Abstract
Background
Low serum concentrations of sex steroids and gonadotropins in men have been associated with increased cardiometabolic risk and mortality, but the clinical correlates of these hormones in men over the late adulthood are less clearly understood.
Methods
We analyzed up to five serial measurements of total testosterone (TT), dehydroepiandrosterone sulfate (DHEAS), follicle stimulating hormone (FSH), luteinizing hormone (LH), and total estradiol (EST) in older men in the original cohort of the Framingham Heart Study to determine the short- (2-years; 1,165 person-observations in 528 individuals) and long-term (up to 10-years follow-up; 2,520 person-observations in 835 individuals with mean baseline age: 71.2 years) clinical correlates of these sex steroids and gonadotropins using multilevel modelling and Generalized Estimating Equations.
Results
Age, body mass index, and pre-existing type 2 diabetes were inversely related to long-term TT concentrations, whereas higher systolic blood pressure showed a positive association. Furthermore, age and pre-existing cardiovascular disease (CVD) were inversely and HDL cholesterol concentrations positively associated with long-term DHEAS concentrations. Analyses of short-term changes revealed age was inversely related to DHEAS, but positively related to FSH and LH concentrations.
Conclusion
Our community-based study identified modifiable correlates of decreasing TT and DHEAS concentrations in elderly men, suggesting that maintenance of a low CVD risk factor burden may mitigate the age-related decline of these hormones over the late adulthood.
Keywords: sex steroids, gonadotropins, testosterone, aging male, Framingham Heart Study
INTRODUCTION
Low serum concentrations of total testosterone (TT) in men have been associated with increased cardiometabolic risk factor burden including a greater prevalence of dyslipidemia (Haring et al. 2011), hypertension (Torkler et al. 2011), metabolic syndrome (MetS) (Haring et al. 2009; Kupelian et al. 2006), type 2 diabetes (Schipf et al. 2011; Vikan et al. 2010), and atherosclerosis (Svartberg et al. 2006; Vikan et al. 2009), as well as mortality risk (Araujo et al. 2007; Haring et al. 2010b; Laughlin et al. 2008). Additionally, prostate cancer patients who undergo long-term androgen deprivation therapy are at greater risk of developing dyslipidemia, insulin resistance, hyperglycaemia, and MetS, suggesting potentially beneficial effects of endogenous TT on cardiovascular disease (CVD) risk factor burden (Hakimian et al. 2008). But prospective studies relating sex steroids to incident CVD have yielded inconsistent results (Muller et al. 2003b) and an initial trial of testosterone therapy was discontinued after it showed adverse cardiovascular events in the treatment group (Basaria et al. 2010). Furthermore, the interpretation of temporality of associations is challenging based on cross-sectional data due to the possibility of reverse causality. Thus, low TT concentrations are associated with incident MetS (Haring et al. 2009) or type 2 diabetes (Schipf et al. 2011; Vikan et al. 2010), whereas these conditions are also associated with decreased TT concentrations (Corona et al. 2010; Laaksonen et al. 2005). Although it has been widely observed that TT and other sex steroids in men decline with age (Feldman et al. 2002; Gray et al. 1991; Harman et al. 2001; Svartberg et al. 2003), other contributing factors have also been proposed (Andersson et al. 2007; Haring et al. 2010a; Travison et al. 2007b), suggesting a likely multifactorial basis for the decline in circulating TT concentrations in aging men (Snyder 2008).
Overall, there is a gap in our current knowledge about whether low TT serum concentrations in men are causal or the consequence of CVD. Also the clinical correlates of longitudinal tracking of TT concentrations in men from the general population have been less well studied (Snyder 2008). Accordingly, we examined the longitudinal profile of sex steroids and gonadotropins in older men from the Framingham Heart Study (FHS) using up to five serial hormone measurements over a 10-year follow-up period, and evaluated the clinical correlates. Specifically, we assessed both the short-term (2-year) change and the long-term (10-year) tracking for these steroid- and non-steroid hormones.
METHODS
Study Population
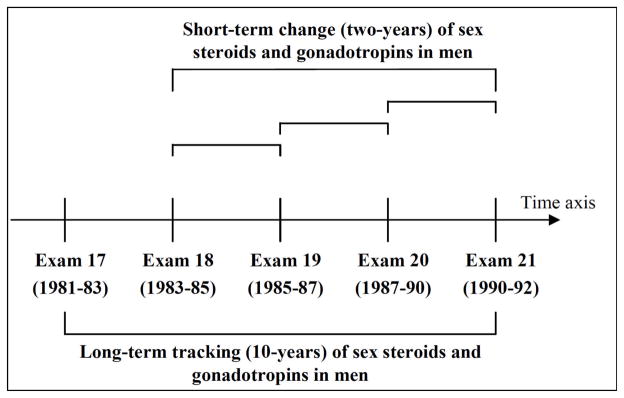
The FHS is a longitudinal epidemiological study that was initiated in 1948 in Framingham, Massachusetts, to investigate risk factors for heart disease in the community. The selection criteria and study design of the original FHS cohort have been described previously (Dawber et al. 1951). Written informed consent was obtained from attendees at each examination and the Institutional Review Board of the Boston University Medical Center approved the study protocol. The present study focused on men who attended at least two of the biennial examination cycles 17 (1981 to 1983) through 21 (1990 to 1992). We excluded observations with missing outcome or covariate information. We did not use information regarding testosterone therapy or hypogonadism as additional exclusion criteria as such data were not systematically collected at these examinations. Correlates of short-term change in sex steroids and gonadotropins (over any 2-year period) were investigated pooling 1,165 person-observations from 528 unique participants who attended two consecutive examinations. Long-term tracking of sex steroids and gonadotropins (over the course of 10 years) was analyzed in 835 unique participants (2,520 person-observations) (Figure 1).
