Abstract
Rationale
Although airway inflammation begins early in life in children with chronic respiratory diseases, current methods to assess this inflammation are invasive and entail significant risk. Measurement of exhaled breath condensate (EBC) purines and other biomarkers offer a less invasive method to assess airway inflammation; however, the feasibility and utility of EBC biomarkers in young children has not been established.
Methods
EBC was collected from children <3 years old with cystic fibrosis or other lung diseases during clinically indicated infant pulmonary function tests (iPFTs). EBC concentrations of the purine biomarkers adenosine, adenosine monophosphate (AMP) and the dilution marker urea were measured using mass spectrometry.
Results
EBC was successfully collected (average volume 330±170 μl) from preschool children (age 2.3 ± 0.8 years) in 15 of 17 iPFTs. No significant changes in oxygen saturation (96.9±1.6 start, 96.8±1.7 end, p=0.389) or respiratory rate (35.2±7.5 start, 34.6±7.9 end, p=0.443) were observed during collection. Adenosine and AMP were successfully measured in 13/15 samples (8 CF). EBC AMP to adenosine ratio (AMP/Ado) negatively correlated with forced expiratory volume at 0.5 seconds (FEV0.5, r=−0.71, p<0.01) and positively with the ratio of residual volume to total lung capacity (RV/TLC, r=0.66, p=0.015). These correlations remained statistically significant in the subset with CF.
Conclusions
EBC can be safely collected and analyzed in preschool children using commercially available equipment. The EBC AMP/Ado ratio correlates with measures of infant lung function and may be a less invasive means of monitoring airway inflammation in this population.
Keywords: Mass spectrometry, infant pulmonary function testing, adenosine, adenosine monophosphate
Introduction
Cystic fibrosis (CF) lung disease begins in infancy and is characterized by persistent airway inflammation even in the absence of respiratory symptoms1-4. Readily measured biomarkers of inflammation are needed to monitor disease progression in this vulnerable population; however assessing airway biomarkers in this population is challenging. The gold standard has classically been collecting bronchoalveolar lavage fluid, but this methodology is too invasive for routine use5,6 . Most preschool children also cannot expectorate sputum even after induction with hypertonic saline7.
Exhaled breath condensate (EBC) is a simple, non-invasive means of monitoring airway disease pathology in older children and adults. Our previous studies13, 14 have demonstrated that mass spectrometry can be utilized to perform quantitative analyses of multiple EBC biomarkers such as the adenyl purines, adenosine and adenosine monophosphate (AMP) with simultaneous measurement of the dilution marker urea. Adenyl purines are released onto the airway surfaces by airway epithelial and inflammatory cells where they act as signaling molecules to regulate host defenses. Multiple studies suggest that airway purines correlate with neutrophilic airway inflammation and EBC purines are elevated in subjects with airways disease such as CF15, asthma28 and chronic obstructive pulmonary disease 22.
While the potential value of EBC biomarkers has been demonstrated in older children and adults, there are few established methods for EBC biomarker evaluation in infants and preschoolers largely because of the challenges in obtaining adequate samples from this uncooperative cohort. Furthermore, many previously described methods for EBC collection in this age group rely on novel devices developed at the institution conducting the study, limiting the ability to generalize methods 8-11. In addition, the small volumes of EBC recovered coupled with the low concentrations of biomarkers make detection challenging5.
In this study we developed a method for measuring biomarkers in EBC collected from young children undergoing clinically indicated infant pulmonary function testing (iPFTs) using a commercially available collection device (RTube, Respiratory Research, Inc., Charlottesville, VA). We focused on purines based on our past success evaluating EBC purines as biomarkers of respiratory disease 13, 22, using mass spectrometry to assess the EBC concentrations of the purines adenosine (Ado) and adenosine monophosphate (AMP). Ado and AMP mediate multiple inflammatory cell responses and have been demonstrated to be potential biomarkers of neutrophilic airway inflammation in older children and adults with CF13-15. Moreover, we examined relationships between lung function indices and purine levels in EBC. Based on known relationships between purines and airway bronchitis in older children and adults, we hypothesized that EBC purines, particularly AMP, would correlate with measures of lung function.
