses.
Table 2.
Multilevel Model of Daily Number of Stressors (Previous Day) and Number of Negative Life Events Predicting Log Awakening Cortisol Level
| Model 1 | Model 2 | |
|---|---|---|
| Fixed Effects | ||
| Intercept | 2.473 (0.070)*** | 2.473 (0.070)*** |
| Age | 0.005 (0.005) | 0.005 (0.005) |
| Education | -0.069 (0.056) | -0.069 (0.056) |
| Taking at least one medication (yes=1) | -0.101 (0.089) | -0.102 (0.088) |
| Months between times 4 and 5 | -0.022 (0.011)t | -0.022 (0.011)t |
| Behavior problems | -0.002 (0.005) | -0.002 (0.005) |
| Number of negative life events | -0.031 (0.035) | -0.031 (0.035) |
| Awakening collection time (BP) | 0.027 (0.046) | 0.027 (0.046) |
| Awakening collection time (WP) | 0.002 (0.031) | 0.004 (0.031) |
| Number of daily stressors-previous day (BP) | -0.059 (0.052) | -0.058 (0.052) |
| Number of daily stressors-previous day (WP) | 0.011 (0.031) | 0.013 (0.031) |
| Negative life events × daily stressors-previous day (WP) | -0.055 (0.023)* | |
| Variance Components | ||
| Between-person intercept (level 2) | 0.082*** (d.f.=73) | 0.083*** (d.f.=73) |
| Within-person (level 1) | 0.173 | 0.168 |
Note. BP=between person; WP=within person.
p<.10
p<.05
**p<.01
p<.001.
Figure 1.
Log awakening cortisol level by number of daily stressors (previous day) and number of negative life events.
Severity of Daily Stressors, Number of Negative Life Events, and Cortisol
Next, we explored the effects of stressor severity from the previous day and number of negative life events previously experienced on maternal cortisol level at awakening for days when respondents reported at least one daily stressor. The correlation of life events and stressor severity from the previous day (r=0.09, p=0.19) indicated the lack of overlap between the two. Results from the main effect model revealed that neither stressor severity from the previous day nor negative life events in the previous period was a significant predictor of maternal cortisol level at awakening (Table 3, Model 1).
Table 3.
Multilevel Model of Severity of Daily Stressors (Previous Day) and Number of Negative Life Events Predicting Log Awakening Cortisol Level
| Model 1 | Model 2 | |
|---|---|---|
| Fixed Effects | ||
| Intercept | 2.509 (0.083)*** | 2.509 (0.082)*** |
| Age | 0.005 (0.006) | 0.005 (0.006) |
| Education | -0.114 (0.067)t | -0.113 (0.067)t |
| Taking at least one medication (yes=1) | -0.133 (0.102) | -0.132 (0.102) |
| Months between times 4 and 5 | -0.024 (0.013)t | -0.024 (0.013)t |
| Behavior problems | -0.006 (0.005) | -0.006 (0.005) |
| Number of negative life events | -0.038 (0.040) | -0.038 (0.040) |
| Awakening collection time (BP) | 0.039 (0.053) | 0.040 (0.053) |
| Awakening collection time (WP) | 0.041 (0.040) | 0.050 (0.039) |
| Severity of daily stressors-previous day (BP) | 0.002 (0.019) | 0.002 (0.019) |
| Severity of daily stressors-previous day (WP) | -0.012 (0.013) | -0.006 (0.013) |
| Life events × daily stressors severity-previous day (WP) | -0.025 (0.010)* | |
| Variance Components | ||
| Between-person intercept (level 2) | 0.091*** (d.f.=71) | 0.094*** (d.f.=71) |
| Within-person (level 1) | 0.175*** | 0.167*** |
Note. BP=between person; WP=within person.
p<.10
p<.05
**p<.01
p<.001.