Figure 1. Study design.
Short-term change in sex steroids and gonadotropins was evaluated in 528 men (1,165 person-observations). Long-term tracking of sex steroids and gonadotropins was performed in 835 men who attended up to five serial examination cycles (2,520 person-observations).
Laboratory measurements
Single non-fasting blood samples were obtained through out the day from attendees at the original cohort’s 17th–20st examination cycles between May 1981 and December 1989, and the biosamples were stored at −20°C until measurement of sex hormones and gonadotropins between October 1984 to March 1990. The maximum storage length (for only a portion of the samples) was about five years. Concentrations of serum TT, DHEAS, follicle stimulating hormone (FSH), luteinizing hormone (LH), and total estradiol (EST) were measured using radioimmunoassay’s (Diagnostic Products Corp., Los Angeles, CA, USA) in the Boston University laboratory of Dr. Sawin. The interassay coefficients of variation in the Sawin laboratory were: for TT, 11%; DHEAS, 11%; FSH, 5%; LH, 6%; and EST, 4%, as previously described (Amin et al. 2000).
Clinical Correlates
At each FHS examination, socio-demographic and behavioral characteristics (such as age, smoking status etc.), as well as medical history and medication use were assessed using standardized interviews. Height and weight were measured and body mass index (BMI) was calculated (kg/m2). Blood pressure (BP) was measured twice in the left arm of the seated subject with a mercury column sphygmomanometer. The average of the two readings was used as the exam BP, and hypertension was defined as systolic BP ≥140 mmHg or a diastolic BP ≥90 mmHg or the self-reported use of antihypertensive medications. Type 2 diabetes was defined by a non-fasting glucose >200 mg/dl, or self-reported use of insulin or oral hypoglycemic medications. Plasma total cholesterol and high-density lipoprotein (HDL) cholesterol concentrations were measured using standard enzymatic methods, as previously described (Mcnamara & Schaefer 1987). Pre-existing CVD was defined according to previously reported standardized FHS criteria (including coronary heart disease, cerebrovascular disease, intermittent claudication, or congestive heart failure) and confirmed with the aid of medical histories, physical examinations at the study clinic, hospitalization records, and communication with personal physicians as previously described (Kannel et al. 1979).
Statistical Analyses
Correlates of short-term change of sex steroids and gonadotropins in men
We naturally logarithmically transformed serum DHEAS, FSH, LH, and EST concentrations to normalize their distributions. Generalized Estimating Equations (GEE) were used to determine clinical correlates of short-term change in sex steroids and gonadotropins during a 2-year follow-up period (Figure 1). In these multivariable models, we related hormone concentrations (dependent variable; each hormone considered individually) to the following clinical covariates (independent variables): age, BMI, systolic BP, antihypertensive treatment, smoking status, type 2 diabetes, total cholesterol, HDL cholesterol, and pre-existing CVD. These variables were chosen based on their previously reported associations with steroid and non-steroid hormone concentrations in the literature (Haring et al. 2010a; Travison et al. 2007a). To account for potential temporal trends in sex steroids and gonadotropins across different examination cycles, we carefully adjusted all statistical analyses for examination cycle. Furthermore, we provided boxplots for the median sex steroid and gonadotropin concentrations over time for a reference age group of individuals aged 65–75 years at each examination cycle, indicating no large variability across the different examination cycles (Supplemental Figure 1). Interaction terms between age and each clinical covariate were investigated using multivariable models. To analyze patterns of missingness we compared characteristics of the study sample according to the number of missing examinations (Supplemental Table 1 and 2).
Effects are presented as regression coefficients and their corresponding 95% confidence interval (95% CI). We examined and detected statistically significant random age effects for DHEAS, FSH, LH, and EST, and modeled age as a random and a fixed effect accordingly. We also examined nonlinear age effects, by including the ‘squared age’ term into the regression models and assessing the p-value for the term. Since the ‘squared age’ term was not statistically significant in any of the models, we did not include it in further regression modeling.
Correlates of long-term tracking of sex steroids and gonadotropins in men
We performed multilevel statistical modeling (SAS PROC MIXED; using an unstructured correlation matrix) to identify clinical correlates of long-term tracking of sex hormone concentrations over a 10-year period. Accommodating participants with missing data at some of the serial examinations over the 10-year follow-up period, this analytical approach allows the maximization of the available number of observations in a longitudinal study design and accounts for a hierarchical data structure that varies on the individual-level. Multivariable models incorporated the same set of covariates and interaction terms as used in the short-term analyses described above. While change in covariates is captured in the long-term follow-up analysis, the short-term analysis used “baseline” covariate data and only the hormone data from the baseline and the subsequent examination cycle. For the paired 2-year data, participants had to attend consecutive examinations while for the 10-year data no such sequential follow-up was mandated (not every individual has to attend every examination), which is why the latter approach provided more person-observations.
