Abstract
Background
The p53 and the PIK3CA/AKT/mTOR pathways are frequently altered in sarcoma with complex genomics such as leiomyosarcoma (LMS) or undifferentiated pleomorphic sarcoma (UPS). The scale of genetic abnormalities in these pathways remains unknown in angiosarcoma.
Methods
We investigated the status of critical genes involved in the p53 and the PIK3CA/AKT/mTOR pathways in a series of 62 AS.
Results
The mutation and deletion rates of TP53 were 4% and 0, respectively. p53 overexpression was detected by immunohistochemistry in 49% of cases and was associated with inferior disease-free survival. Although p14 inactivation or HDM2 overexpression are frequent in LMS and UPS and could substitute for TP53 mutation or deletion, such alterations were rare in angiosarcomas. pS6K and/or p-4eBP1 overexpression was observed in 42% of cases suggesting frequent activation of the PIK3CA/AKT/mTOR pathway in angiosarcomas. Activation was not related to intragenic deletion of PTEN, an aberration that is frequent in LMS and UPS, but absent in angiosarcomas.
Conclusion
Angiosarcomas constitute a distinct subgroup among sarcomas with complex genomics. Although TP53 mutation and PTEN deletion are frequent in LMS and UPS, these aberrations are rarely involved in the pathogenesis of AS.
Keywords: angiosarcoma, TP53, PIK3CA, PTEN, MDM2, P14, BRAF, NRAS
INTRODUCTION
Angiosarcomas (AS) represent a rare (<2%) subgroup of soft tissue sarcomas (STS) characterized by an aggressive clinical behavior and occurring not only in different anatomical locations, but also in distinct clinical settings, such as de novo (primary AS), or after radiation therapy or chronic lymphedema (secondary AS) 1. Radical surgery and adjuvant radiotherapy when indicated represent the cornerstone of treatment for patients with localized disease. However, despite an adequate loco-regional treatment, half or more of patients will develop metastatic relapse and will die of disease 2. The genetic aberrations involved in AS genesis are still poorly understood. Until recently, data were limited to case reports or small case series showing a complex pattern of numerical and structural genetic aberrations 3-11. We and others have recently identified MYC amplification as a recurrent genetic aberration in secondary AS 12, 13, but no specific recurrent event has been reported in primary AS to date.
Alterations of TP53 or PTEN are among the most frequent genetic events in human cancer and the role of their combined inactivation in tumorigenesis has been well examined in several contexts, including lymphoma, prostate cancer, glioblastoma, and medulloblastoma 14-20. Interestingly, the p53 and the PIK3CA/AKT/mTOR pathways are frequently altered in sarcoma with complex karyotypes. Mutations and/or deletions of the TP53 gene have been recently shown to occur in approximately 80% of leiomyosarcomas (LMS), 70% of undifferentiated pleomorphic sarcoma (UPS), and 60% of pleomorphic liposarcomas 21-23. Large deletions on chromosome 10 including the tumor suppressor gene PTEN have also been observed in approximately 40-50% of LMS and 20-25% of UPS 24, 25. Moreover, in vitro and in vivo studies 25, 26 suggest the crucial role of the PIK3CA/AKT/mTORpathway in LMS genesis. Due to its rarity, studies regarding the incidence of p53 and PIK3CA/AKT/mTOR pathway aberrations in AS have used heterogeneous methods and are based on single case reports or small case series 27-35. Therefore, we investigated the status of key genes involved in these pathways in a series of primary and secondary AS by sequencing, array CGH and immunohistochemistry.
PATIENTS AND METHODS
Patient and samples
Sixty-two AS (30 primary and 32 secondary) were included in this study on the basis of availability of tumor material for genetic studies. The clinico-pathologic characteristics are listed in Table 1. Diagnoses were established according to the World Health Organization Classification of Tumors 1. This study was approved by the institutional review board (IRB-protocol 02-060).
Table 1.
Clinical and pathologic characteristics of angiosarcoma patients
| Case ID | Age | Gender | Previous radiation therapy |
Chronic Lymphedema |
Primary location |
|---|---|---|---|---|---|
| AS3δ | 58 | M | No | No | Femur |
| AS4 | 63 | F | Yes | No | Breast |
| AS5 | 82 | F | No | No | Thigh |
| AS6 | 49 | F | No | No | Humerus |
| AS9δ | 70 | M | No | No | Thigh |
| AS10δ | 70 | F | Yes | No | Breast |
| AS11δ | 50 | M | No | No | Spleen |
| AS12 | 66 | M | No | No | Kidney |
| AS13 | 67 | F | No | Yes | Forearm |
| AS15δ | 61 | F | Yes | No | Breast |
| AS14 | 40 | F | No | No | Breast |
| AS16 | 34 | M | No | No | Pelvis |
| AS17 | 74 | F | Yes | No | Breast |
| AS19 | 62 | F | Yes | No | Breast |
| AS20δ/AS21 | 56 | F | Yes | No | Breast |
| AS22 | 67 | F | No | No | Breast |
| AS25 | 70 | F | Yes | No | Chest wall |
| AS26 | 34 | F | No | No | Breast |
| AS27 | 37 | F | No | No | Breast |
| AS28 | 54 | M | No | No | Pelvis |
| AS29δ | 75 | F | Yes | No | Breast |
| AS30δ | 38 | F | Yes | No | Head and Neck |
| AS31 | 79 | M | No | No | Scalp |
| AS32δ | 76 | F | Yes | No | Breast |
| AS33 | 69 | M | No | No | Thigh |
| AS34 | 43 | F | No | No | Breast |
| AS36 | 77 | F | No | No | Breast |
| AS37 | 71 | F | No | No | Mediastinum |
| AS38δ | 74 | F | No | Yes | Forearm |
| AS39δ | 84 | M | No | Yes | Arm |
| AS44 | 63 | F | Yes | No | Breast |
| AS45 | 66 | F | Yes | No | Breast |
| AS47 | 65 | F | Yes | No | Breast |
| AS48 | 74 | F | Yes | No | Breast |
| AS49 | 43 | F | Yes | No | Chest wall |
| AS56 | 64 | F | No | No | Breast |
| AS57 | 66 | F | Yes | No | Thigh |
| AS58 | 74 | M | No | No | Scalp |
| AS59 | 79 | M | No | No | Nostril |
| AS61 | 83 | F | Yes | No | Breast |
| AS62 | 52 | F | No | No | Liver |
| AS66 | 77 | M | No | No | Femur |
| AS68δ | 80 | F | Yes | No | Breast |
| AS69 | 60 | F | Yes | No | Breast |
| AS70δ | 38 | F | No | No | Breast |
| AS71 | 41 | F | Yes | No | Spleen |
| AS72 | 66 | F | No | Yes | Breast |
| AS73δ | 83 | F | Yes | No | Breast |
| AS75 | 80 | M | No | No | Head and Neck |
| AS76δ | 76 | M | Yes | No | Bladder |
| AS77 | 73 | M | No | No | Retroperitoneum |
| AS79 | 48 | F | No | No | Spine |
| AS83 | 65 | M | Yes | No | Breast |
| AS85 | 76 | F | Yes | No | Breast |
| AS100 | 60 | M | No | No | Chest wall |
| AS101 | 62 | F | Yes | No | Breast |
| AS109 | 63 | M | No | No | Spleen |
| AS117 | 38 | F | Yes | No | Head and Neck |
| AS123δ | 76 | F | Yes | No | Breast |
| AS124δ | 57 | M | No | No | Head and Neck |
| AS125δ | 73 | F | No | No | Breast |
tumors studied by aCGH.
