Abstract
Elevated levels of endogenous Na/K-ATPase (NKA) inhibitors, cardiotonic steroids (CTSs) including marinobufagenin (MBG), contribute to pathogenesis of preeclampsia (PE) and represent a target for immunoneutralization by Digibind (Ovine Digoxin Immune Antibody, Glaxo-Smith Kline). Because Digibind is no longer commercially available, we studied whether DigiFab (BTG International Ltd, UK) can substitute Digibind for immunoneutralization of CTS in patients with PE. We compared DigiFab, Digibind, and anti-MBG monoclonal antibody (mAb) with respect to their ability to interact with CTS in PE plasma and to restore NKA activity in erythrocytes from patients with PE. Using immunoassays based on DigiFab, Digibind, and anti-MBG mAb, we studied the elution profile of CTS following high-performance liquid chromatography (HPLC) fractionation of PE plasma. Totally, 7 patients with mild PE (28 ± 2 years; gestational age, 39 ± 0.5 weeks; blood pressure 156 ± 5/94 ± 2 mm Hg) and 6 normotensive pregnant participants (28 ± 1 years; gestational age, 39 ± 0.4 weeks; blood pressure 111 ± 2/73 ± 2 mm Hg) were enrolled. Preeclampsia was associated with a substantial inhibition of erythrocyte NKA (1.47 ± 0.17 vs 2.65 ± 0.16 µmol Pi/mL per h in control group, P < .001). Ex vivo, at 10 µg/mL concentration, which is consistent with the clinical dosing of Digibind administered previously in PE, DigiFab and Digibind as well as anti-MBG mAb (0.5 µg/mL) restored erythrocyte NKA activity. Following HPLC fractionation of pooled PE and control plasma, PE-associated increase in CTS material was detected by Digibind (176 vs 75 pmoles), DigiFab (221 vs 70 pmoles), and anti-MBG mAb (1056 vs 421 pmoles). Therefore, because DigiFab interacts with CTS from PE plasma and reverses PE-induced NKA inhibition, it can substitute Digibind for immunoneutralization of CTS in patients with PE.
Keywords: preeclampsia, cardiotonic steroids, Na/K-ATPase, immunotherapy, monoclonal antibody, digibind, DigiFab, marinobufagenin
Introduction
Preeclampsia (PE) represents one of the most serious complications of pregnancy, leading to maternal and fetal morbidity and mortality. The mechanisms of its pathogenesis are not well understood, and there is no effective cure or prophylaxis of this obstetric malady.1 One of the factors implicated in pathogenesis of PE are endogenous digitalis-like Na/K-ATPase (NKA) inhibitors, that is cardiotonic steroids (CTSs).2–4 Specifically, elevated levels of endogenous CTS, including a bufadienolide marinobufagenin (MBG), contribute to the pathogenesis of PE via induction of vasoconstriction5–7 and vascular fibrosis8 as well as by the impairment of proliferation and invasion of the cytotrophoblast.9,10
Marinobufagenin induces natriuresis via inhibition of α-1 NKA in renal epithelium and reduction in sodium reabsorption.11 Exaggerated MBG production, however, causes inhibition of smooth muscle NKA, leading to vasoconstriction.7 Pregnancy is associated with water and sodium retention12 and with moderately elevated plasma MBG concentrations.5,6,13 While in normal pregnancy moderately elevated MBG levels do not cause vasoconstriction, in patients with PE plasma levels of MBG exhibit dramatic elevations5–7 and represent a potential target for therapy. Immunoneutralization of CTS is considered to be a novel approach in the treatment of PE.14 During the last 20 years, several successful cases of the treatment of PE by Digibind were reported.15–18 The antihypertensive activity of Digibind is based on the binding of CTS and reversal of the vasoconstriction, and administration of Digibind to patients with PE is associated with restoration of activity of erythrocyte NKA, a target enzyme for CTS.19 Accordingly, ex vivo anti-MBG mAb and Digibind also restore PE-induced inhibition of erythrocyte NKA.6,7 Furthermore, in NaCl-supplemented pregnant rats exhibiting symptoms of PE, antihypertensive effect of Digibind and anti-MBG mAb is associated with restoration of the NKA in the vasculature.13 In 2010, Digibind production was terminated by its manufacturer and DigiFab (BTG International Ltd, UK) became the only available antidigoxin antibody, approved by Food and Drug Administration for administration to patients for the reversal of digoxin toxicity. The aim of our study was to investigate whether DigiFab