Abstract
BACKGROUND
In people with diabetes and peripheral neuropathy (DM+PN), injury risk is not clearly known for weight bearing (WB) vs. non-weight bearing (NWB) exercise. In-shoe peak plantar pressures (PPP) often are used as a surrogate indicator of injury to the insensitive foot.
OBJECTIVE
Compare PPPs in people with DM+PN during selected WB and NWB exercises.
METHODS
15 subjects with DM+PN participated. PPPs were recorded for the forefoot, midfoot, and heel during level walking and compared to; WB exercises - treadmill walking, heel and toe raises, sit to stands, stair climbing, single leg standing; and NWB exercises - stationary bicycling, balance ball exercise and plantar flexion exercise.
RESULTS
Compared to level walking; mean forefoot PPP during treadmill walking was 13% higher, but this difference was eliminated when walking speed was used as a covariate. Mean PPPs were similar or substantially lower for other exercises, except for higher forefoot PPP with heel raise exercises.
CONCLUSIONS
Slow progression and regular monitoring of insensitive feet are recommended for all exercises, but especially for heel raises, and increases in walking speed. The remaining WB and NWB exercises pose no greater risk to the insensitive foot due to increases in PPP compared to level walking.
Keywords: Diabetes, Peripheral Neuropathy, Plantar pressure, Exercise
People with diabetes and peripheral neuropathy (DM+PN) have significant amounts of lower extremity impairments and disability. Insensate feet pose an additional threat for skin ulcers or injury from unnoticed trauma which can lead to amputations.1-3 The usefulness of weight bearing activities, like walking and resistance exercises, in the control of diabetes has been described by a number of researchers.4,5 The American College of Sports Medicine (ACSM) and American Diabetes Association (ADA), until recently, discouraged weight bearing exercises in people with DM+PN due to increased risk of skin injury and ulceration.6 Several studies, however, have provided evidence in contradiction of this traditional belief that those with DM+PN should avoid weight-bearing exercise.7-9 A recent randomized controlled trial showed that a progressive increase in weight bearing activities in people with DM+PN did not raise the risk of ulceration.8 Based on this growing body of evidence, the most recent guidelines from the ACSM and ADA promote “moderate walking” for those with DM+PN.10 Additional research is needed, however, to understand the potential risk of skin injury due to repetitive stresses during various weight-bearing and non weight-bearing exercises.
In – shoe plantar pressure measurement is often used as a proxy to identify areas of high pressure and potential injury to insensate feet.3,11,12 Some studies have measured plantar pressures during level walking and daily life activities like stair climbing, ramp walking and turning in people with DM+PN.13,14 Burnfield and colleagues quantified peak plantar pressures in healthy adults across five different cardiovascular exercises – treadmill walking, running, elliptical training, stair climbing and recumbent biking.15 However, to the best of our knowledge, no such study has been done in people with DM+PN to examine the plantar pressures during exercises.
The purpose of this study was to determine the in-shoe plantar pressures during selected forms of weight bearing (WB) and non-weight bearing (NWB) exercises in people with DM+PN. We hypothesized that the plantar pressures during weight bearing exercises will be higher than non weight bearing exercises, but not greater than level walking. Understanding the plantar pressures during various exercises can help to design optimal exercise programs and purposeful loading of plantar tissues for this population with lower extremity impairments that are at risk of unnoticed foot injury.
METHOD
Participants
Fifteen subjects participated in this study [8 men/7 women; mean age 67.4 (12.8); BMI 34.6 (7.1)]. Participants were recruited from the Diabetic Foot Center, Diabetes Research and Training Center and Volunteers for Health at Washington University School of Medicine, and BJC health system in St. Louis, MO. This study was part of a larger randomized control study investigating the effect of exercise on DM+PN. Subjects who had a history of severe foot deformity, current foot ulcer, Charcot neuroarthropathy or partial foot amputation that would require custom therapeutic footwear and anyone who was physically unable to tolerate one and half hour of activity testing, were excluded. All subjects had mild to moderate fore foot deformity (i.e., hammer or claw toe). One subject with bilateral fifth toe amputation was included because the metatarsals were intact in both feet and this individual did not require custom footwear. Three subjects had a history of foot ulcer which had been healed for a minimum of six months. All subjects read and signed the consent form approved by the Human Research Protection Office at Washington University in St. Louis. Patient demographics are illustrated in Table 1.
