Summary
In the context of tendon and ligament repair, mechanical loading and the presence of joint synovial fluid are known to profoundly influence the form and function of the repair tissue and potentially the host response to biomaterials. Previously we demonstrated that a xenograft extra cellular matrix (ECM) scaffold implanted in the rat shoulder elicited a unique host response from that seen in the body wall. However, the host response to xenografts implanted in shoulders with a tendon/capsule injury was not different from xenografts implanted in shoulders with no injury. In the current study, we hypothesized that varying clinically relevant surgical and environmental factors would introduce significant differences in host response to xenograft implantation at the shoulder. Contrary to our hypothesis, we found no significant differences in host response between any shoulder implantation conditions or between shoulder and body wall implantation in the rat model. These findings suggest that there is no advantage to using an orthotopic shoulder model to investigate the host response to rotator cuff scaffold materials in the rat model, and due to the insensitivity of its host response to various clinically relevant surgical conditions, may suggest that the rat does not provide a surrogate for directly translating the host response to biomaterials to the human application.
Keywords: fascia lata ECM, host response, shoulder, gene expression, linear regression of efficiency
Introduction
Via their natural composition, 3D structure, and/or remodeling byproducts, ECM scaffolds are believed to provide a chemically and structurally instructive environment for host cells, thus improving the biology of repair healing1–3. The host response and remodeling of ECM scaffolds were investigated using subcutaneous 4–7 or abdominal wall 8–11 implantation models. Although these evaluations did not necessarily take place at the intended site of clinical application, it is assumed that they allowed for fundamental evaluation of material-tissue interactions. In the context of tendon and ligament repair, however, mechanical loading 12–14 and the presence of joint synovial fluid15, 16 are known to profoundly influence the form and function of the repair tissue and potentially the host response to biomaterials. While ECM scaffolds are used for tendon and ligament repair in human patients17, 18, the host response and remodeling of ECM scaffolds have only been minimally investigated in tendon and ligament sites.19–22
In our previous study22, we compared the host response to a xenograft ECM scaffold among various implantation sites and injury conditions in a rat model, examining the expression of several pro-inflammatory and pro-remodeling genes identified in previous musculoskeletal injury and treatment models4, 8, 23–27. We showed that at 28 days a xenograft implanted in the rat shoulder elicited a unique host response from that seen in the body wall22. However, we were unable to demonstrate an expected difference in the host response to xenografts implanted in shoulders with a tendon/capsule injury compared to shoulders with no injury. Therefore, we questioned whether the difference in host response between the shoulder and body wall was the result of artifact introduced by the surgical procedures used for the repairs. For example, the shoulder grafts were about twice the size of the capsule and tendon defect, thus differences in host response due to the presence of the injury may have been masked. Further, portions of the grafts that were outside of the suture attachment point and thus incapable of experiencing any tensile loads were included in the gene analysis. As well, the humeral bone tunnel was disproportionately larger than in a human undergoing rotator cuff repair; therefore, any influence of bone healing on the host response to the graft have may been exaggerated22.
Hence, the primary objective of the current study was to assess if more clinically relevant surgical factors influence the host response to xenograft implantation in the shoulder (e.g., bony fixation, mechanical load, and/or synovial fluid). We also examined the extent to which environmental factors (e.g., subcutaneous fascia defect vs. muscle pocket implantation site) influence the host response to xenograft implantation in the body wall. Finally, we investigated whether the more clinically relevant xenograft implantation in shoulders elicits a unique host response from that seen in the body wall as shown previously22. We hypothesized that varying clinically relevant surgical and environmental factors will introduce significant differences in host response to xenograft implantation at the shoulder and at the body wall. We further hypothesized that the host response to xenograft implantation will be significantly different between the shoulder and body wall, for at least some of the surgical conditions investigated.
Methods
Study Design
16 male Lewis rats (retired breeders, > 400 g) were used. Each rat underwent xenograft implantation in both shoulders. A full factorial study design was used for the 4 shoulder surgical models (Table 1, n = 8/experimental group). 11 of the rats were also randomly assigned to receive two xenograft implants in the lumbar region (Table 1). Rats were euthanized at 28 days, and samples harvested and analyzed for the expression of 16 host response genes and 10 matrix-related genes (Table 2).