Array Comparative Genomic Hybridization (CGH)
Genomic DNA was isolated from frozen tumor tissue by phenol/chloroform extraction and quality was confirmed by spectrophotometry and electrophoresis. Array CGH analysis was performed as described previously (Thomas et al., 2011). Briefly, tumor and reference DNA samples were labeled with Cyanine-3-dUTP and Cyanine-5-dUTP, respectively, by random priming (Agilent Enzymatic Labeling Kit, Agilent Technologies, Santa Clara, CA). The reference sample comprised a pool of DNA from multiple clinically healthy donors (Promega, Madison, WI) of the same gender as the AS patient. The labeled probes were combined and hybridized to a ~180,000-feature, genome-wide oligonucleotide CGH array (design 052252, Agilent Technologies). Arrays were scanned at 3μm resolution using an Agilent G2565CA scanner. Image data were processed with Feature Extraction version 10.10 and Genomic Workbench version 6.5 (Agilent Technologies). Data were filtered to exclude probes exhibiting non-uniform hybridization or signal saturation and were normalized using the centralization algorithm with a threshold of six. The ADM2 algorithm was used to define DNA copy number aberrations using a ‘three probes minimum’ filter and a threshold of six with a fuzzy zero correction, with genomic gain and loss defined as log2 test:reference DNA signal intensity >0.2 and <−0.2, respectively. A genomic copy number amplification was defined as a region within which the log2 ratio of tumor DNA to reference DNA exceeded 2.0.
Real-Time Quantitative Reverse Transcription-PCR
One μg of total RNA was reverse-transcribed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA) at 25°C for 10 min, 37°C for 120 min, 85°C for five min and hold at 4°C. Twenty ng/μl of resultant cDNA was used in a quantitative-PCR using an 7500 Real-Time PCR System (Applied Biosystems, Carlsbad, CA) and pre-designed TaqMan ABI Gene expression Assays (Hs_00234753_m1 for HDM2; Hs_99999189_m1 for P14/CDKN2A). Amplification was carried out at 95°C for 10 min, and 40 cycles (95°C for 15 sec, 60°C for one min). To calculate the efficiency of the PCR and to assess the sensitivity of each assay, we also performed a five point standard curve (80, 26.67, 8.88, 2.96, and 0.98 ng/ul). Triplicates CT values were averaged and amounts of target were interpolated from the standard curves and normalized to GAPDH (reference gene).
Mutation Screening
The mutational status of TP53 (exon 2 to exon 11), PIK3CA (exons 3, 10, 20), BRAF (exon 15) and NRAS (exons 2 and 3) was assessed by direct sequencing of genomic DNA. Protocols and primers are available on request. Sequence analysis was performed with Applied Biosystems Sequence ScannerTM v1.0.
Enhanced Mismatch Mutation Detection Analysis (EMMA) and Copy Number Analysis by Quantitative Multiplex PCR (QMP)
A screening of genomic variants of PTEN was performed by using Enhanced Mismatch Mutation Detection Analysis (EMMA), an electrophoretic heteroduplex analysis based on the use of a matrix increasing the electrophoretic mobility between homoduplex and heteroduplex 36. Primers were designed to include flanking intronic sequences (at least 50 base-pairs from splices sites) and to avoid known polymorphism and prevent mis-priming (primer sequences are available upon request). PTEN coding sequences were divided into nine amplicons, analyzed in three multiplex PCRs. EMMA was performed as previously described using EMMA polymer and according to the manufacturer’s instructions 37. Amplicons showing abnormal profiles were sequenced and analyzed on the ABI PRISM 3130XL Genetic Analyser using the Collection and Sequence Analysis software package (Applied Biosystems, Courtaboeuf, France). Nucleotide position was indicated on the basis of the coding sequence NM_000314.4 and according to the recommended guidelines (available at http://www.emqn.org/emqn.php and www.hgvs.org/mutnomen).