Table 1. Subject Characteristics.
| Number of participants | 15 |
|---|---|
| Age (yrs) | 67.4 (12.8) |
| BMI (kg/m2) | 34.6 (7.1) |
| Gender (M/F) | 8/7 |
| Duration of DM (yrs) | 13.7 (8.5) |
| History of ulcer | N=3 |
| Footwear |
Standard shoe without custom insole N= 9
Standard shoe with custom insole N= 3 Prescribed shoe with custom insole N= 3 |
|
Vibration Perception Threshold (Volts) |
45.1 (8.4) |
| Level walking speed (m/s) | 0.85 (0.08) |
| Treadmill walking speed (m/s) | 1.01 (0.28)* |
Data represented as means (SD) unless otherwise indicated.
indicates a difference in the level walking and treadmill walking speeds based on t-test results. (P < 0.05)
Assessments
Peripheral Neuropathy
Sensation was tested using Semmes-Weinstein Monofilaments and a Biothesiometer. Peripheral neuropathy was characterized by the inability to sense the 5.07 Semmes-Weinstein monofilament on at least one spot on the plantar surface of the foot and a vibration perception threshold at the plantar great toe of more than 25 volts, as measured by the Biothesiometer which has a maximum value of 50 volts.9,16 The mean vibration perception threshold for this group was 45.1 (8.4) volts (Table 1) indicating a severe level of sensory neuropathy.
Footwear Assessment
Pressure testing was conducted in the subject’s own footwear after ensuring the size and fit of the shoe were adequate using previously described criteria.17,18 Therapeutic footwear is generally an important treatment component for people with DM+PN who have a history of ulceration.17 In people with DM+PN that have no history of ulcer, some studies have shown that custom therapeutic footwear and inserts do not provide additional reduction of ulcer risk as compared to subjects’ usual footwear.18,19 Therefore, we conducted the study in subjects’ habitual footwear that was checked for excessive wear, fit of foot to the shoe (appropriate length, width, and depth of toe box), and accommodation of bony deformities. For description purposes, the footwear was classified into three categories: prescribed shoes with insoles, and standard, well fitting shoes with or without custom insoles. Nine subjects wore standard shoes without custom insoles, three wore standard shoes with custom insoles, and three wore prescribed shoes with custom insoles (Table 1).
Plantar Pressure testing
Plantar pressures on both feet were collected during selected exercise conditions using the F-SCAN system (Tekscan, South Boston, MA, USA) with methods that have been previously described and validated.20,21 Each pressure sensor was 0.15 mm thick, contained 954 sensels and measured pressure over a range 0-862 KPa. A new pressure sensor, cut to each subject’s shoe size, was placed inside each subject’s shoes. The sensors were connected to a computer via a 20 feet long cable. The sensors were calibrated, at the beginning and midway through the test or as needed, according to the manufacturer guidelines and standardized techniques.20 Plantar pressures were collected at 50 Hz. Two trials of level walking across a distance of 6.1-m (20 feet) with at least five steps each were collected at 50 Hz to record pressure data and calculate walking speed. The mean walking speed was 0.85 m/s (0.08). Data were then collected during WB exercises - treadmill walking (ICON Health and Fitness, West, Logan, UT, USA), heel and toe raise exercise, sit to stands, stair climbing (using step stools, 5.5″ step height and 7.5″ step width), single leg standing (15 seconds), and NWB exercises - stationary bicycling (upright (ICON Health and Fitness, West, Logan, UT, USA) and recumbent (Schwinn, Olney, IL, USA), balance ball (Theraband ®, Hygenic Corporation, Akron, Ohio, USA) exercise (lifting one leg at a time with arms crossed across the chest) and plantar flexion with a resistance band (Theraband ®, Hygenic Corporation, Akron, Ohio, USA).
Each participant was allowed to use the treadmill, upright bike and recumbent bike exercises before the test, to ensure appropriate seat alignment and self-selected speed settings, which was comfortable for the participant. The participant was asked to score his/her workout on a Borg scale of 6-20 in order to get a rate of perceived exertion. They were instructed (and monitored) to exercise at level 12-13 (somewhat hard) on the Borg scale.6 These instructions were given to establish consistent intensity levels over a “typical” 30-45 minute workout session for the treadmill, upright bike and recumbent bike exercises. Before the exercise session, each participant’s height, weight, date of birth, medical history, resting blood pressure and resting heart rate was recorded.