Table 1.
Study design: The 6 experimental groups and their distribution among animals and anatomic sites. The distribution of the xenograft sourced from two human donors is also denoted.
| Rat # | Fascia Donor | Experimental Group | |||
|---|---|---|---|---|---|
| Right Shoulder | Left Shoulder | Right Lumbar | Left Lumbar | ||
| 1 | A | 3 | 4 | 5 | 6 |
| 2 | A | 1 | 4 | 6 | 5 |
| 3 | B | 4 | 4 | 5 | 6 |
| 4 | B | 4 | 3 | 5 | 6 |
| 5 | B | 1 | 2 | na | na |
| 6 | A | 1 | 3 | na | na |
| 7 | A | 4 | 2 | na | na |
| 8 | B | 3 | 3 | 6 | 5 |
| 9 | B | 2 | 3 | na | na |
| 10 | A | 2 | 2 | 6 | 5 |
| 11 | A | 1 | 1 | 5 | 6 |
| 12 | B | 3 | 2 | 6 | 5 |
| 13 | B | 2 | 4 | 5 | 6 |
| 14 | A | 3 | 3 | na | na |
| 15 | A | 4 | 1 | 6 | 5 |
| 16 | B | 2 | 1 | 6 | 5 |
Experimental Group:
No capsule/tendon injury, bone tunnel, tension
No capsule/tendon injury, bone anchor, tension
Capsule/tendon injury, bone anchor, tension
Capsule/tendon injury, bone anchor, no tension
Lumbar fascia defect, overlay
Lumbar muscle pocket
Table 2.
Gene expression current study: Normalized copy number, ln transformed, mean and std dev for the 26 genes compared between shoulder and body wall implantation sites at 28 days.
| GENE | 28 Day Gene Expression | |||
|---|---|---|---|---|
| Category | Name | Abbr. | Shoulder Xenograft Mean (SD) |
Body Wall Xenograft Mean (SD) |
| Pro-inflammatory | Interleukin-1α | IL-1α | −8.66(4.51) | −9.50(4.34) |
| Interleukin-1β | IL-1β | −6.93(3.49) | −7.24(4.15) | |
| Interleukin-6 | IL-6 | −11.12(3.61) | −12.11(3.40) | |
| Interleukin-8 | IL-8 | −13.99(3.40) | −15.13(2.41) | |
| Interleukin-12β | IL-12β | −10.25(3.08) | −9.90(3.02) | |
| Interleukin-18 | IL-18 | −7.02(4.55) | −6.80(4.16) | |
| Interferon-γ | INFγ | −13.19(4.25) | −12.78(6.32) | |
| inducible Nitric Oxide Synthase | iNOS | −6.81(3.30) | −6.51(3.48) | |
| Tumor Necrosis Factor-α | TNFα | −10.13(4.86) | −9.73(2.58) | |
| Pro-remodeling | Arginase | ARG | −13.96(3.12) | −14.11(3.07) |
| Interleukin-1 receptor antagonist | IL-1ra | −5.52(2.86) | −4.85(3.64) | |
| Interleukin-4 | IL-4 | −12.83(4.11) | −12.97(4.29) | |
| Interleukin-5 | IL-5 | −18.21(4.80) | −17.06(3.87) | |
| Interleukin-10 | IL-10 | −12.18(3.23) | −12.24(2.86) | |
| Interleukin-13 | IL-13 | −13.00(3.32) | −12.58(3.40) | |
| Transforming Growth Factor-β | TGF-β | −6.89(3.54) | −7.01(2.83) | |
| ECM/Tendon | Decorin | DEC | −3.40(3.96) | −3.50(3.87) |
| Collagen Type I | Col I | 1.18(3.72) | 0.15(3.92) | |
| Collagen Type III | Col III | −8.09(3.84) | −7.95(3.35) | |
| Thrombospondin-4 | TSP4 | −11.42(3.27) | −11.27(3.12) | |
| Scleraxis | SCX | −10.36(3.99) | −11.54(4.36) | |
| Tenomodulin | TND | −7.51(5.20) | −8.44(5.30) | |
| Enzyme | Cathepsin K | Cat K | −6.33(3.87) | −4.85(4.54) |
| Matrix Metalloproteinase-2 | MMP2 | −10.37(4.26) | −10.55(4.49) | |
| Matrix Metalloproteinase-3 | MMP3 | −6.95(3.97) | −7.07(3.96) | |
| Matrix Metalloproteinase-13 | MMP13 | −5.59(2.93) | −5.74(3.81) | |
Human Fascia Xenografts
54 fascia lata implants (0.5 × 0.5 cm) were obtained from 2 human donors (Table 1). The fascia was processed similarly to the antibiotic only treatment method (“ABX”) described previously, in which processed fascia lata demonstrated minimal genetic material and minimal inter-donor variability in biochemical or mechanical properties28. All grafts within a given rat were derived from fascia from the same human donor.
