Abstract
Two currently licensed live oral rotavirus vaccines (Rotarix® and RotaTeq®) are highly efficacious against severe rotavirus diarrhea. However, the efficacy of such vaccines in selected low-income African and Asian countries is much lower than that in middle or high-income countries. Additionally, these two vaccines have recently been associated with rare case of intussusception in vaccinated infants. We developed a novel recombinant subunit parenteral rotavirus vaccine which may be more effective in low-income countries and also avert the potential problem of intussusception. Truncated recombinant VP8* (ΔVP8*) protein of human rotavirus strain Wa P[8], DS-1 P[4] or 1076 P[6] expressed in E. coli was highly soluble and was generated in high yield. Guinea pigs hyperimmunized intramuscularly with each of the ΔVP8* proteins (i.e., (P[8], P[4] or P[6]) developed high levels of homotypic as well as variable levels of heterotypic neutralizing antibodies. Moreover, the selected ΔVP8* proteins when administered to mice at a clinically relevant dosage, route and schedule, elicited high levels of serum anti-VP8* IgG and/or neutralizing antibodies. Our data indicated that the ΔVP8* proteins may be a plausible additional candidate as new parenteral rotavirus vaccines.
Keywords: Rotavirus, Vaccine, Subunit vaccine, VP8* protein, P type
1. Introduction
Rotaviruses are the most common cause of severe gastroenteritis among children under 5 years of age in both developed and developing countries [1, 2]. In general, because natural rotavirus infections induce efficient protection against subsequent severe rotavirus diseases [3], concerted efforts have been made to develop an attenuated live oral rotavirus vaccine. Currently, two live oral rotavirus vaccines, Rotarix® (GlaxoSmithKline) [4] and RotaTeq® (Merck) [5], have been licensed in many countries. These two vaccines have been demonstrated to be safe and efficacious in preventing severe rotavirus diarrhea among children in middle-income and high-income countries, however, the immunogenicity and efficacy of these vaccines in selected low-income countries of Africa, Asia and Central America where the vaccines are needed most was low [6–9]. In addition, a small increased risk of intussusception shortly after the first vaccine administration was detected during postmarketing surveillance of both vaccines [10]. Recently, the presence of porcine circovirus (PCV) type 1 DNA in Rotarix® vaccine and fragments of DNA of PCV type 1 and type 2 in RotaTeq® vaccine was confirmed [11]. More recently, PCV type 1 in Rotarix® was demonstrated to be infectious [12] and was able to replicate productively in human hepatocellular carcinoma cells [13]. Recently, in response to at least 8 cases of vaccine-acquired rotavirus infection in infants with severe combined immunodeficiency (SCID) reported in the literature and to the Vaccine Adverse Event Reporting System [14,15] (i. e., seven received RotaTeq® and one received Rotarix®, and all had diarrhea, and in addition, most had additional infections), SCID has been added by the FDA and recommended by the CDC and the Advisory Committee on Immunization Practices as a contraindication for administration of either licensed oral rotavirus vaccine in the US [16]. Moreover, live rotavirus vaccine strains could generate reassortant rotaviruses between vaccine strains and circulating wild-type strains or among vaccine strains if a mixed infection occurs in a vaccinated child, which could lead to the generation of potentially virulent strains as reported recently [17].
Because of concerns associated with live oral rotavirus vaccines and potential advantages of non-living rotavirus vaccines (e.g., no involvement of gastrointestinal factors; no environmental interference; and coherence with other routine childhood vaccines), alternative strategies have been pursued including the development of an inactivated rotavirus vaccine [18,19], triple- and double-layered virus-like particle vaccines [20,21] and recombinant subunit VP6 protein vaccine [22,23]. Evidence from licensed vaccines has also shown that parenteral vaccines are successful in preventing orally transmitted diseases such as poliovirus, hepatitis A and B viruses, Vibrio cholera or Salmonella typhi [24–28].