The order of WB and NWB exercises were randomized. A brief demonstration and practice session was given prior to each exercise. Appropriate rest periods were given between exercises. Data were collected for at least ten walking and bicycle rotation cycles, during the middle 1 to 2 minutes of exercise on the treadmill and stationary bicycles. Plantar pressures were collected for a period of 15 seconds during single leg standing. For all other exercises, a minimum of five trials were collected. Heart rate was monitored during all exercises and walking speed on the treadmill was recorded because walking speed is known to affect plantar pressures.22
Data analysis
Plantar pressure data were analyzed using the F-Scan Mobile Research software (ver. 5.72; Tekscan, South Boston, MA, USA). Each foot was divided into three primary anatomical areas that might be affected differently by the various exercises defined as; forefoot (distal 40% of the total length), midfoot (middle 30% of the total length) and heel (proximal 30% of the total length). The peak plantar pressures (PPP) were calculated during WB and NWB exercises for each anatomical region of the foot. A mean of three trials during the middle portion of the exercise was used to calculate the mean PPP for each anatomical region on each foot.
Statistical analyses were performed using Systat software (Systat Software, Inc., Chicago, IL, USA) for Windows (ver. 13.0). Descriptive statistics (mean, standard deviation, range) were used to characterize PPPs across the different anatomical regions during exercises. There were no differences between right and left foot mean PPPs, hence only mean PPPs for the right foot were included in further analysis. Data was analyzed for normality using the Shapiro-Wilk test for normality. Mean PPPs during exercises were compared to the mean PPP during level walking. A repeated measures analysis of variance (ANOVA) was conducted for the dependent variable (peak plantar pressure) to determine significant differences across exercise conditions, for each anatomical region. Post-hoc t-tests were performed when the ANOVA indicated significant differences in PPPs between exercise conditions; i.e., protected t-test under the umbrella of a significant F test.22 Post-hoc, within each anatomical foot region, the mean PPP was compared between each exercise condition and level walking. One participant was removed from the ANOVA analyses due to missing data (could not perform heel raise exercise). In addition, a post-hoc repeated measures analysis of covariance was conducted for the comparison of mean PPPs between treadmill walking and level walking to determine if walking speed (covariate) affected the results. The statistical significance was set at P<0.05 for all testing.
RESULTS
The mean PPPs were significantly different across the selected exercise conditions for each area of the foot (P<0.01, Table 2).
Table 2. Results of plantar 6 pressures during exercise.
| N | Forefoot PPP | Midfoot PPP | Heel PPP | ||||
|---|---|---|---|---|---|---|---|
| (KPa) | % change |
(KPa) | % change |
(KPa) | % change |
||
| Level Walking | 15 | 230.5 (62.1) | 113.5(66.6) | 169.5 (47.4) | |||
| Treadmill | 15 | 260.9(79.4)* | 13.2 | 121.9 (57.6) | 7.4 | 170.7 (43.7) | 0.7 |
| Heel Raises | 14 | 316.7(90.5)# | 27.2 | 136.2 (112.8) | 20.2 | 108.9 (59.4)# | −35.8 |
| Toe Raises | 15 | 58.1 (44.7)# | −74.8 | 58.8 (23.3)# | −48.2 | 205.3 (66.1) | 21.1 |
| Single leg standing | 15 | 149.7(115.8)* | −35.1 | 123.2 (49.7) | 8.5 | 163.3 (61) | −3.7 |
| Stair Climbing | 15 | 202.4 (76.4)* | −12.2 | 119.2 (55.1) | 4.9 | 120.1 (51)# | −29.2 |
| Sit to stand | 15 | 93.1 (53)# | −59.6 | 59.9 (27.1)# | −47.2 | 109.8 (37.9)# | −35.2 |
| Recumbent Bike | 15 | 59.3 (29.6)# | −74.3 | 47.8 (26.7)# | −57.9 | 28.3 (14.5)# | −83.2 |
| Upright Bike | 15 | 38.3 (23.8)# | −83.4 | 36.4 (21.2)# | −67.9 | 38 (24.9)# | −77.6 |
| Plantar Flexion | 15 | 38.2 (14)# | -83.4 | 19.8 (10.1)# | -82.5 | 34.4 (15.7)# | −79.5 |
|
Balance Ball
Exercise |
15 | 46.5 (20.4)# | −79.8 | 23.5 (11.1)# | −79.3 | 52.4 (21)# | −69.1 |
| Pvalues | 14 | <0.01 | <0.01 | <0.01 | |||
Data is represented as mean (SD) unless otherwise indicated.
% change = % increase or decrease from level walking
P values are from the repeated measures ANOVA results (calculated from complete data on 14 subjects) for the peak plantar pressures during different exercises in each anatomical region.