Graphical representation of long-term sex steroids in men
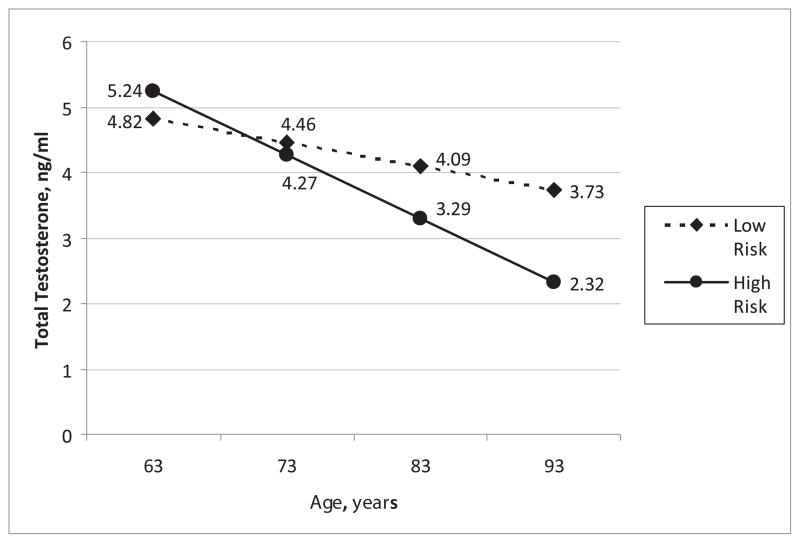
To illustrate the impact of an individual’s risk factor burden on long-term TT concentrations, graphical displays show the adjusted (for all significant variables from the long-term analyses) mean TT concentrations with increasing age stratified by “high” vs. “low” risk factor burden. The covariates used to define high and low risk factor status (age, BMI, systolic BP, type 2 diabetes, and smoking) were selected on the basis of their statistically significant associations with TT concentrations in the final regression model. All statistical analyses were performed using SAS statistical software (SAS Institute Inc., Cary, NC, USA), and the figures were generated using Excel (Microsoft Office 2003, Redmond, WA, USA).
RESULTS
The baseline characteristics of our sample of older men are displayed in Table 1.
Table 1.
Baseline characteristics of the study population with complete 10-year follow-up.*
| Characteristic | |
|---|---|
| Age, years | 71.2 ± 6.4 |
| Body Mass Index, kg/m2 | 26.6 ± 3.8 |
| Current Smoker, % | 16.5 |
| Systolic Blood Pressure, mmHg | 141.6 ± 18.5 |
| Diastolic Blood Pressure, mmHg | 78.0 ± 10.0 |
| Antihypertensive Medication, % | 41.2 |
| Hypertension, % | 68.4 |
| Type 2 Diabetes, % | 12.7 |
| HDL Cholesterol, mg/dl | 44.3 ± 13.6 |
| Total Cholesterol, mg/dl | 216.6 ± 37.4 |
| Ratio Total : HDL Cholesterol, mg/dl | 5.3 ± 1.6 |
| Prevalence of Cardiovascular Disease, % | 34.3 |
| Total Testosterone, ng/ml | 4.9 (3.9; 5.9) |
| DHEAS, mg/dl | 91.2 (56.8; 137.0) |
| FSH, IU/l | 9.0 (6.2; 13.4) |
| Luteinizing Hormone, IU/l | 8.6 (6.2; 12.0) |
| Total Estradiol, pg/ml | 30.2 (21.3; 39.2) |
Data are percentages, mean ± SD, or median (Q1; Q3).
Baseline characteristics are presented for the sample with the largest available data for sex steroids and gonadotropins: total testosterone, N = 834; dehydroepiandrosterone sulfate (DHEAS), N = 657, follicle stimulating hormone (FSH), N = 835, luteinizing hormone, N = 835; estradiol, N = 834. Values for these hormones are reported based on availability of each hormone.
Correlates of short-term change in sex steroids and gonadotropins in men
We identified age as the main correlate of short-term change in sex steroids and gonadotropins (Table 2), inversely associated with change in log-DHEAS (β per one year increase in age, −0.007; 95% CI, −0.012 to −0.002) and positively associated with change in log-FSH and log-LH concentrations (β per one year increase in age, 0.005; 95% CI, 0.001 to 0.008 and 0.007; 95% CI, 0.002 to 0.012, respectively). BMI was inversely associated with change in TT concentrations (β per one unit increase in BMI, −0.02 ng/ml; 95% CI, −0.04 to −0.007 ng/ml), and systolic BP was positively associated with change in log-DHEAS concentrations (β per 10 mmHg increase in systolic BP, 0.02; 95% CI, 0.01 to 0.03).
Table 2.
Directionality of correlates of short-term change and long-term tracking of sex steroids and gonadotropins in men based on multivariable analyses.
| Correlates | Short-term change (over any 2-year period) | Long-term tracking (complete 10-year period) |
|---|---|---|
| Age | ↓ DHEAS, ↑ FSH, ↑ LH | ↓ TT, ↓ DHEAS, ↑ LH |
| Body Mass Index | ↓ TT | ↓ TT |
| Current Smoking | ↑ LH | |
| Systolic Blood Pressure | ↑ DHEAS | ↑ TT, ↑ EST |
| Antihypertensive Medication | ↑ EST | |
| Type 2 Diabetes, | ↓ TT | |
| HDL Cholesterol, | ↑ DHEAS | |
| Pre-existing Cardiovascular Disease | ↓ DHEAS |
TT, total testosterone; DHEAS, dehydroepiandrosterone sulfate; FSH, follicle-stimulating hormone; LH, luteinizing hormone; EST, total estradiol.