Surgical Procedure
Surgical procedures were approved by the IACUC at the Cleveland Clinic. For bilateral shoulder implantation, via a posterior skin incision and following acromionectomy, a fascia xenograft was attached to the supraspinatus and infraspinatus at the myotendinous junction with two 5-0 Prolene (Ethicon Inc., Somerville, NJ) mattress sutures. In the bone tunnel, no injury group (Table 1, Group 1), the graft was tied to the humerus via a transosseous tunnel with a 5-0 Prolene mattress suture22. In shoulders designated for the capsule/tendon defect (Table 1, Groups 3, 4), the supraspinatus and infraspinatus tendons were released from the humerus and both the tendons and underlying capsule were excised. In the bone anchor groups (Table 1, Groups 2, 3, 4), a customized stainless steel eye bolt (T2M-3005, Scale Hardware, Ft. Lauderdale, FL) was inserted into the humerus just lateral to the apex of the tendons’ insertion sites, and the xenograft was tied through the eyelet of the bolt with a 5-0 Prolene mattress suture (Fig. 1). In shoulders designated for no tension (Table 1, Group 4), the 5-0 Prolene suture was run through the islet of the anchor, and tied with ~5 to 7 mm additional length. In all shoulders, the overlying muscles were approximated with single figure of eight 4-0 Vicryl (Ethicon Inc) suture and the skin closed with staples.
Figure 1.
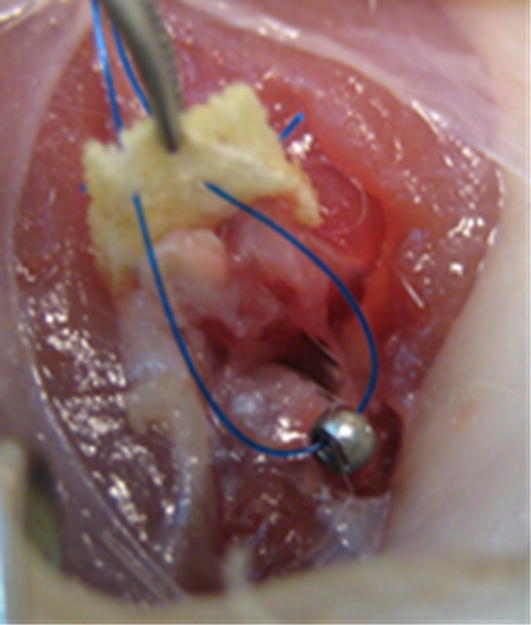
Bone anchor: For Groups 2, 3, 4 (Table 1), a customized stainless steel eye bolt (T2M-3005, Scale Hardware, Ft. Lauderdale, FL) was inserted into the humerus just lateral to the apex of the tendons’ insertion sites, and the xenograft was tied through the eyelet of the bolt with a 5-0 Prolene mattress suture.
For lumbar wall implantation, a single 3 cm dorsal midline incision was made in the skin, and the areolar layer was detached from the spinal processes. For the subcutaneous fascia defect with overlay implantation (Table 1, Group 5), a full-thickness 0.5 × 0.5 cm fascial defect was created in the lumbar fascia without violating the underlying paraspinal muscles. A fascia xenograft was secured into the defect at its corners using 5-0 Prolene simple sutures. For muscle pocket implantation (Table 1, Group 6), an incision was made in the lumbar fascia on the contralateral side. The underlying muscle layers were bluntly dissected, and a fascia xenograft was laid flat into the muscle pocket and sutured to the overlying paraspinal muscles with a 5-0 Prolene mattress suture. The lumbar fascia was then closed with two simple interrupted 5-0 Prolene sutures. The aereolar layer was subsequently closed with continuous 4-0 Vicryl suture, and the skin closed with staples.