PPP = Peak Plantar Pressure
indicates difference from level walking in each anatomical region based on post hoc t-tests from ANOVA results (P<0.05)
indicates difference from level walking in each anatomical region based on post hoc t-tests from ANOVA results (P <0.01)
Comparison of forefoot plantar pressure across exercises
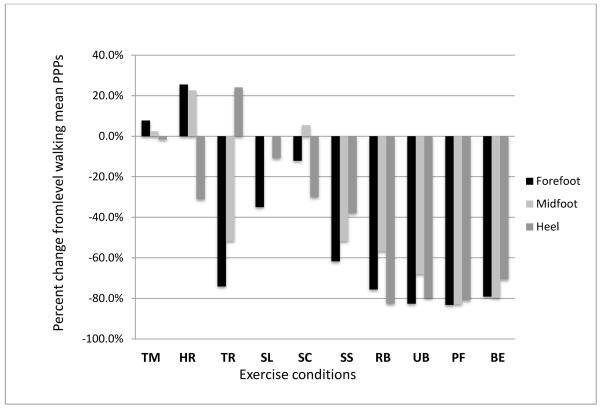
The mean forefoot PPP during level walking was 230.5 (62.1) KPa (Table 2). During treadmill walking, the mean PPP increased 13.2% (P<0.05), when compared to level walking, but these differences were eliminated when walking speed was used as a covariate (P>0.05). The mean forefoot PPP was 27.2% (P<0.01) higher during heel raise exercise as compared to level walking. Forefoot mean PPPs decreased during toe raise exercise, −74.8% (P<0.01); single leg standing, −35.1% (P<0.05); stair climbing, −12.1% (P<0.05); sit to stand, −59.6% (P<0.01); recumbent bike, −74.3% (P<0.01); upright bike, −83.4% (P<0.01); plantar flexion exercise, - 83.4% (P<0.01) and balance ball exercise, −79.8% (P<0.01) when compared to level walking (Table 2, Fig 1).
Figure 1. Plantar pressures compared to level walking.
Data represented as percentages.
PPP=Peak plantar pressure; TM=treadmill walking; HR=heel raise; TR=toe raise; SL=single leg standing; SC=stair climbing; SS=sit to stand; RB=recumbent biking; UB=upright biking; PF=plantar flexion exercise; BE=balance ball exercise.
Comparison of midfoot plantar pressure across exercises
The mean midfoot PPP during level walking was 113.5 (66.6) KPa (Table 2). The mean midfoot PPPs for treadmill walking, heel raise exercise, single leg standing, and stair climbing, were not different when compared to level walking (P >0.05, Table 2). When compared to level walking, midfoot PPPs decreased for toe raise exercise, −48.2% (P<0.01); sit to stand, −47.2% (P<0.01); recumbent bike, −57.9% (P<0.01); upright bike, −67.9% (P<0.01); plantar flexion exercise; −82.5% (P<0.01) and balance ball exercise, −79.3% (P<0.01, Table 2, Fig 1).
Comparison of heel plantar pressures across exercises
The mean heel PPP during level walking was 169.5 (47.4) KPa. In the WB exercises, heel PPP was unchanged for treadmill walking, toe raise exercise, and for single leg standing compared to level walking (P >0.05). The mean heel PPP was reduced for heel raise exercise, - 35.8% (P<0.01); stair climbing, −29.2% (P<0.01) and sit to stand, −35.2% (P<0.01) when compared to level walking. In the NWB exercises, mean heel PPPs were reduced for all exercises; recumbent bike, −83.2% (P<0.01); upright bike, −77.6% (P<0.01); plantar flexion exercise, -79.5% (P<0.01) and balance ball exercise, -69.1% (P<0.01) as compared to level walking (Table 2, Fig 1).
DISCUSSION
To the best of our knowledge, this is the first study to examine plantar pressures during exercises in people with DM+PN. The results from this study show that the mean forefoot PPP during treadmill waking was 13% higher than during level walking. However, when walking speed was used as a covariate, this difference no longer remained significant. Compared to level walking, heel raise exercise led to 27% higher mean PPP at the forefoot region. For all other WB exercises, i.e. toe raise exercise, single leg standing, stair climbing and sit to stand exercise, mean PPPs were no different than the mean PPP during level walking. Mean PPPs for all the NWB exercises; i.e. stationary biking, plantar flexion exercise and balance ball exercise were significantly lower (58 % to 83 %) compared to WB exercises and level walking.