Correlates of long-term tracking of sex steroids and gonadotropins in men
Age, BMI, smoking, systolic BP, hypertension treatment, type 2 diabetes, HDL cholesterol concentrations, and pre-existing CVD were identified as significant correlates of changes in sex steroids and gonadotropins over the 10-year follow-up period (Table 2 and 3). With regard to long-term TT concentrations, we found inverse associations with age, BMI, and type 2 diabetes, and a positive association with systolic BP, respectively. The interaction between age and smoking was statistically significant, indicating that the inverse effect of age on TT concentrations varies according to smoking status (smokers have a greater age-related decline in TT concentrations compared to non-smokers). Consistent with the analyses of short-term change, age was inversely associated with log-DHEAS concentrations and positively associated with log-LH concentrations in long-term analyses. Furthermore, HDL cholesterol concentrations were positively and pre-existing CVD inversely associated with log-DHEAS concentrations in long-term analyses. Other correlates and their directionality are listed in Table 2 and 3. Finally, Figure 2 illustrates the conjoint effect of multiple risk factors (categorized into two groups for example) on TT concentrations over the 10-year period.
Table 3.
Correlates of long-term tracking of sex steroids and gonadotropins in men based on multivariable analyses.
| TT | DHEAS | FSH | LH | EST | |
|---|---|---|---|---|---|
|
| |||||
| Correlates | Beta coef. (95% CI) | Beta coef. (95% CI) | Beta coef. (95% CI) | Beta coef. (95% CI) | Beta coef. (95% CI) |
| Age | −0.04 (−0.05, −0.02)* | −0.03 (−0.04, −0.02)* | −0.01 (−0.04, 0.02) | 0.03 (0.02, 0.04)* | |
| Body Mass Index | −0.04 (−0.06, −0.02)* | ||||
| Current Smoking | 0.17 (−0.06, 0.41) | 0.09 (0.02, 0.17)* | |||
| Systolic Blood Pressure | 0.004 (0.001, 0.007)* | −0.0004 (−0.002, 0.0007) | 0.002 (0.001, 0.004)* | ||
| Antihypertensive Medication | 0.08 (0.02, 0.14)* | ||||
| Type 2 Diabetes, | −0.23 (−0.45, −0.02)* | ||||
| HDL Cholesterol, | 0.003 (0.0002, 0.006)* | ||||
| Pre-existing CVD | −0.10 (−0.17, −0.03)* | ||||
| Age* Systolic Blood Pressure | 0.0003 (0.0001, 0.0005)* | ||||
| Age* Smoking | −0.06 (−0.09, −0.03)* | ||||
p<0.05
CVD, cardiovascular disease, TT, total testosterone; DHEAS, dehydroepiandrosterone sulfate; FSH, follicle-stimulating hormone; LH, luteinizing hormone; EST, total estradiol. Any non-significant covariates were retained in the model if they contributed to a significant interaction term.
Age was centered at the mean of all participants at all exams (73 years) to reduce multicollinearity between regression coefficients.
DHEAS, EST, FSH, and LH were naturally log-transformed, therefore the coefficient (coef.) indicates an eβ-fold change in the respective sex steroid or gonadotropin. For example: β for age (log-DHEAS) = −0.03 → e−0.03 = 0.97 fold decrease in DHEAS concentration per year increase. TT was used untransformed, therefore a one-unit increase in body mass index resulted in a 0.04 ng/ml decrease in TT concentration.
The effect of variables that are included in statistically significant interaction terms needs to be interpreted taking into account the respective interaction terms. For example, the effect of age on TT depends on smoking status (due to the presence of the statistically significant age*smoking interaction term in the model), therefore a one-year increase in age results in a 0.1 ng/ml decrease in TT among smokers and a 0.04 ng/ml decrease in TT among non-smokers.
Figure 2. Adjusted mean total testosterone concentrations with increasing age.
Long-term tracking of total testosterone concentrations in men with low and high CVD risk factor burden. Low risk factor burden: body mass index, 25 kg/m2; systolic blood pressure, 130 mmHg; non-smoker; no pre-existing type 2 diabetes; high risk factor burden: body mass index, 30 kg/m2; systolic blood pressure, 150 mmHg; smoker; pre-existing type 2 diabetes.
DISCUSSION
Our longitudinal study identified various clinical correlates of short-term changes and long-term tracking of sex steroids and gonadotropins in older men, respectively. Analyses of short-term changes revealed age inversely related to DHEAS, but positively related to FSH and LH concentrations. Age, BMI, and type 2 diabetes were inversely related to long-term TT concentrations, whereas higher systolic BP showed a positive association. Thus, our study offers important insights into the correlates of long-term progression of sex steroids and gonadotropins in community-dwelling older men over their late adulthood.