Methods
Subjects
Subjects were preschool children (<3 years old) scheduled for clinically indicated iPFTs. All subjects were at clinical baseline at the time of testing with the exception of one subject who had collections performed before and after treatment of a CF pulmonary exacerbation. Subjects were categorized as CF if they met CF Foundation guidelines for the diagnosis16. Subjects were excluded if they woke before EBC collection could be attempted. This study was IRB approved and informed consent was obtained from subject parents prior to sample collection.
Infant Lung Function Testing
Infant pulmonary function tests were performed according to the guidelines of the American Thoracic Society and European Respiratory Society using the nSpire Infant Pulmonary Laboratory (nSpire Health, Inc., Longmont, CO)17,18. Patients were sedated with oral chloral hydrate based on the UNC Hospitals Sedation Guidelines; each subject’s heart rate, oxygen saturation and respiratory rate were monitored and documented throughout the procedure. Z-scores were calculated using previously reported reference equations19, 20.
EBC Collection
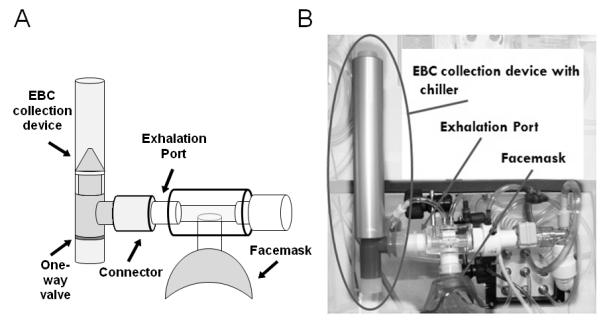
Collection of EBC from infants and preschoolers was performed by connecting the RTube EBC collection device (Respiratory Research, Inc., Charlottesville, VA) to the exhalation port of the nSpire Infant Pulmonary Laboratory, with the RTube chilled using a chiller sleeve held at −20°C prior to collection (Figure 1). EBC was collected during 10 minutes of tidal breathing either at the end of the procedure or during the 15 minute waiting period after administration of albuterol in those subjects receiving post-bronchodilator testing (13 of 15 subjects). Pulse oximetry and respiratory rates were recorded throughout the collection. EBC samples were extracted from the RTube and stored at −80°C until analyzed.
Figure 1.
Mass spectrometric analysis
Mass spectrometry was used to measure EBC concentrations of adenosine, AMP, and urea as previously described13,14. Briefly, an internal standard solution containing stable isotope was added to an aliquot of EBC at a ratio of 1:20 of the EBC volume, then the EBC plus internal standard solution was lyophilized to dryness and re-suspended in 25 μl of HPLC-grade water yielding an average of 14.4±5.2 fold increase in concentration. Ten μl of the lyophilized solution was injected onto a C18 column (Acquity UPLC HSS T3 1.8 μm), separated using 0.1% formic acid and methanol gradients, then analyzed on a triple quadrupole mass spectrometer (TSQ Quantum Ultra Mass Spectrometer, ThermoFinnigan, San Jose, CA) using selected reaction monitoring. All samples were assessed in duplicate and the mean was used for analysis. Of the 15 samples analyzed, two were eliminated from further analysis: one with low internal standard concentrations suggesting technical error and another as an outlier with biomarker concentrations >4 standard deviations from the mean, suggesting contamination. In a third EBC sample in which AMP was below detection limits, the AMP concentration was assigned as ½ the lowest measured concentration in other samples.
Statistical analyses
were performed using parametric statistical methods (Pearson for correlations, Student’s T-test for between group differences) using GraphPad Prism 5.0. EBC values were log transformed prior to analysis, based on data distributions observed in previous, larger studies13. All data is presented as means ± standard deviations.