can substitute Digibind for immunoneutralization of CTS in patients with PE. For this, we compared DigiFab and Digibind with respect to their ability to ex vivo reverse PE-induced inhibition of erythrocyte NKA. Next, using competitive immunoassays based on DigiFab and Digibind, we studied the profile of elution of CTS following fractionation of normal pregnant and PE plasma on reverse-phase high-performance liquid chromatography (HPLC) column. In our experiments, we also compared the effects of DigiFab and Digibind with that of 3E9 anti-MBG monoclonal antibody (mAb) which recently was shown to reverse PE-induced NKA inhibition7 and to potently reverse the deleterious effects of CTS in chronic renal failure.20
Methods
The protocol for this study was approved by the Ethical Committee of Almazov Federal Heart, Blood and Endocrinology Center and by the Institutional Review Board of Medstar Research Institute, Washington, District of Columbia. Thirteen participants who were admitted to the Institute of Neonatology and Pediatrics, Almazov Federal Heart, Blood and Endocrinology Center in St Petersburg, Russia, were consented and enrolled in the study. Diagnosis of mild PE in 7 patients was based on the criteria of American Congress of Obstetrics and Gynecology.21 This definition includes systolic blood pressure of at least 140 mm Hg or diastolic blood pressure of at least 90 mm Hg, at least on 2 occasions 6 hours apart and new onset proteinuria (urinary protein excretion more than 0.3 g/24 h or a urinary protein concentration of more than 1 g/L in at least 2 random urine specimens collected 6 hours or more apart) during pregnancy after the 20th week of gestation. Exclusion criteria included patients with a clinical need for digitalis drugs, antecedent history of essential hypertension, chronic cardiovascular, renal, hepatic, or endocrine disorders. We specifically enrolled 6 age-matched normotensive uncomplicated pregnancies to serve as the control group.
Ten milliliters of venous blood were collected in heparinized tubes. Four milliliters of blood were centrifuged at 1500g for 15 minutes and plasma samples were kept at −80°C. Aliquots of 250 μL of plasma from PE and control participants were extracted on Sep-Pak C-18 reverse-phase cartridges (Waters, Milford, Massachusetts) and eluted with 80% acetonitrile. Individual samples were used for measurement of CTS using competitive fluoroimmunoassays based on a murine anti-MBG 4G4 mAb, rabbit polyclonal antiouabain antibody “Anti-OU-M-2005,” and rabbit polyclonal antidigoxin antibody (Sigma Chemicals, St Louis, Missouri) as described recently in detail.7,22 The cross-reactivity of 4G4 mAb is expressed in percentage as follows: MBG—100; marinobufotoxin—43; cinobufotalin—40; telocinobufagin—14; resibufagenin—0.5; bufalin—0.08; cinobufagin—0.07; digoxin—0.03; ouabain—0.005; digoxigenin—0.004; proscillaridin A, digitoxin, aldosterone, progesterone, prednisone, corticosterone, and thyroglobulin—<0.001. The cross-reactivity of antidigoxin polyclonal antibody is expressed in percentage as follows: digoxin—100, ouabain—<0.01, MBG—0.2, digitoxin—10, bufalin—0.01, and cinobufagin—<0.01. The cross-reactivity of anti-OU-M-2005 ouabain antibody is expressed in percentage as follows: ouabain—100, ouabagenin—52, digoxin—1.8, digitoxin—0.47, progesterone—0.002, prednisone—0.001, proscillaridin—0.03, bufalin—0.10, aldosterone—0.04, telocinobufagin—0.02, resibufagin—0.15, marinobufotoxin—0.06, cinobufagin—0.02, and MBG—0.036.
Six milliliters of blood were used for the measurement of erythrocyte NKA activity in the presence and in the absence of 3E9 anti-MBG mAb, Digibind, and DigiFab as previously reported in detail.6,7 The amount of Digibind and DigiFab for the ex vivo incubation with blood was 10 μg/mL, which corresponds to the dose used clinically in PE.16–18 Anti-MBG 3E9 mAb was used in concentration, which reversed half maximal inhibitory concentration (IC50) for the inhibition of rat α-1 NKA from renal medulla by MBG.7 Cross-reactivity of 3E9 anti-MBG mAb is expressed in percentage as follows: MBG—100; marinobufotoxin—4; telocinobufagin—7; cinobufotalin—44; cinobufagin—1.4; resibufagenin—0.5; bufalin—0.3; proscillaridin A—3; ouabain—0.02; ouabagenin—<0.001; digoxin—1.8; digitoxin—0.7; aldosterone—<0.01; progesterone—<0.01; and prednisone—<0.001.