Our study confirms previous longitudinal data reporting age as the main correlate of sex steroids and gonadotropins in men (Cappola et al. 2009; Gray et al. 1991; Harman et al. 2001; Lapauw et al. 2008; Leifke et al. 2000; Morley et al. 1997; Muller et al. 2003a; Wu et al. 2008), showing inverse associations with TT (Harman et al. 2001) and DHEAS (Cappola et al. 2009) concentrations, and positive associations with FSH (Lapauw et al. 2008; Morley et al. 1997) and LH (Lapauw et al. 2008; Morley et al. 1997; Wu et al. 2008) concentrations. The fact that age was the only consistent correlate in our analyses of short-term changes may be explained by the relatively short follow-up time of two years, limiting our ability to elucidate the effects of other covariates on sex steroids and gonadotropins in men. But besides aging alone, the clinical correlates of long-term TT concentrations identified in this study are consistent with previous studies reporting visceral obesity (Derby et al. 2006; Mohr et al. 2006; Travison et al. 2007a; Wu et al. 2008), MetS (Haring et al. 2010a; Laaksonen et al. 2005), type 2 diabetes (Haring et al. 2010a; Vermeulen et al. 1996), and comorbidity (Derby et al. 2006; Haring et al. 2010a; Svartberg et al. 2003) related to decreased TT concentrations. In contrast to previous studies reporting an inverse association between TT concentrations and BP among middle-aged men (Barrett-Connor & Khaw 1988; Torkler et al. 2011; Yarnell et al. 1993), the present study is the first to report a positive association between systolic BP and TT among elderly men, while others did not observe any association at all (Khaw et al. 2007; Zmuda et al. 1997). However, these inconsistencies may relate to differences in study design, study sample, characteristics of the study population including age and underlying comorbidity, or confounders adjusted for. Concerning DHEAS concentrations, a nine-year follow-up of 989 older men and women (mean age 85.2 years) from the Cardiovascular Health Study also showed that pre-existing CVD was associated with greater incident DHEAS decline (Sanders et al. 2010). Furthermore, our finding of a positive association between HDL cholesterol and DHEAS concentrations in long-term analyses was consistent with previous results from small cross-sectional studies (Haffner et al. 1993; Yasui et al. 2008).
Special attention belongs to our finding that BMI showed an inverse association with TT concentrations. It has been previously shown that a four to five kg/m2 increase in BMI is associated with declines in TT concentrations comparable to that associated with approximately 10 years of aging (Travison et al. 2007a). Furthermore, we observed consistent with previous longitudinal studies that pre-existing type 2 diabetes (Corona et al. 2010; Laaksonen et al. 2005) and pre-existing CVD (Sanders et al. 2010) are associated with lower TT and DHEAS concentrations. Given the previously shown associations of low TT and DHEAS concentrations with incident type 2 diabetes (Schipf et al. 2010; Vikan et al. 2010) and pre-existing CVD (Barrett-Connor et al. 1986), respectively, the present results provide further evidence for bidirectional influences between sex steroids and chronic diseases (Yeap 2009).
Our reported positive associations between age and LH concentrations and also between smoking and LH concentrations confirms previous cross-sectional findings from the European Male Aging Study among 3,220 men aged 40 to 79 years (Wu et al. 2008) and extends them using serial longitudinal observations. Since LH stimulates the Leydig cells to secrete testosterone and the quantity of testosterone secreted increases approximately in direct proportion to the amount of LH available, the effects of smoking are tightly linked for both steroid and non-steroid hormones in men (Mendelson et al. 2003); which could possibly explain the observed positive associations between current smoking, LH (Wu et al. 2008), and TT (Vermeulen et al. 1996; Wu et al. 2008).
Strengths and Limitations
The strengths of the present investigation include the use of multilevel modelling in a unique community-based sample of older men with up to five serial measurements of sex steroids and gonadotropins over a 10-year period. Some important limitations also have to be mentioned. First, we used single serum samples and radioimmunoassay’s to measure circulating steroid and non-steroid hormone concentrations. However, since we investigated relative changes instead of absolute, the necessity of repeated testing at single time points to characterize borderline hormone concentrations does not apply to our study aims (Rosner et al. 2007). Furthermore, we sought to limit artifactual changes in sex steroids and gonadotropins across serial examinations by performing measurements in one central laboratory, following the same collection protocol, and using the same laboratory assays for serum samples that were stored at −70°C. Additionally, we carefully adjusted all our analyses for “examination cycle” to account for potential measurement bias. Second, we did not measure sex-hormone binding globulin and were therefore not able to examine its correlates and interplay with the identified correlates of steroid and non-steroid hormone concentrations in men. Third, the external validity or generalizability of our findings to other populations, age groups, or ethnicities is limited due to a community-based study sample of predominantly white older Caucasian men.
Conclusions
Given the accumulating evidence suggesting that low sex steroid concentrations in men may be associated with greater cardiometabolic risk, it is crucial to characterize their correlates. But the identified correlates influencing long-term steroid and non-steroid hormone concentrations in older men also constitute major cardiometabolic risk factors associated with CVD onset and progression. Thus, reverse causation might explain some of the observed associations in the literature, wherein adverse cardiometabolic risk factor profiles influence (lower) TT concentrations, which in turn may affect cardiometabolic risk factor burden. To further elucidate the potential role of low TT as a causal CVD risk factor (and the direction of causality), future research from large randomized controlled clinical trials of testosterone replacement therapy is needed. However, the present findings assert that prevention strategies should focus on health maintenance including a low cardiometabolic risk factor burden, instead of testosterone replacement therapy for improving CV health and lowering CVD risk.
Supplementary Material
Acknowledgments
SOURCES OF FUNDING
This work was supported by the National Heart Blood and Lung Institute (contract N01-HC-25195).
Footnotes
DISCLOSURES
None.