We then examined how appraisal of the within-person stressor severity from the previous day interacted with number of negative life events in predicting awakening cortisol level. Results showed a significant interaction effect of within-person stressor severity from the previous day and number of negative life events previously experienced for awakening cortisol level (Table 3, Model 2 and Figure 2). We also examined how the association between stressor severity-previous day and awakening cortisol level varies with previous exposure to life events by estimating the simple slopes of stressor severity on awakening cortisol at different levels of negative life events. Results showed that there was no significant association between the stressor severity-previous day and awakening cortisol level for mothers who experienced no negative life events (severity slope estimated at zero negative life events: b = 0.032, SE = 0.022, p > 0.10). In contrast, for mothers who experienced a greater number of negative life events in the previous period, there was a significant negative association between stressor severity-previous day and awakening cortisol level (severity slope estimated at four negative life events: b = -0.068, SE = 0.026, p < 0.01). Again, these patterns are consistent with our hypotheses.
Figure 2.
Log awakening cortisol level by severity of daily stressors (previous day) and number of negative life events.
Lastly, we examined the three-way interaction effect of negative life events, number of daily stressors-previous day, and stressor severity-previous day on awakening cortisol level. However, the three-way interaction effect was not significant.
Discussion
This study took a biopsychosocial approach to the study of how parenting a child with a lifelong disability affects the physiological functioning of midlife and older parents. It adds to recent findings that have shown that the stress of parenting a son or daughter with developmental disabilities is more likely to result in chronic state of low hormone release (Seltzer et al., 2010). These previous studies focused on stressors directly related to the symptoms (e.g., behavior problems) of the grown child with developmental disabilities. Less attention has been directed towards the impact of “normative” and everyday stressors on physiological functioning. The current study extends prior conclusions by investigating the extended effect of negative life events and daily stressors on the awakening cortisol level found the next morning in mothers of individuals with ASD.
Findings from this study resonate with the cumulative advantage/disadvantage framework and the stress proliferation literature (see O'Rand, 2009 for a review) by highlighting the importance of historical context to later events and conditions, as related to the pathways of health and well-being. In this study, the physiological toll on maternal caregivers was evident only when both the effects of negative life events experienced in a previous period and daily stressors yesterday were considered together. Among mothers who did not experience any negative life events in the previous period, those exposed to daily stressors responded with increased level of cortisol on the following morning, consistent with typical cortisol reactions to acute stress (e.g., Dickerson & Kemeny, 2004). At a trend level towards significance, we observed a flatter cortisol level in mothers who were exposed to daily stressors provided that they also experienced at least four negative life events in the previous period. In this study, the severity of the stressor was less of an influence on the next day's awakening cortisol level for mothers who did not experience any negative life events in the previous period than for mothers who reported a greater number of negative life events. Together, the results showed that daily stress has differential implications for physiological functioning, depending on personal contextual factors (e.g., prior experience of negative life events).
The observed diminished cortisol level in this study is in line with past findings that showed a pattern of blunted or flattening cortisol response in individuals with PTSD (Yehuda, 2000) and those with stress-related disorders (e.g., burnout, fatigue; Heim et al., 2000; Neeck et al., 1990). Most importantly, the low cortisol level contributes to the growing number of studies demonstrating that blunted or flattening cortisol response appears to be a distinct characteristic of parents of adolescents and adults with DD (Seltzer et al., 2009; Seltzer et al., 2010) across different types of stressors (e.g., general vs. child specific stressors). As elucidated by Fries and colleagues (2005), one pathway in which blunted or flattening cortisol response develops is from exposure to prolonged periods of stress as well as from the body's difficulty to self-adjusting. In our study, midlife mothers of grown children with ASD who experienced high levels of negative life events may not have the resources to handle the severe stress in their everyday lives. In this challenging and taxing context, daily stressors may represent the proverbial “straw that broke the camel's back”.