Quantitative multiplex PCR (QMP) was carried out with 200 ng of tumor DNA sample, measured on a NanoDrop (ND-1000, Labtech International, France). All primers were designed in order to include flanking intronic sequences and prevent the detection of the processed pseudogene (PTEN1) that presents 98.6% homology to the cDNA of PTEN (the sequences of primers are available upon request). Amplicon sizes ranged from 111-347 bp. Exons of the PTEN gene were analyzed by two assays consisting of four and five PTEN amplicons, one amplicon of two genes proximal to PTEN (PAPSS2 and KILLIN) and amplicons of the MSH2 and BRCA1 genes used as internal control for copy number analysis. Multiplex PCRs were performed with the Qiagen Multiplex PCR kit, according to the manufacturer’s recommendations (Qiagen, Courtaboeuf, France), and 1 μl of 10X primer mix (final concentration from one to 10 μmol/L of each primer), one of them carrying a 6-FAM label. Eight per cent DMSO was added for the assay including PTEN exon 1 and KILLIN amplicons. Amplification was performed as recommended by the manufacturer (Qiagen) and 24 cycles were performed for quantitative PCR. DNA fragments generated by QMP were separated by capillary electrophoresis on the ABI 3130XL Genetic Analyser (Applied Biosystems). Data were analyzed with the GeneMapper Software V4.0 and copy number variation was evaluated for each amplicon by calculating the relative peak height for each sample with respect to the mean value of the relative peak height observed in all samples.
Immunohistochemistry
Immunohistochemistry (IHC) for p53 (1:800, DO7, Dako, Carpinteria, CA), HDM2 (1:50, clone# 1F2,Calbiochem), p14 (1:1000, clone 4C6/4, Cell Signaling, Danvers, MA), p-S6K (1:50, clone Ser235/236, Cell Signaling), p-4eBP1 (1:500, clone Thr37/46, Cell Signaling), and p44/p-ERK (1:50; clone Thr202/Tyr204, Cell Signaling) was performed according to manufacturer’s recommendations. The immunoreactivity scoring was counted as the percentage of nuclear staining per 10 high-power fields, in several areas, regardless of staining intensity. A 20% cutoff value for detection of positive nuclear reactivities was selected for all antibodies. 38, 39
Statistical analysis
Descriptive statistics were used to show the distribution of variables in the population. Differences between groups were evaluated by χ2 test or Fisher’s exact test for categorical variables and t test for continuous variables. The statistical analysis of baseline demographics and clinical outcome is based on all data available up to the cut-off date, 31 August 2011. Disease-free survival was defined as the length of time after diagnosis during which patients had no evidence of disease and was estimated with the use of the Kaplan–Meier method.
RESULTS
TP53 gene alterations are very rare in angiosarcomas
TP53 deletions have been reported to be frequent events in sarcomas with complex karyotypes, especially LMS and UPS. To assess the frequency of this aberration in AS, we analyzed 18 tumors (6 primary and 12 secondary) by array CGH, none of which exhibited copy number loss of the TP53 gene. We then analyzed the mutational status of TP53 by sequencing exons 2-11 on genomic DNA in 52 tumors. We found a TP53 mutation in only two cases (4%) (A138S exon 5, R238Q exon 7), which were not present in the corresponding germline DNA from the same patient. Both cases with TP53 mutation occurred in patients with a secondary breast AS developed in a previously irradiated field (AS10, AS17). Both tumors carried MYC amplification and one of them a KDR A1065T exon 24 mutation (AS17), as previously reported 40. Moreover, we observed a R72P polymorphism in TP53 exon 4 in 30 cases (58%), including 19 secondary and 11 primary AS. P53 protein expression was then assessed by IHC in 46 cases. Twenty-three tumors (50%) were negative for p53 expression and 23 (50%) were positive (including the two tumors with TP53 mutation). There was no significant difference of p53 IHC status between primary and secondary AS. Moreover, there was no correlation between the presence of the R72P polymorphism and protein expression.
p53 protein expression correlates with disease-free survival in angiosarcoma
Since p53 protein status assessed by IHC was previously associated with outcome in several tumor types, including sarcomas with complex genomics, we decided to analyze its correlation with clinical behavior in AS. We found that p53 positivity (defined as >20% nuclear positivity) was significantly associated with worse disease-free survival: 3.4 months (95% CI: 0.8-6) versus 14.9 months (95% CI: 2.5-27.4), p= 0.002 (Figure 1).
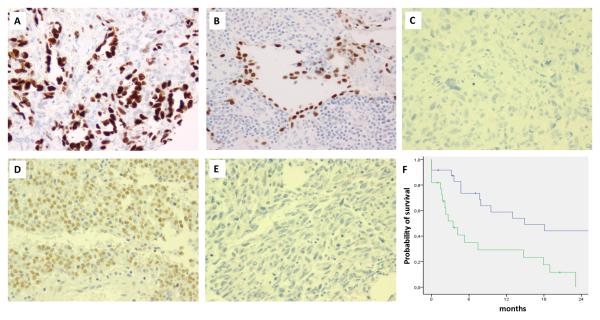
Figure 1.
Immunohistochemical (IHC) results for p53 (A,B,C), p14 (D), HDM2 (E) and prognostic impact of p53 expression (F). (A,B) Diffuse and strong nuclear reactivity for p53 in a secondary AS associated with a TP53 mutation and KDR mutation (A: AS17, 200x) and a primary AS without TP53 mutation (B: AS59, 200x). (C) Absence of expression of p53 in a case of secondary AS (AS19, 200x). (D) Diffuse nuclear reactivity for p14 in a secondary AS (AS20, 200x). (E) Absence of expression of HDM2 in a case of secondary AS (AS39, 200x). (F) Kaplan Meier curve for disease-free survival according to p53 IHC status (blue line: negative; green line: positive).
p14 inactivation or HDM2 overexpression does not substitute for TP53 inactivation in angiosarcoma
Since p14 is implicated in p53 degradation by proteasome through its interaction with HDM2, we analyzed the p14 and MDM2 copy number alterations by array CGH and their expression level (real-time PCR, IHC) in order to identify the potential loss of p14 expression or HDM2 overexpression, which could compensate for a lack of TP53 inactivation. However, no deletion of the CDKN2A locus encoding for P14 or MDM2 amplification was observed by array CGH. Real-time RT-PCR was performed for 19 tumors (10 secondary AS and nine primary AS) and did not reveal any significant difference in P14 or MDM2 expression between primary and secondary AS or between AS and other vascular tumors (data not shown). Immunohistochemistry was performed for 43 tumors. We observed P14 and HDM2 expression in 36 tumors out of 40 (89%) and 6 tumors out of 40 (15%) with interpretable results, respectively. We found a concordance between the presence/absence of p14 overexpression and absence/presence of HDM2 expression in 81% of cases.