Rats were euthanized (n=16) at 28 days, and the xenografts harvested and placed immediately in Trizol (Ambion, Carlsbad CA) at 4°C for gene expression analysis.
RNA Harvest and RT-PCR
Tissue samples were processed as described previously22, 29. All genes were measured in triplicate using SYBR® GreenER™ qPCR SuperMix (ABI, Foster City, CA) on a ABI-7500 Real Time PCR System (ABI). The primer sets for all experimental genes were published previously22. We utilized 18S as a housekeeping gene (Forward primer: GCTGGAATTACCGCGGCGGCTGCTG; Reverse primer: CGGCTACCACATCCAAGGAAGGCA). A universal quantitation standard, genomic lambda DNA (New England Biolabs Inc., Ipswich, MA) (Forward primer: TACCGGCGCGACGATGATGC; Reverse primer: GGTCCCGGCACCTTTCACCG) was included on each plate.
The Linear Regression of Efficiency (LRE) method uses the 4-parameter sigmoid function to model PCR amplification. Then based on the linear relationship between amplification rate and amplicon quantity, quantification is accomplished using linear regression analysis. Using a universal standard (lambda genomic DNA) to calculate an optical calibration factor allows for calculation of absolute copy number without the need for standard curves30–32.
Individual amplification curves were analyzed via the LRE analysis method30–32 using a custom MatLab code (http://www.mathworks.com/matlabcentral/fileexchange/35174-lre-analysis-of-real-time-pcr-data) to calculate copy number as follows. For every amplification curve, the cycle fluorescence (FC) values were used to calculate the efficiency at each cycle (EC) according to the equation: . An LRE window was then defined, to start in the linear phase of the amplification curve and end where the plot of cycle efficiency versus cycle fluorescence was no longer linear (r2 < 0.85). For each point within the LRE window, the fluorescence at cycle zero (F0) was predicted with a kinetic-based sigmoid model31 and then averaged to give a F0 for the curve. The F0 for each genomic Lambda DNA curve, its quantity (0.1 nanograms per reaction), and the amplicon size (AS), were used to calculate an optical calibration factor (OCF) for each curve using the equation: and the triplicates averaged to give an OCF for the plate. The F0 for each experimental curve was then converted to copy number using the plate OCF, and the triplicates averaged to give a sample copy number. Samples with undetectable gene expression were assigned a copy number 10-fold lower than the lowest measured value for a given gene. All sample copy numbers were divided by their respective 18S copy number to give a normalized copy number for each sample.
Statistical Analysis
Normalized copy numbers were natural log (ln) transformed to correct for the skew of their distributions. To account for the rat-to-rat variability, a two factor ANOVA was used with rat as a random factor. A Bonferroni adjustment for multiple comparison was made such that a p-value of 0.002 (0.05/26 genes evaluated) for the gene expression analysis was considered significant. Where ANOVA showed significant differences (i.e., p < 0.002), a Tukey HSD post hoc test (p = 0.05) was used to identify differences between experimental groups. JMP Pro 9 software (SAS Institute Inc.) was used for statistical analysis.
Results
Current Study
All animals tolerated the surgeries well and recovered without complication. At 28 days, no significant differences were detected in the expression of any gene between any of the shoulder implantation models: (1) no injury, bone tunnel, tension, (2) no injury, bone anchor, tension, (3) injury, bone anchor, tension, and (4) injury, bone anchor, no tension. Similarly, no significant differences were found between the body wall implantation models: (5) lumbar fascia defect, overlay and (6) lumbar muscle pocket. Data were therefore reduced to two groups: shoulder xenografts (1, 2, 3, and 4) and body wall xenografts (5 and 6). No significant differences were found in the expression of any pro-inflammatory, pro-remodeling, ECM/tendon or ECM catabolic gene between the shoulder xenografts and body wall xenografts (Table 2).