Plasma samples of 250 μL from each group were pooled, extracted on C-18 cartridges as above, dried, reconstituted in 10% acetonitrile, and fractionated on Agilent 1100 series HPLC system using Agilent Zorbax Eclipse XDB-C18 (Agilent Technologies, Palo Alto, California), 4.6 × 150 mm, 5 μm particle size, 80 Å column, flow rate 1 mL/min, in linear (10%-85.5%) gradient of acetonitrile against 0.1% trifluoroacetic acid for 45 minutes. Thirty 1.5-minute fractions were collected and analyzed for MBG immunoreactivity using assays based on 4G4 anti-MBG mAb (above) and on 3E9 anti-MBG mAb, an antibody reported previously to reduce blood pressure in experimental hypertension and to ex vivo reverse PE-induced NKA inhibition,7 and digoxin-like immunoreactivity using assays based on Digibind and DigiFab. For the digoxin immunoassay, we labeled the primary antibodies, Digibind and DigiFab using a Europium-labeling kit (Perkin Elmer, Wellesley, Massachusetts), as reported previously in detail for Digibind.7,23 The assays are based on a competition between the CTS in the sample and digoxin–bovine serum albumin conjugate immobilized on the bottom of immunoprecipitation strips for a limited number of binding sites of the Digibind or DigiFab. The sensitivity of Digibind and DigiFab immunoassays for digoxin is 0.01 nmol/L, data on cross-immunoreactivity of Digibind and DigiFab are presented in Table 1.
Table 1.
Cross-immunoreactivity of Digibind and DigiFab
| Cross-reactant | % Cross-reactivity | |
|---|---|---|
| Digibind | DigiFab | |
| Digoxin | 100 | 100 |
| Digitoxin | 1.4 | 1.3 |
| Digoxigenin | 76 | 100 |
| Ouabain | 0.4 | 0.14 |
| Ouabagenin | 0.1 | 0.025 |
| MBG | 0.2 | 0.29 |
| Marinobufotoxin | 0.06 | 0.02 |
| Telocinobufagin | 1.0 | 0.34 |
| Bufalin | 2.7 | 0.9 |
| Cinobufagin | 0.02 | 0.03 |
| Cinobufotalin | 0.2 | 0.006 |
| Resibufagenin | 1 | 0.02 |
| Proscillaridin-A | 1.46 | 0.2 |
| Prednisone | <0.001 | <0.001 |
| Progesterone | <0.001 | <0.001 |
Abbreviation: MBG, marinobufagenin.
All chemicals were from Sigma-Aldrich (St Louis, Missouri). Digibind was obtained from Glaxo-Smith Kline (Research Triangle Park, North Carolina) and DigiFab from BTG International Ltd (London, UK). Marinobufagenin (>98% purity by HPLC) was purified from the secrete of parotid glands of Bufo marinus toads,24 and anti-MBG 3E9 and 4G4 mAb were developed as reported recently in detail.7
The results are presented as mean ± standard error of the mean. Data were analyzed using 1-way analysis of variance ([ANOVA] intergroup analysis) or by repeated measures ANOVA (intragroup analysis) followed by Newman-Keuls test, and by 2-tailed t test when applicable (Graph Pad Prism Software, San Diego, California). A 2-sided P value of less than .05 was considered to be statistically significant.
Results
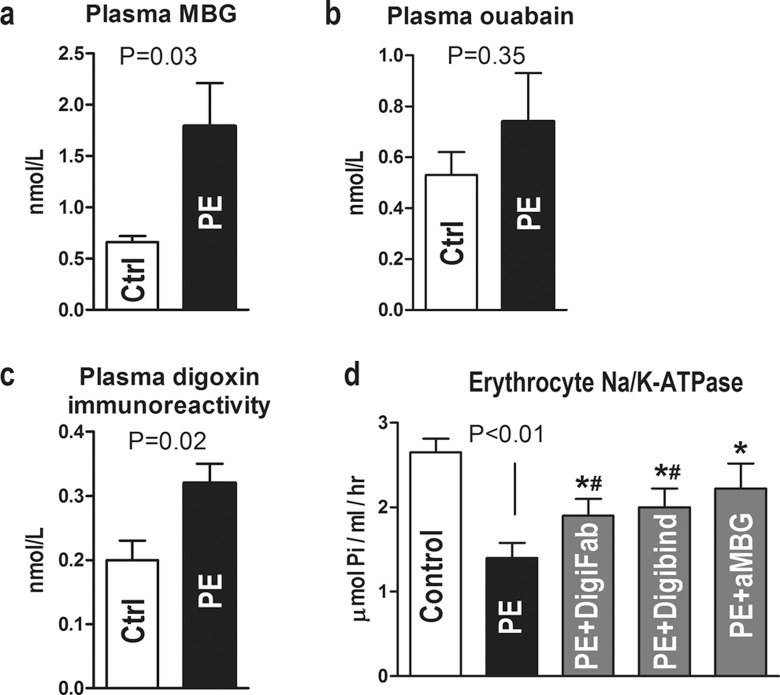
Maternal demographics and data on levels of blood pressure are presented in Table 2. Data on levels of plasma MBG and erythrocyte NKA activity in patients with PE and in control participants are summarized in Figure 1. In patients with PE, elevated blood pressure was accompanied by increase in plasma MBG (Figure 1A) and a concomitant 50% inhibition of NKA activity in erythrocytes (Figure 1D). Ex vivo incubation of erythrocytes in the presence of Digibind, DigiFab, and of 3E9 anti-MBG mAb restored the activity of NKA (Figure 1D). As illustrated in Figure 1B, plasma levels of ouabain immunoreactivity in patients with PE did not differ significantly from that in control group, while the levels of digoxin-like immunoreactivity exhibited significant elevation (Figure 1C).