In this study, forefoot PPP during treadmill walking was 13% higher than during level walking. Studies have shown that walking speed affects plantar pressures; higher speeds are associated with higher PPPs.23 Our participants walked at a mean walking speed of 0.85 m/s (0.08) versus 1.01 (0.28) m/s during treadmill walking (P < 0.05, Table 1). Accordingly, when walking speed was used as a covariate in the repeated measures analysis, the difference in the forefoot mean PPP between level walking and treadmill walking was not significant (P>0.05). Our results for the mean forefoot and midfoot PPPs were similar to those measured by Burnfield et al in healthy older adults during treadmill walking (Forefoot; 260.9 KPa vs. 256 KPa and Midfoot; 121.9 KPa vs. 125 KPa; Table 2); while, mean heel PPP was lower in our participants (170.7 KPa vs. 215 KPa, Table 2).15 Past research has shown that people with DM+PN walk slower compared to their healthy, age matched controls.24 Likewise, participants in our study walked slower compared to the participants in the Burnfield et al study (1.01 m/s versus 1.5 m/s). Although PPP at the forefoot is known to be higher in people with DM+PN than those without DM+PN;1 lower heel PPP recorded in this study may be associated with slower walking speeds when compared to the Burnfield et al study. Because PPPs were similar for treadmill walking compared to over ground level walking in people with DM+PN, we believe the treadmill is a safe alternative, but neuropathic feet should be monitored especially carefully with an increase in walking speed.
Compared to level walking, heel raise exercises resulted in higher PPPs at the forefoot (27%). During heel raise exercises, the contact area between the foot and surface decreases, resulting in high, localized PPPs. However, the benefit of these exercises in this population should not be overlooked.25 Strength of the plantar flexor muscles decreases in people with DM+PN and in older adults.24 Ankle plantar flexor muscle strength is particularly important during chair rising, stair climbing and walking.26 Additionally, in people with DM+PN, weakness of dorsiflexor muscles may contribute to early “slapping of the foot” during gait, specifically when the foot makes contact with the surface,27 justifying the inclusion of toe raise exercises to improve the dorsiflexor muscles strength. Thus, based on these results, heel and toe exercises may be included in an exercise program for people with DM+PN. We acknowledge that individual characteristics (for example, deformity and open lesions) may be a contraindication for these exercises.
For the remaining WB exercises, i.e., stair climbing, sit to stand and balance exercises, the mean PPPs were no different from level walking. For stair climbing, mean PPPs we recorded were similar to those reported in two previous studies,13,14 but were higher than the plantar pressures measured by Burnfield et al.15 One reason for this difference may be because our participants walked with minimal to no hand support. For the sit to stand exercise, our results are similar to those reported previously by Rozema et al.28 In both these studies, mean PPPs during rising from a chair and sitting down were lower than level walking. Studies suggest that individuals with diabetes have some amount of physical disability reflected in their inability to climb a flight stairs or rise from a chair.26 Thus, including stair climbing and sit to stand exercises in the exercise routine could improve functional capabilities of people with DM+PN. For the balance exercises, relatively low PPPs recorded in our study match those reported by Mao et al, measured in a group of Tai-chi practitioners performing single leg standing.29 In both studies, mean PPPs for single leg standing were lower than level walking at the forefoot and heel. Balance impairments and risk of fall are high in people with DM+PN.30, 31 The ability to stand on one leg is an important predictor for falls32 and single leg standing can be used as part of an exercise program.25 Evidence exists indicating that balance can be improved with exercise in people with DM+PN.25, 33,34
As expected, mean PPPs during NWB exercises were much lower than during WB exercises and level walking. The mean PPPs measured during recumbent biking were similar to those measured by Burnfield et al in healthy older adults.15 The mean PPPs during upright biking and recumbent biking were substantially lower than level walking indicating that the stationary biking is a good method to provide aerobic exercise without stress to the plantar foot. The mean PPPs, for the plantar flexion exercise and alternating ‘marching’ of the feet while being seated on a balance ball, were approximately 80% less than for level walking in all foot regions. Seated balance exercises on a balance ball are beneficial as they activate core abdominal muscles which may help improve balance control.25 As noted above, strength of plantar flexor muscles is reduced in people with DM+PN.24 Resistance exercises with an elastic band may be used as an alternative to heel raise exercise, when reduction in plantar pressure is advised; for example when a patient has a forefoot ulcer or severe deformity.
The rationale for fostering WB exercises in people with DM+PN can further be explained by Mueller and Maluf’s “Physical Stress Theory”.35 When the intensity of exercises (magnitude, duration and number of repetitions) is gradually increased; the tissue (in the case of this study, skin) would become more tolerant to subsequent stresses. Further support to provide WB exercise for people with DM+PN is provided by LeMaster et al who found that increased WB activities did not increase the risk of foot ulceration.8 Recent ACSM and ADA guidelines also recommend WB exercises in people with DM+PN.10 Thus, careful monitoring and slow progression of exercises could help to reduce the incidence of skin breakdown in people with DM+PN who do not have an active ulcer, but additional research is needed to test this speculation.