One implication of our findings is the pressing need for services to support midlife and aging mothers of individuals with ASD. Currently, much of the services and supports are targeted to families of children with ASD, and less has been directed towards parents of adults with ASD. More services are needed to help these midlife and older mothers improve their coping strategies to better handle the complex, multifaceted demands that go beyond their son or daughter's ASD symptoms. Additionally, appropriate intervention is needed to help reduce distress in families raising adult children with disabilities. Much of the current autism intervention research is aimed at parents of young children and early adolescents with ASD, the children themselves, or a combination (e.g., Keen, Couzens, Muspratt, & Rodger, 2010; Landa, Holman, O'Neill, & Stuart, 2011; Strauss et al., 2012). It is important, therefore, that researchers develop empirically based interventions to help midlife and aging parents as they navigate the challenges, as well as the opportunities, associated with their child's transition into adulthood and midlife.
A study limitation is that we did not have a comparison group of mothers with typically developing children. Therefore, we cannot rule out whether the observed effects are specific to caregivers of adolescents and adults with ASD or more general to the population. Another limitation is the time lapse between the assessments of negative life events and daily stressors. Although we controlled for the length of time between the assessments of life events and daily stressors, we cannot rule out whether additional negative life events occurred between the two time points. While the time frame between the two measures is imperfect, the Life Events Checklist offered the opportunity to examine how chronic stressors affect the relation of daily stressors to physiological functioning. We also did not have information on the duration or timing of the reported negative life events (Wheaton, 1990). This study solely focused on mothers who resided with their adolescent and adult children, and did not include mothers of adults in other types of living arrangements. Prior literature has shown that the stress of caring for an adult child with a developmental disability typically decreases after the adult child moves out of the home (Krauss, Seltzer, & Jacobson, 2005). It is possible that other living arrangements would decrease stressor exposure for the maternal caregivers. We focused on midlife and aging mothers of adolescents and adults with ASD. Now that a pattern of blunted or flattening cortisol response is well established for this population by this and other research, it would be important to evaluate the physiological functioning of both younger and older mothers of children with ASD and other types of developmental disabilities to determine the life course onset and development of atypical cortisol responses to daily and global stress, and its effects on aging. Furthermore, it would be worthwhile for future studies to examine whether protective factors, such as availability and effectiveness of support systems, may help to buffer the wear-and-tear toll on the neuroendocrine axis of these caregivers.
In spite of some limitations, our study has a number of important strengths. The daily diary approach provides a less biased account of well-being and reactions without decay of recollection over time (Nisbett & Wilson, 1977). The daily diary methodology also enabled us to more accurately capture the temporal order effects of daily stressors on the next day's cortisol activity. Instead of bringing participants into a controlled laboratory setting where they provide saliva samples in response to challenge tasks (e.g., Dickerson & Kemeny, 2004), participants provided saliva samples in their everyday settings. By utilizing a naturalistic field approach, we hoped to gain better insights into the stress-responsive physiology system of mothers with respect to naturally occurring stressors both acutely and over time.
In conclusion, this study adds to a growing literature examining cortisol responses in midlife and older mothers of adolescents and adults with ASD. Collectively, the findings suggest that the wear-and-tear toll on the neuroendocrine axis is comparable to that of job strains (e.g., Steptoe et al., 1998) and illnesses with chronic symptoms such as fibromyalgia (e.g., Riva, Mork, Westgaard, Rø, & Lundberg, 2010). Further, in our sample of middle-aged and older maternal caregivers of adolescents and adult children with ASD, the impact of severe daily stressors on awakening cortisol level is only evident in the context of negative life events.
Acknowledgements
This study was supported by grants from the National Institute on Aging to support longitudinal research on families of adolescents and adults with autism (R01 AG08768, M. M. Seltzer, PI). Support also was obtained from the National Institute of Child Health and Human Development for grant T32 HD07489 to M.M. Seltzer, PI, and for grant P30 HD03352 to M. M. Seltzer, PI.