Rotavirus outer capsid proteins VP7 (which defines G type) and VP4 (which defines P type) are independent protective antigens. Rotavirus infectivity requires proteolytic cleavage of the VP4 and the subsequent formation of VP8* and VP5* proteins. Initially, we tried to express various portions of the VP5*, however, such bacterially-expressed truncated VP5* proteins were all insoluble, and therefore, we expressed the VP8* protein. The VP8* protein of VP4 has been expressed in various systems and demonstrated to induce rotavirus-specific neutralizing antibodies and/or protection in a mouse model [29–37]. The objective of this study was to generate and characterize a truncated recombinant subunit VP8* protein vaccine candidate containing amino acid residues 64 (or 65)-223 with P[8], P[4] or P[6] specificity expressed in E. coli and to evaluate its vaccine potential. This VP8* region was selected since all the VP8*-specific neutralizing monoclonal antibodies have been mapped to this region [38]; and Wa VP8*(64-223) has previously been expressed in E. coli and analyzed by X-ray crystallography [39].
2. Materials and Methods
2.1. Viruses and cell culture
Human rotavirus strains Wa (G1P[8]) [40], DS-1 (G2P[4]) [41] and 1076 (G2P[6]) [42] were grown in primary African green monkey kidney cells (Diagnostic Hybrids, Athens, OH). Eagle’s minimum essential medium supplemented with 0.5 μg/ml of trypsin (Sigma γ-irradiated trypsin), 100 IU/ml of Penicillin, 100 μg/ml of Streptomycin and 2.5 μg/ml of Amphotericin B was used as maintenance medium.
2.2. Vaccine plasmid construction
Truncated VP8* (ΔVP8*) gene cDNA of human rotavirus Wa, DS-1 or 1076 strain was obtained by a reverse transcription-polymerase chain reaction (RT-PCR) procedure from viral RNA extracted using TRIzol-LS (Invitrogen). The primers used were designed according to the genomic sequences of RNA segment 4 of each rotavirus strain [GenBank accession numbers: FJ423116 (Wa), EF672577 (DS-1) and M88480 (1076)]. Oligonucleotide sequences included restriction endonuclease sites Nde I and Sac I to facilitate the cloning of the inserts in multiple clone sites in an expression vector. The primers for constructing ΔVP8* were as follows: 5′-TACTCATATGTTAGATGGTCCTTATCAGCCAAC- 3′ (Wa ΔVP8* sense), 5′-TAGAGCTCTATCACAGACCATTATTAATATATTCATTAC-3′ (Wa ΔVP8* antisense), 5′-TACTCATATGGTTTTAGATGGTCCTTATCAAC-3′ (DS-1 ΔVP8* sense) and 5′-TAGAGCTCTATCATAAACCATTATTGATATACTCG -3′ (DS-1 ΔVP8* antisense), 5′-TACTCATATGGTACTCGATGGTCCTTATCAACC-3′ (1076 ΔVP8* sense) and 5′-TAGAGCTCTATCATAACCCAGTATTTATATATTCATT ACAC-3′ (1076 ΔVP8* antisense). Nde I and Sac I sites, respectively (underlined), and two stop codons were introduced into each antisense primer (in bold). VP8* cDNA was synthesized by using Superscript III (Invitrogen) and reaction parameters were as follows: 50–200 ng of genomic RNA was denatured with a final concentration of 15% DMSO and incubated at 94°C for 3 min, followed by chilling immediately. The first strand cDNA was synthesized following manufacturer’s instructions. The products of RT reaction were used as templates for PCR using an iProof High-Fidelity PCR system (Bio-Rad) to amplify the truncated VP8* fragments of rotaviruses with P[8], P[4] or P[6] specificity. The amplified VP8* fragment was cloned into the bacterial expression vector pET28a (Novagen) containing a 6×histidine region for affinity column isolation of recombinant protein. Each PCR product was purified by a QIA-quick gel extraction kit (Qiagen) by agarose gel electrophoresis. Each purified PCR product was cloned into the Nde I and Sac I multiple clone site of the pET28a vector, yielding pET28a-His-ΔVP8*s which encoded amino-acid (aa) residues 65-223 of Wa VP8*, aa 64-223 of DS-1 VP8* or aa 64-223 of 1076 VP8*. Amplification of the recombinant plasmids was conducted by transformation into XL-10 Gold E. coli cells (Stratagene). The integrity and fidelity of amplification were confirmed by DNA sequencing.