Table 2.
Characteristics of Study Participants and Levels of Blood Pressurea
| Normal Pregnancy (n = 6) | Preeclampsia (n = 7) | |
|---|---|---|
| Maternal age, years | 28 ± 1 | 28 ± 2 |
| Gestational weeks at delivery | 39.0 ± 0.4 | 39 ± 0.5 |
| No of primigravida, % | 6 (100%) | 6 (86%) |
| Cesarean section (n) | 3 | 2 |
| Vaginal delivery (n) | 3 | 5 |
| Infant birth weight, g | 3796 ± 121 | 3401 ± 201 |
| Systolic blood pressure, mm Hg | 111 ± 2 | 156 ± 5b |
| Diastolic blood pressure, mm Hg | 73 ± 2 | 94 ± 2b |
Abbreviation: SEM, standard error of the mean.
aValues are expressed as means ± SEM.
b P < .001 versus normal pregnant participants, 2-tailed t test.
Figure 1.
Plasma levels of marinobufagenin (MBG) (a), endogenous ouabain (b), and digoxin-like immunoreactivity (c) in normotensive pregnant participants (Ctrl) and in patients with preeclampsia (PE). d, Erythrocyte Na/K-ATPase in normal pregnant participants and in patients with preeclampsia, and effect of ex vivo treatment with DigiFab, Digibind, and 3E9 anti-MBG mAb (aMBG) on erythrocyte Na/K-ATPase. *P < .01 versus preeclampsia (PE); # P < .01 versus control by repeated measures analysis of variance (ANOVA) and Newman-Keuls test.
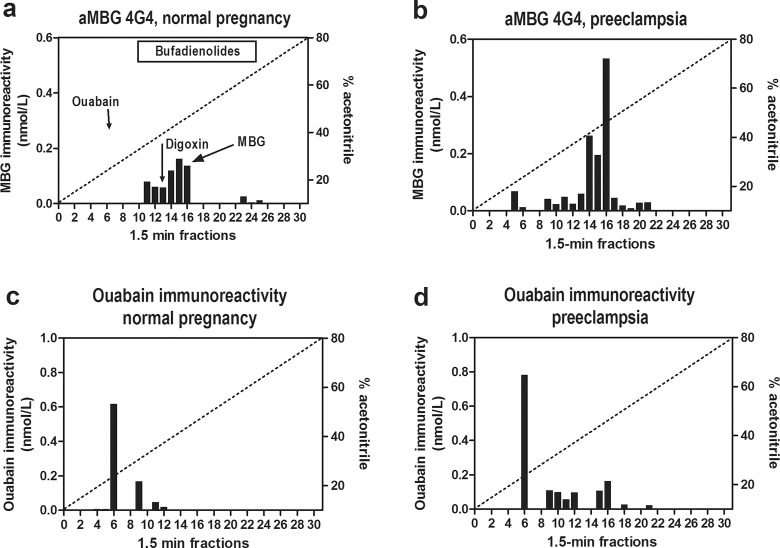
The retention times of cardenolide and bufadienolide CTS standards on Agilent Zorbax Eclipse XDB-C18 HPLC column were as follows: ouabain—8.5 minutes (fraction 6), digoxin—18.0 minutes (fraction 13), telocinobufagin—20.5 minutes (fraction 14), MBG—22.6 minutes (fraction 16), and marinobufotoxin—22.7 minutes (fraction 16), cinobufatalin—22.9 minutes (fraction 16), bufalin—24.5 minutes (fraction 17), and resibufagenin—27.7 minutes (fraction 19). As presented in Figure 2, following HPLC fractionation of normal and PE plasma maximum ouabain-immunoreactivity coeluted with ouabain standard. Levels of ouabain-like immunoreactivity in HPLC fractions from control plasma did not differ significantly from that in preeclamptic plasma (620 and 780 pmoles, respectively; Figure 2C and D).
Figure 2.