Contributor Information
David M. Almeida, Human Development and Family Studies, The Pennsylvania State University
Christopher L. Coe, Psychology, University of Wisconsin-Madison
References
- Abbeduto L, Seltzer MM, Shattuck P, Krauss M, Orsmond G, Murphy M. Psychological well-being and coping in mothers of youths with Autism, Down syndrome, or fragile X syndrome. American Journal of Mental Retardation. 2004;109:237–54. doi: 10.1352/0895-8017(2004)109<237:PWACIM>2.0.CO;2. doi:10.1111/j.1365-2788.2006.00907. [DOI] [PubMed] [Google Scholar]
- Abidin R. Parenting Stress Index. 2nd ed. Pediatric Psychology Press; Charlottesville, VA: 1986. [Google Scholar]
- Adam EK, Gunnar MR. Relationship functioning and home and work demands predict individual differences in diurnal cortisol patterns in women. Psychoneuroendocrinology. 2001;26:189–208. doi: 10.1016/s0306-4530(00)00045-7. doi:10.1016/S0306-4530(00)00045-7. [DOI] [PubMed] [Google Scholar]
- Almeida DM. Resilience and vulnerability to daily stressors assessed via diary methods. Current Directions in Psychological Science. 2005;14:64–8. doi:10.1111/j.09637214.2005.00336.x. [Google Scholar]
- Almeida DM, Piazza JR, Stawski RS. Inter-individual differences and intra-individual variability in the cortisol awakening response: An examination of age and gender. Psychology and Aging. 2009;24:819–27. doi: 10.1037/a0017910. doi:10.1037/a0017910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida DM, Wethington E, Kessler RC. The daily inventory of stressful experiences (DISE): An interview-based approach for measuring daily stressors. Assessment. 2002;9:41–55. doi: 10.1177/1073191102091006. doi:10.1177/1073191102091006. [DOI] [PubMed] [Google Scholar]
- Backhaus J, Junghanns K, Hohagen F. Sleep disturbances are correlated with decreased morning awakening salivary cortisol. Psychoneuroendocrinology. 2004;29:1184–1191. doi: 10.1016/j.psyneuen.2004.01.010. doi: 10.1016/j.psyneuen.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Barker ET, Hartley SL, Seltzer MM, Floyd FJ, Greenberg JS, Orsmond GI. Trajectories of emotional well-being in mothers of adolescents and adults with Autism. Developmental Psychology. 2011;47:551–61. doi: 10.1037/a0021268. doi:10.1037/a0021268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya MR, Molloy GJ, Steptoe A. Depression is associated with flatter cortisol rhythms in patients with coronary artery disease. Journal of Psychosomatic Research. 2008;65:107–13. doi: 10.1016/j.jpsychores.2008.03.012. doi:10.1016/j.jpsychores.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Bloch M, Peleg I, Koren D, Aner H, Klein E. Long-term effects of early parental loss due to divorce on the HPA. Hormones and Behavior. 2007;51:516–23. doi: 10.1016/j.yhbeh.2007.01.009. doi:10.1016/j.yhbeh.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Braddock D, Hemp R, Rizzolo M. The state of the states in developmental disabilities. Department of Psychiatry and Coleman Institute for Cognitive Disabilities, University of Colorado; Boulder: 2008. [Google Scholar]
- Bruininks RH, Woodcock RW, Weatherman RF, Hill B. Scales of Independent Behavior-Revised. Riverside; Itasca, IL: 1996. [Google Scholar]
- Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: A systematic review and meta-analysis. Biological Psychology. 2009;80:265–278. doi: 10.1016/j.biopsycho.2008.10.004. doi: 10.1016/j.biopsycho.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Dahlgren A, Kecklund G, Theorell T, Akerstedt T. Day-today variation in saliva cortisol—Relation with sleep, stress and self-rated health. Biological Psychology. 2009;82:149–155. doi: 10.1016/j.biopsycho.2009.07.001. doi:10.1016/j.biopsycho.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Davis NO, Carter AS. Parenting stress in mothers and fathers of toddlers with Autism Spectrum Disorders: Associations with child characteristics. Journal of Autism and Developmental Disorders. 2008;38:1278–91. doi: 10.1007/s10803-007-0512-z. doi:10.1007/s10803-007-0512-z. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–91. doi: 10.1037/0033-2909.130.3.355. doi:10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dixon WJ, Yuen KK. Trimming and winsorization: A review. Statistical Papers. 1974;15:157–70. doi:10.1007/BF02922904. [Google Scholar]