No PTEN intragenic mutations or deletions are detected in angiosarcoma
PTEN deletion is a frequent event in sarcomas with complex karyotypes. In order to assess the incidence of this genomic aberration in AS, we analyzed a series of 26 tumors using QMP to detect gross genomic rearrangements and by array CGH to detect smaller deletions. Six cases revealed a duplication of all the exons of PTEN, whereas eight cases revealed deletion of all the exons. No case with intragenic rearrangement or deletion was identified. These results indicate the presence of large genomic imbalance involving chromosome 10 but do not favor PTEN as a target gene. We then used EMMA in order to detect point mutations that may inactivate the function of PTEN and substitute the absence of specific deletion. We did not find PTEN mutations in this series of AS. We analyzed the expression of PTEN gene using published expression data from a series of 21 AS and 3 other vascular tumors previously studied on the U133A Affymetrix platform40. Although the expression of PTEN was relatively low, it was uniform across the samples and similar between AS and other vascular tumors.
The PIK3CA/AKT/mTOR pathway is activated in a subset of angiosarcoma
Previous studies suggested a crucial role of the PIK3CA/AKT/mTOR pathway in the malignant transformation of endothelial cells. Although, PTEN did not appear to be specifically altered in AS we hypothesized that the PIK3CA/AKT/mTOR pathway was activated through alternative mechanisms in at least a subset of AS. We therefore performed further immunohistochemical analyses to evaluate the expression of p-S6K and p-4eBP1 (4E-binding protein 1), two downstream targets of mTORC1. We also evaluated the activation of the ERK pathway due to its multiple interactions with the PIK3CA/AKT/mTOR pathway and its potential involvement in the phosphorylation of S6 ribosomal protein. We found that 17 tumors out of 40 AS (42%) were positive for pS6K and/or p-4eBP1. We observed also that 12 tumors of 39 (31%) were positive for p44/p-ERK. Eleven of the 17 tumors positive for p-S6K and/or p-4eBP1 were also positive for p44/p-ERK (Pearson correlation coefficient 0.6; P=0.02). Then we investigated the incidence of point mutations in PIK3CA by screening this gene for 13 common hot spot mutations in exons 3, 10 and 20 in 33 cases of AS (22 secondary and 11 primary). We found a recurrent truncating frame shift mutation in exon 10 of the PI3KCA gene (S553fs) in 13 cases (39%) including five primary and eight secondary AS. This mutation was then confirmed by PCR using mutant-specific allele exon 10 primers (Fwd: 5′-CAGTTACTATTCTGTGACTGGTGT-3′; Rev: 5′-AGATTTTCTATGGACCACAGG-3′, Fig. S1). In order to explore further the biological significance of this unusual mutation, we sequenced the cDNA of three mutated cases with available RNA. However, no mutation was identified suggesting that the mutant PIK3CA transcript undergoes a nonsense-mediated decay and therefore is not detectable at the cDNA level (Fig. S1). Moreover, there was no correlation between the PI3KCA mutational status and the p-S6K and/or p-4eBP1 immunohistochemistry results.
NRAS and BRAF are not mutated in angiosarcomas
Given the high proportion of AS being positive for p44/ERK IHC in our series and the potential role of RAS dysregulation in abnormal vascular growth control, we hypothesized that a subset of AS are associated with mutation of the BRAF or NRAS genes. We screened BRAF exon 15 and NRAS exons 2 and 3 in 52 cases of AS, however, no mutations were identified at these hot spots.
DISCUSSION
Recent studies investigating the TP53 pathway in large series of sarcomas with complex genomics (LMS, UPS and pleomorphic liposarcomas) have shown that TP53 alterations are highly recurrent in this group of disease with up to 80% of cases exhibiting at least one TP53 alteration (mutation and/or deletion) 21-23. Our results showing deletion and mutation rates of 0% and 4%, respectively, indicate that AS display a different TP53 alteration profile than the majority of other sarcomas with complex karyotypes. Our findings are in agreement with previous smaller scale reports suggesting that TP53 mutations are uncommon in AS 28, 30.
Interestingly, a P72R polymorphism in exon 4 was detected in 58% of the analyzed cases. Unlike the deleterious tumor-associated P53 mutations, which occur in about 98% of cases in the DNA-binding-domain of the protein, single nucleotide polymorphisms are expected to be phenotypically silent. The R72P polymorphism occurs in the polyproline domain, which lies between the N-terminal transactivating domain and the DNA-binding domain of p53. This polymorphism has been associated with a modulation of the pro-apoptotic potential of p53 and cancer susceptibility 41. However, the level of evidence is poor due to important methodological issues and several meta-analyses failed to demonstrate a risk between the R72P polymorphism and any cancer 41. Interestingly, a high incidence of this polymorphism has been recently reported in atypical vascular lesions occurring after radiotherapy 35. In our series, we have observed this SNP in both primary and secondary AS suggesting the absence of any link between this polymorphism and the etiology of AS.
The rarity of deleterious TP53 mutations or deletions in AS does not necessarily imply that the TP53 pathway is intact and functional in these tumors. p14 acts on the TP53 pathway by interacting with HDM2, an E3 ubiquitin ligase targeting p53 for degradation by the proteasome. This results in a stabilization and activation of p53. Loss of p14 expression and/or HDM2 overexpression are frequent in sarcomas and have been shown to substitute for p53 inactivation in tumors without TP53 gene aberrations. Our results indicate that these genetic aberrations are not commonly involved in AS genesis. Despite these results, we observed a high proportion of AS displaying p53 overexpression. Normal p53 protein has an extremely short half-life and it is not detected by IHC. However, following genotoxic stress, the p53 protein level increases rapidly due to stabilization through post-transcriptional mechanisms. In contrast, mutation of the TP53 gene results in nuclear accumulation of the abnormal protein, which has a longer half-life and can be detected by IHC. Based on the assumption that only stable (presumably mutated) protein will be expressed, TP53 IHC is often used as a surrogate to predict the presence of TP53 mutations. In our study, however, we observed a high level of discordance between the IHC and the sequencing results. Although the two cases harboring a TP53 mutation were found to be positive upon immunostaining, 21 cases harboring wild-type TP53 stained also positive. Such discrepancies have already been reported in several studies suggesting that IHC is not a reliable tool for TP53 mutation screening42, 43. One hypothesis is that this overexpression is the result of specific oncogenic stresses leading to stabilization of the wild-type protein. In this case, we would expect to observe an improved outcome as the result of increased tumor apoptosis, which was not the case. A second hypothesis is that the nuclear accumulation of p53 is dependent on mechanisms different from mutation and reflects functional changes. Interestingly, p53 overexpression by IHC has been identified as a strong prognostic factor in many human cancers, including soft-tissue sarcomas 42, 43. Our data indicate that this biomarker has also a prognostic value in AS.