Fractionation of endogenous cardiotonic steroids (CTSs) from normal and preeclamptic plasma on Zorbax Eclipse XDB-C18 HPLC columns. a and b, Pattern of elution of endogenous marinobufagenin (MBG)-immunoreactive material determined by assay based on anti-MBG 4G4 mAb. Retention times of MBG, ouabain, and digoxin standards are indicated by arrows. c and d, Pattern of elution of endogenous ouabain-immunoreactive material. Bar on the top of panel “a” indicates retention times of bufadienolide standards.
In addition to measuring MBG in C18-extracted plasma samples (Figure 1A), levels of MBG immunoreactivity were determined in HPLC fractions from control and PE plasma using immunoassays based on 2 anti-MBG mAbs, 4G4 which is used to measure plasma MBG levels and 3E9 which potently reverses effects of endogenous MBG in vivo.7,20 Data are presented in Figure 2A and B (assay based on 4G4 anti-MBG mAb) and in Figure 3E and F (assay based on 3E9 anti-MBG mAb). As presented in Figure 2A, following HPLC fractionation of control plasma MBG immunoreactive material eluted in fractions 11-16. Immunoassay based on 4G4 mAb detected a 2-fold increase in MBG in HPLC-fractionated PE plasma (648 vs 310 pmoles in HPLC fractions from normal pregnant plasma) with a maximum of MBG immunoreactivity coeluting with MBG standard in fraction 16 (Figure 2C).
Figure 3.
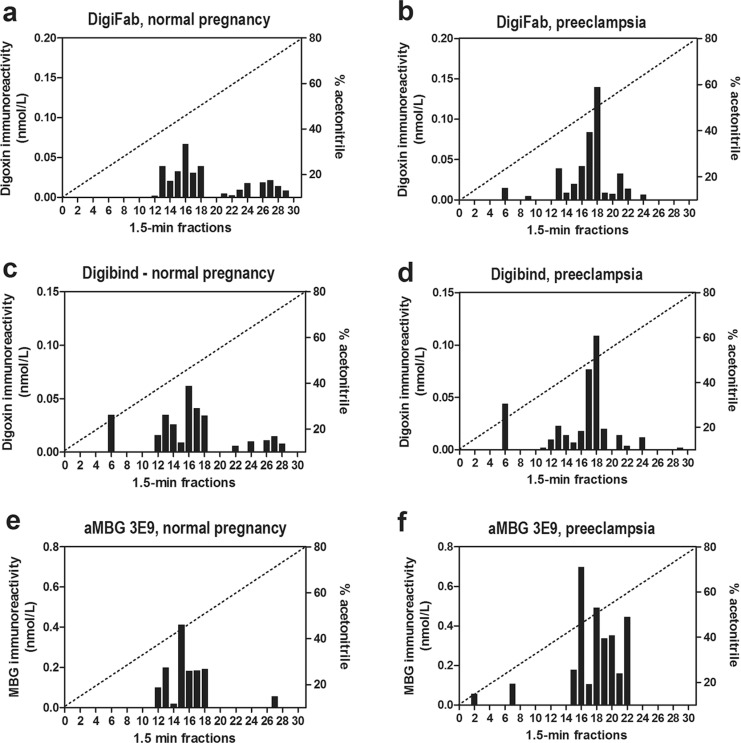
Fractionation of endogenous cardiotonic steroids (CTSs) from normal and preeclamptic plasma on Zorbax Eclipse XDB-C18 high-performance liquid chromatographic (HPLC) columns. a-d, Pattern of elution of digoxin-immunoreactivity measured by assays based on Digibind and DigiFab. e-f, Pattern of elution of endogenous marinobufagenin (MBG)-immunoreactivity measured by immunoassay based on anti-MBG 3E9 mAb.
Figure 3 illustrates data on interaction of antibodies used to reverse PE-induced NKA inhibition, DigiFab, Digibind, and 3E9 anti-MBG mAb, with CTS following fractionation of normal pregnant and PE plasma on reverse-phase HPLC column. As presented in Figure 3A, C and E, following fractionation of control plasma, assays based on Digibind, DigiFab, and 3E9 anti-MBG mAb detected CTS in fractions 14-18. In HPLC-fractionated PE plasma material measured using Digibind and DigiFab eluted in fractions 17-22 with maximum in fraction 18 (Figure 3B and D). Marinobufagenin-immunoreactive material from PE plasma measured by 3E9 mAb eluted in fractions 16-22 with a maximum eluting in fraction 16 (Figure 3F). The total amount of MBG-immunoreactive material determined by 3E9 anti-MBG mAb in HPLC fractions following fractionation of PE plasma was 2.5-fold greater than that in control samples (1056 vs 421 pmoles; Figure 3E and F). The PE-associated increase in CTS material was detected by Digibind (176 vs 75 pmoles; Figure 3C and D) and DigiFab (221 vs 70 pmoles; Figure 3A and B) and magnitude of PE-induced increase in the levels of digoxin-like immunoreactivity in HPLC fractions was similar to the magnitude of increase in the levels of MBG.