Comparison to Previous Study
The findings of this study contradict those of our previous study where xenograft implantation in the rat shoulder elicited a unique host response from that seen in the body wall at 28 days22. Hence we reanalyzed the gene expression data from the previous study according to the LRE method to allow for direct comparison to this study. An average of the optical calibration factors from all plates in the current study (n = 55) was used in the re-analysis. Only where significant differences in the re-analyzed data existed was the gene expression data plotted along with the corresponding data from the current study (Fig. 2).
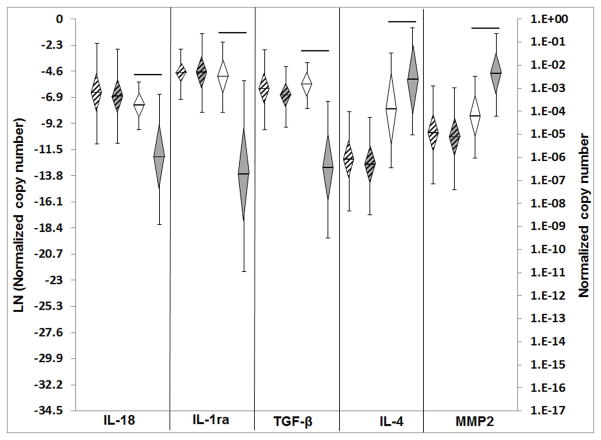
Figure 2.
Gene expression current study versus reanalysis of previous study22: Normalized copy number, ln transformed, plotted as mean, error bars = std dev, diamond = 95% confidence interval. White diamonds with strips – shoulder xenografts current study (n=32), grey diamonds with strips – body wall xenografts current study (n=32), white diamonds without strips shoulder xenografts previous study (n=16), grey diamonds without stripes – body wall xenografts previous study (n=16). Significant differences between groups are denoted by the solid horizontal lines.
In the previous study, 6 of 9 pro-inflammatory genes (IL-1α, IL-1β, IL-6, IL-18, iNOS, TNFα) demonstrated significantly higher expression levels in the shoulder xenografts than in the body wall xenografts22. Upon LRE reanalysis, IL-18 was the only pro-inflammatory gene whose expression remained significantly higher in the shoulder xenografts than the body wall xenografts (Fig. 2).
In the previous study, 3 of 7 pro-remodeling genes demonstrated significantly different expression levels in the shoulder xenografts than in the body wall xenografts (IL-1ra and TGFβ higher, and IL-5 lower)22. Upon LRE analysis, the expression of two of these (IL-1ra, TGFβ) remained significantly higher in shoulder xenografts than in the body wall xenografts (Fig. 1). IL-5 expression levels were no longer different; however, upon reanalysis IL-4 was found to be expressed significantly lower in shoulder xenografts than in body wall xenografts (Fig. 2).
In the previous study, 2 of 6 ECM/tendon genes (SCX, TNMD) demonstrated significantly higher expression levels in the shoulder xenografts than in the body wall xenografts22. Upon LRE analysis, no differences in ECM/tendon gene expression were found between the shoulder xenografts and the body wall xenografts.
In the previous study, 2 of 4 ECM catabolic genes (Cat K, MMP3) demonstrated significantly higher expression levels in the shoulder xenografts than in the body wall xenografts22. Upon LRE analysis, neither of these genes had expression levels significantly different between the shoulder xenografts and the body wall xenografts, however, another ECM catabolic gene (MMP2) was found to be significantly lower in the shoulder xenografts than in the body wall xenografts (Fig. 2).
Discussion
In our previous work with this animal model, we did not detect an expected difference in the host response to xenografts implanted in shoulders with a tendon/capsule injury compared to shoulders with no injury. We questioned whether these findings were the result of artifact introduced by the surgical procedures and designed the current study to assess if more clinically relevant surgical and environmental factors would influence the host response to xenograft implantation in the shoulder or the body wall. Contrary to our hypothesis, variations in clinically relevant surgical factors in the shoulder (e.g., bony fixation, mechanical load, and/or synovial fluid) or body wall (e.g., subcutaneous or muscle surrounding tissue) did not result in differences in host response at these sites for any of the genes evaluated. Furthermore, no differences in host response to xenograft implantation were found between the shoulder and body wall sites, even though 14 of 26 genes were found to be different between shoulder and body wall previously22.