We have also shown that in contrast with other sarcomas with complex genomics, PTEN is neither deleted nor mutated in AS. Moreover, our expression data showing similar level of PTEN expression across the AS cases and other vascular tumors suggest that epigenetic silencing or transcriptional defect of PTEN is unlikely. All together, our results are in agreement with previous reports showing that constitutional biallelic inactivation of PTEN was associated with vascular malformation, but not with malignant vascular tumors 44.
Despite the lack of PTEN alteration in AS, we have identified an activation of the PIK3CA/AKT/mTOR pathway in a significant proportion of cases. Several studies have already demonstrated the crucial role of PIK3CA/AKT/mTOR signaling in endothelial cell homeostasis 45. Moreover, embryonal fibroblasts transformed with the PI3KCA gene have been shown to induce AS at the site of injection in chicken 46, 47. Interestingly, we have found a recurrent frameshift mutation affecting the helical domain of PI3KCA in about 40% of cases. The same mutation has been recently reported at a low incidence rate in one case of large B cell lymphoma 48 and one case of synovial sarcoma 49. Our results showing that this mutation is not detectable at cDNA level strongly suggest that this aberration does not have functional consequence. Therefore, further studies are needed to identify the mechanisms involved in the activation of PIK3CA/AKT/mTOR activation in AS. Indeed, the patterns of regulation of the PIK3CA/AKT/mTOR pathway in cancer cells are very complex and cross-talk with several other pathways. For instance, it is now recognized that PI3KCA and MAPK signals converge upstream and downstream of mTORC1 50. Interestingly, we found a good correlation between the activation of these two pathways in our series with the majority of tumors positive for p-S6K and/or p-4eBP1 being also positive for p44/p-ERK. Overall, our findings may have therapeutic implications, specifically that PI3KCA and/or mTOR antagonists represent a promising approach in the systemic treatment of AS, as already suggested by a recent in vitro study and preliminary clinical data 51, 52. They suggest also that, besides the inhibition of VEGFR receptors, the inhibition of MAPK signaling may be a relevant approach in AS as suggested by preclinical data 53 and may also contribute to the recently described clinical activity of multi-target tyrosine kinase inhibitors such as sorafenib in AS 54. Moreover, synergy is suggested with the combination of mTOR and MAPK inhibitors 50.
In summary, we have shown here that AS represents a distinct subgroup among sarcomas with complex karyotypes. Although TP53 mutation and PTEN deletion are frequent in LMS and UPS, these aberrations are not involved in the pathogenesis of AS. However, AS are characterized by a complex pattern of alterations of the TP53 and PIK3CA/AKT/mTOR pathways that is not dependent on the MYC amplification status. These results underscore the complex coordinated regulation of the survival and growth-control pathways in AS. Our results represent an important basis for further investigations with the aim of determining how the activities of these pathways are coordinated in AS and for the design of new therapeutic strategies.
Supplementary Material
Figure S1. Detection of truncating frame shift mutation in exon 10 of the PIK3CA gene (S553fs) by DNA PCR and direct sequencing (A), further confirmed by specific mutant allele PCR (B), but not detected by RT-PCR at cDNA level (C).
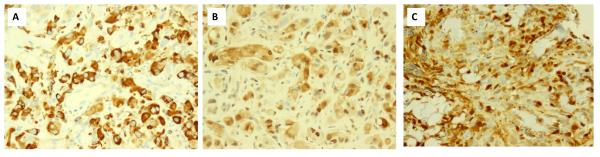
Figure 2.
Immunohistochemical (IHC) results for p-S6K (A), p-4eBP1 (B) and p44/p-ERK (C). Diffuse and strong reactivity for pS6K (A, 200x), p-4eBP1 (B, 200x) and p44/p-ERK (C, 200x) in a primary angiosarcoma (AS9).
ACKNOWLEDGMENTS
We thank Myriam Ardo Dia and Delphine Lafon for their helpful technical assistance; and Milagros Soto for editorial assistance.
Supported by: PO1 CA047179-15A2 (CRA), P50 CA 140146-01 (CRA), Cycle for survival (CRA), Fulbright program - French American Commission (AI), Fondation Monahan, French National Cancer Institute: Soutien pour la formation à la recherche translationnelle en cancérologie 2010 (AI) .