Discussion
The main observations of this study are that DigiFab, affinity-purified Fab fragments of antidigoxin antibody, detects PE-induced increase in plasma levels of CTS and ex vivo reverse PE-induced inhibition of erythrocyte NKA similar to that of Digibind. These data suggest that DigiFab is capable to immunoneutralize CTS in PE and other conditions in which elevated levels of CTS exhibit deleterious effects.
Digitalis-like CTSs act as endogenous ligands of the NKA and exhibit effects mediated by pumping and/or by signaling effects of this enzyme.4,11 According to the concept of natriuretic hormone, CTSs are produced with the adaptive aim to regulate sodium excretion via inhibition of the NKA in the renal tubules.4 In patients with salt-sensitive hypertension, exaggerated CTS response, however, produces inhibition of the sodium pump in the vasculature leading to vasoconstriction.4,11 In addition, an increasing body of evidence indicates that CTS bind to NKA and elicit cell signaling events leading to oxidative stress, hypertrophic growth and fibrosis, apoptosis, and cell differentiation.4 Previous studies have demonstrated that in mammals, plasma levels of CTS, including MBG and endogenous ouabain, are moderately elevated throughout pregnancy indicating that these hormones are implicated in the physiology of gestation.5–7,13 In PE, however, plasma and placental levels of CTS, and especially MBG, become substantially elevated and associated with heightened arterial pressure and inhibition of erythrocyte NKA.5–7,13 Moreover, in PE, placental vascular bed exhibits heightened sensitivity to CTS-induced vasoconstriction.25 Recent evidence, however, indicates that the contribution of CTS to PE is not limited to only vasoconstriction. Marinobufagenin in PE has been demonstrated to contribute to the impairment of vascular relaxation causing vascular fibrosis via inhibition of Fli-1, a nuclear transcription factor and a known negative regulator of collagen synthesis.8 In addition, MBG, acting via activation of Jnk, p38, and Src leads to increased apoptosis and secretion of interleukin 6 that has been reported to impair the proliferation, migration, and invasion of cytotrophoblast cell.9,10 Most recently, elevated levels of MBG in pregnant NaCl-supplemented rats were shown to be associated with reduced number of nephrons in the offspring which is known to be a factor predisposing to the subsequent development of hypertension.26
The above features make CTS attractive targets for therapy of PE, but interference with CTS at the level of cell signaling could interfere with other signaling pathways implicated in physiology of gestation.27,28 Therefore, in PE, immunoneutralization appears to be a promising approach to CTS antagonism. Because CTS share some of their antigenic properties with digitalis and digoxin, and antibodies raised to them have been used in humans to treat PE, the idea of immunoneutralization of CTS with Digibind has emerged.13 Goodlin was the first to report the successful use of Digibind in a patient with PE.14 Following individual case reports,15,16 Digibind has been reported to reduce the blood pressure in cohort of participants with postpartum PE.17 Data from a recent phase II B randomized, double blinded, placebo controlled clinical trial of Digibind in women with severe antepartum PE suggest that CTS-induced abnormalities could be reversed by Digibind.18 The fact that in our previous6,7 and present studies, PE-induced inhibition of erythrocyte NKA was ex vivo reversed by immunoneutralization of CTS demonstrates that in PE, there is a causative link between elevated levels of endogenous digitalis and sodium pump inhibition. In 2011, production of Digibind was terminated and currently DigiFab remains the only antidigoxin antibody available for human administration. Our present data suggest that with respect to reversal to PE-induced NKA inhibition, DigiFab can substitute Digibind.