While this study provides some insight to the mean PPPs during WB and NWB exercises, some limitations should be considered. Firstly, PPP measurement is used as a surrogate measure to predict skin breakdown and ulcer formation. Skin breakdown depends on multiple factors including foot deformity, altered sensation, limited joint mobility and abnormal pressures.1-3,23,36 Our study cohort included a variety of individuals who had mild to moderate foot deformities, decreased sensation and possible limited joint mobility. We acknowledge that the sample size is small (n=15) and there were a few outliers in our data. Nonetheless, the results were essentially the same with the use of non parametric statistics (Friedman test and post-hoc Wilcoxon signed rank test) or parametric statistics (ANOVA). Due to the high effect size observed between level walking and the different exercise conditions, we believe that our results are a good representation of the PPPs in people with DM+PN, who do not have an active ulcer or severe foot deformity. Our results may not be generalized, however, to people with DM+PN that have severe joint deformity, Charcot neuroarthropathy, or partial foot amputation. Plantar pressures are known to be higher in these individuals. Further studies are needed to examine the effect of WB exercises in these individuals. Additionally, we did not measure cumulative forces over a typical 30-45 minute exercise session. However, we would not expect plantar pressures to change substantially during that amount of time the participants were tested, and we believed that these subjects would be unable to tolerate a full intervention of every exercise. Nonetheless, we acknowledge that increased risk for plantar ulceration is dependent on both magnitude and duration of exercise. Clinicians should take both these variables into account when prescribing exercises to people with DM+PN.
In conclusion, the results of this study provide some guidance regarding risk of injury to the insensitive foot during various exercises. The mean forefoot PPP during treadmill walking and during heel raise exercises were higher than during level walking. After adjusting for the difference of walking speed between treadmill and level walking, the mean forefoot PPP was no different from level walking. For all other WB and NWB exercises, mean PPPs were either similar or significantly lower than level walking. Toe raise exercise, single leg standing, stair climbing and sit to stand exercises pose no greater risk, i.e. no greater PPP, to the insensitive foot compared to level walking. Based on these results, we recommend slow progression and careful monitoring of the feet for all exercises, but especially for heel raise exercises; and increases in walking speed. NWB exercises (stationary bicycling, resistance band exercises and balance ball exercises) may be recommended for people with DM+PN who have severe foot deformity or an acute ulcer, as these exercises provide greater reductions in plantar pressures than WB exercises.
Acknowledgments
This work was supported by grant funding from NIH NCMRR R21 HD058938 (Mueller). Assistance with subject recruitment was provided by grant NIH UL1 RR024992 (Mueller).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
The authors deny any conflict of interest present in this work.
Contributor Information
Kshamata M. Shah, Movement Science Program, Program in Physical Therapy, Washington University School of Medicine.
Michael J. Mueller, Washington University School of Medicine, Program in Physical Therapy and Department of Radiology.
References
- 1.Brand PW. The diabetic foot. In: Ellenberg M, Rifkin H, editors. Diabetes Mellitus: Theory and Practice. 3rd Ed Medical Examination Publishing Co Inc.; New Hyde Park, NY: 1983. pp. 829–849. [Google Scholar]
- 2.Mueller MJ, Diamond JE, Delitto A, Sinacore DR. Insensitivity, limited joint mobility, and plantar ulcers in patients with diabetes mellitus. Physical Therapy. 1989;69:453–462. doi: 10.1093/ptj/69.6.453. [DOI] [PubMed] [Google Scholar]
- 3.Veves A, Murray HJ, Young MJ, Boulton AJ. The risk of foot ulceration in diabetic patients with high foot pressure: a prospective study. Diabetologia. 1992;35(7):660–663. doi: 10.1007/BF00400259. [DOI] [PubMed] [Google Scholar]