2.3. Expression of recombinant protein
The expression plasmids pET28a-His- ΔVP8* with different specificities were transformed into competent E. coli BL21(DE3)pLysS cells by heat shock. A single colony was inoculated into fresh LB broth containing 50μg/ml kanamycin. When absorbance at 600 nm reached 0.5, the expression of His-ΔVP8* was induced by the addition of 1 mM isopropyl-1-thio-β-D-galactoside at 18°C for 5 h. The recombinant E. coli cells harboring ΔVP8* proteins were harvested and stored at −80°C until use.
2.4. Western blot
Proteins were analyzed by 4–12% NuPAGE gel and transferred electrophoretically onto a nitrocellulose membrane (Whatman). The membrane was blocked with TBS containing 5% non-fat milk (W/V) and incubated for 1 h at room temperature followed by rinsing twice with TBS. The membrane was then incubated at 4°C overnight with a hyperimmune guinea pig antiserum raised against strain Wa (P[8]), DS-1 (P[4]) or ST3 (P[6]) diluted at 1:150 with blocking buffer. Following washing three times with TBS containing 0.05% (V/V) Tween-20 (TBS-T), the membrane was incubated with a peroxidase-conjugated goat anti-guinea pig IgG (H+L) (KPL) (1:3,000) for 1 h at room temperature. Following rinsing five times with TBS-T, the membrane was developed by the addition of 3,3′-diaminobenzidine substrate (Sigma).
2.5. Purification of truncated VP8* protein
BugBuster Master Mix (Novagen) containing a protease inhibitor cocktail (Roche) was used to destroy recombinant E. coli cell walls and the soluble product of each fraction was harvested and stored at −80°C until use. The ΔVP8* proteins were purified by using ProBond nickel-NTA agarose affinity chromatography (Invitrogen) as described in the manufacturer’s protocol. The presence of recombinant proteins and their purity were confirmed by SDS-PAGE. The concentration of purified recombinant proteins was measured by BCA assay (Thermo) according to the manufacturer’s instructions. The level of endotoxin in each purified protein was quantified by using Toxinsensor™ Chromogenic LAL Endotoxin Assay Kit (GenScript) according to the manufacturer’s protocols. The recombinant proteins were stored at −80°C until use.
2.6. Immunization of guinea pigs or mice
Outbred female Hartley guinea pigs weighing 500–550 grams were purchased from Charles River Laboratories (Wilmington, MA). Guinea pigs were maintained under animal biosafety level 2 conditions in isolator cages for the entire period of each experiment. All animal experiments were done at the NIH, in compliance with the guidelines of the Institutional Animal Care and Use Committee of the National Institute of Allergy and Infectious Diseases or the National Institute of Child Health and Human Development. Since the immunogenicity of the recombinant truncated VP8* protein was unknown, initially we immunized intramuscularly (IM) two guinea pigs each with 250 μg of the ΔVP8* with P[8], P[4] or P[6] specificity with complete and incomplete Freund’s adjuvants as previously reported [43]. Later, however, we found that the presence of Freund’s adjuvants did not increase the immunogenicity of the ΔVP8* protein significantly. In a dosage-immunogenicity study, 2 or 4 guinea pigs were injected IM with 10 (or 20, 40, 80 or 160) μg of P[8] ΔVP8* 3 times at 2-week intervals without Freund’s adjuvants. Blood samples were collected prior to each immunization as well as 7 days after the third immunization. To evaluate adjuvant effects of aluminum compounds, 10 and 20 μg of Wa P[8] ΔVP8* were mixed with aluminum phosphate (ADJU-PHOS™, Brenntag Biosector) or aluminum hydroxide (ALHYDROGEL® 2%, Brenntag Biosector) at an aluminum content of 100 μg/dose and injected IM into guinea pigs 3 times at 2-week intervals.
For evaluation of immunogenicity in mice, we followed a protocol that resembles infant vaccine administration. Eight weanling 5–6 week-old general-purpose female mice from the NIH colony were injected subcutaneously with 5ug/dose of purified DS-1 P[4] ΔVP8* protein in PBS, 3 times at 2-week intervals. Mice were exsanguinated 1 week after the last injection. Hyperimmune antisera were prepared as described [44].
2.7. Serology
The neutralizing antibody titer of each serum sample was determined by 60% plaque reduction neutralization (PRN) assay as previously described [45], except that the virus-serum mixture was left in the agarose overlay after a one hour adsorption period. Mouse anti-VP8* IgG was measured by enzyme-linked immunosorbent assay (ELISA) using DS-1 P[4] ΔVP8* or full-length Wa P[8] VP8* in PBS as a coating antigen (1 μg/well). Hyperimmune antisera were used as a reference and antibody levels were expressed in ELISA units (EU). The lowest detectable level was assigned 1 EU.
