Abstract
It is well known that uremia causes an increase in the serum anion gap; however, whether changes in the anion gap occur earlier in the course of chronic kidney disease is not known. Here we investigated whether different measures of the anion gap, as a marker of kidney function, are associated with mortality. To do this we analyzed the available laboratory data of 11,957 adults in the National Health and Nutrition Examination Survey 1999–2004 to calculate anion gap using the traditional method, or one that was albumin-adjusted, as well as a full anion gap reflecting other electrolytes. A significant elevation in the traditional anion gap was seen only with an estimated glomerular filtration rate (eGFR) less than 45 mL/min/1.73m2, whereas increases in the albumin-adjusted and full anion gap were found with eGFRs less than 60 or 90mL/min/1.73m2, respectively. Higher levels of each anion gap were associated with an increased risk of all-cause mortality after adjustment for age, gender, race/ethnicity, and eGFR. After adjustment for additional covariates including body-mass index and comorbidities, higher levels of the albumin-adjusted and full anion gap were associated with mortality (relative hazard for highest compared to the lowest quartile were 1.62 and 1.64, respectively). Thus, higher levels of anion gap are present in individuals with less advanced kidney disease than previously recognized, and are associated with increased risk of mortality. Further study is needed to identify the unmeasured anions and to determine their physiologic significance.
INTRODUCTION
It is well known that uremia causes an increase in the serum anion gap (AG) due to the accumulation of a variety of solutes. Whether changes in the AG occur earlier in the course of chronic kidney disease (CKD) has been little explored. Previous studies in persons with CKD have demonstrated an increase in the AG only with relatively advanced kidney disease.1–3 Studies of the general population have supported this view.4, 5 However, variations in the serum albumin concentration affect the AG,6 and these studies have not accounted for the hypoalbuminemia that commonly accompanies progressive kidney disease.4 Therefore, the AG may increase earlier in the course of CKD than has been previously recognized. Such changes may be of prognostic significance as higher levels of the AG have been associated with hypertension, insulin resistance, and low cardiorespiratory fitness in nationally representative populations largely free of advanced kidney disease.7–9 Calculation of the AG after accounting for electrolyte measurements that are not traditionally included, in addition to the serum albumin, could also yield a measurement with greater specificity for the accumulation of organic anions.
We hypothesized that after accounting for changes in albumin and other electrolytes: (1) higher levels of AG would be present in persons with relatively preserved glomerular filtration rate (GFR), and (2) higher AG would be associated with increased risk of mortality in individuals without advanced kidney disease. We tested these hypotheses using data from participants in the National Health and Nutrition Examination Survey (NHANES) 1999–2004.
RESULTS
Participant Characteristics
The AG was calculated in the traditional manner (traditional), after adjustment for serum albumin (albumin-adjusted), and after adjustment for serum albumin and other electrolytes (full) (depicted graphically in Figure 1). The mean levels of AG were 12.08 (standard error (SE) 0.15), 1.20 (SE 0.15), and 5.45 (SE 0.16) mEq/L for the traditional, albumin-adjusted, and full AG, respectively. Participants with higher traditional AG were more likely to have lower education and physical activity levels, more likely to have hypertension and diabetes, and had higher levels of hemoglobin, serum albumin and calcium, and lower serum bicarbonate (eTable 1). Participants with higher albumin-adjusted or full AG were older, more likely to be women, had higher BMI and lower education and physical activity levels, and were more likely to have hypertension, diabetes, cardiovascular disease (CVD), low estimated GFR (eGFR), and microalbuminuria, and had lower serum bicarbonate (eTable 2 and Table 1, respectively). Participants with higher full AG also had higher levels of hemoglobin and lower serum albumin and phosphate. Each 1 mEq/L increment in the traditional, albumin-adjusted, and full AG was associated with a 0.42 (95% CI 0.38–0.47), 0.47 (95% CI 0.43–0.51), and 0.46 (95% CI 0.41–0.50) mEq/L lower serum bicarbonate, respectively.
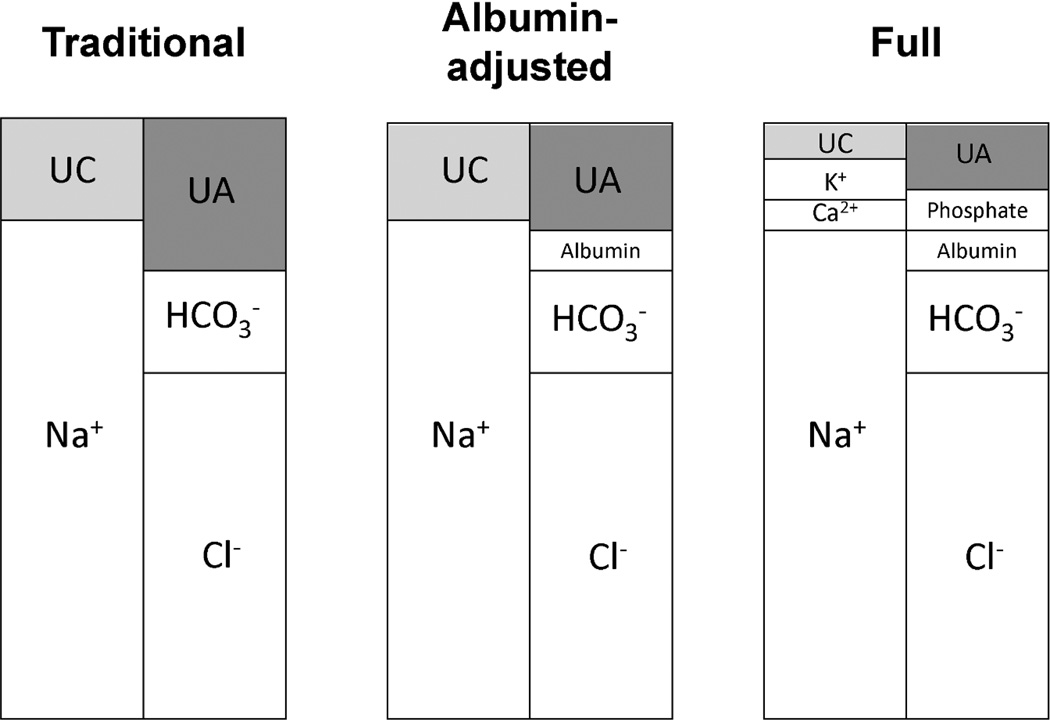
Figure 1.
Graphical depiction of components of the anion gap (AG) in each version of the calculation. Anion gap = Unmeasured anions (UA) – Unmeasured cations (UC). UA and UC are determined by the anions and cations that are accounted for in the calculation. Each panel depicts the components included in each AG calculation. The following definitions were used: Traditional AG=serum sodium(mEq/L) − (serum chloride(mEq/L) + serum bicarbonate(mEq/L)); Albumin-adjusted AG=Traditional AG − (2.5 × serum albumin(g/dL)); Full AG=Albumin-adjusted AG + serum potassium(mEq/L) + ionized calcium(mEq/L) − serum phosphate(mEq/L). The traditional AG calculation includes only Na+, Cl−, and HCO3−. As additional anions and cations are accounted for in the calculation of the albumin-adjusted and full AG, the unmeasured components (UA and UC) become smaller.
Table 1.