Results
Collection and measurement of EBC biomarkers
EBC collection was attempted during 17 iPFT studies on 16 subjects, including 11 with CF and 5 with other chronic respiratory diseases (3 primary ciliary dyskinesia (PCD)21, 1 recurrent cough, 1 surfactant protein deficiency post lung transplant). Demographic data are summarized in Table 1. All subjects were at clinical baseline at the time of study, with the exception of one subject who had EBC collection with iPFTs before and after treatment of a CF pulmonary exacerbation. Pathogens were identified on most recent respiratory culture in 47% of subjects. The most common pathogens were Pseudomonas (n=4), Staphylococcus (n=2) and Haemophilus (n=2) species and were recovered in both CF and non-CF groups.
| All | CF | non-CF | |
|---|---|---|---|
| n =* | 17 | 12 | 5 |
| Age (years)** | 2.3±0.8 | 2.1±0.9 | 2.6±0.4 |
| Gender, male (%) | 6 (35%) | 4 (33%) | 2 (40%) |
| Pathogens on culture*** | 8 (47%) | 5 (42%) | 3 (60%) |
Data expressed as mean in years ± standard deviation
Most common pathogens were Pseudomonas (n=4), Staphylococcus (n=2) and Haemophilus (n=2) species and were recovered in both CF and non-CF groups.
EBC was successfully collected in 15 out of 17 attempts, including the one subject who had collections before and after antibiotic treatment for a CF pulmonary exacerbation. Successful EBC collection was defined as a condensate volume greater than or equal to 100 μL based on our experience with EBC analysis. All but two of the successful collections were obtained after albuterol administration. There were no obvious differences notable between the biomarker concentrations in these samples. EBC collection was not associated with any significant changes in oxygen saturation or respiratory rate (Table 2).
Table 2.
EBC collection
| Number of attempts (n) | 17 |
| Number of successful collections | 15 |
| EBC volume, successful collections | 360±130 μl |
| Oxygen saturations | |
| Start collection | 96.9±1.6 |
| End collection | 96.8±1.7 |
| P-value | 0.389 |
| Respiratory rates | |
| Start collection | 35.2±7.5 |
| End collection | 34.6±7.9 |
| P-value | 0.443 |
Data expressed as means ± standard deviation
Airway purines are detected in small volumes of EBC and correlate with infant lung function
Biomarkers were successfully measured via mass spectrometry in 13 of the 15 EBC samples. Based on analysis of standard curves for these experiments, we would estimate limits of detection at 5μM for urea and 1nM for AMP and Ado. Our biomarker concentrations were generally near these limits of detection, ranging from 3.4 to 124 μM for urea (27.2±33.42), 1.0 to 7.8 nM for adenosine (4.15±4.35), and 1.0 to 13.5 nM for AMP (5.87±7.97).
Relationships between EBC biomarkers and iPFT measures
To assess the relationship between EBC biomarkers and iPFT measures, controlling for the known variable incorporation of airway secretions within EBC was needed14. Although we previously utilized ratios of biomarkers to the dilution marker urea to control for this variability13, 14, 22, the measured EBC urea concentrations in this study were often below reliable quantification limits suggesting poor dependability as a dilution marker. Indeed, the adenosine to urea (Ado/Urea) and AMP/Urea ratios were not correlated with any iPFT measures (not shown).