- 4.Boulé NG, Kenny GP, Haddad E, Wells GA, Sigal RJ. Meta-analysis of the effect of structured exercise training on cardiorespiratory fitness in type 2 diabetes mellitus. Diabetologia. 2003;46:1071–1081. doi: 10.1007/s00125-003-1160-2. [DOI] [PubMed] [Google Scholar]
- 5.Castaneda C, Layne JE, Munoz-Orians L, Gordon PL, Walsmith J, Foldvari M, Roubenoff R, Tucker KL, Nelson ME. A randomized controlled trial of resistance exercise training to improve glycemic control in older adults with type 2 diabetes. Diabetes Care. 2002;25:2335–2341. doi: 10.2337/diacare.25.12.2335. [DOI] [PubMed] [Google Scholar]
- 6.Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C, White RD. Physical activity/exercise and type 2 diabetes: a consensus statement from the American Diabetes Association. Diabetes Care. 2006;29(6):1433–1438. doi: 10.2337/dc06-9910. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong DG, Lavery LA, Holtz-Neiderer K, Mohler MJ, Wendel CS, Nixon BP, Boulton AJ. Variability in activity may precede diabetic foot ulceration. Diabetes Care. 2004;27(8):1980–1984. doi: 10.2337/diacare.27.8.1980. [DOI] [PubMed] [Google Scholar]
- 8.Lemaster JW, Mueller MJ, Rieber GE, Mehr DR, Madsen RW, Conn VS. Effect of Weight-Bearing Activity on Foot Ulcer Incidence in People With Diabetic Peripheral Neuropathy: Feet First Randomized Controlled Trial. Physical Therapy. 2008;88(11):1385–1398. doi: 10.2522/ptj.20080019. [DOI] [PubMed] [Google Scholar]
- 9.Maluf KS, Mueller MJ. Comparison of physical activity and cumulative plantar tissue stress among subjects with and without diabetes mellitus and a history of recurrent plantar ulcers. Clinical Biomechanics. 2003;18(7):567–575. doi: 10.1016/s0268-0033(03)00118-9. [DOI] [PubMed] [Google Scholar]
- 10.Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, Chasan- Taber L, Albright AL, Braun B. American College of Sports Medicine; American Diabetes Association. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care. 2010;33(12):e147–e167. doi: 10.2337/dc10-9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavanagh PR, Hewitt FG, Perr JE. In-shoe plantar pressure measurement: a review. Foot. 1992;2:185–94. [Google Scholar]
- 12.Mueller MJ. Use of an in-shoe pressure measurement system in the management of patients with neuropathic ulcers or metatarsalgia. Journal of Orthopaedic & Sports Physical Therapy. 1995;21(6):328–336. doi: 10.2519/jospt.1995.21.6.318. [DOI] [PubMed] [Google Scholar]
- 13.Guldemond NA, Leffers P, Sanders AP, Schaper NC, Nieman F, Walenkamp GH. Daily-life activities and in-shoe forefoot plantar pressure in patients with diabetes. Diabetes Research and Clinical Practice. 2007;77(2):203–209. doi: 10.1016/j.diabres.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Maluf K, Morley RE, Jr, Richter EJ, Klaesner JW, Mueller MJ. Foot pressures during level walking are strongly associated with pressures during other ambulatory activities in subjects with diabetic neuropathy. Archives of Physical Medical and Rehabilitation. 2004;85(2):253–260. doi: 10.1016/j.apmr.2003.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Burnfield JM, Jorde AG, Augustin TR, Augustin TA, Bashford GR. Variations in plantar pressure variables across five cardiovascular exercises. Medicine & Science in Sports & Exercise. 2007;39(11):2012–2020. doi: 10.1249/mss.0b013e318148bdfa. [DOI] [PubMed] [Google Scholar]
- 16.Diamond JE, Mueller MJ, Delitto A, Sinacore DR. Reliability of a diabetic foot evaluation. Physical Therapy. 1989;69:797–802. doi: 10.1093/ptj/69.10.797. [DOI] [PubMed] [Google Scholar]
- 17.American Diabetes Association PS Preventive Foot Care in Diabetes. Diabetes Care. 2004;27:S63–S64. doi: 10.2337/diacare.27.2007.s63. [DOI] [PubMed] [Google Scholar]
- 18.Reiber GE, Smith DG, Wallace CM, Vath CA, Sullivan K, Hayes S, Yu O, Martin D, Maciejewski ML. Footwear used by individuals with diabetes and a history of foot ulcer. Journal of Rehabilitation Research & Development. 2002;39(5):615–622. [PubMed] [Google Scholar]
- 19.Wooldridge J, Moreno L. Evaluation of the costs to Medicare of covering therapeutic shoes for diabetic patients. Diabetes Care. 1994;17(6):541–547. doi: 10.2337/diacare.17.6.541. [DOI] [PubMed] [Google Scholar]