Because several cardenolide and bufadienolide CTS exist in mammalian tissues, a question arises concerning the identity of targets of DigiFab and Digibind in PE. In the present study, Digibind and DigiFab, as well as anti-MBG mAb did not detect increases in the level of the material coeluting with ouabain (HPLC fraction 6), which agrees with our previous data demonstrating that bufadienlide CTS, rather than endogenous ouabain, is implicated in the pathogenesis of PE.7,8 In competitive immunoassays, both DigiFab and Digibind detected substantial increases in CTS levels in HPLC fractions17–22. While 4G4 mAb detected increased levels of MBG mainly in HPLC fraction16, 3E9 anti-MBG mAb was capable to detect PE-induced elevation of CTS in HPLC fractions16–22, which overlaps with the material detected by assays based on DigiFab and Digibind. Thus, DigiFab and Digibind cross-reacted with MBG-immunoreactive material eluting in fractions18–22 and corresponding to the elution time of several other bufadienolides, bufalin, resibufagenin, and cinobufagin. This pattern closely resembles that observed in our previous study investigating interactions of Digibind with CTS from preeclamptic placentae.23 In our previous study, following HPLC fractionation of chloroform extract of PE placentae, 3E9 mAb in addition to MBG per se, cross-reacted with more hydrophobic material having retention time greater than MBG. In the present study, PE-induced elevation in CTS levels in HPLC fractions eluting later than MBG was detected by 3E9 mAb, DigiFab, and Digibind.23 Thus, our previous findings and our present data warrant search for yet unidentified hydrophobic bufadienolide CTS. The fact that 3E9 mAb cross-reacted with several HPLC fractions from PE plasma may at least in part explain very high in vivo potency of this antibody. Thus, in rats with chronic renal failure, a single injection of 3E9 mAb induced a hypotensive effects lasting for 7 days and reduced levels of oxidative stress and fibrosis in the myocardium.20
In summary, our results demonstrate that DigiFab and Digibind detect identical CTS in PE plasma, exhibit comparable cross-immunoreactivity with CTS, and exert similar capacity to ex vivo reverse PE-induced NKA inhibition. Because DigiFab appears clinically similar to Digibind in its ability to immunoneutralize CTS, it holds promise for treatment of PE and other conditions in which CTS exhibits deleterious effects, including cerebral salt-wasting syndrome29 and chronic kidney disease.20,30
Footnotes
Author’s Note: Valentina V. Ishkaraeva-Yakovleva and Olga V. Fedorova equally contributed to this work.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Intramural Research Program, National Institute on Aging, National Institutes of Health (OVF and AYB), by a grant from Ministry of Science and Education of Russian Federation Nr. 14.740.11.0928, and by Glenveigh Pharmaceuticals (CDA), Chattanooga, Tennessee.
References
- 1. Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365(9461):785–799 [DOI] [PubMed] [Google Scholar]
- 2. Gusdon JP, Jr, Buckalew VM, Jr, Hennessy JF. A digoxin-like immunoreactive substance in preeclampsia. Am J Obstet Gynecol. 1984;150(1):83–85 [DOI] [PubMed] [Google Scholar]
- 3. Graves SW, Valdes R, Jr, Brown BA, Knight AB, Craig HR. Endogenous digoxin-immunoreactive substance in human pregnancies. J Clin Endocrinol Metab. 1984;58(4):748–751 [DOI] [PubMed] [Google Scholar]
- 4. Bagrov AY, Shapiro JI. Endogenous digitalis: pathophysiologic roles and therapeutic applications. Nat Clin Pract Nephrol. 2008;4(7):378–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lopatin DA, Ailamazian EK, Dmitrieva RI, et al. Circulating bufodienolide and cardenolide sodium pump inhibitors in preeclampsia. J Hypertens. 1999;17(8):1179–1187 [DOI] [PubMed] [Google Scholar]
- 6. Averina IV, Tapilskaya NI, Reznik VA, et al. Endogenous Na/K-ATPase inhibitors in patients with preeclampsia. Cell Mol Biol. 2006;52(8):19–23 [PubMed] [Google Scholar]
- 7. Fedorova OV, Simbirtsev AS, Kolodkin NI, et al. Monoclonal antibody to an endogenous bufadienolide, marinobufagenin, reverses preeclampsia-induced Na/K-ATPase inhibition and lowers blood pressure in NaCl-sensitive hypertension. J Hypertens. 2008;26(12):2414–2425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nikitina ER, Mikhailov AV, Nikandrova ES, et al. In preeclampsia endogenous cardiotonic steroids induce vascular fibrosis and impair relaxation of umbilical arteries. J Hypertens. 2011;29(4):769–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. LaMarca HL, Morris CA, Pettit GR, Nagowa T, Puschett JB. Marinobufagenin impairs first trimester cytotrophoblast differentiation. Placenta. 2006;27(9-10):984–988 [DOI] [PubMed] [Google Scholar]