Participant Characteristics by Quartiles of Full Anion Gap in 11,957 participants of NHANES 1999–2004
| Full Anion Gap (mEq/L) | |||||
|---|---|---|---|---|---|
| Characteristic | < 3.62 | 3.62 – 5.35 | 5.36 – 7.23 | > 7.23 | P |
| Number | 2783 | 3044 | 3033 | 3097 | |
| Age (years) | 44.1 (0.3) | 46.1 (0.5) | 46.6 (0.5) | 47.9 (0.5) | <0.001 |
| Women (%) | 47.1 (1.0) | 51.4 (1.3) | 51.9 (1.0) | 52.6 (1.0) | <0.001 |
| Race/Ethnicity (%) | 0.46 | ||||
| Non-Hispanic White | 72.6 (2.1) | 72.5 (2.0) | 71.9 (2.4) | 72.6 (2.7) | |
| Mexican American | 7.8 (1.1) | 7.7 (1.1) | 7.0 (1.1) | 6.5 (1.3) | |
| Non-Hispanic Black | 9.6 (1.1) | 11.7 (1.2) | 10.1 (1.0) | 9.3 (1.8) | |
| Body-mass index (kg/m2) | <0.001 | ||||
| < 20 | 5.8 (0.5) | 5.3 (0.6) | 5.0 (0.6) | 5.1 (0.5) | |
| 20 – 25 | 34.2 (1.3) | 29.6 (0.9) | 28.7 (1.1) | 24.0 (1.1) | |
| 25 – 29 | 36.0 (0.9) | 34.2 (1.2) | 35.2 (1.4) | 34.0 (1.0) | |
| 30 – 35 | 15.7 (0.7) | 19.7 (1.0) | 18.5 (0.8) | 20.1 (0.8) | |
| > 35 | 8.2 (0.7) | 11.3 (0.9) | 12.5 (0.9) | 16.7 (0.9) | |
| Less than high-school diploma (%) | 16.6 (1.2) | 18.5 (0.9) | 22.0 (1.0) | 23.1 (1.2) | <0.001 |
| Activity Level (MET-min/wk, %) | <0.001 | ||||
| 0 | 12.7 (0.8) | 15.0 (0.8) | 17.8 (0.9) | 20.7 (1.7) | |
| < 500 | 20.8 (1.1) | 22.8 (0.8) | 20.1 (1.2) | 19.9 (0.9) | |
| 500 – 2000 | 37.0 (1.1) | 37.3 (0.9) | 34.3 (1.1) | 32.0 (1.3) | |
| > 2000 | 29.5 (1.2) | 24.9 (1.2) | 27.9 (1.3) | 27.4 (1.6) | |
| Smoking (%) | 0.13 | ||||
| Never | 53.1 (1.1) | 50.4 (1.2) | 47.6 (1.3) | 48.6 (2.4) | |
| Former | 24.5 (1.1) | 24.3 (0.9) | 26.0 (1.1) | 26.0 (1.4) | |
| Current | 22.4 (1.3) | 25.3 (1.1) | 26.4 (1.2) | 25.4 (1.4) | |
| Hypertension (%) | 32.6 (1.3) | 39.3 (1.3) | 41.5 (1.2) | 47.2 (1.6) | <0.001 |
| Diabetes mellitus (%) | 4.0 (0.5) | 6.4 (0.7) | 8.2 (0.6) | 9.9 (0.7) | <0.001 |
| Cardiovascular disease (%) | 5.3 (0.6) | 8.5 (0.8) | 9.2 (0.7) | 10.7 (0.7) | <0.001 |
| eGFR (mL/min/1.73m2) | <0.001 | ||||
| 15 – 59 | 3.7 (0.4) | 5.7 (0.6) | 6.7 (0.5) | 9.2 (0.6) | |
| 60 – 89 | 34.9 (1.4) | 34.7 (1.4) | 35.9 (1.2) | 35.4 (0.9) | |
| 90 – 119 | 50.7 (1.3) | 49.4 (1.5) | 48.0 (1.3) | 46.7 (1.2) | |
| ≥ 120 | 10.6 (0.8) | 10.2 (0.7) | 9.4 (0.7) | 8.8 (0.8) | |
| UACR > 30 mg/g (%) | 5.8 (0.6) | 8.7 (0.7) | 9.6 (0.6) | 12.9 (0.8) | <0.001 |
| Hemoglobin (g/dL) | 14.49 (0.05) | 14.44 (0.06) | 14.55 (0.05) | 14.64 (0.07) | 0.02 |
| Serum bicarbonate (mEq/L) | 25.5 (0.1) | 24.6 (0.1) | 23.8 (0.1) | 22.6 (0.1) | <0.001 |
| Serum albumin (g/dL) | 4.40 (0.01) | 4.32 (0.01) | 4.34 (0.01) | 4.34 (0.01) | 0.03 |
| Serum calcium (mg/dL) | 9.49 (0.02) | 9.49 (0.01) | 9.47 (0.02) | 9.49 (0.02) | 0.61 |
| Serum phosphate (mg/dL) | 3.70 (0.02) | 3.71 (0.01) | 3.63 (0.02) | 3.59 (0.02) | <0.001 |
Abbreviations: MET, metabolic equivalent; eGFR, estimated glomerular filtration rate; UACR, urinary albumin-creatinine ratio.
Data are expressed as mean (SE) or percent (SE).
Association of Anion Gap with Glomerular Filtration Rate
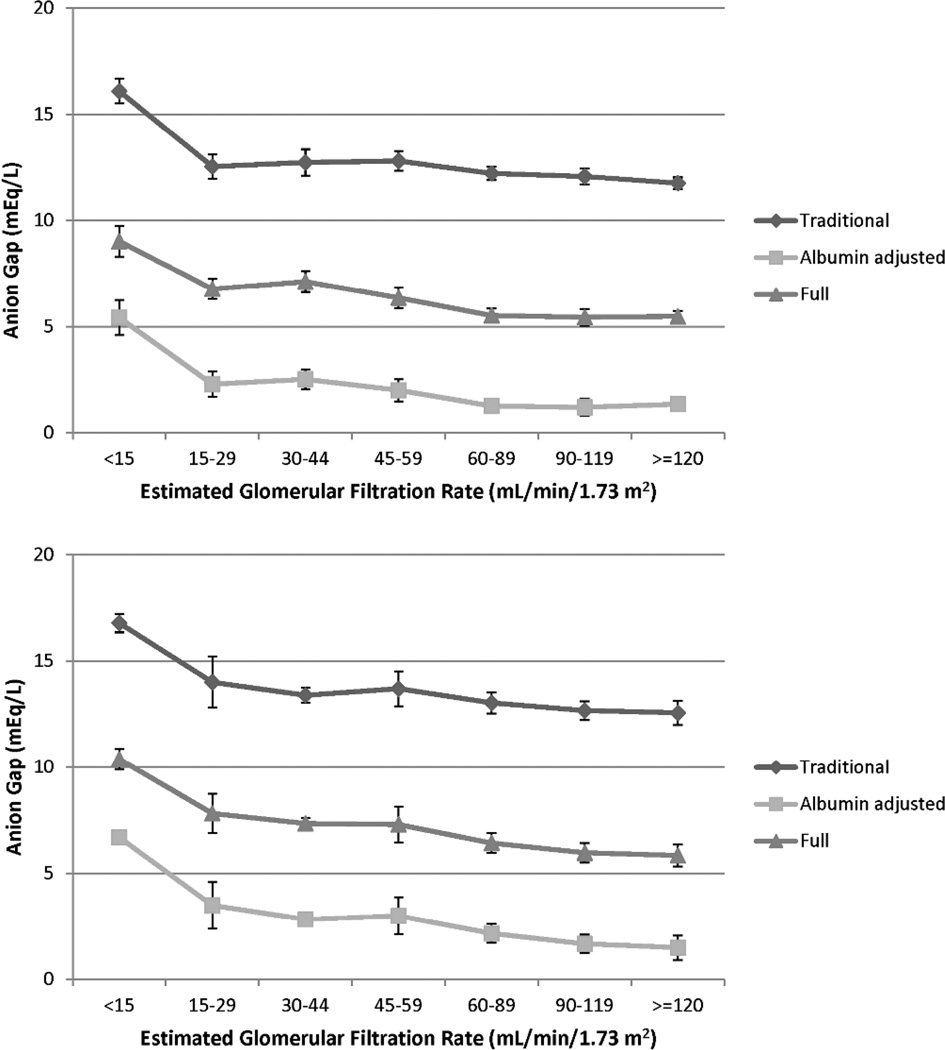
Compared to participants with Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) –defined eGFR 90–119 mL/min/1.73m2, the traditional AG was significantly elevated only among those with eGFR 30–44 mL/min/1.73m2 (Table 2, upper panel). Using the albumin-adjusted and full definitions, there was a graded rise in AG across eGFR categories beginning with eGFR 45–59 and 60–89 mL/min/1.73m2, respectively. When eGFRcys was calculated using cystatin C levels in 4,132 participants, there was a graded rise in AG with lower eGFRcys for all 3 AG definitions (Table 2, lower panel). Compared to participants with eGFRcys 90–119 mL/min/1.73m2, higher AG was seen beginning with eGFRcys 30–44 mL/min/1.73m2 for the traditional AG and beginning with eGFRcys 60–89 mL/min/1.73m2 for the albumin-adjusted and full AG. A similar pattern was seen for the association of age-adjusted AG with eGFR and eGFRcys categories (Figure 2).