REFERENCES
- 1.Fletcher CD, Unni K, Mertens F. World Health Organization Classification of Tumours Pathology and Genetics of Tumours of Soft Tissue and Bone (2002) IARC Press. G; Lyon: 2002. [Google Scholar]
- 2.Fayette J, Martin E, Piperno-Neumann S, Le Cesne A, Robert C, Bonvalot S, Ranchere D, Pouillart P, Coindre JM, Blay JY. Angiosarcomas, a heterogeneous group of sarcomas with specific behavior depending on primary site: a retrospective study of 161 cases. Ann Oncol. 2007;18:2030–6. doi: 10.1093/annonc/mdm381. [DOI] [PubMed] [Google Scholar]
- 3.Mandahl N, Jin YS, Heim S, Willen H, Wennerberg J, Biorklund A, Mitelman F. Trisomy 5 and loss of the Y chromosome as the sole cytogenetic anomalies in a cavernous hemangioma/angiosarcoma. Genes Chromosomes Cancer. 1990;1:315–6. doi: 10.1002/gcc.2870010410. [DOI] [PubMed] [Google Scholar]
- 4.Kindblom LG, Stenman G, Angervall L. Morphological and cytogenetic studies of angiosarcoma in Stewart-Treves syndrome. Virchows Arch A Pathol Anat Histopathol. 1991;419:439–45. doi: 10.1007/BF01605079. [DOI] [PubMed] [Google Scholar]
- 5.Gil-Benso R, Lopez-Gines C, Soriano P, Almenar S, Vazquez C, Llombart-Bosch A. Cytogenetic study of angiosarcoma of the breast. Genes Chromosomes Cancer. 1994;10:210–2. doi: 10.1002/gcc.2870100311. [DOI] [PubMed] [Google Scholar]
- 6.Van den Berg E, Van Oven MW, de Jong B, Dam A, Wiersema J, Dijkhuizen T, Hoekstra HJ, Molenaar WM. Comparison of cytogenetic abnormalities and deoxyribonucleic acid ploidy of benign, borderline malignant, and different grades of malignant soft tissue tumors. Lab Invest. 1994;70:307–13. [PubMed] [Google Scholar]
- 7.Cerilli LA, Huffman HT, Anand A. Primary renal angiosarcoma: a case report with immunohistochemical, ultrastructural, and cytogenetic features and review of the literature. Arch Pathol Lab Med. 1998;122:929–35. [PubMed] [Google Scholar]
- 8.Wong KF, So CC, Wong N, Siu LL, Kwong YL, Chan JK. Sinonasal angiosarcoma with marrow involvement at presentation mimicking malignant lymphoma: cytogenetic analysis using multiple techniques. Cancer Genet Cytogenet. 2001;129:64–8. doi: 10.1016/s0165-4608(01)00431-9. [DOI] [PubMed] [Google Scholar]
- 9.Zu Y, Perle MA, Yan Z, Liu J, Kumar A, Waisman J. Chromosomal abnormalities and p53 gene mutation in a cardiac angiosarcoma. Appl Immunohistochem Mol Morphol. 2001;9:24–8. [PubMed] [Google Scholar]
- 10.Baumhoer D, Gunawan B, Becker H, Fuzesi L. Comparative genomic hybridization in four angiosarcomas of the female breast. Gynecol Oncol. 2005;97:348–52. doi: 10.1016/j.ygyno.2004.12.046. [DOI] [PubMed] [Google Scholar]
- 11.Cao Y, Zou SM, Zhang KT, Lu N, Liu Y, Feng L, Wen P, Han NJ, Lin DM. Genetic alterations in pulmonary epithelioid hemangioendothelioma and epithelioid angiosarcoma. Histol Histopathol. 2011;26:491–6. doi: 10.14670/HH-26.491. [DOI] [PubMed] [Google Scholar]
- 12.Manner J, Radlwimmer B, Hohenberger P, Mossinger K, Kuffer S, Sauer C, Belharazem D, Zettl A, Coindre JM, Hallermann C, Hartmann JT, Katenkamp D, Katenkamp K, Schoffski P, Sciot R, Wozniak A, Lichter P, Marx A, Strobel P. MYC high level gene amplification is a distinctive feature of angiosarcomas after irradiation or chronic lymphedema. Am J Pathol. 2010;176:34–9. doi: 10.2353/ajpath.2010.090637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo T, Chang NE, Singer S, Maki RG, Antonescu CR. Consistent MYC and FLT4 gene amplification in radiation-induced angiosarcoma but not in other radiation-associated atypical vascular lesions. Genes Chromosomes Cancer. 2011;50:25–33. doi: 10.1002/gcc.20827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mao JH, Wu D, Perez-Losada J, Nagase H, DelRosario R, Balmain A. Genetic interactions between Pten and p53 in radiation-induced lymphoma development. Oncogene. 2003;22:8379–85. doi: 10.1038/sj.onc.1207083. [DOI] [PubMed] [Google Scholar]
- 15.Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, Koutcher JA, Scher HI, Ludwig T, Gerald W, Cordon-Cardo C, Pandolfi PP. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436:725–30. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cully M, You H, Levine AJ, Mak TW. Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer. 2006;6:184–92. doi: 10.1038/nrc1819. [DOI] [PubMed] [Google Scholar]
- 17.Hambardzumyan D, Becher OJ, Rosenblum MK, Pandolfi PP, Manova-Todorova K, Holland EC. PI3K pathway regulates survival of cancer stem cells residing in the perivascular niche following radiation in medulloblastoma in vivo. Genes Dev. 2008;22:436–48. doi: 10.1101/gad.1627008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwon CH, Zhao D, Chen J, Alcantara S, Li Y, Burns DK, Mason RP, Lee EY, Wu H, Parada LF. Pten haploinsufficiency accelerates formation of high-grade astrocytomas. Cancer Res. 2008;68:3286–94. doi: 10.1158/0008-5472.CAN-07-6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng H, Ying H, Yan H, Kimmelman AC, Hiller DJ, Chen AJ, Perry SR, Tonon G, Chu GC, Ding Z, Stommel JM, Dunn KL, Wiedemeyer R, You MJ, Brennan C, Wang YA, Ligon KL, Wong WH, Chin L, DePinho RA. p53 and Pten control neural and glioma stem/progenitor cell renewal and differentiation. Nature. 