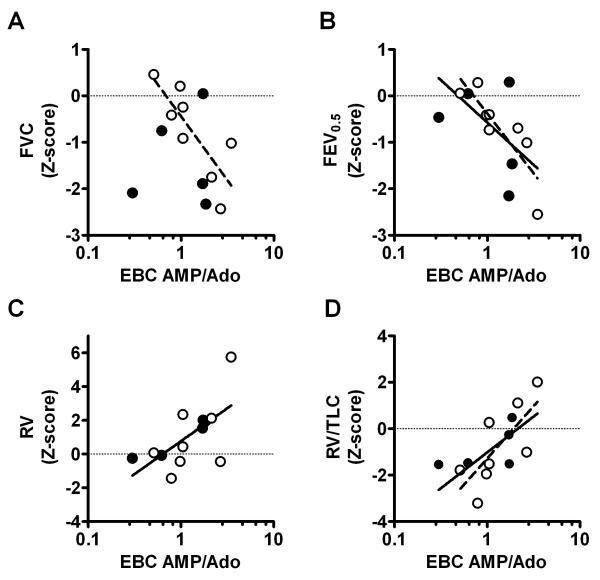
Alternatively, ratios of biomarkers to each other can also be used to control for dilution. We examined ratios of AMP/Ado since this ratio has been previously shown to correlate with neutrophilic inflammation in bronchoalveolar lavage fluid. 15 In spirometric flow measures, the EBC AMP/Ado ratio negatively correlated with forced vital capacity (FVC) in the subset with CF (r=−0.72 p=0.043), although not in the group as a whole (Figure 2A). EBC AMP/Ado negatively correlated with forced expiratory volume in 0.5 seconds (FEV0.5) in both the group as a whole (r=−0.71, p<0.01) and the subset with CF (r=−0.90, p<0.01) (Figure 2B). EBC AMP/Ado was not significantly correlated with forced expiratory flows between 25 and 75% of volume (FEF25-75) or flows at 75% volume (FEF75) in the entire group or the subset with CF. Of note, the ratio of AMP/Ado was log transformed based on our previous experience with larger EBC biomarker data sets. All findings remain significant even without log transformation prior to analysis.
Figure 2.
In plethysmographic volume measures, EBC AMP/Ado was positively correlated with residual volume (RV) (r=0.64, p<0.020, Figure 2C) and the ratio of residual volume to total lung capacity (RV/TLC) (r =0.66, p=0.015, Figure 2D) in the group as a whole. The latter relationship remained statistically significant in the subset with CF (r=0.74, p=0.037), with the correlation between EBC AMP/Ado. We did not observe any correlations between EBC AMP/Ado and functional residual capacity (FRC) or total lung capacity (TLC) in the whole group or the subset with CF.
We did not observe a significant difference between EBC AMP/Ado in the CF and non-CF groups (p=0.769). Interestingly, in the one subject in whom we had paired values, the EBC AMP/Ado ratio was lower in the sample obtained after intravenous antibiotic treatment relative to the sample obtained before treatment.
Discussion
EBC can be safely collected from sedated infants undergoing iPFTs using commercially available equipment. Consistent with previous studies23-25, EBC collection was well tolerated without any measurable changes in respiratory rate or oxygen saturation. The EBC AMP/Ado ratio correlated with infant lung function indices of airway obstruction. Additionally, EBC volumes were sufficient to measure our purine biomarkers.
More significantly, we observed that the EBC AMP/Ado ratio correlates with infant lung function indices of airway obstruction. The simplest interpretation is that the EBC AMP/Ado is a biomarker of neutrophilic inflammation, which has been shown to correlate with iPFT measures in children with CF experiencing an exacerbation26. In particular, the observed relationship between EBC AMP/Ado and plethysmographic findings are similar to those reported by Peterson-Carmichael et al., who found that RV/TLC correlated directly with % neutrophils in BALF. However, the relationships between inflammatory markers and spirometric measures were somewhat different in the two studies, these differences could reflect the relatively small sample size in both studies. Additionally, mid-expiratory flows are among the most sensitive, but also most variable measures of small airways obstruction. The coefficient of variation in our study was higher for FEF25-75 than for any other lung function measure. This variability can make it difficult to observe relationships when the sample size is small, and we suspect that was the case here. Ultimately, their conclusion was similar to ours in that markers of neutrophilic inflammation are correlated with increased airways obstruction. Furthermore, we have previously demonstrated that the AMP/Ado ratio in bronchoalveolar lavage fluid from older children correlated with percent neutrophils as well as with FEV115; our findings in preschoolers may represent the same pathophysiology.