Because differences in host response were observed between the shoulder and body wall sites in the previous study and not in the current study, we reanalyzed the previous gene expression data according to the methods of the current study in an attempt to reconcile the disparate findings. The LRE method calculates the reaction efficiency for each amplification curve individually. Thus, unlike the previously used standard curve method, LRE accounts for PCR efficiency differences between samples, or between samples and the standard curve itself. Furthermore, the LRE method allows for detection of gene expression values at high cycle numbers that fall below the standard curve and are considered undetectable using the standard curve method. Specifically, 347 of 792 analyses were defined as undetectable previously whereas only 117 of 792 analyses were assigned an undetectable gene expression value upon reanalysis by LRE. Having far fewer undetected values after reanalysis reduced skew and increased the mean gene expression level for several genes. Further, to match the current study, only the shoulder and body wall groups from the previous study (two groups) were statistically compared in the ANOVA. The consequence of both LRE and statistical reanalysis of the previous data reduced the number of significantly different genes between shoulder and body wall from 14 of 26 in the previous study to 5 of 26 after reanalysis.
Reanalysis of the previous data brings the findings of the previous and current studies into much greater agreement. The 5 genes that remain different between shoulder and body wall from the previous study even after reanalysis (IL-18, IL-1ra, TGFβ, Il-4 and MMP2) may represent real differences between the sites unique to the previous study. For instance, in the previous study only, the amount of suture used for fixation relative to the size of the graft was less for body wall xenografts than for shoulder xenografts, possibly explaining the modest differences in gene expression profile between the two sites. Because many cytokines are temporally expressed4, 8, 23–27, interpretation of these gene expression differences would require repeating the study under the same conditions with various time points, prospective LRE analysis, and additional histologic, immunologic and functional outcome measures. However, our confidence in the results from the current study that showed no differences in the expression of any gene between the shoulder and body wall xenografts is strengthened by noting that even if adjustment for multiple comparisons was not made and a p-value of 0.05 was used for the ANOVA, there were still no significant differences between shoulder and body wall sites for any of the 26 genes analyzed. Hence the reanalyzed previous data taken together with the current data lead us to conclude that differences in host response to xenograft implantation between the shoulder and body wall are negligible in the rat model utilized in this study.
This study demonstrates some of the challenges of real time PCR analysis and underscores the need to understand the assumptions and sensitivity of the analysis methods used. We demonstrate here how analysis of the same PCR data using standard curves and LRE yields different results, likely because the LRE method does not rely on any assumptions of reaction efficiency and is more sensitive to detection of low expressing genes. We showed that the choice of gene expression analysis methods led to results that suggested two different conclusions: (1) shoulder sites do give different host response to xenograft implantation than body wall sites (previous study analysis methods); or (2) shoulder sites do not give different host response to xenograft implantation than body wall sites (current study analysis methods). Because we have more confidence in the accuracy of the LRE analysis method, we intend to employ the LRE analysis method for our future gene expression studies to eliminate the need for assumptions concerning the efficiency of the PCR reaction and to increase detection sensitivity.
A limitation to this study is the single time point evaluated, which did not allow for elucidating differences between implantation sites at early time points or beyond 28 days. In particular, a longer duration would allow the functional consequence of the gene expression profiles on long-term graft remodeling to be investigated. Furthermore, only a finite number of genes were examined, and no histologic, biochemical, biomechanical, or immunological findings are reported. Nonetheless, using 26 genes to examine host response to xenograft implantation at 28 days, we found that variations in clinically relevant surgical and environmental factors in the shoulder and the body wall did not result in significant gene expression differences within or between these sites in the rat model. This result was unexpected as bony fixation, mechanical load, and/or synovial fluid are known to influence the healing of a primary tendon repair back to bone12–16. Hence, the insensitivity of the rat host response to these various surgical conditions could also suggest that neither the shoulder nor the abdominal wall sites in the rat provide a model for defining the role of these surgical variables (i.e. bony fixation, mechanical load and/or synovial fluid) in the host response to biomaterials.