References
- Amin S, Zhang Y, Sawin CT, Evans SR, Hannan MT, Kiel DP, Wilson PW, Felson DT. Association of hypogonadism and estradiol levels with bone mineral density in elderly men from the Framingham study. Ann Intern Med. 2000;133:951–963. doi: 10.7326/0003-4819-133-12-200012190-00010. [DOI] [PubMed] [Google Scholar]
- Andersson AM, Jensen TK, Juul A, Petersen JH, Jorgensen T, Skakkebaek NE. Secular decline in male testosterone and sex hormone binding globulin serum levels in Danish population surveys. The Journal of clinical endocrinology and metabolism. 2007;92:4696–4705. doi: 10.1210/jc.2006-2633. [DOI] [PubMed] [Google Scholar]
- Araujo AB, Kupelian V, Page ST, Handelsman DJ, Bremner WJ, McKinlay JB. Sex steroids and all-cause and cause-specific mortality in men. Archives of internal medicine. 2007;167:1252–1260. doi: 10.1001/archinte.167.12.1252. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Khaw KT. Endogenous sex hormones and cardiovascular disease in men. A prospective population-based study. Circulation. 1988;78:539–545. doi: 10.1161/01.cir.78.3.539. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Khaw KT, Yen SS. A prospective study of dehydroepiandrosterone sulfate, mortality, and cardiovascular disease. The New England journal of medicine. 1986;315:1519–1524. doi: 10.1056/NEJM198612113152405. [DOI] [PubMed] [Google Scholar]
- Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, Jette AM, Eder R, Tennstedt S, Ulloor J, Zhang A, Choong K, Lakshman KM, Mazer NA, Miciek R, Krasnoff J, Elmi A, Knapp PE, Brooks B, Appleman E, Aggarwal S, Bhasin G, Hede-Brierley L, Bhatia A, Collins L, LeBrasseur N, Fiore LD, Bhasin S. Adverse events associated with testosterone administration. The New England journal of medicine. 2010;363:109–122. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappola AR, O’Meara ES, Guo W, Bartz TM, Fried LP, Newman AB. Trajectories of dehydroepiandrosterone sulfate predict mortality in older adults: the cardiovascular health study. The journals of gerontology. 2009;64:1268–1274. doi: 10.1093/gerona/glp129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona G, Monami M, Rastrelli G, Aversa A, Sforza A, Lenzi A, Forti G, Mannucci E, Maggi M. Type 2 diabetes mellitus and testosterone: a meta-analysis study. International journal of andrology. 2010 doi: 10.1111/j.1365-2605.2010.01117.x. [DOI] [PubMed] [Google Scholar]
- Dawber TR, Meadors GF, Moore FE., Jr Epidemiological approaches to heart disease: the Framingham Study. American journal of public health and the nation’s health. 1951;41:279–281. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derby CA, Zilber S, Brambilla D, Morales KH, McKinlay JB. Body mass index, waist circumference and waist to hip ratio and change in sex steroid hormones: the Massachusetts Male Ageing Study. Clin Endocrinol (Oxf) 2006;65:125–131. doi: 10.1111/j.1365-2265.2006.02560.x. [DOI] [PubMed] [Google Scholar]
- Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, Bremner WJ, McKinlay JB. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. The Journal of clinical endocrinology and metabolism. 2002;87:589–598. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- Gray A, Feldman HA, McKinlay JB, Longcope C. Age, disease, and changing sex hormone levels in middle-aged men: results of the Massachusetts Male Aging Study. The Journal of clinical endocrinology and metabolism. 1991;73:1016–1025. doi: 10.1210/jcem-73-5-1016. [DOI] [PubMed] [Google Scholar]
- Haffner SM, Mykkanen L, Valdez RA, Katz MS. Relationship of sex hormones to lipids and lipoproteins in nondiabetic men. The Journal of clinical endocrinology and metabolism. 1993;77:1610–1615. doi: 10.1210/jcem.77.6.8263149. [DOI] [PubMed] [Google Scholar]
- Hakimian P, Blute M, Jr, Kashanian J, Chan S, Silver D, Shabsigh R. Metabolic and cardiovascular effects of androgen deprivation therapy. BJU international. 2008;102:1509–1514. doi: 10.1111/j.1464-410X.2008.07933.x. [DOI] [PubMed] [Google Scholar]
- Haring R, Baumeister SE, Völzke H, Dorr M, Felix SB, Kroemer HK, Nauck M, Wallaschofski H. Prospective Association of Low Total Testosterone Concentrations with an Adverse Lipid Profile and Increased Incident Dyslipidemia. Eur J Cardiovasc Prev Rehabil. 2011;18:86–96. doi: 10.1097/HJR.0b013e32833c1a8d. [DOI] [PubMed] [Google Scholar]
- Haring R, Ittermann T, Volzke H, Krebs A, Zygmunt M, Felix SB, Grabe HJ, Nauck M, Wallaschofski H. Prevalence, incidence and risk factors of testosterone deficiency in a population-based cohort of men: results from the study of health in Pomerania. Aging Male. 2010a;13:247–257. doi: 10.3109/13685538.2010.487553. [DOI] [PubMed] [Google Scholar]