2.9. Statistical analysis
For comparison of (i) geometric mean serum neutralizing antibody titers between different dosages of P[8] ΔVP8* protein and (ii) serum IgG titers elicited by the DS-1P[4] ΔVP8* in mice, the student t-test was used. Statistical significance was assessed at P<0.05.
3. Results
3.1. Expression and purification of recombinant ΔVP8* protein of Wa P[8], DS-1 P[4], or 1076 P[6] strain
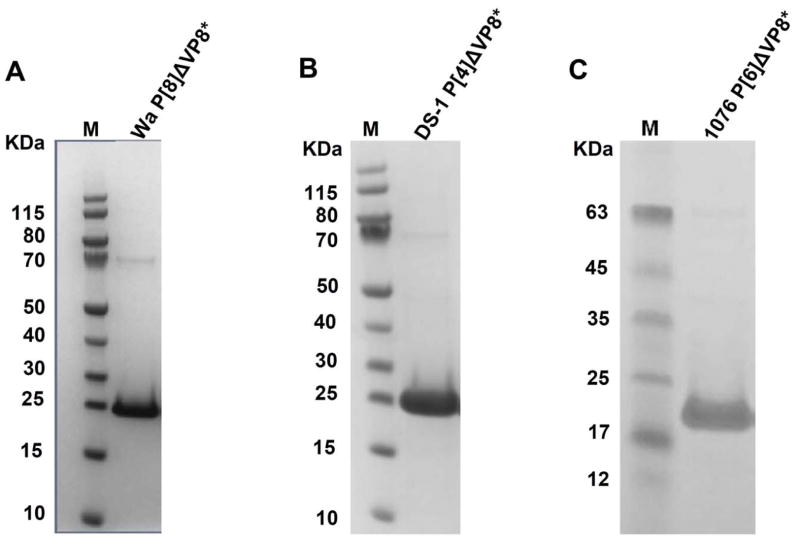
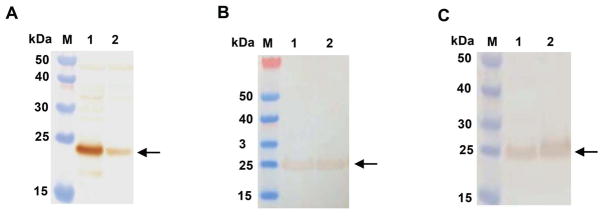
Each of 3 recombinant ΔVP8* proteins (i.e., Wa P[8], DS-1 P[4] or 1076 P[6]) was expressed both in soluble and insoluble (inclusion bodies) forms. We found that the lower induction temperature yielded more soluble proteins (data not shown). The expression of recombinant ΔVP8* protein was confirmed by SDS-PAGE (Fig. 1) and Western blot analyses (Fig. 2). The yield of purified DS-1 P[4] ΔVP8* protein reached ~70mg per liter (L) of culture followed by 1076 P[6] ΔVP8* (~50 mg/L culture) and Wa P[8] ΔVP8* (~40 mg/L culture), although a significant amount of soluble protein was lost during the washing of the column with washing buffer containing various concentrations of imidazole. The yield could be improved further upon optimizing the parameters of induction and purification. The average endotoxin level of three purified ΔVP8* proteins was 1.8 EU/ml (1.813 EU/ml for Wa P[8] ΔVP8*, 1.804 EU/ml for DS-1 P[4]ΔVP8* and 1.818 EU/ml for 1076 P[6] ΔVP8*, respectively) which was far less than the recommended endotoxin level (i.e., a level of <20 EU/ml for recombinant subunit vaccines)[46].
Figure 1.
SDS-PAGE analysis of purified recombinant ΔVP8* proteins Wa P[8] (panel A), DS-1 P[4] (panel B) and 1076 P[6] (panel C). The ΔVP8* proteins were expressed in E. coli, induced at 18°C (Wa P[8] and 1076 P[6]) or at 37°C (DS-1 P[4]) and purified as described in Materials and Methods. Lane M contains molecular size markers.
Figure 2.