- Dressendörfer RA, Kirschbaum C, Rohde W, Stahl F, Strasburger CJ. Synthesis of a cortisol-biotin conjugate and evaluation as tracer in an immunoassay for salivary cortisol measurement. Journal of Steroid Biochemistry and Molecular Biology. 1992;43:683–92. doi: 10.1016/0960-0760(92)90294-s. doi:10.1016/0960-0760(92)90294-S. [DOI] [PubMed] [Google Scholar]
- Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): Facts and future directions. International Journal of Psychophysiology. 2009;72:67–73. doi: 10.1016/j.ijpsycho.2008.03.014. doi:10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Fries E, Hesse J, Hellhammer J, Hellhammer D. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30:1010–16. doi: 10.1016/j.psyneuen.2005.04.006. doi:10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Granger D, Hibel L, Fortunato C, Kapelewski C. Medication effects on salivary cortisol: Tactics and strategy to minimize impact in behavioral and developmental science. Psychoneuroendocrinology. 2009;34:1437–48. doi: 10.1016/j.psyneuen.2009.06.017. doi:10.1016/j.psyneuen.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Happe F, Charlton RA. Aging in Autism Spectrum Disorders: A mini-review. Gerontology. 2011 doi: 10.1159/000329720. Advance online publication. doi:10.1159/000329720. [DOI] [PubMed] [Google Scholar]
- Heim C, Ehler U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. doi:10.1016/S0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Hoffman L, Stawski R. Persons as contexts: Evaluating between-person and within-person effects in longitudinal analysis. Research in Human Development. 2009;6:97–100. doi:10.1080/15427600902911189. [Google Scholar]
- Holmes TH, Rahe RH. Holmes-Rahe life changes scale. Journal of Psychosomatic Research. 1967;11:213–18. doi: 10.1016/0022-3999(67)90010-4. [DOI] [PubMed] [Google Scholar]
- Janicki MP, Dalton AJ, Henderson CM, Davidson PW. Mortality and morbidity among older adults with intellectual disability: Health services considerations. Disability Rehabilitation. 1999;21:284–294. doi: 10.1080/096382899297710. [DOI] [PubMed] [Google Scholar]
- Keen D, Couzens D, Muspratt S, Rodger S. The effects of a parent-focused intervention for children with a recent diagnosis of autism spectrum disorder on parenting stress and competence. Research in Autism Spectrum Disorders. 2010;4:229–241. [Google Scholar]
- Keenan DM, Licinio J, Veldhuis JD. A feedback controlled ensemble model of the stress-responsive hypothalamo-pituitary-adrenal axis. Proceedings of the National Academy of Sciences. 2001;98:4028–33. doi: 10.1073/pnas.051624198. doi:10.1073/pnas.051624198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research. Psychoneuroendocrinology. 1994;19:313–33. doi: 10.1016/0306-4530(94)90013-2. doi:10.1016/0306-4530(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Krauss MW, Seltzer MM, Jacobson HT. Adults with Autism living at home or in non-family settings: Positive and negative aspects of residential status. Journal of Intellectual Disability Research. 2005;49:111–24. doi: 10.1111/j.1365-2788.2004.00599.x. doi:10.1111/j.1365-2788.2004.00599.x. [DOI] [PubMed] [Google Scholar]