Acknowledgments
This work was funded by the NIH (AR056633-01). One or more of the authors receives royalties from the Musculoskeletal Transplant Foundation.
References
- 1.Badylak SE. The extracellular matrix as a scaffold for tissue reconstruction. Semin Cell Dev Biol. 2002;13:377–383. doi: 10.1016/s1084952102000940. [DOI] [PubMed] [Google Scholar]
- 2.Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009;5:1–13. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Reing JE, Zhang L, Myers-Irvin J, et al. Degradation products of extracellular matrix affect cell migration and proliferation. Tissue Eng Part A. 2009;15:605–614. doi: 10.1089/ten.tea.2007.0425. [DOI] [PubMed] [Google Scholar]
- 4.Allman AJ, McPherson TB, Badylak SF, et al. Xenogeneic extracellular matrix grafts elicit a TH2-restricted immune response. Transplantation. 2001;71:1631–1640. doi: 10.1097/00007890-200106150-00024. [DOI] [PubMed] [Google Scholar]
- 5.Kidd KR, Dal Ponte DB, Kellar RS, Williams SK. A comparative evaluation of the tissue responses associated with polymeric implants in the rat and mouse. J Biomed Mater Res. 2002;59:682–689. doi: 10.1002/jbm.10032. [DOI] [PubMed] [Google Scholar]
- 6.Laschke MW, Haufel JM, Thorlacius H, Menger MD. New experimental approach to study host tissue response to surgical mesh materials in vivo. J Biomed Mater Res A. 2005;74:696–704. doi: 10.1002/jbm.a.30371. [DOI] [PubMed] [Google Scholar]
- 7.Patil SD, Papadmitrakopoulos F, Burgess DJ. Concurrent delivery of dexamethasone and VEGF for localized inflammation control and angiogenesis. J Control Release. 2007;117:68–79. doi: 10.1016/j.jconrel.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Brown BN, Valentin JE, Stewart-Akers AM, et al. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials. 2009;30:1482–1491. doi: 10.1016/j.biomaterials.2008.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cook JL, Fox DB, Kuroki K, et al. In vitro and in vivo comparison of five biomaterials used for orthopedic soft tissue augmentation. Am J Vet Res. 2008;69:148–156. doi: 10.2460/ajvr.69.1.148. [DOI] [PubMed] [Google Scholar]
- 10.Thomsen P, Bjursten LM, Ericson LE. Implants in the abdominal wall of the rat. Scand J Plast Reconstr Surg. 1986;20:173–182. doi: 10.3109/02844318609006316. [DOI] [PubMed] [Google Scholar]
- 11.Valentin JE, Badylak JS, McCabe GP, Badylak SF. Extracellular matrix bioscaffolds for orthopaedic applications. A comparative histologic study. J Bone Joint Surg Am. 2006;88:2673–2686. doi: 10.2106/JBJS.E.01008. [DOI] [PubMed] [Google Scholar]
- 12.Gimbel JA, Van Kleunen JP, Williams GR, et al. Long durations of immobilization in the rat result in enhanced mechanical properties of the healing supraspinatus tendon insertion site. J Biomech Eng. 2007;129:400–404. doi: 10.1115/1.2721075. [DOI] [PubMed] [Google Scholar]
- 13.Thomopoulos S, Das R, Birman V, et al. Fibrocartilage tissue engineering: the role of the stress environment on cell morphology and matrix expression. Tissue Eng Part A. 2011;17:1039–1053. doi: 10.1089/ten.tea.2009.0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomopoulos S, Williams GR, Soslowsky LJ. Tendon to bone healing: Differences in biomechanical, structural, and compositional properties due to a range of activity levels. J Biomech Eng. 2003;125:106–113. doi: 10.1115/1.1536660. [DOI] [PubMed] [Google Scholar]
- 15.Amiel D, Harwood FL, Gelberman RH, et al. Autogenous intrasynovial and extrasynovial tendon grafts: an experimental study of pro alpha 1(I) collagen mRNA expression in dogs. J Orthop Res. 1995;13:459–463. doi: 10.1002/jor.1100130321. [DOI] [PubMed] [Google Scholar]