Table 2.
Levels of Anion Gap by Categories of CKD-EPI and Cystatin C-based eGFR in participants of NHANES 1999–2004
| Anion gap by CKD-EPI eGFR category in 11,957 participants | ||||||
|---|---|---|---|---|---|---|
| Traditional Anion Gap (mEq/L) | Albumin-adjusted Anion Gap (mEq/L) |
Full Anion Gap (mEq/L) | ||||
| Mean (SE) | Change | Mean (SE) | Change | Mean (SE) | Change | |
| eGFR | ||||||
| ≥ 120 (n=1330) | 12.05 (0.16) | −0.03 (−0.28 to 0.22) | 1.14 (0.16) | 0.005 (−0.25 to 0.26) | 5.24 (0.16) | −0.11 (−0.37 to 0.15) |
| 90 – 119 (n=5288) | 12.08 (0.17) | ref | 1.13 (0.17) | ref | 5.35 (0.17) | ref |
| 60 – 89 (n=4210) | 12.04 (0.15) | −0.04 (−0.17 to 0.10) | 1.20 (0.15) | 0.07 (−0.06 to 0.20) | 5.49 (0.16) | 0.14 (0.01 to 0.27) |
| 45 – 59 (n=792) | 12.21 (0.18) | 0.13 (−0.16 to 0.42) | 1.67 (0.17) | 0.54 (0.27 to 0.81) | 6.07 (0.18) | 0.72 (0.43 to 1.00) |
| 30 – 44 (n=259) | 12.65 (0.24) | 0.56 (0.28 to 0.85) | 2.29 (0.25) | 1.15 (0.87 to 1.43) | 6.78 (0.25) | 1.43 (1.15 to 1.70) |
| 15 – 29 (n=78) | 12.17 (0.26) | 0.09 (−0.46 to 0.63) | 2.02 (0.33) | 0.89 (0.25 to 1.53) | 6.55 (0.30) | 1.20 (0.63 to 1.76) |
| P trend* | 0.006 | <0.001 | <0.001 | |||
| <15 (n=24) | 16.02 (0.66) | 3.94 (2.66 to 5.23) | 5.53 (0.57) | 4.39 (3.26 to 5.53) | 9.20 (0.70) | 3.85 (2.49 to 5.21) |
| Anion gap by cystatin C eGFR category in 4,132 participants | ||||||
|---|---|---|---|---|---|---|
| Traditional Anion Gap (mEq/L) | Albumin-adjusted Anion Gap (mEq/L) |
Full Anion Gap (mEq/L) | ||||
| Mean (SE) | Change | Mean (SE) | Change | Mean (SE) | Change | |
| eGFR | ||||||
| ≥ 120 (n=307) | 12.81 (0.24) | 0.11 (−0.34 to 0.56) | 1.60 (0.26) | −0.04 (−0.48 to 0.39) | 5.86 (0.26) | −0.06 (−0.50 to 0.37) |
| 90 – 119 (n=1169) | 12.70 (0.23) | ref | 1.64 (0.23) | ref | 5.92 (0.24) | ref |
| 60 – 89 (n=1865) | 12.91 (0.24) | 0.21 (−0.04 to 0.46) | 2.08 (0.22) | 0.43 (0.20 to 0.67) | 6.39 (0.22) | 0.47 (0.21 to 0.72) |
| 45 – 59 (n=537) | 13.07 (0.36) | 0.37 (−0.22 to 0.95) | 2.47 (0.35) | 0.83 (0.30 to 1.35) | 6.82 (0.37) | 0.90 (0.33 to 1.46) |
| 30 – 44 (n=208) | 13.20 (0.23) | 0.50 (0.15 to 0.86) | 2.83 (0.25) | 1.19 (0.87 to 1.51) | 7.34 (0.24) | 1.42 (1.10 to 1.74) |
| 15 – 29 (n=52) | 13.67 (0.37) | 0.97 (0.28 to 1.67) | 3.24 (0.40) | 1.59 (0.85 to 2.34) | 7.72 (0.35) | 1.80 (1.17 to 2.42) |
| P trend* | 0.03 | <0.001 | <0.001 | |||
| <15 (n=15) | 17.36 (0.66) | 4.66 (3.29 to 6.02) | 6.69 (0.40) | 5.04 (4.14 to 5.95) | 10.90 (0.60) | 4.98 (3.73 to 6.23) |
Abbreviations: CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; SE, standard error.
P values calculated without including participants with eGFR <15 mL/min/1.73m2 in the analysis.
Figure 2.
Mean age-standardized anion gap by categories of creatinine-based Chronic Kidney Disease Epidemiology Collaboration estimated glomerular filtration rate in 11,957 participants (upper panel) and cystatin C-based estimated glomerular filtration rate in 4,132 participants (lower panel) of NHANES 1999–2004.