Although our previous studies have shown that AMP is elevated in EBC from older children in CF, we have not observed elevated AMP/Ado ratio in CF relative to healthy controls. It is possible that this discrepancy reflects differences between acute and chronic airway inflammation. Our previous study involved an older CF population (average age 12.6±3.7 years)13 with more established, chronic airway inflammation. Such chronic inflammation has been associated with elevated EBC adenosine in many diseases22, 27, and we observed a trend towards elevated EBC Ado/Urea in the CF population in our previous study13. Many of these older children with CF also exhibited symptoms of asthma, which is a common co-morbidity associated with increased airway adenosine. Increased airway adenosine from chronic airway inflammation and/or asthma may have reduced the AMP/Ado ratio even in the context of elevated AMP from neutrophilic inflammation.
Limitations to this study include a relatively small number subjects, which while sufficient to demonstrate feasibility may have been too small to detect subtle relationships. Additionally, the timing of collection was chosen to minimize interference with the iPFTs, either after testing was complete or during the gap between pre and post-albuterol testing. Therefore, we cannot exclude the possibility that the iPFT procedure and/or albuterol administration affected our EBC purine measurements. At present, EBC collection is primarily an additional tool for those centers with the specialized equipment and personnel to perform iPFTs, however, the value lies in the fact that it adds a potential measure of lower airways inflammation that can otherwise only be obtained via bronchoscopy in this young population.
The positive findings from this study may be sufficient to justify modifying the iPFT protocol in future studies to allow EBC collection prior to testing; thereby, eliminating these potential confounders. Follow-up studies examining EBC biomarkers before and after antibiotic treatment and before and after albuterol would be necessary to validate preliminary evidence in this study. Furthermore, future studies exploring collection from non-sedated infants and preschoolers could certainly broaden the applicability of the EBC approach. Additionally, EBC AMP/Ado measurements could serve as an outcome measure in clinical trials that include iPFTs, especially for therapies predicted to improve airway inflammation. Additionally, biomarkers may add information about other aspects of lower airways disease; e.g., specific infections. Moreover, new techniques in sample collection (RTube connected to facemask) and analysis (more sensitive MS) may allow transition of this technology to the outpatient setting.
In summary, our results show that EBC can be safely collected from young children undergoing iPFTS and that EBC purine biomarkers may have utility in assessing lower airway inflammation in this vulnerable population. While promising, additional investigations are needed to validate the utility of EBC purines and other biomarkers in infants and preschoolers.
Acknowledgments
Funded by: CFF PATEL10A0, CFF DAVIS08Y2, NHLBI grant 1K23HL089708 and NIEHS grant P30 ES-10126,
Literature Cited
- 1.Armstrong DS, Hook SM, Jamsen KM, Nixon GM, Carzino R, Carlin JB, Robertson CF, Grimwood K. Lower airway inflammation in infants with cystic fibrosis detected by newborn screening. Pediatr Pulmonol. 2005;40(6):500–510. doi: 10.1002/ppul.20294. [DOI] [PubMed] [Google Scholar]