- 16.Bedi A, Kawamura S, Ying L, Rodeo SA. Differences in Tendon Graft Healing Between the Intra-articular and Extra-articular Ends of a Bone Tunnel. HSS J. 2009;5:51–57. doi: 10.1007/s11420-008-9096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barber FA, Burns JP, Deutsch A, et al. A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy. 2012;28:8–15. doi: 10.1016/j.arthro.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 18.Lee DK. A preliminary study on the effects of acellular tissue graft augmentation in acute Achilles tendon ruptures. J Foot Ankle Surg. 2008;47:8–12. doi: 10.1053/j.jfas.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 19.Dejardin LM, Arnoczky SP, Ewers BJ, et al. Tissue-engineered rotator cuff tendon using porcine small intestine submucosa - Histologic and mechanical evaluation in dogs. Am J Sports Med. 2001;29:175–184. doi: 10.1177/03635465010290021001. [DOI] [PubMed] [Google Scholar]
- 20.Schlegel TF, Hawkins RJ, Lewis CW, et al. The effects of augmentation with Swine small intestine submucosa on tendon healing under tension: histologic and mechanical evaluations in sheep. Am J Sports Med. 2006;34:275–280. doi: 10.1177/0363546505279912. [DOI] [PubMed] [Google Scholar]
- 21.Nicholson GP, Breur GJ, Van Sickle D, et al. Evaluation of a cross-linked acellular porcine dermal patch for rotator cuff repair augmentation in an ovine model. J Shoulder Elbow Surg. 2007;16:S184–190. doi: 10.1016/j.jse.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Leigh DR, Baker AR, Mesiha M, et al. Effect of implantation site and injury condition on host response to human-derived fascia lata ECM in a rat model. J Orthop Res. 2012;30:461–467. doi: 10.1002/jor.21529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kapoor M, Martel-Pelletier J, Lajeunesse D, et al. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 24.Kon T, Cho TJ, Aizawa T, et al. Expression of osteoprotegerin, receptor activator of NF-kappaB ligand (osteoprotegerin ligand) and related proinflammatory cytokines during fracture healing. J Bone Miner Res. 2001;16:1004–1014. doi: 10.1359/jbmr.2001.16.6.1004. [DOI] [PubMed] [Google Scholar]
- 25.Lange J, Sapozhnikova A, Lu C, et al. Action of IL-1beta during fracture healing. J Orthop Res. 2010;28:778–784. doi: 10.1002/jor.21061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mountziaris PM, Mikos AG. Modulation of the inflammatory response for enhanced bone tissue regeneration. Tissue Eng Part B Rev. 2008;14:179–186. doi: 10.1089/ten.teb.2008.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pape HC, Marcucio R, Humphrey C, et al. Trauma-induced inflammation and fracture healing. J Orthop Trauma. 2010;24:522–525. doi: 10.1097/BOT.0b013e3181ed1361. [DOI] [PubMed] [Google Scholar]
- 28.Derwin KA, Baker AR, Spragg RK, et al. Regional variability, processing methods, and biophysical properties of human fascia lata extracellular matrix. J Biomed Mater Res A. 2008;84:500–507. doi: 10.1002/jbm.a.31455. [DOI] [PubMed] [Google Scholar]
- 29.Leigh DR, Abreu EL, Derwin KA. Changes in gene expression of individual matrix metalloproteinases differ in response to mechanical unloading of tendon fascicles in explant culture. J Orthop Res. 2008;26:1306–1312. doi: 10.1002/jor.20650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rutledge RG. Sigmoidal curve-fitting redefines quantitative real-time PCR with the prospective of developing automated high-throughput applications. Nucleic Acids Res. 2004;32:e178. doi: 10.1093/nar/gnh177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rutledge RG, Stewart D. A kinetic-based sigmoidal model for the polymerase chain reaction and its application to high-capacity absolute quantitative real-time PCR. BMC Biotechnol. 2008;8:47. doi: 10.1186/1472-6750-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rutledge RG, Stewart D. Assessing the performance capabilities of LRE-based assays for absolute quantitative real-time PCR. PLoS One. 2010;5:e9731. doi: 10.1371/journal.pone.0009731. [DOI] [PMC free article] [PubMed] [Google Scholar]