Western blot analysis of recombinant ΔVP8* proteins of Wa P[8] (probed with guinea pig anti-Wa strain antiserum, panel A), DS-1 P[4] (probed with guinea pig anti-DS-1 antiserum, panel B) and 1076 P[6] (probed with guinea pig anti-ST3 antiserum, panel C) as described in Materials and Methods. Lanes M contains molecular size markers; lane 1 contains supernatant of lysed recombinant E. coli cells and lane 2 contains pellets of lysed recombinant E. coli cells. Arrows indicate the recombinant ΔVP8* proteins.
3.2. Immunogenicity of recombinant ΔVP8* proteins
Guinea pigs hyperimmunized with purified Wa P[8] ΔVP8* elicited high levels of VP8*-homotypic (range 1:7,680 to 1:5,120) as well as VP8*-heterotypic (1:5,120 vs P[4]) neutralizing antibodies (Table 1). In addition, low levels (1:320 and 1:80) of VP8*-heterotypic neutralizing antibodies were induced against P[6] and P[10], respectively. Similarly, guinea pigs hyperimmunized with purified DS-1 P[4] ΔVP8* elicited high levels of homotypic (1:5,120) and heterotypic (1:1,280 vs P[8]), as well as moderate to low levels (1:320 and1:80 vs P[6] and P[10], respectively) neutralizing antibodies. Guinea pigs hyperimmunized with 1076 P[6] ΔVP8*, developed high levels of VP8*-homotypic (1:1,280) as well as lower heterotypic neutralizing antibodies (1:320 to 1:40 vs P[8] and P[10], respectively). It was of interest that these serological relationships among four different P type strains (i.e., P[4], P[6], P[8] and P[10]) correlated well with molecular relationships among them: Wa P[8] strain shares the highest amino acid identity of 82.4% with DS-1 P[4] strain followed by 64.2% with 1076 P[6] strain and 46.8% with 69M P[10] strain.
Table 1.
Neutralization characteristics of hyperimmune guinea pig antisera raised against ΔVP8* protein with P[8], P[4] or P[6] specificity
| Rotavirus
|
antibody titera of serum raised to indicated subunit protein
|
|||
|---|---|---|---|---|
| Strain | P and G type | Wa P[8]ΔVP8* | DS-1 P[4] ΔVP8* | 1076 P[6] ΔVP8* |
| Wa | P[8]G1 | 7680b | 1280 | 320 |
| P | P[8]G3 | 5120 | 1280 | 160 |
| WI61 | P[8]G9 | 5120 | 1280 | 160 |
| DS-1 | P[4]G2 | 5120 | 5120 | 240 |
| M37 | P[6]G1 | 320 | 320 | 1280 |
| 69M | P[10]G8 | 80 | 80 | 40 |
60% plaque reduction neutralizing antibody titer (reciprocal)
Homotypic values in bold
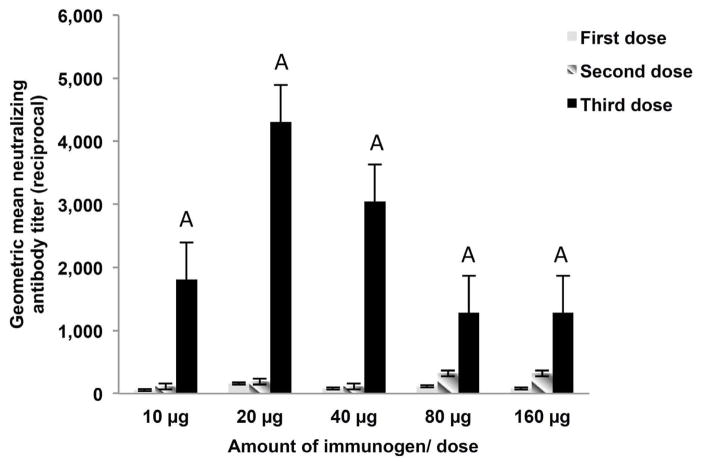
In a dosage-immunogenicity study, two IM immunizations of 10μg (or 20, 40, 80 or 160 μg) Wa P[8] ΔVP8*/ injection without adjuvant did not induce in guinea pigs any detectable levels of neutralizing antibodies (Fig. 3). However, after the third dose at 10–40 ug/injection, guinea pigs developed high level of homologous neutralizing antibodies (GM range from 1:1,810 to 1:4,305). Unexpectedly, levels of neutralizing antibodies at higher dosages (80 μg to 160 μg/injection) were lower (1:1,280), though not statistically significant (P>0.05). The addition of aluminum phosphate or aluminum hydroxide adjuvant to Wa P[8] ΔVP8* did not enhance the immune response significantly (data not shown).