- 10. Uddin MN, Horvat D, Glaser SS, Mitchell BM, Puschett JB. Examination of the cellular mechanisms by which marinobufagenin inhibits cytotrophoblast function. J Biol Chem. 2008;283(26):17946–17953 [DOI] [PubMed] [Google Scholar]
- 11. Bagrov AY, Shapiro JI, Fedorova OV. Endogenous cardiotonic steroids: physiology, pharmacology, and novel therapeutic targets. Pharmacol Rev. 2009;61(1):9–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Masilamani S, Castro L, Baylis C. Pregnant rats are refractory to the natriuretic actions of atrial natriuretic peptide. Am J Physiol. 1994;267(6 Pt 2):R1611–R1616 [DOI] [PubMed] [Google Scholar]
- 13. Goodlin RC. Will treatment with digoxin antibody benefit pregnant patients with toxemia and elevated digoxin like factor? Med Hypotheses. 1987;24(1):107–110 [DOI] [PubMed] [Google Scholar]
- 14. Goodlin RC. Antidigoxin antibodies in eclampsia. N Engl J Med. 1988;318(8):518–559 [DOI] [PubMed] [Google Scholar]
- 15. Adair CD, Buckalew V, Taylor K, et al. Elevated endoxin-like factor complicating a multifetal second trimester pregnancy: treatment with digoxin-binding immunoglobulin. Am J Nephrol. 1996;16(6):529–531 [DOI] [PubMed] [Google Scholar]
- 16. Adair CD, Luper A, Rose JC, Russell G, Veille JC, Buckalew VM. The hemodynamic effects of intravenous digoxin-binding fab immunoglobulin in severe preeclampsia: a double-blind, randomized, clinical trial. J Perinatol. 2009;29(4):284–289 [DOI] [PubMed] [Google Scholar]
- 17. Adair CD, Buckalew VM, Kipikasa J, Torres C, Stallings SP, Briery CM. Repeated dosing of digoxin-fragmented antibody in preterm eclampsia. J Perinatol. 2009;29(2):163–165 [DOI] [PubMed] [Google Scholar]
- 18. Adair CD, Buckalew VM, Graves SW, et al. Digoxin immune fab treatment for severe preeclampsia. Am J Perinatol. 2010;27(8):655–662 [DOI] [PubMed] [Google Scholar]
- 19. Adair CD, Haupert GT, Jr, Koh HP, Wang Y, Veille JC, Buckalew V. Erythrocyte sodium/potassium ATPase activity in severe preeclampsia. J Perinatol. 2009;29(4):280–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Haller ST, Kennedy DJ, Shidyak A, et al. Monoclonal antibody against marinobufagenin reverses cardiac fibrosis in rats with chronic renal failure [published online ahead of print March 1, 2012]. Am J Hypertens. 2012. PMID 22378033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. National Institutes of Health Working Group on Hypertension in Pregnancy. Classification of Hypertensive Disorders of Pregnancy. Bethesda, MD: US Department of Health and Human Services; 1991 [Google Scholar]
- 22. Fedorova OV, Kolodkin NI, Agalakova NI, et al. Antibody to marinobufagenin lowers blood pressure in pregnant rats on a high NaCl intake. J Hypertens. 2005;23(4):835–842 [DOI] [PubMed] [Google Scholar]
- 23. Fedorova OV, Tapilskaya NI, Bzhelyansky AM, et al. Interaction of Digibind with endogenous cardiotonic steroids from preeclamptic placentae. J Hypertens. 2010;28(2):361–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bagrov AY, Fedorova OV, Roukoyatkina NI, Ukhanova MV, Zhabko EP. Digitalis-like and vasoconstrictor properties of endogenous digoxin like factor from Bufo marinus toad. Eur J Pharmacol. 1993;234(2-3):165–172 [DOI] [PubMed] [Google Scholar]
- 25. Armler E, Cester N, Salvolini E, et al. Human hypertensive placenta contains an increased amount of Na, K-ATPase with higher affinity for cardiac glycosides. Cell Biol Int. 1994;18(7):723–727 [DOI] [PubMed] [Google Scholar]
- 26. Koleganova N, Piecha G, Ritz E, et al. Both high and low maternal salt intake in pregnancy alter kidney development in the offspring. Am J Physiol Renal Physiol. 2011;301(2):F344–F354 [DOI] [PubMed] [Google Scholar]
- 27. Uzumcu M, Westfall SD, Dirks KA, Skinner MK. Embryonic testis cord formation and mesonephric cell migration requires the phosphotidylinositol 3-kinase signaling pathway. Biol Reprod. 2002;67(6):1927–1935 [DOI] [PubMed] [Google Scholar]
- 28. Kita N, Mitsushita J, Ohira S, et al. Expression and activation of MAP kinases, ERK1/2, in the human villous trophoblasts. Placenta. 2003;24(2-3):164–172 [DOI] [PubMed] [Google Scholar]
- 29. Menezes JC, Troster EJ, Dichtchekenian V. Digoxin antibody decreases natriuresis and diuresis in cerebral hemorrhage. Intensive Care Med. 2003;29(12):2291–2296 [DOI] [PubMed] [Google Scholar]
- 30. Kolmakova EV, Haller ST, Kennedy DJ, et al. Endogenous cardiotonic steroids in chronic renal failure. Nephrol Dial Transplant. 2011;26(9):2912–2919 [DOI] [PMC free article] [PubMed] [Google Scholar]