Association of Anion Gap with All-Cause Mortality
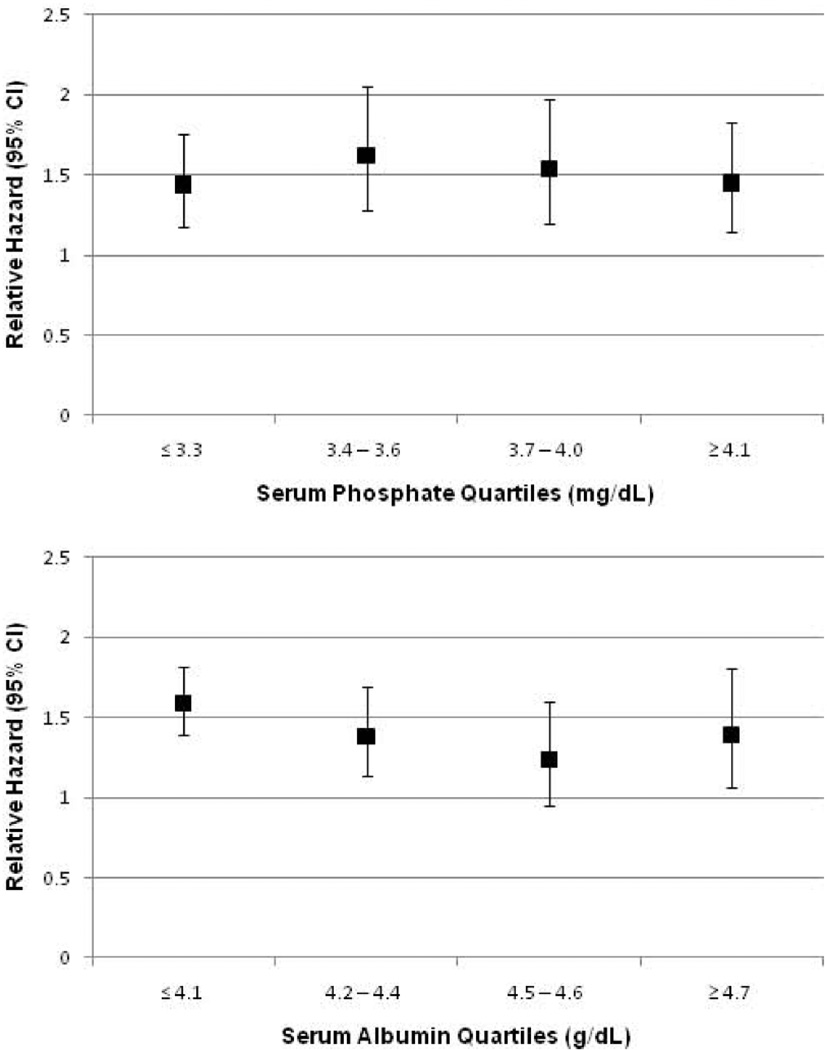
During a median 57 months follow-up time (interquartile range 39–74), 729 participants died. When examined as continuous variables, higher levels of each AG were associated with increased risk of all-cause mortality in unadjusted analyses and after adjustment for age, sex, race/ethnicity, and eGFR (Table 3). In fully adjusted models, only the albumin-adjusted and full AG were associated with mortality as continuous variables. Compared to participants in the lowest quartiles of albumin-adjusted and full AG, there was a significantly increased risk of mortality among those in the highest quartile of each in unadjusted models and after adjustment for age, sex, and race/ethnicity, which was little changed by additional adjustment for eGFR. In fully adjusted models, there was no association of traditional AG with mortality but there remained a significant association of higher albumin-adjusted and full AG with all-cause mortality (relative hazard (RH) for highest versus lowest quartile, 1.52 (95% confidence interval (CI) 1.17–1.97) for albumin-adjusted AG and 1.53 (95% CI 1.11–2.11) for full AG, respectively.) Additional analyses exploring the effect of albuminuria demonstrated that log-transformed urine albumin-creatinine ratio (UACR) was a confounder of the association of each AG with mortality (eTable 3). The association of full AG with mortality did not vary across quartiles of serum phosphate or albumin, respectively (Figure 3). After adding serum albumin and phosphate to the fully adjusted model, the RH for mortality per standard deviation higher full AG was 1.17 (95% CI 1.01–1.36); for participants in the highest quartile of full AG, compared with the lowest, the RH was 1.48 (95% CI 1.08–2.02).
Table 3.
Relative Hazard of All-Cause Mortality by Anion Gap Quartile in 11,957 participants of NHANES 1999–2004
| Relative Hazard (95% CI) | ||||
|---|---|---|---|---|
| Anion Gap | Model 1 | Model 2 | Model 3 | Model 4 |
| Traditional | ||||
| Continuous* | 1.15 (1.02–1.29) | 1.15 (1.03–1.29) | 1.14 (1.02–1.27) | 1.08 (0.94–1.24) |
| Quartile 1 | Ref | Ref | Ref | Ref |
| Quartile 2 | 0.86 (0.66–1.12) | 0.86 (0.67–1.10) | 0.86 (0.67–1.10) | 0.83 (0.64–1.07) |
| Quartile 3 | 1.01 (0.76–1.35) | 0.99 (0.75–1.32) | 0.98 (0.74–1.30) | 0.90 (0.66–1.23) |
| Quartile 4 | 1.30 (0.96–1.74) | 1.32 (1.01–1.72) | 1.29 (1.00–1.67) | 1.20 (0.89–1.61) |
| P for trend | 0.04 | 0.02 | 0.03 | 0.15 |
| Albumin adjusted | ||||
| Continuous* | 1.40 (1.25–1.58) | 1.28 (1.14–1.44) | 1.27 (1.13–1.42) | 1.19 (1.03–1.38) |
| Quartile 1 | Ref | Ref | Ref | Ref |
| Quartile 2 | 1.36 (1.05–1.76) | 1.29 (0.99–1.67) | 1.27 (0.99–1.64) | 1.24 (0.97–1.59) |
| Quartile 3 | 1.30 (1.00–1.70) | 1.19 (0.91–1.56) | 1.18 (0.91–1.53) | 1.07 (0.82–1.38) |
| Quartile 4 | 2.17 (1.62–2.91) | 1.82 (1.37–2.41) | 1.76 (1.34–2.32) | 1.62 (1.19–2.21) |
| P for trend | <0.001 | <0.001 | <0.001 | 0.007 |
| Full | ||||
| Continuous* | 1.50 (1.33–1.68) | 1.29 (1.15–1.45) | 1.28 (1.14–1.43) | 1.20 (1.03–1.41) |
| Quartile 1 | Ref | Ref | Ref | Ref |
| Quartile 2 | 1.67 (1.22–2.29) | 1.40 (1.01–1.94) | 1.36 (0.98–1.89) | 1.31 (0.97–1.79) |
| Quartile 3 | 1.59 (1.19–2.13) | 1.28 (0.95–1.72) | 1.26 (0.94–1.68) | 1.19 (0.90–1.57) |
| Quartile 4 | 2.68 (1.87–3.83) | 1.88 (1.31–2.70) | 1.82 (1.28–2.59) | 1.64 (1.17–2.31) |
| P for trend | <0.001 | 0.001 | 0.001 | 0.01 |
Abbreviations: CI, Confidence Interval.
Bold values indicate p<0.05.
Quartile cutoff levels for each anion gap definition: Traditional: Q1 ≤10; Q2 10.1–12; Q3 12.1–14; Q4 ≥14.1mEq/L; Albumin adjusted: Q1 ≤ −0.50; Q2 −0.45–1.05; Q3 1.10–2.95; Q4 ≥3 mEq/L; Full: Q1 ≤3.61; Q2 3.62–5.34; Q3 5.35–7.23; Q4 >7.23 mEq/L.
Per standard deviation higher anion gap (traditional SD = 2.55 mEq/L; Albumin adjusted SD = 2.47 mEq/L; Full SD = 2.49 mEq/L).
Model 1: unadjusted
Model 2: adjusted for age, sex, race/ethnicity
Model 3: adjusted for age, sex, race/ethnicity, and eGFR categories
Model 4: adjusted for age, sex, race/ethnicity, eGFR categories, BMI, education, activity level, smoking status, diagnosis of diabetes mellitus, hypertension, cardiovascular disease, log-transformed urine albumin-creatinine ratio, serum bicarbonate, and hemoglobin
Figure 3.
Unadjusted relative hazard of all-cause mortality per one standard deviation higher full anion gap (SD = 2.49 mEq/L) within quartiles of serum phosphate (upper panel) and albumin (lower panel) in 11,957 participants of NHANES 1999–2004.
Sensitivity Analyses
After excluding participants with eGFR <60 mL/min/1.73m2, higher levels of the albumin-adjusted and full AG were associated with an increased risk of mortality (Table 4). These results were unchanged after excluding participants with either eGFR <60 mL/min/1.73m2 or macroalbuminuria. A similar, although non-significant, trend remained after further excluding participants with microalbuminuria. To explore if our findings would differ with adjustment for cystatin C-based versus creatinine-based eGFR, we examined mortality in the subgroup of participants with measurement of cystatin C. Our estimates were similarly attenuated by adjustment for CKD-EPI eGFR as by adjustment for eGFRcys (Table 5). Finally, we examined the independent association of serum bicarbonate with mortality. Higher serum bicarbonate was associated with an increased risk of mortality in unadjusted models, and this association was no longer significant after adjustment for age, sex, and race/ethnicity (eTable 4).