Figure 3.
Dosage-immunogenicity experiment of recombinant Wa P[8] ΔVP8* protein in guinea pigs. Guinea pigs were immunized IM with 10 μg (n=4), 20 μg (n=4), 40 μg (n=4), 80 μg (n=2) or 160 μg (n=2)/dose of the purified P[8]ΔVP8* protein 3 times at 2-week intervals as described in Materials and Methods. The error bars represent standard errors of the means. Columns with letter A do not differ significantly.
Mice immunized with DS-1 ΔVP8* developed high levels of homotypic (i.e., DS-1 P[4] ΔVP8*) as well as moderate to low levels of heterotypic (i.e., Wa P[8] ΔVP8*) IgG antibodies (Table 2). In addition, selected mouse sera (mouse # 7 and #8) showed both homologous (1:160 vs DS-1 strain) and heterologous (1:80 vs Wa strain) neutralizing activities.
Table 2.
IgG enzyme-linked immunosorbent assay unit (EU) of serum samples derived from mice immunized subcutaneously three times with DS-1 P[4]ΔVP8* protein
| Mouse # | IgG antibody in EU to indicated protein
|
|
|---|---|---|
| DS-1 P[4•] VP8* | Wa P[8] VP8*c | |
| 1 | 692 | 111 |
| 2 | 271 | 24 |
| 3 | 28 | 7 |
| 4 | 175 | 179 |
| 5 | 25 | 47 |
| 6 | 69 | 22 |
| 7 | 126 (160a) | 16 (80b) |
| 8 | 830 (160a) | 16 (80b) |
| Geometic mean | 140 | 31 |
Reciprocal of 60% PRN antibody titer to DS-1 strain
Reciprocal of 60% PRN antibody titer to Wa strain
Full-length Wa VP8* was used to coat ELISA plate.
Note: Serum samples from mice 1–6 were not tested by neutralization assay.
4. Discussion
In consideration of various concerns associated with live oral rotavirus vaccines, we generated recombinant subunit rotavirus vaccine candidates (i.e., ΔVP8* protein with P[8], P[4] or P[6] specificity) designed to be delivered parenterally since parenteral vaccines in general are free from various concerns associated with live oral vaccines and may work better in underdeveloped regions where the vaccine is needed most. Three doses of 10 μg or 20 μg of our ΔVP8* parenteral vaccines without adjuvant elicited in guinea pigs high levels of homotypic as well as variable levels of heterotypic neutralizing antibodies against different strains. In addition, weanling mice immunized with DS-1 P[4]ΔVP8* vaccine by clinically relevant dosage, route and schedule developed high levels of VP8*-specific serum IgG antibodies. Thus, we have shown that our ΔVP8* proteins may be plausible parenteral rotavirus vaccines.
In general, the yield of recombinant protein vaccine candidates prepared from the E. coli expression system is significantly higher than that prepared from other expression systems including baculovirus-insect cell expression and mammalian cell expression systems. More importantly, the E. coli system costs less and takes a shorter time to generate recombinant proteins than other systems. Rotavirus ΔVP8* is not a glycoprotein and can be expressed very well in E. coli. Previously, Kovacs-Nolan et al. (2001) generated in E. coli a full-length P[8] VP8* of Wa strain in the form of a fusion protein with GST protein and obtained a soluble protein of 1.8mg/L bacterial culture [33]. Similarly, Bellido et al. (2009) expressed the Brucella spp. lumazine synthase-truncated bovine rotavirus VP8* chimeric protein in E. coli and obtained ~15 mg/L of a soluble protein [35]. In the current study, we obtained a soluble ΔVP8* protein in high yields, ranging from ~70mg/L of DS-1 P[4] VP8* to ~40mg/L of Wa P[8] VP8*.