- Haring R, Volzke H, Felix SB, Schipf S, Dorr M, Rosskopf D, Nauck M, Schofl C, Wallaschofski H. Prediction of metabolic syndrome by low serum testosterone levels in men: results from the study of health in Pomerania. Diabetes. 2009;58:2027–2031. doi: 10.2337/db09-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haring R, Volzke H, Steveling A, Krebs A, Felix SB, Schofl C, Dorr M, Nauck M, Wallaschofski H. Low serum testosterone levels are associated with increased risk of mortality in a population-based cohort of men aged 20–79. European heart journal. 2010b;31:1494–1501. doi: 10.1093/eurheartj/ehq009. [DOI] [PubMed] [Google Scholar]
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. The Journal of clinical endocrinology and metabolism. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. American journal of epidemiology. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- Khaw KT, Dowsett M, Folkerd E, Bingham S, Wareham N, Luben R, Welch A, Day N. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European prospective investigation into cancer in Norfolk (EPIC-Norfolk) Prospective Population Study. Circulation. 2007;116:2694–2701. doi: 10.1161/CIRCULATIONAHA.107.719005. [DOI] [PubMed] [Google Scholar]
- Kupelian V, Page ST, Araujo AB, Travison TG, Bremner WJ, McKinlay JB. Low sex hormone-binding globulin, total testosterone, and symptomatic androgen deficiency are associated with development of the metabolic syndrome in nonobese men. The Journal of clinical endocrinology and metabolism. 2006;91:843–850. doi: 10.1210/jc.2005-1326. [DOI] [PubMed] [Google Scholar]
- Laaksonen DE, Niskanen L, Punnonen K, Nyyssonen K, Tuomainen TP, Valkonen VP, Salonen JT. The metabolic syndrome and smoking in relation to hypogonadism in middle-aged men: a prospective cohort study. The Journal of clinical endocrinology and metabolism. 2005;90:712–719. doi: 10.1210/jc.2004-0970. [DOI] [PubMed] [Google Scholar]
- Lapauw B, Goemaere S, Zmierczak H, Van Pottelbergh I, Mahmoud A, Taes Y, De Bacquer D, Vansteelandt S, Kaufman JM. The decline of serum testosterone levels in community-dwelling men over 70 years of age: descriptive data and predictors of longitudinal changes. European journal of endocrinology/European Federation of Endocrine Societies. 2008;159:459–468. doi: 10.1530/EJE-07-0873. [DOI] [PubMed] [Google Scholar]
- Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. The Journal of clinical endocrinology and metabolism. 2008;93:68–75. doi: 10.1210/jc.2007-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leifke E, Gorenoi V, Wichers C, Von Zur Muhlen A, Von Buren E, Brabant G. Age-related changes of serum sex hormones, insulin-like growth factor-1 and sex-hormone binding globulin levels in men: cross-sectional data from a healthy male cohort. Clin Endocrinol (Oxf) 2000;53:689–695. doi: 10.1046/j.1365-2265.2000.01159.x. [DOI] [PubMed] [Google Scholar]
- McNamara JR, Schaefer EJ. Automated enzymatic standardized lipid analyses for plasma and lipoprotein fractions. Clinica chimica acta; international journal of clinical chemistry. 1987;166:1–8. doi: 10.1016/0009-8981(87)90188-4. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Sholar MB, Mutschler NH, Jaszyna-Gasior M, Goletiani NV, Siegel AJ, Mello NK. Effects of intravenous cocaine and cigarette smoking on luteinizing hormone, testosterone, and prolactin in men. The Journal of pharmacology and experimental therapeutics. 2003;307:339–348. doi: 10.1124/jpet.103.052928. [DOI] [PubMed] [Google Scholar]
- Mohr BA, Bhasin S, Link CL, O’Donnell AB, McKinlay JB. The effect of changes in adiposity on testosterone levels in older men: longitudinal results from the Massachusetts Male Aging Study. European journal of endocrinology/European Federation of Endocrine Societies. 2006;155:443–452. doi: 10.1530/eje.1.02241. [DOI] [PubMed] [Google Scholar]
- Morley JE, Kaiser FE, Perry HM, 3rd, Patrick P, Morley PM, Stauber PM, Vellas B, Baumgartner RN, Garry PJ. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metabolism: clinical and experimental. 1997;46:410–413. doi: 10.1016/s0026-0495(97)90057-3. [DOI] [PubMed] [Google Scholar]
- Muller M, den Tonkelaar I, Thijssen JH, Grobbee DE, van der Schouw YT. Endogenous sex hormones in men aged 40–80 years. European journal of endocrinology/European Federation of Endocrine Societies. 2003a;149:583–589. doi: 10.1530/eje.0.1490583. [DOI] [PubMed] [Google Scholar]
- Muller M, van der Schouw YT, Thijssen JH, Grobbee DE. Endogenous sex hormones and cardiovascular disease in men. The Journal of clinical endocrinology and metabolism. 2003b;88:5076–5086. doi: 10.1210/jc.2003-030611. [DOI] [PubMed] [Google Scholar]
- Rosner W, Auchus RJ, Azziz R, Sluss PM, Raff H. Utility, Limitations, and Pitfalls in Measuring Testosterone: An Endocrine Society Position Statement. The Journal of clinical endocrinology and metabolism. 2007;92:405–413. doi: 10.1210/jc.2006-1864. [DOI] [PubMed] [Google Scholar]