Table 4.
Sensitivity Analyses of Associations of Anion Gap with All-Cause Mortality in 11,957 participants of NHANES 1999–2004
| Relative Hazard among Participants with eGFR ≥60 mL/min/1.73m2 (n=10,828; 467 deaths) | |||
|---|---|---|---|
| Relative Hazard (95% CI) | |||
| Anion Gap | Traditional | Albumin adjusted | Full |
| Continuous* | 1.17 (1.00–1.37) | 1.31 (1.13–1.52) | 1.30 (1.12–1.51) |
| Quartile 1 | Ref | Ref | Ref |
| Quartile 2 | 0.76 (0.51–1.13) | 1.38 (0.92–2.05) | 1.34 (0.88–2.04) |
| Quartile 3 | 0.92 (0.62–1.37) | 1.02 (0.71–1.48) | 1.07 (0.72–1.60) |
| Quartile 4 | 1.34 (0.91–1.96) | 1.86 (1.24–2.78) | 1.79 (1.17–2.75) |
| P for trend | 0.06 | 0.007 | 0.01 |
| Relative Hazard among Participants with eGFR ≥60 mL/min/1.73m2 and UACR <300 mg/g (n=10,697; 446 deaths) | |||
|---|---|---|---|
| Relative Hazard (95% CI) | |||
| Anion Gap | Traditional | Albumin adjusted | Full |
| Continuous* | 1.14 (0.97–1.34) | 1.28 (1.10–1.50) | 1.27 (1.09–1.48) |
| Quartile 1 | Ref | Ref | Ref |
| Quartile 2 | 0.76 (0.51–1.14) | 1.37 (0.92–2.03) | 1.32 (0.86–2.00) |
| Quartile 3 | 0.93 (0.64–1.37) | 1.03 (0.70–1.50) | 1.06 (0.71–1.60) |
| Quartile 4 | 1.28 (0.87–1.88) | 1.84 (1.24–2.73) | 1.76 (1.16–2.68) |
| P for trend | 0.10 | 0.008 | 0.01 |
| Relative Hazard among Participants with eGFR ≥60 mL/min/1.73m2 and UACR <30 mg/g (n=9,700; 324 deaths) | |||
|---|---|---|---|
| Relative Hazard (95% CI) | |||
| Anion Gap | Traditional | Albumin adjusted | Full |
| Continuous* | 1.07 (0.86–1.33) | 1.21 (0.98–1.48) | 1.20 (0.99–1.46) |
| Quartile 1 | Ref | Ref | Ref |
| Quartile 2 | 0.70 (0.43–1.14) | 1.45 (0.89–2.36) | 1.24 (0.78–1.99) |
| Quartile 3 | 0.75 (0.50–1.12) | 0.99 (0.63–1.57) | 1.05 (0.66–1.67) |
| Quartile 4 | 1.10 (0.63–1.91) | 1.55 (0.91–2.63) | 1.45 (0.85–2.48) |
| P for trend | 0.63 | 0.21 | 0.24 |
Abbreviations: CI, Confidence Interval; eGFR, estimated glomerular filtration rate; UACR, urinary albumin-creatinine ratio.
Bold values indicate p<0.05.
Quartile cutoff levels for each anion gap definition: Traditional: Q1 ≤10; Q2 10.1–12; Q3 12.1–14; Q4 ≥14.1mEq/L; Albumin adjusted: Q1 ≤ −0.50; Q2 −0.45–1.05; Q3 1.10–2.95; Q4 ≥3 mEq/L; Full: Q1 ≤3.61; Q2 3.62–5.34; Q3 5.35–7.23; Q4 >7.23 mEq/L.
Per standard deviation higher anion gap (traditional SD = 2.55 mEq/L; Albumin adjusted SD = 2.47 mEq/L; Full SD = 2.49 mEq/L).
Models adjusted for age, sex, race/ethnicity, eGFR categories, BMI, education, activity level, smoking status, diagnosis of diabetes mellitus, hypertension, cardiovascular disease, log-transformed urine albumin-creatinine ratio, serum bicarbonate, and hemoglobin.
Table 5.
Sensitivity Analysis of All-Cause Mortality Using Different eGFR Adjustments in 4,132 Participants of NHANES 1999–2002 with cystatin C Measurements (491 deaths)
| Relative Hazard (95% CI) | |||
|---|---|---|---|
| Anion Gap | Unadjusted | CKD-EPI eGFR | Cystatin C eGFR |
| Traditional | |||
| Continuous* | 1.11 (0.92–1.35) | 1.14 (0.95–1.36) | 1.11 (0.93–1.32) |
| Quartile 1 | Ref | Ref | Ref |
| Quartile 2 | 0.74 (0.48–1.13) | 0.83 (0.53–1.29) | 0.80 (0.52–1.23) |
| Quartile 3 | 0.90 (0.55–1.50) | 0.96 (0.57–1.61) | 0.94 (0.57–1.54) |
| Quartile 4 | 1.14 (0.77–1.69) | 1.26 (0.86–1.86) | 1.18 (0.81–1.72) |
| P for trend | 0.33 | 0.15 | 0.24 |
| Albumin adjusted | |||
| Continuous* | 1.34 (1.09–1.64) | 1.24 (1.05–1.47) | 1.20 (1.01–1.42) |
| Quartile 1 | Ref | Ref | Ref |
| Quartile 2 | 0.75 (0.45–1.25) | 0.76 (0.47–1.26) | 0.77 (0.48–1.24) |
| Quartile 3 | 0.92 (0.57–1.49) | 0.90 (0.56–1.44) | 0.87 (0.56–1.36) |
| Quartile 4 | 1.69 (1.12–2.55) | 1.42 (0.96–2.10) | 1.34 (0.92–1.94) |
| P for trend | 0.009 | 0.03 | 0.07 |
| Full | |||
| Continuous* | 1.45 (1.16–1.80) | 1.26 (1.06–1.51) | 1.23 (1.02–1.47) |
| Quartile 1 | Ref | Ref | Ref |
| Quartile 2 | 0.65 (0.37–1.14) | 0.65 (0.39–1.08) | 0.65 (0.39–1.07) |
| Quartile 3 | 0.89 (0.54–1.49) | 0.80 (0.48–1.32) | 0.76 (0.47–1.23) |
| Quartile 4 | 1.81 (1.16–2.84) | 1.33 (0.88–2.00) | 1.24 (0.83–1.84) |
| P for trend | 0.004 | 0.05 | 0.11 |
Abbreviations: CI, Confidence Interval; eGFR, estimated glomerular filtration rate.
Bold values indicate p<0.05.
Quartile cutoff levels for each anion gap definition: Traditional: Q1 ≤11; Q2 11.1–13; Q3 13.1–14.6; Q4 ≥14.7mEq/L; Albumin adjusted: Q1 ≤0.2; Q2 0.25–1.95; Q3 2.00–3.60; Q4 ≥3.65 mEq/L; Full: Q1 <4.41; Q2 4.41–6.24; Q3 6.25–7.94; Q4 ≥7.95 mEq/L.
Per standard deviation higher anion gap (traditional SD = 2.55 mEq/L; Albumin adjusted SD = 2.47 mEq/L; Full SD = 2.49 mEq/L).
Models are unadjusted, or adjusted for age, sex, race/ethnicity, and CKD-EPI or eGFRcys categories, as noted in the Table.