2008;455:1129–33. doi: 10.1038/nature07443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puzio-Kuter AM, Castillo-Martin M, Kinkade CW, Wang X, Shen TH, Matos T, Shen MM, Cordon-Cardo C, Abate-Shen C. Inactivation of p53 and Pten promotes invasive bladder cancer. Genes Dev. 2009;23:675–80. doi: 10.1101/gad.1772909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perot G, Chibon F, Montero A, Lagarde P, de The H, Terrier P, Guillou L, Ranchere D, Coindre JM, Aurias A. Constant p53 pathway inactivation in a large series of soft tissue sarcomas with complex genetics. Am J Pathol. 2010;177:2080–90. doi: 10.2353/ajpath.2010.100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghadimi MP, Liu P, Peng T, Bolshakov S, Young ED, Torres KE, Colombo C, Hoffman A, Broccoli D, Hornick JL, Lazar AJ, Pisters P, Pollock RE, Lev D. Pleomorphic liposarcoma: Clinical observations and molecular variables. Cancer. 2011 doi: 10.1002/cncr.26195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ito M, Barys L, O’Reilly T, Young S, Gorbatcheva B, Monahan J, Zumstein-Mecker S, Choong PF, Dickinson I, Crowe P, Hemmings C, Desai J, Thomas DM, Lisztwan J. Comprehensive mapping of p53 pathway alterations reveals an apparent role for both SNP309 and MDM2 amplification in sarcomagenesis. Clin Cancer Res. 2011;17:416–26. doi: 10.1158/1078-0432.CCR-10-2050. [DOI] [PubMed] [Google Scholar]
- 24.Yang J, Du X, Chen K, Ylipaa A, Lazar AJ, Trent J, Lev D, Pollock R, Hao X, Hunt K, Zhang W. Genetic aberrations in soft tissue leiomyosarcoma. Cancer Lett. 2009;275:1–8. doi: 10.1016/j.canlet.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibault L. PhD thesis. University Paris Descartes; Paris: 2010. Recurrent genomic alterations in sarcomas with complex genomics: PTEN and DKK1 loss of expression, mechanisms and biological consequences. (Print) [Google Scholar]
- 26.Hernando E, Charytonowicz E, Dudas ME, Menendez S, Matushansky I, Mills J, Socci ND, Behrendt N, Ma L, Maki RG, Pandolfi PP, Cordon-Cardo C. The AKT-mTOR pathway plays a critical role in the development of leiomyosarcomas. Nat Med. 2007;13:748–53. doi: 10.1038/nm1560. [DOI] [PubMed] [Google Scholar]
- 27.Hollstein M, Marion MJ, Lehman T, Welsh J, Harris CC, Martel-Planche G, Kusters I, Montesano R. p53 mutations at A:T base pairs in angiosarcomas of vinyl chloride-exposed factory workers. Carcinogenesis. 1994;15:1–3. doi: 10.1093/carcin/15.1.1. [DOI] [PubMed] [Google Scholar]
- 28.Soini Y, Welsh JA, Ishak KG, Bennett WP. p53 mutations in primary hepatic angiosarcomas not associated with vinyl chloride exposure. Carcinogenesis. 1995;16:2879–81. doi: 10.1093/carcin/16.11.2879. [DOI] [PubMed] [Google Scholar]
- 29.Naka N, Tomita Y, Nakanishi H, Araki N, Hongyo T, Ochi T, Aozasa K. Mutations of p53 tumor-suppressor gene in angiosarcoma. Int J Cancer. 1997;71:952–5. doi: 10.1002/(sici)1097-0215(19970611)71:6<952::aid-ijc7>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 30.Zietz C, Rossle M, Haas C, Sendelhofert A, Hirschmann A, Sturzl M, Lohrs U. MDM-2 oncoprotein overexpression, p53 gene mutation, and VEGF up-regulation in angiosarcomas. Am J Pathol. 1998;153:1425–33. doi: 10.1016/S0002-9440(10)65729-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amo Y, Masuzawa M, Hamada Y, Katsuoka K. Serum concentrations of vascular endothelial growth factor in angiosarcomas with and without p53 gene mutation. Acta Derm Venereol. 2002;82:373–4. doi: 10.1080/000155502320624122. [DOI] [PubMed] [Google Scholar]
- 32.Weihrauch M, Bader M, Lehnert G, Koch B, Wittekind C, Wrbitzky R, Tannapfel A. Mutation analysis of K-ras-2 in liver angiosarcoma and adjacent nonneoplastic liver tissue from patients occupationally exposed to vinyl chloride. Environ Mol Mutagen. 2002;40:36–40. doi: 10.1002/em.10084. [DOI] [PubMed] [Google Scholar]
- 33.Domfeh AB, Fichera M, Hunt JL. Allelic loss of 3 different tumor suppressor gene loci in benign and malignant endothelial tumors of the head and neck. Arch Pathol Lab Med. 2006;130:1184–7. doi: 10.5858/2006-130-1184-ALODTS. [DOI] [PubMed] [Google Scholar]
- 34.Tate G, Suzuki T, Mitsuya T. Mutation of the PTEN gene in a human hepatic angiosarcoma. Cancer Genet Cytogenet. 2007;178:160–2. doi: 10.1016/j.cancergencyto.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 35.Santi R, Cetica V, Franchi A, Pepi M, Cesinaro AM, Miracco C, Paglierani M, De Giorgi V, Delfino C, Difonzo EM, Pimpinelli N, Bianchi S, Sardi I, Santucci M, Massi D. Tumour suppressor gene TP53 mutations in atypical vascular lesions of breast skin following radiotherapy. Histopathology. 2011;58:455–66. doi: 10.1111/j.1365-2559.2011.03770.x. [DOI] [PubMed] [Google Scholar]
- 36.Weber J, Miserere S, Champ J, Looten R, Stoppa-Lyonnet D, Viovy JL, Houdayer C. High-throughput simultaneous detection of point mutations and large-scale rearrangements by CE. Electrophoresis. 2007;28:4282–8. doi: 10.1002/elps.200700010. [DOI] [PubMed] [Google Scholar]