- Sanders JL, Boudreau RM, Cappola AR, Arnold AM, Robbins J, Cushman M, Newman AB. Cardiovascular disease is associated with greater incident dehydroepiandrosterone sulfate decline in the oldest old: the cardiovascular health study all stars study. J Am Geriatr Soc. 2010;58:421–426. doi: 10.1111/j.1532-5415.2010.02724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipf S, Haring R, Friedrich N, Nauck M, Lau K, Alte D, Stang A, Völzke H, Wallaschofski H. Low total testosterone is associated with increased risk of incident type 2 diabetes mellitus in men: results from the Study of Health in Pomerania (SHIP) Aging Male. 2011;14:168–175. doi: 10.3109/13685538.2010.524955. [DOI] [PubMed] [Google Scholar]
- Schipf S, Haring R, Friedrich N, Nauck MA, Lau K, Alte D, Stang A, Volzke H, Wallaschofski H. Low Total Testosterone is Associated with Increased Risk of Incident Type 2 Diabetes Mellitus in Men: Results from the Study of Health in Pomerania (SHIP) Aging Male. 2010 doi: 10.3109/13685538.2010.524955. [DOI] [PubMed] [Google Scholar]
- Snyder PJ. Decreasing testosterone with increasing age: more factors, more questions. The Journal of clinical endocrinology and metabolism. 2008;93:2477–2478. doi: 10.1210/jc.2008-0922. [DOI] [PubMed] [Google Scholar]
- Svartberg J, Midtby M, Bonaa KH, Sundsfjord J, Joakimsen RM, Jorde R. The associations of age, lifestyle factors and chronic disease with testosterone in men: the Tromso Study. European journal of endocrinology/European Federation of Endocrine Societies. 2003;149:145–152. doi: 10.1530/eje.0.1490145. [DOI] [PubMed] [Google Scholar]
- Svartberg J, von Muhlen D, Mathiesen E, Joakimsen O, Bonaa KH, Stensland-Bugge E. Low testosterone levels are associated with carotid atherosclerosis in men. J Intern Med. 2006;259:576–582. doi: 10.1111/j.1365-2796.2006.01637.x. [DOI] [PubMed] [Google Scholar]
- Torkler S, Wallaschofski H, Baumeister SE, Volzke H, Dorr M, Felix S, Rettig R, Nauck M, Haring R. Inverse association between total testosterone concentrations, incident hypertension and blood pressure. Aging Male. 2011;14:176–182. doi: 10.3109/13685538.2010.529194. [DOI] [PubMed] [Google Scholar]
- Travison TG, Araujo AB, Kupelian V, O’Donnell AB, McKinlay JB. The relative contributions of aging, health, and lifestyle factors to serum testosterone decline in men. The Journal of clinical endocrinology and metabolism. 2007a;92:549–555. doi: 10.1210/jc.2006-1859. [DOI] [PubMed] [Google Scholar]
- Travison TG, Araujo AB, O’Donnell AB, Kupelian V, McKinlay JB. A population-level decline in serum testosterone levels in American men. The Journal of clinical endocrinology and metabolism. 2007b;92:196–202. doi: 10.1210/jc.2006-1375. [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Kaufman JM, Giagulli VA. Influence of some biological indexes on sex hormone-binding globulin and androgen levels in aging or obese males. The Journal of clinical endocrinology and metabolism. 1996;81:1821–1826. doi: 10.1210/jcem.81.5.8626841. [DOI] [PubMed] [Google Scholar]
- Vikan T, Johnsen SH, Schirmer H, Njolstad I, Svartberg J. Endogenous testosterone and the prospective association with carotid atherosclerosis in men: the Tromso study. European journal of epidemiology. 2009;24:289–295. doi: 10.1007/s10654-009-9322-2. [DOI] [PubMed] [Google Scholar]
- Vikan T, Schirmer H, Njolstad I, Svartberg J. Low testosterone and sex hormone-binding globulin levels and high estradiol levels are independent predictors of type 2 diabetes in men. European journal of endocrinology/European Federation of Endocrine Societies. 2010;162:747–754. doi: 10.1530/EJE-09-0943. [DOI] [PubMed] [Google Scholar]
- Wu FC, Tajar A, Pye SR, Silman AJ, Finn JD, O’Neill TW, Bartfai G, Casanueva F, Forti G, Giwercman A, Huhtaniemi IT, Kula K, Punab M, Boonen S, Vanderschueren D. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. The Journal of clinical endocrinology and metabolism. 2008;93:2737–2745. doi: 10.1210/jc.2007-1972. [DOI] [PubMed] [Google Scholar]
- Yarnell JW, Beswick AD, Sweetnam PM, Riad-Fahmy D. Endogenous sex hormones and ischemic heart disease in men. The Caerphilly prospective study. Arterioscler Thromb. 1993;13:517–520. doi: 10.1161/01.atv.13.4.517. [DOI] [PubMed] [Google Scholar]
- Yasui T, Uemura H, Irahara M, Arai M, Kojimahara N, Okabe R, Ishii Y, Tashiro S, Sato H. Associations of endogenous sex hormones and sex hormone-binding globulin with lipid profiles in aged Japanese men and women. Clinica chimica acta; international journal of clinical chemistry. 2008;398:43–47. doi: 10.1016/j.cca.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Yeap BB. Are declining testosterone levels a major risk factor for ill-health in aging men? Int J Impot Res. 2009;21:24–36. doi: 10.1038/ijir.2008.60. [DOI] [PubMed] [Google Scholar]
- Zmuda JM, Cauley JA, Kriska A, Glynn NW, Gutai JP, Kuller LH. Longitudinal relation between endogenous testosterone and cardiovascular disease risk factors in middle-aged men. A 13-year follow-up of former Multiple Risk Factor Intervention Trial participants. American journal of epidemiology. 1997;146:609–617. doi: 10.1093/oxfordjournals.aje.a009326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.