DISCUSSION
Our results demonstrate that higher levels of AG are present in persons with less advanced kidney disease than has been previously recognized, and that this association is dependent upon the definition of AG utilized. Changes in serum albumin account for much of the difference between our results and previous studies, although including other electrolytes in the calculation further increased the magnitude of the association of AG levels with eGFR. Measurement of eGFR using cystatin C improved the robustness of the association, with a linear increase in AG seen with eGFRcys below 90 mL/min/1.73m2. After accounting for serum albumin and other electrolytes, higher levels of AG were associated with an increased risk of all-cause mortality, independent of multiple factors including eGFR and albuminuria. Estimation of GFR using cystatin C did not appear to alter these findings. These results suggest that small yet measurable increases in typically unmeasured anions may be present earlier in the course of kidney disease than is currently appreciated, and that these compounds may be markers for, or have a causal role in, the association of CKD with an increased risk for mortality.
We may speculate about the composition of higher levels of AG based on previous literature. In a study of 57 hospitalized patients, lactate and ketoanions accounted for 62 percent of the increment in the traditional AG, and changes in proteins, potassium, phosphate, and calcium accounted for an additional 15 percent.10 An average of 23 percent of the increased AG was unaccounted for, suggesting the possibility of unidentified anions or changes in other normal plasma components. This study, however, examined hospitalized patients with levels of traditional AG greater than 16 mEq/L, in marked contrast to our study of community-dwelling participants with a mean traditional AG of 12.08 mEq/L. Therefore, it is unclear to what degree these prior results can be extrapolated to our findings.
Differences in AG may be due to solutes known to accumulate in advanced kidney disease.11 Tubular secretion is an important contributor to the clearance of a number of compounds whose levels rise in uremia, such as the negatively-charged hippurate.12 Loss of tubular function causes elevated concentrations of various organic anions,13 and the accumulation of organic solutes is known to contribute to the high AG metabolic acidosis seen in advanced kidney disease.14 Whether impairment of tubular function occurs earlier in the course of CKD is unknown. Disruption of renal organic anion secretion could result in higher concentrations of endogenous organic anions, as demonstrated in a mouse knockout model of organic anion transporter 1 (OAT1).13 Mild impairment of proximal tubular function early in the course of kidney disease could result in small increases in the levels of such solutes. Over 70 years ago Shannon postulated renal tubular excretion as a mechanism for minimizing levels of endogenous toxins, such that deficiencies in tubular function could account for some of the symptomatology of kidney disease.15 Nevertheless, tubular handling of organic solutes remains a relatively understudied aspect of kidney function, especially as it relates to the clinical phenotype of persons with CKD. Alternatively, mild impairment in glomerular filtration may be sufficient to account for the retention of non-chloride anions that were not included in our calculation of the full AG, as acid retention has been demonstrated in subjects with only mild reductions in eGFR.16 Alterations in circulating proteins other than albumin could also produce changes in the AG. Thus the AG may represent a cumulative measure of the changes in a number of circulating factors that occur even with mild impairment of kidney function. Indeed, our results were unchanged after the exclusion of participants with eGFR <60 mL/min/1.73m2, suggesting that the AG, as a marker for the retention of various solutes and possibly a measure of tubular function, may provide a more sensitive measure of impaired kidney function among individuals with eGFR ≥60 mL/min/1.73m2.
Higher AG may also reflect increased concentrations of organic anions present in persons with obesity, insulin resistance, and hypertension. In the general population, higher levels of the traditional AG have been associated with hypertension and insulin resistance.7, 8 The association with insulin resistance, in particular, was of greater magnitude in obese individuals. This suggests a relationship between obesity, insulin resistance, and the metabolic syndrome and the solutes accounting for higher levels of AG. The attenuation of mortality risk by adjustment for BMI, activity level, diabetes, hypertension, and CVD may be reflective of this relationship. If so, our results indicate that measurements of organic solutes may be predictive of mortality in such individuals. Alternatively, as obesity and the metabolic syndrome are risk factors for the development of CKD and end-stage renal disease,17, 18 the attenuation of our estimates could signal subtle impairment of kidney function, manifested as higher AG, in persons with components of the metabolic syndrome.
The association of albumin-adjusted and full AG with mortality could be reflective of the known inverse association of serum albumin with mortality risk, as by definition, lower albumin levels result in higher albumin-adjusted and full AG. However, the difference in serum albumin across full AG quartiles, while statistically significant, was quite small. In addition, the association of full AG with mortality did not vary across quartiles of serum albumin. Similarly, the association of full AG with mortality did not vary across serum phosphate quartiles, and participants with higher phosphate levels, which have been associated with mortality, would have lower AG. Furthermore, adjustment for serum albumin and phosphate did not alter our results. Therefore, independent associations of albumin or phosphate seem an unlikely explanation for our findings.
Similarly, the association of higher AG with increased mortality does not appear to be mediated by the effects of acidosis. Serum bicarbonate was not independently associated with mortality, and the estimates of mortality risk associated with the AG were independent of serum bicarbonate. Therefore, these data do not support a role for alkali therapy for an elevated AG per se. Rather, such therapeutic decisions should be based on the degree of acidosis and the evidence base informing the treatment of acidosis. Similarly, we cannot comment on whether individuals with a high albumin-adjusted or full AG but normal serum bicarbonate have a low-grade metabolic acidosis that could be amenable to alkali therapy. As alkali may be beneficial even in early CKD,19 this should be the subject of future research.
Several important limitations of our analysis should be noted. Estimation of the charge contribution of serum albumin, calcium, and phosphate could have been performed more precisely had values of blood pH or ionized calcium been available. However, the participants in this study were sampled from the general population, and the vast majority would be expected to have pH values within the normal range. Differences of 0.05–0.10 pH units within the range of physiologic pH would have negligible effects on our estimates of charge contribution. We employed the generally accepted adjustments for serum albumin and phosphate and tested different assumptions for the estimation of ionized calcium, which had no effect on our results. Thus we believe that differences in full AG levels represent differences in the concentrations of solutes not included in our calculation, rather than simply imprecise charge estimation. Our calculations did not account for changes in serum sulfate, which is known to accumulate in uremia, because it was not measured in NHANES. We also did not have measures of magnesium. However, the lack of accounting for possibly higher magnesium levels in persons with CKD would have resulted in falsely low AG levels, which would have reduced the likelihood of detecting significant associations with eGFR or mortality. In addition, levels of AG were determined from single measurements, and variability in sample handling may affect bicarbonate levels.20–22 GFR estimation using cystatin C levels may be affected by factors such as inflammation.23 Finally, as this was an observational study, we cannot infer causality regarding the associations seen.
In conclusion, higher levels of AG are present in persons with less advanced kidney disease than has been previously recognized, and are associated with an increased risk of mortality independent of eGFR and albuminuria. Further research is needed to identify the molecules accounting for higher levels of AG in persons without advanced kidney disease and to determine their physiologic significance.
METHODS
Study Population
NHANES 1999–2004 was a nationally representative survey of the noninstitutionalized civilian population in the United States.24 A stratified, multistage, probability sampling design was used to select participants. Overall, 13,269 adults ≥20 years of age completed the interview and examination components and had complete laboratory data for each of the AG definitions. We excluded participants who were pregnant (n=716), missing mortality data (n=17), had an eGFR <15 mL/min/1.73m2 (n=42), or missing covariate data (n=537). Thus 11,957 participants were available for analysis. In analyses of the association of AG with eGFR, participants with eGFR <15 mL/min/1.73m2 were included for comparison. The Committee on Clinical Investigation at the Albert Einstein College of Medicine determined this analysis to be exempt.