- 2.Davis SD, Fordham LA, Brody AS, Noah TL, Retsch-Bogart GZ, Qaqish BF, Yankaskas BC, Johnson RC, Leigh MW. Computed tomography reflects lower airway inflammation and tracks changes in early cystic fibrosis. Am J Respir Crit Care Med. 2007;175(9):943–950. doi: 10.1164/rccm.200603-343OC. [DOI] [PubMed] [Google Scholar]
- 3.Khan TZ, Wagener JS, Bost T, Martinez J, Accurso FJ, Riches DW. Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med. 1995;151(4):1075–1082. doi: 10.1164/ajrccm/151.4.1075. [DOI] [PubMed] [Google Scholar]
- 4.Sly PD, Brennan S, Gangell C, de Klerk N, Murray C, Mott L, Stick SM, Robinson PJ, Robertson CF, Ranganathan SC. Australian Respiratory Early Surveillance Team for Cystic F. Lung disease at diagnosis in infants with cystic fibrosis detected by newborn screening. Am J Respir Crit Care Med. 2009;180(2):146–152. doi: 10.1164/rccm.200901-0069OC. [DOI] [PubMed] [Google Scholar]
- 5.Effros R, Dunning MB, III, Biller J, Shaker R. The promise and perils of exhaled breath condensates. American Journal of Physiology-Lung Cell Mol Physiol. 2004;287:L1073–L1080. doi: 10.1152/ajplung.00069.2004. [DOI] [PubMed] [Google Scholar]
- 6.Kabra SK, Alok A, Kapil A, et al. Can throat swab after physiotherapy replace sputum identification of microbial pathogens in children with CF? Indian Journal of Pediatrics. 2004;71(1):21–23. doi: 10.1007/BF02725650. [DOI] [PubMed] [Google Scholar]
- 7.Ho SA, Ball R, Morrison LJ, Brownlee KG, Conway SP. Clinical value of obtaining sputum and cough swab samples following inhaled hypertonic saline in children with cystic fibrosis. Pediatr Pulmonol. 2004;38(1):82–87. doi: 10.1002/ppul.20035. [DOI] [PubMed] [Google Scholar]
- 8.Vogelberg C, Wurfel C, Knoetzsch A, et al. Exhaled breath condensate pH in infants and children with acute and recurrent wheezy bronchitis. Pediatric Pulmonology. 2007;42:1166–1172. doi: 10.1002/ppul.20712. [DOI] [PubMed] [Google Scholar]
- 9.Walsh BKMD, Pajewski T, Yu Y, Gaston BM, Hunt JF. Exhaled-breath condensate pH can be safely and continuously monitored in mechanically ventilated patients. Respiratory Care. 2006;51(10):1125–1131. [PubMed] [Google Scholar]
- 10.Muller WG, Morini F, Eaton S, et al. Safety and feasibility of exhaled breath condensate collection in ventilated infants and children. European Respiratory Journal. 2006;28:479–485. doi: 10.1183/09031936.06.00063505. [DOI] [PubMed] [Google Scholar]
- 11.Moeller A, Franklin P, Hall GL, et al. Measuring exhaled breath condensates in infants. Pediatric Pulmonology. 2006;41:184–187. doi: 10.1002/ppul.20362. [DOI] [PubMed] [Google Scholar]
- 12.Effros RM, Dunning MB, III, Biller J, Shaker R. The promise and perils of exhaled breath condensates. American Journal of Physiology-Lung Cell Mol Physiol. 2004;287:L1073–L1080. doi: 10.1152/ajplung.00069.2004. [DOI] [PubMed] [Google Scholar]
- 13.Esther CR, Jr., Boysen G, Olsen BM, Collins LB, Ghio AJ, Swenberg JW, Boucher RC. Mass spectrometric analysis of biomarkers and dilution markers in exhaled breath condensate reveals elevated purines in asthma and cystic fibrosis. Am J Physiol Lung Cell Mol Physiol. 2009;296(6):L987–993. doi: 10.1152/ajplung.90512.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esther CR, Jr., Jasin HM, Collins LB, Swenberg JA, Boysen G. A mass spectrometric method to simultaneously measure a biomarker and dilution marker in exhaled breath condensate. Rapid Commun Mass Spectrom. 2008;22(5):701–705. doi: 10.1002/rcm.