- 20.Mueller MJ, Strube MJ. Generalizability of in-shoe peak pressure measures using the F-scan system. Clinical Biomechanics. 1996;11(3):159–164. doi: 10.1016/0268-0033(95)00047-x. [DOI] [PubMed] [Google Scholar]
- 21.Pitei DL, Lord M, Foster A, Wilson S, Watkins PJ, Edmonds ME. Plantar pressures are elevated in the neuroischemic and the neuropathic diabetic foot. Diabetes Care. 1999;22(12):1966–1970. doi: 10.2337/diacare.22.12.1966. [DOI] [PubMed] [Google Scholar]
- 22.Rosenthal R, Rosnow RL. Essentials of Behavioral Research: Methods and Data Analysis. Vol. 478. New York: McGraw-Hill Book Company; New York: 1984. [Google Scholar]
- 23.Morag E, Cavanagh PR. Structural and functional predictors of regional peak pressures under the foot during walking. Journal of Biomechanics. 1999;32(4):359–370. doi: 10.1016/s0021-9290(98)00188-2. [DOI] [PubMed] [Google Scholar]
- 24.Mueller MJ, Minor SD, Sahrmann SA, Schaaf JA, Strube MJ. Differences in the gait characteristics of patients with diabetes and peripheral neuropathy compared with age-matched controls. Physical Therapy. 1994;74(4):299–313. doi: 10.1093/ptj/74.4.299. [DOI] [PubMed] [Google Scholar]
- 25.Song CH, Petrofsky JS, Lee SW, Lee KJ, Yim JE. Effects of an exercise program on balance and trunk proprioception in older adults with diabetic neuropathies. Diabetes Technology & Therapeutics. 2011;13(8):803–811. doi: 10.1089/dia.2011.0036. [DOI] [PubMed] [Google Scholar]
- 26.Gregg EW, Beckles GL, Williamson DF, Leveille SG, Langlois JA, Engelgau MM, Narayan KM. Diabetes and physical disability among older U.S. adults. Diabetes Care. 2000;23(9):1272–1277. doi: 10.2337/diacare.23.9.1272. [DOI] [PubMed] [Google Scholar]
- 27.Abboud RJ, Rowley DI, Newton RW. Lower limb muscle dysfunction may contribute to foot ulceration in diabetic patients. Clinical Biomechanics. 2000;15(1):37–45. doi: 10.1016/s0268-0033(99)00038-8. [DOI] [PubMed] [Google Scholar]
- 28.Rozema A, Ulbrecht JS, Pammer SE, Cavanagh PR. In-shoe plantar pressures during activities of daily living: implications for therapeutic footwear design. Foot & Ankle International. 1996;17(6):352–359. doi: 10.1177/107110079601700611. [DOI] [PubMed] [Google Scholar]
- 29.Mao DW, Li JX, Hong Y. The duration and plantar pressure distribution during one- leg stance in Tai Chi exercise. Clinical Biomechanics. 2006;21(6):640–645. doi: 10.1016/j.clinbiomech.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Nardone A, Grasso M, Schieppati M. Balance control in peripheral neuropathy: are patients equally unstable under static and dynamic conditions? Gait & Posture. 2006;23(3):364–373. doi: 10.1016/j.gaitpost.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Wallace C, Reiber GE, LeMaster JW, Smith DG, Sullivan K, Hayes S, Vath C. Incidence of falls, risk factors for falls, and fall-related fractures in individuals with diabetes and a prior foot ulcer. Diabetes Care. 2002;25(11):1983–1986. doi: 10.2337/diacare.25.11.1983. [DOI] [PubMed] [Google Scholar]
- 32.Richardson JK, Ashton-Miller JA, Lee SG, Jacobs K. Moderate Peripheral Neuropathy Impairs and Unipedal Balance in the Elderly. Archives of Physical Medicine and Rehabilitation. 1996;77:1152–1156. doi: 10.1016/s0003-9993(96)90139-2. [DOI] [PubMed] [Google Scholar]
- 33.Morrison S, Colberg SR, Mariano M, Parson HK, Vinik AI. Balance Training Reduces Falls Risk in Older Individuals With Type 2 Diabetes. Diabetes Care. 2010;33(4):748–750. doi: 10.2337/dc09-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richardson JK, Sandman D, Vela S. A focused exercise regimen improves clinical measures of balance in patients with peripheral neuropathy. Archives of Physical Medicine and Rehabilitation. 2001;82(2):205–209. doi: 10.1053/apmr.2001.19742. [DOI] [PubMed] [Google Scholar]
- 35.Mueller MJ, Maluf KS. Tissue adaptation to physical stress: a proposed “Physical Stress Theory” to guide physical therapist practice, education, and research. Physical Therapy. 2002;82(4):383–403. [PubMed] [Google Scholar]
- 36.Rao S, Saltzman CL, Yack HJ. Relationships between segmental foot mobility and plantar loading in individuals with and without diabetes and neuropathy. Gait & Posture. 2010;31(2):251–255. doi: 10.1016/j.gaitpost.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]