Data Collection
Information on education, physical activity, smoking, and comorbidities was obtained by self-report. Race/ethnicity was self-identified. Participants were asked about the frequency and duration of walking or bicycling, home or yard work, and moderate or vigorous leisure time physical activity performed within the past 30 days. These responses were used to calculate metabolic equivalents (MET-min/wk)25 and to classify activity level as 0, <500, 500–2000, or >2000 MET-min/wk. Smoking was classified as never, former, or current smoker. Hypertension was defined as a systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, physician diagnosis, and/or antihypertensive medication use.26 Diabetes mellitus was defined as a physician diagnosis while not pregnant or the current use of insulin or oral hypoglycemic medications. CVD was defined by self-report of a physician diagnosis of congestive heart failure, coronary heart disease, angina, myocardial infarction, or stroke.
Serum chemistry values were measured using the Hitachi 917 multichannel analyzer (Roche Diagnostics, Indianapolis, IN) in 1999–2001 and the Beckman Synchron LX20 (Beckman Coulter Inc., Brea, CA) in 2002–2004. Serum sodium, potassium, and chloride were measured with an ion-selective electrode (ISE), albumin by the bromocresol purple method, phosphate via reaction with ammonium molybdate, and calcium via reaction with o-cresolphthalein complexone in 1999–2001 and by ISE in 2002–2004. Serum bicarbonate was measured via the phosphoenolpyruvate carboxylase method from 1999–2001 and with a pH-sensitive electrode in 2002–2004. The AG was calculated in 3 ways, using ionic contributions of electrolytes and albumin based on the published literature (Figure 1): Traditional AG=serum sodium(mEq/L) – (serum chloride(mEq/L) + serum bicarbonate(mEq/L)); Albumin-adjusted AG=Traditional AG − (2.5 × serum albumin(g/dL));27 and Full AG=Albumin-adjusted AG + serum potassium(mEq/L) + ionized calcium(mEq/L) − serum phosphate(mEq/L), where we assumed 50% of calcium existed in the ionized form and a valence of 1.8 for inorganic phosphate at physiologic pH,28 such that ionized calcium(mEq/L)=[0.5×(total calcium(mg/dL) + 0.8× (4 − serum albumin(g/dL))]/2) and serum phosphate(mEq/L)=(0.323 × serum phosphate(mg/dL)) × 1.8. Given the imprecision with adjustment of total calcium for albumin and the variation of the ionized fraction by pH, all analyses were repeated without adjustment of total calcium for albumin and also assuming an ionized fraction of 40% or 60%. As the results did not differ, only the analyses with the initial definition of full AG are presented.
Serum creatinine was measured by a modified kinetic Jaffé reaction. Values from 1999–2000 were calibrated to the Cleveland Clinic laboratory standard by multiplying by 1.013 and then adding 0.147. Correction of values from 2001–2004 was not necessary. eGFR was calculated using the CKD-EPI equation.29 Serum cystatin C was measured in a sub-sample of participants in 1999–2002 using a particle-enhanced nephelometric assay. eGFRcys was calculated using age, sex, race, and cystatin C.23 Appropriate sample weights were used to account for the smaller sample in which cystatin C levels were available.
Outcome Variables
All-cause mortality was ascertained through December 31, 2006 using public-use linked mortality files.30 Mortality status was determined primarily through probabilistic record matching with the National Death Index. Complete details of the matching methodology are available.31 The public-use files were subjected to data perturbation techniques due to concerns regarding participant anonymity. For selected decedent records, the date of death and cause of death were perturbed. Vital status was not perturbed. Due to the perturbation of cause-of-death data, we examined only all-cause mortality. Analyses performed by the National Center for Health Statistics demonstrate consistent and nearly identical numerical results when examining all-cause mortality with Cox proportional hazards models using the public-use data compared with non-perturbed restricted-use data.32
Statistical Analysis
All analyses used NHANES-appropriate sampling weights and accounted for the complex multistage cluster design using the “survey” command in Stata 11.1 (Stata Corporation, College Station, TX, USA). The distributions of participant characteristics were examined by quartiles of each AG. The association of serum bicarbonate with each AG was also examined using linear regression. The mean traditional, albumin-adjusted, and full AG were calculated within categories of eGFR and eGFRcys. Age-standardized mean values of each AG were then calculated for each eGFR and eGFRcys category using 2000 U.S. census data. Cox proportional hazards models were created to examine associations with all-cause mortality. Each AG was analyzed as a continuous variable and within quartiles to examine non-linear associations with mortality. Models were created examining associations with mortality in the overall cohort without adjustment for additional covariates and then including age, sex, race/ethnicity, and eGFR categories as covariates. For survival analyses, eGFR was categorized as ≥120, 90–119, 60–89, and 15–59 mL/min/1.73m2 due to the relatively small number of participants with eGFR <60 mL/min/1.73m2. Additional models were created adding log-transformed UACR to the above covariates. Multivariable models were then created to include as covariates other potential confounders or mediators of the association of AG with mortality, including education (as a marker of socioeconomic status), BMI categories, activity level, smoking status, diagnosis of diabetes mellitus, hypertension, CVD, serum bicarbonate, and hemoglobin. We examined the possibility that associations of the albumin-adjusted and full AG with mortality were driven by the inclusion in our calculations of serum albumin in both and serum phosphate in the latter, as both serum albumin and phosphate have been associated with all-cause mortality.33–35 We therefore examined the associations of the full AG with mortality within quartiles of serum albumin and phosphate, respectively, as the full AG incorporates both albumin and phosphate in the calculation. We then entered serum albumin and phosphate into an exploratory model including the above covariates to determine if associations of full AG with mortality were independent of these measures. The proportional hazards assumption was shown to be accurate by visual inspection of log-log plots.
Sensitivity Analyses
To determine whether our results were driven by participants with CKD, we examined associations of each type of AG with mortality in participants with eGFR ≥60 mL/min/1.73m2, after excluding those with either eGFR <60 mL/min/1.73m2 or macroalbuminuria (defined as UACR ≥300 mg/g), and then after further excluding participants with microalbuminuria (defined as UACR ≥30 mg/g). To explore differences in confounding related to cystatin C-based versus creatinine-based eGFR, we repeated our survival analyses in the subgroup of participants with cystatin C measurements. We recalculated quartiles of each AG in this subgroup, and then examined the association of each AG with mortality in unadjusted models and then in models adjusted for age, sex, race/ethnicity, and either eGFR or eGFRcys categories. Finally, we examined the independent association of serum bicarbonate with mortality, modeling it as a continuous variable and within quartiles.
Supplementary Material
Acknowledgments
This research was supported by National Institutes of Health (NIH) grants K23DK078774 to Dr. Melamed, R21DK077326, R01DK087783 and RO1DK080123 to Dr. Hostetter, and CTSA grants UL1RR025750, KL2RR025749 and TL1RR025748 from the National Center for Research Resources, a component of the NIH.
Footnotes
Disclosures
Dr. Hostetter has consulted for Bristol Myers Squibb, Eli Lilly, Genzyme, and Wyeth. Neither of the other authors has any financial conflicts to disclose.