- Landa RJ, Holman KC, O'Neill AH, Stuart EA. Intervention targeting development of socially synchronous engagement in toddlers with autism spectrum disorder: A randomized controlled trial. Journal of Child Psychology and Psychiatry. 2011;52:13–21. doi: 10.1111/j.1469-7610.2010.02288.x. doi:10.1111/j.1469-7610.2010.02288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, Couteur AL. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–85. doi: 10.1007/BF02172145. doi:10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Biron CA, Brunson KW, Bulloch K, Chambers WH, Dhabhar FS, et al. The role of adrenocorticoids as modulators of immune function in health and disease: neural, endocrine and immune interactions. Brain Research Reviews. 1997;23:79–133. doi: 10.1016/s0165-0173(96)00012-4. doi:10.1016/S0165-0173(96)00012-4. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Pyschological Bulletin. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. doi:10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Mikolajczak M, Quoidbach J, Vanootighem V, Lambert F, Lahaye M, Fillée C, de Timary P. Cortisol awakening response (CAR)'s flexibility leads to larger and more consistent associations with psychological factors than CAR magnitude. Psychoneuroendocrinology. 2010;35:752–757. doi: 10.1016/j.psyneuen.2009.11.003. doi: 10.1016/j.psyneuen.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Morgan CA, Cho T, Hazlett G, Coric V, Morgan J. The impact of burnout on human physiology and on operational performance: a prospective study of soldiers enrolled in the combat diver qualification course. Yale Journal of Biology and Medicine. 2002;75:199–205. [PMC free article] [PubMed] [Google Scholar]
- Mroczek DK, Almeida DM. The effect of daily stress, personality, and age on daily negative affect. Journal of Personality. 2004;72:355–78. doi: 10.1111/j.0022-3506.2004.00265.x. doi:10.1111/j.00223506.2004.00265.x. [DOI] [PubMed] [Google Scholar]
- Neeck G, Federlin K, Graef FV, Rusch D, Schmidt KL. Adrenal secretion of cortisol in patients with rheumatoid arthritis. Journal of Rheumatoloy. 1990;17:24–9. [PubMed] [Google Scholar]
- Neugarten BL. Time, age, and the life cycle. The American Journal of Psychiatry. 1979;136:887–94. doi: 10.1176/ajp.136.7.887. [DOI] [PubMed] [Google Scholar]
- Nicolson NA. Childhood parental loss and cortisol levels in adult men. Psychoneuroendocrinology. 2004;29:1012–8. doi: 10.1016/j.psyneuen.2003.09.005. doi:10.1016/j.psyneuen.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Nisbett RE, Wilson TD. The halo effect: Evidence for unconscious alteration of judgments. Journal of Personality and Social Psychology. 1977;35:250–56. doi:10.1037//0022-3514.35.4.250. [Google Scholar]
- O'Rand A. Cumulative processes in the life course. In: Elder GH Jr., Giele JZ, editors. The craft of life course research. Guilford Press; New York: 2009. pp. 121–40. [Google Scholar]
- Pearlin LI, Schieman S, Fazio E, Meersman SC. Stress, health, and the life course: Some conceptual perspectives. Journal of Health and Social Behavior. 2005;46:205–19. doi: 10.1177/002214650504600206. doi:10.1177/002214650504600206. [DOI] [PubMed] [Google Scholar]
- Pruchno R. Enmeshed lives: Adult children with developmental disabilities and their aging mothers. Psychology and Aging. 2003;18:851–7. doi: 10.1037/0882-7974.18.4.851. doi:10.1037/0882-7974.18.4.851. [DOI] [PubMed] [Google Scholar]
- Riva R, Mork P, Westgaard R, Rø M, Lundberg U. Fibromyalgia syndrome is associated with hypocortisolism. International Journal of Behavioral Medicine. 2010;17:223–233. doi: 10.1007/s12529-010-9097-6. doi:10.1007/s12529-010-9097-6. [DOI] [PubMed] [Google Scholar]
- Rutter M, Couteur AL, Lord C. ADI-R. Autism Diagnostic Interview-Revised. WPS edition Western Psychological Services; Los Angeles: 2003. [Google Scholar]