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esther CR, Jr., Alexis NE, Clas ML, Lazarowski ER, Donaldson SH, Ribeiro CM, Moore CG, Davis SD, Boucher RC. Extracellular purines are biomarkers of neutrophilic airway inflammation. Eur Respir J. 2008;31(5):949–956. doi: 10.1183/09031936.00089807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farrell PM, Rosenstein BJ, et al. Guidelines for diagnosis of cystic fibrosis in newborns through older adults:Cystic Fibrosis Foundation Consensus Report. Journal of Pediatrics. 2008;153(2):S4–S14. doi: 10.1016/j.jpeds.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stocks J, Godfrey S, Beardsmore C, et al. Plethysmographic measurements of lung volume and airway resistance ERS/ATS task force on standars for infant respiratory function testing. European Respiratory Journal. 2001;17(2):302–312. doi: 10.1183/09031936.01.17203020. [DOI] [PubMed] [Google Scholar]
- 18.ATS/ERS ATS/ERS statement: Raised volume forced expirations in infants. American Journal of Respiratory and Critical Care Medicine. 2005;172(11):1463–1471. doi: 10.1164/rccm.200408-1141ST. [DOI] [PubMed] [Google Scholar]
- 19.Davis SD, Rosenfeld M, Kerby GS, Brumback L, Kloster MH, Acton JD, Colin AA, Conrad CK, Hart MA, Hiatt PW, Mogayzel PJ, Johnson RC, Wilcox SL, Castile RG. Multicenter evaluation of infant lung function tests as cystic fibrosis clinical trial endpoints. Am J Respir Crit Care Med. 2010;182(11):1387–1397. doi: 10.1164/rccm.200908-1236OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones M, Castile R, Davis S, Kisling J, Filbrun D, Flucke R, Goldstein A, Emsley C, Ambrosius W, Tepper RS. Forced expiratory flows and volumes in infants. Normative data and lung growth. Am J Respir Crit Care Med. 2000;161(2 Pt 1):353–359. doi: 10.1164/ajrccm.161.2.9903026. [DOI] [PubMed] [Google Scholar]
- 21.Leigh MWZM, Knowles MR. Primary ciliary dyskinesia: Improving diagnostic approach. Current Opinions in Pediatrics. 2009;21(3):320–325. doi: 10.1097/MOP.0b013e328329cddb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Esther CR, Jr., Lazaar AL, Bordonali E, Qaqish B, Boucher RC. Elevated airway purines in chronic obstructive pulmonary disease. Chest. 2011 doi: 10.1378/chest.10-2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vogelberg C, Wurfel C, Knoetzsch A, Kahlert A, Range U, Leupold W. Exhaled breath condensate pH in infants and children with acute and recurrent wheezy bronchitis. Pediatr Pulmonol. 2007;42(12):1166–1172. doi: 10.1002/ppul.20712. [DOI] [PubMed] [Google Scholar]
- 24.Moeller A, Franklin P, Hall GL, Horak F, Jr., Wildhaber JH, Stick SM. Measuring exhaled breath condensates in infants. Pediatr Pulmonol. 2006;41(2):184–187. doi: 10.1002/ppul.20362. [DOI] [PubMed] [Google Scholar]
- 25.Walsh BK, Mackey DJ, Pajewski T, Yu Y, Gaston BM, Hunt JF. Exhaled-breath condensate pH can be safely and continuously monitored in mechanically ventilated patients. Respir Care. 2006;51(10):1125–1131. [PubMed] [Google Scholar]
- 26.Peterson-Carmichael SL, Harris WT, Goel R, Noah TL, Johnson R, Leigh MW, Davis SD. Association of lower airway inflammation with physiologic findings in young children with cystic fibrosis. Pediatr Pulmonol. 2009;44(5):503–511. doi: 10.1002/ppul.21044. [DOI] [PubMed] [Google Scholar]
- 27.Huszar E, Vass G, Vizi E, Csoma Z, Barat E, Vilagos G Molnar, Herjavecz I, Horvath I. Adenosine in exhaled breath condensate in healthy volunteers and in patients with asthma. Eur Respir J. 2002;20(6):1393–1398. doi: 10.1183/09031936.02.00005002. [DOI] [PubMed] [Google Scholar]