REFERENCES
- 1.Hakim RM, Lazarus JM. Biochemical parameters in chronic renal failure. Am J Kidney Dis. 1988;11:238–247. doi: 10.1016/s0272-6386(88)80156-2. [DOI] [PubMed] [Google Scholar]
- 2.Wallia R, Greenberg A, Piraino B, et al. Serum electrolyte patterns in end-stage renal disease. Am J Kidney Dis. 1986;8:98–104. doi: 10.1016/s0272-6386(86)80119-6. [DOI] [PubMed] [Google Scholar]
- 3.Widmer B, Gerhardt RE, Harrington JT, et al. Serum electrolyte and acid base composition. The influence of graded degrees of chronic renal failure. Arch Intern Med. 1979;139:1099–1102. [PubMed] [Google Scholar]
- 4.Eustace JA, Astor B, Muntner PM, et al. Prevalence of acidosis and inflammation and their association with low serum albumin in chronic kidney disease. Kidney Int. 2004;65:1031–1040. doi: 10.1111/j.1523-1755.2004.00481.x. [DOI] [PubMed] [Google Scholar]
- 5.Hsu CY, Chertow GM. Elevations of serum phosphorus and potassium in mild to moderate chronic renal insufficiency. Nephrol Dial Transplant. 2002;17:1419–1425. doi: 10.1093/ndt/17.8.1419. [DOI] [PubMed] [Google Scholar]
- 6.Figge J, Jabor A, Kazda A, et al. Anion gap and hypoalbuminemia. Crit Care Med. 1998;26:1807–1810. doi: 10.1097/00003246-199811000-00019. [DOI] [PubMed] [Google Scholar]
- 7.Farwell WR, Taylor EN. Serum bicarbonate, anion gap and insulin resistance in the National Health and Nutrition Examination Survey. Diabet Med. 2008;25:798–804. doi: 10.1111/j.1464-5491.2008.02471.x. [DOI] [PubMed] [Google Scholar]
- 8.Taylor EN, Forman JP, Farwell WR. Serum anion gap and blood pressure in the national health and nutrition examination survey. Hypertension. 2007;50:320–324. doi: 10.1161/HYPERTENSIONAHA.107.092643. [DOI] [PubMed] [Google Scholar]
- 9.Abramowitz MK, Hostetter TH, Melamed ML. Lower serum bicarbonate and a higher anion gap are associated with lower cardiorespiratory fitness in young adults. Kidney International. 2012 Feb 1; doi: 10.1038/ki.2011.479. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gabow PA, Kaehny WD, Fennessey PV, et al. Diagnostic importance of an increased serum anion gap. N Engl J Med. 1980;303:854–858. doi: 10.1056/NEJM198010093031505. [DOI] [PubMed] [Google Scholar]
- 11.Vanholder R, De Smet R, Glorieux G, et al. Review on uremic toxins: classification, concentration, and interindividual variability. Kidney Int. 2003;63:1934–1943. doi: 10.1046/j.1523-1755.2003.00924.x. [DOI] [PubMed] [Google Scholar]
- 12.Abramowitz MK, Meyer TW, Hostetter TH. The pathophysiology of uremia. In: Himmelfarb J, Sayegh MH, editors. Chronic kidney disease, dialysis, and transplantation : companion to Brenner & Rector's the kidney. 3rd edn. Philadelphia: Saunders; 2011. pp. 251–264. [Google Scholar]
- 13.Eraly SA, Vallon V, Vaughn DA, et al. Decreased renal organic anion secretion and plasma accumulation of endogenous organic anions in OAT1 knock-out mice. J Biol Chem. 2006;281:5072–5083. doi: 10.1074/jbc.M508050200. [DOI] [PubMed] [Google Scholar]
- 14.Relman AS. Renal Acidosis and Renal Excretion of Acid in Health and Disease. Adv Intern Med. 1964;12:295–347. [PubMed] [Google Scholar]
- 15.Shannon JA. Renal tubular excretion. Physiol Rev. 1939;19:63–93. [Google Scholar]
- 16.Wesson DE, Simoni J, Broglio K, et al. Acid retention accompanies reduced GFR in humans and increases plasma levels of endothelin and aldosterone. Am J Physiol Renal Physiol. 2011;300:F830–F837. doi: 10.1152/ajprenal.00587.2010. [DOI] [PubMed] [Google Scholar]
- 17.Hsu CY, McCulloch CE, Iribarren C, et al. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144:21–28. doi: 10.7326/0003-4819-144-1-200601030-00006. [DOI] [PubMed] [Google Scholar]
- 18.Kurella M, Lo JC, Chertow GM. Metabolic syndrome and the risk for chronic kidney disease among nondiabetic adults. J Am Soc Nephrol. 2005;16:2134–2140. doi: 10.1681/ASN.2005010106. [DOI] [PubMed] [Google Scholar]
- 19.Mahajan A, Simoni J, Sheather SJ, et al. Daily oral sodium bicarbonate preserves glomerular filtration rate by slowing its decline in early hypertensive nephropathy. Kidney Int. 2010;78:303–309. doi: 10.1038/ki.2010.129. [DOI] [PubMed] [Google Scholar]
- 20.Bray SH, Tung RL, Jones ER. The magnitude of metabolic acidosis is dependent on differences in bicarbonate assays. Am J Kidney Dis. 1996;28:700–703. doi: 10.1016/s0272-6386(96)90251-6. [DOI] [PubMed] [Google Scholar]
- 21.Kirschbaum B. Spurious metabolic acidosis in hemodialysis patients. Am J Kidney Dis. 2000;35:1068–1071. doi: 10.1016/s0272-6386(00)70041-2. [DOI] [PubMed] [Google Scholar]
- 22.Laski ME. Penny wise and bicarbonate foolish. Am J Kidney Dis. 2000;35:1224–1225. doi: 10.1016/s0272-6386(00)70063-1. [DOI] [PubMed] [Google Scholar]
- 23.Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; [Accessed December 21, 2010]. About the National Health and Nutrition Examination Survey. Available at: http://www.cdc.gov/nchs/nhanes/about_nhanes.htm. [Google Scholar]
- 25.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 26.Muntner P, Woodward M, Mann DM, et al. Comparison of the Framingham Heart Study hypertension model with blood pressure alone in the prediction of risk of hypertension: the Multi-Ethnic Study of Atherosclerosis. Hypertension. 2010;55:1339–1345. doi: 10.1161/HYPERTENSIONAHA.109.149609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kraut JA, Madias NE. Serum anion gap: its uses and limitations in clinical medicine. Clin J Am Soc Nephrol. 2007;2:162–174. doi: 10.2215/CJN.03020906. [DOI] [PubMed] [Google Scholar]
- 28.Bansal VK. Serum Inorganic Phosphorus. In: Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd edn. Boston: Butterworths; 1990. pp. 895–899. [PubMed] [Google Scholar]
- 29.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; [Accessed July 27, 2010]. National Health and Nutrition Examination Survey Data. Available at: http://www.cdc.gov/nchs/data_access/data_linkage/mortality/nhanes_99_04_linkage_public_use.htm. [Google Scholar]
- 31.Hyattsville, MD: [Accessed July 27, 2010]. National Health and Nutrition Examination Survey (NHANES) 1999–2004 Linked Mortality Files, Mortality follow-up through 2006: Matching Methodology, May 2009. Available at: http://www.cdc.gov/nchs/data/datalinkage/nh99+_mortality_matching_methodology_final.pdf. [Google Scholar]
- 32.Hyattsville, MD: [Accessed July 27, 2010]. Comparative analysis of the NHANES (1999–2004) public-use and restricted-use linked mortality files: 2010 public-use data release. National Center for Health Statistics. May 2010. Available at: http://www.cdc.gov/nchs/data_access/data_linkage/mortality/nhanes_99_04_linkage.htm. [Google Scholar]
- 33.Fried LP, Kronmal RA, Newman AB, et al. Risk factors for 5-year mortality in older adults: the Cardiovascular Health Study. JAMA. 1998;279:585–592. doi: 10.1001/jama.279.8.585. [DOI] [PubMed] [Google Scholar]
- 34.Menon V, Greene T, Wang X, et al. C-reactive protein and albumin as predictors of all-cause and cardiovascular mortality in chronic kidney disease. Kidney Int. 2005;68:766–772. doi: 10.1111/j.1523-1755.2005.00455.x. [DOI] [PubMed] [Google Scholar]
- 35.Tonelli M, Sacks F, Pfeffer M, et al. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation. 2005;112:2627–2633. doi: 10.1161/CIRCULATIONAHA.105.553198. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.