- Seltzer MM, Almeida DM, Greenberg JS, Savla J, Hong J, Taylor JL. Psychosocial and biological makers of daily lives of midlife parents of children with disabilities. Journal of Health and Social Behavior. 2009;50:1–15. doi: 10.1177/002214650905000101. doi:10.1177/002214650905000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer MM, Barker ET, Greenberg JS, Hong J, Coe CL, Almeida DM. Differential sensitivity to life stress in FMR1 premutation carrier of mothers of children with fragile X syndrome. Health Psychology. 2011 doi: 10.1037/a0026528. Advance online publication. doi: 10.1037/a0026528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer MM, Floyd FJ, Song J, Greenberg JS, Hong J. Midlife and aging parents of adults with intellectual and developmental disabilities: Impacts of lifelong parenting. American Journal of Intellectual and Developmental Disabilities. 2011;116:479–499. doi: 10.1352/1944-7558-116.6.479. doi:10.1352/1944-7558-116.6.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer MM, Greenberg JS, Hong J, Smith L, Almeida D, Coe C, et al. Maternal cortisol levels and behavior problems in adolescents and adults with ASD. Journal of Autism and Developmental Disorders. 2010;40:457–69. doi: 10.1007/s10803-009-0887-0. doi:10.1007/s10803-009-0887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer MM, Greenberg JS, Taylor JL, Smith L, Orsmond GI, Esbensen A, Hong J. Adolescents and adults with autism spectrum disorders. In: Amaral DG, Dawson G, Geschwind D, editors. Autism spectrum disorders. Oxford University Press; New York: 2011. pp. 241–252. [Google Scholar]
- Seltzer MM, Krauss MW, Orsmond GI, Vestal C. Families of adolescents and adults with autism: Uncharted territory. In: Glidden LM, editor. International Review of Research on Mental Retardation. Vol. 23. Academic Press; San Diego: 2000. [Google Scholar]
- Seltzer MM, Krauss MW, Shattuck PT, Orsmond G, Swe A, Lord C. The symptoms of Autism Spectrum Disorders in adolescence and adulthood. Journal of Autism and Developmental Disorders. 2003;33:565–81. doi: 10.1023/b:jadd.0000005995.02453.0b. doi:10.1023/B:JADD.0000005995.02453.0b. [DOI] [PubMed] [Google Scholar]
- Smith L, Hong J, Seltzer MM, Greenberg JS, Almeida DM, Bishop S. Daily experiences among mothers of adolescents and adults with ASD. Journal of Autism and Developmental Disorders. 2010;40:167–78. doi: 10.1007/s10803-009-0844-y. doi:10.1007/s10803-009-0844-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Wardle J, Lipsey Z, Mills R, Oliver G, Jarvis M, Kirschbaum C. A longitudinal study of work load and variations in psychological well-being, cortisol, smoking, and alcohol consumption. Annals of Behavioral Medicine. 1998;20:84–91. doi: 10.1007/BF02884453. doi:10.1007/BF02884453. [DOI] [PubMed] [Google Scholar]
- Strauss K, Vicari S, Valeri G, D'Elia L, Arima S, Fava L. Parent inclusion in early intensive behavioral intervention: The influence of parental stress, parent treatment fidelity and parent-mediated generalization of behavior targets on child outcomes. Research In Developmental Disabilities. 2012;33:688–703. doi: 10.1016/j.ridd.2011.11.008. doi:10.1016/j.ridd.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Wheaton B. Life transitions, role histories, and mental health. American Sociological Review. 1990;55:209–23. doi:10.2307/2095627. [Google Scholar]
- Wust S, Wolf J, Hellhammer DH, Federenko L, Schommer N, Kirschbaum C. The cortisol awakening response-normal values and confounds. Noise & Health. 2000;2:79–88. [PubMed] [Google Scholar]
- Yamaki K, Hsieh K, Heller T. Health profile of aging family caregivers supporting adults with intellectual and developmental disabilities. Intellectual and Developmental Disabilities. 2009;47:425–35. doi: 10.1352/1934-9556-47.6.425. doi:10.1352/1934-9556-47.6.425. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Biology of post-traumatic stress disorder. Journal of Clinical Psychiatry. 2000;61:14–21. doi:10.1016/S0193-953X(02)00027-8. [PubMed] [Google Scholar]