- 37.Caux-Moncoutier V, Castera L, Tirapo C, Michaux D, Remon MA, Lauge A, Rouleau E, De Pauw A, Buecher B, Gauthier-Villars M, Viovy JL, Stoppa-Lyonnet D, Houdayer C. EMMA, a cost- and time-effective diagnostic method for simultaneous detection of point mutations and large-scale genomic rearrangements: application to BRCA1 and BRCA2 in 1,525 patients. Hum Mutat. 2011;32:325–34. doi: 10.1002/humu.21414. [DOI] [PubMed] [Google Scholar]
- 38.Cordon-Cardo C, Reuter VE. Alterations of tumor suppressor genes in bladder cancer. Semin Diagn Pathol. 1997;14:123–32. [PubMed] [Google Scholar]
- 39.Mallofre C, Castillo M, Morente V, Sole M. Immunohistochemical expression of CK20, p53, and Ki-67 as objective markers of urothelial dysplasia. Mod Pathol. 2003;16:187–91. doi: 10.1097/01.MP.0000056628.38714.5D. [DOI] [PubMed] [Google Scholar]
- 40.Antonescu CR, Yoshida A, Guo T, Chang NE, Zhang L, Agaram NP, Qin LX, Brennan MF, Singer S, Maki RG. KDR activating mutations in human angiosarcomas are sensitive to specific kinase inhibitors. Cancer Res. 2009;69:7175–9. doi: 10.1158/0008-5472.CAN-09-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whibley C, Pharoah PD, Hollstein M. p53 polymorphisms: cancer implications. Nat Rev Cancer. 2009;9:95–107. doi: 10.1038/nrc2584. [DOI] [PubMed] [Google Scholar]
- 42.Das P, Kotilingam D, Korchin B, Liu J, Yu D, Lazar AJ, Pollock RE, Lev D. High prevalence of p53 exon 4 mutations in soft tissue sarcoma. Cancer. 2007;109:2323–33. doi: 10.1002/cncr.22680. [DOI] [PubMed] [Google Scholar]
- 43.Robles AI, Harris CC. Clinical outcomes and correlates of TP53 mutations and cancer. Cold Spring Harb Perspect Biol. 2010;2:a001016. doi: 10.1101/cshperspect.a001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caux F, Plauchu H, Chibon F, Faivre L, Fain O, Vabres P, Bonnet F, Selma ZB, Laroche L, Gerard M, Longy M. Segmental overgrowth, lipomatosis, arteriovenous malformation and epidermal nevus (SOLAMEN) syndrome is related to mosaic PTEN nullizygosity. Eur J Hum Genet. 2007;15:767–73. doi: 10.1038/sj.ejhg.5201823. [DOI] [PubMed] [Google Scholar]
- 45.Dimmeler S, Zeiher AM. Akt takes center stage in angiogenesis signaling. Circ Res. 2000;86:4–5. doi: 10.1161/01.res.86.1.4. [DOI] [PubMed] [Google Scholar]
- 46.Chang HW, Aoki M, Fruman D, Auger KR, Bellacosa A, Tsichlis PN, Cantley LC, Roberts TM, Vogt PK. Transformation of chicken cells by the gene encoding the catalytic subunit of PI 3-kinase. Science. 1997;276:1848–50. doi: 10.1126/science.276.5320.1848. [DOI] [PubMed] [Google Scholar]
- 47.Aoki M, Batista O, Bellacosa A, Tsichlis P, Vogt PK. The akt kinase: molecular determinants of oncogenicity. Proc Natl Acad Sci U S A. 1998;95:14950–5. doi: 10.1073/pnas.95.25.14950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baohua Y, Xiaoyan Z, Tiecheng Z, Tao Q, Daren S. Mutations of the PIK3CA gene in diffuse large B cell lymphoma. Diagn Mol Pathol. 2008;17:159–65. doi: 10.1097/PDM.0b013e31815d0588. [DOI] [PubMed] [Google Scholar]
- 49.Barretina J, Taylor BS, Banerji S, Ramos AH, Lagos-Quintana M, Decarolis PL, Shah K, Socci ND, Weir BA, Ho A, Chiang DY, Reva B, Mermel CH, Getz G, Antipin Y, Beroukhim R, Major JE, Hatton C, Nicoletti R, Hanna M, Sharpe T, Fennell TJ, Cibulskis K, Onofrio RC, Saito T, Shukla N, Lau C, Nelander S, Silver SJ, Sougnez C, Viale A, Winckler W, Maki RG, Garraway LA, Lash A, Greulich H, Root DE, Sellers WR, Schwartz GK, Antonescu CR, Lander ES, Varmus HE, Ladanyi M, Sander C, Meyerson M, Singer S. Subtype-specific genomic alterations define new targets for soft-tissue sarcoma therapy. Nat Genet. 2010;42:715–21. doi: 10.1038/ng.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carracedo A, Pandolfi PP. The PTEN-PI3K pathway: of feedbacks and cross-talks. Oncogene. 2008;27:5527–41. doi: 10.1038/onc.2008.247. [DOI] [PubMed] [Google Scholar]
- 51.Schuetze SM, Baker LH, Maki RG. Sirolimus reduced tumor-related morbidity and resulted in biochemical and radiographic response in patients with progressive sarcoma. J Clin Oncol. 2006;24:9503. [Google Scholar]
- 52.Bundscherer A, Vogt T, Kohl G, Landthaler M, Hafner C. Antiproliferative effects of rapamycin and celecoxib in angiosarcoma cell lines. Anticancer Res. 2010;30:4017–23. [PubMed] [Google Scholar]
- 53.LaMontagne KR, Jr., Moses MA, Wiederschain D, Mahajan S, Holden J, Ghazizadeh H, Frank DA, Arbiser JL. Inhibition of MAP kinase kinase causes morphological reversion and dissociation between soft agar growth and in vivo tumorigenesis in angiosarcoma cells. Am J Pathol. 2000;157:1937–45. doi: 10.1016/s0002-9440(10)64832-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maki RG, D’Adamo DR, Keohan ML, Saulle M, Schuetze SM, Undevia SD, Livingston MB, Cooney MM, Hensley ML, Mita MM, Takimoto CH, Kraft AS, Elias AD, Brockstein B, Blachere NE, Edgar MA, Schwartz LH, Qin LX, Antonescu CR, Schwartz GK. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol. 2009;27:3133–40. doi: 10.1200/JCO.2008.20.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Detection of truncating frame shift mutation in exon 10 of the PIK3CA gene (S553fs) by DNA PCR and direct sequencing (A), further confirmed by specific mutant allele PCR (B), but not detected by RT-PCR at cDNA level (C).