Although immunity to rotavirus is not completely understood, since rotavirus primarily infects mature villus epithelial cells of the small intestine, mucosal immunity is considered to play a critical role in the prevention of diseases caused by rotavirus infection [47–51]. Administration of live oral rotavirus vaccines that mimics natural rotavirus infection has been well documented to be effective in inducing protection against rotavirus disease, presumably because it can elicit both mucosal and systemic immune responses. On the other hand, parenteral administration of a subunit vaccine, in general, elicits primarily a systemic immune response. It is noteworthy, however, that the role of serum antibodies in preventing rotavirus infections has been demonstrated in various animal models [52–54]. Recently, Westerman and his colleagues (2005) demonstrated that passively transferred circulating antibodies with certain levels of rotavirus-specific IgG or neutralizing activities were associated with mucosal immunity against rotavirus infection in a non-human primate model [55]. These findings have provided clear implications for the development of parenteral rotavirus vaccines.
It has been well established that rotavirus G-P combinations G1P[8], G2P[4], G3P[8] and G4P[8] are of global epidemiologic importance with G1P[8] being the most important [56]. Currently available two live oral rotavirus vaccines are selected or designed to provide antigenic coverage to such epidemiologically important G and P types since data obtained from experimental animal studies as well as vaccine clinical trials and volunteer studies appear to suggest that the induction of serotype-specific immunity may be important for optimal protection [57,58]. It is of note that recently, unusual G and P types causing a high incidence of human infection have been reported in various regions of the world including G8, G9, G10, G12, and P[6] [59–66] that are not covered serotypically by Rotarix® and RotaTeq®. Since in nature, almost all human rotavirus G types have been detected in combination with P[8], P[4] or P[6] specificity, the P[8], P[4] and P[6] ΔVP8* proteins generated in this study could be used at a minimum singly or preferably in multivalent formulations of two or more components to provide antigenic coverage to almost all the G (VP7) types of global as well as regional epidemiologic importance.
Highlights.
We generated rotavirus subunit ΔVP8* vaccine with P[8], P[6] or P[4] specificity.
Each ΔVP8* protein was highly soluble and was generated in high yield in E. coli.
Each ΔVP8* protein induced high levels of neutralizing antibodies in guinea pigs.
These ΔVP8* parenteral vaccines may be more effective in low-income countries.
Acknowledgments
We thank Elias Gonzales for technical assistance. We extend our appreciation to Dr. Albert Z. Kapikian for his support to this project. This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases (NIAID) and the National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH), USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Estes MK, Kapikian AZ, In Knipe DM, Griffin DE, Lamb RA, Straus SE, et al. Fields Virology. 5. Vol. 2. Philadelphia, PA: Lippincott Williams&Wilkins; 2007. Rotaviruses; pp. 1917–4. [Google Scholar]
- 2.Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Expert Opin Biol Ther. 2009;9(9):1235–1240. doi: 10.1016/S1473-3099(11)70253-5. [DOI] [PubMed] [Google Scholar]
- 3.Velazquez FR, Matson DO, Calva JJ, Guerrero L, Morrow AL, Carter-Campbell S, et al. Rotavirus infections in infants as protection against subsequent infections. N Engl J Med. 1996;335(14):1022–8. doi: 10.1056/NEJM199610033351404. [DOI] [PubMed] [Google Scholar]
- 4.McCormack PL, Keam SJ. Rotavirus vaccine RIX4414 (Rotarix): a review of its use in the prevention of rotavirus gastroenteritis. Paediatr Drugs. 2009;11(1):75–88. doi: 10.2165/0148581-200911010-00025. [DOI] [PubMed] [Google Scholar]
- 5.Chandran A, Santosham M. RotaTeq: a three-dose oral pentavalent reassortant rotavirus vaccine. Expert Rev Vaccines. 2008;7(10):1475–80. doi: 10.1586/14760584.7.10.1475. [DOI] [PubMed] [Google Scholar]
- 6.Madhi SA, Cunliffe NA, Steele D, Witte D, Kirsten M, Louw C, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med. 2010;362(4):289–98. doi: 10.1056/NEJMoa0904797. [DOI] [PubMed] [Google Scholar]
- 7.Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, Feikin DR, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376(9741):606–14. doi: 10.1016/S0140-6736(10)60889-6. [DOI] [PubMed] [Google Scholar]
- 8.Zaman K, Dang DA, Victor JC, Shin S, Yunus M, Dallas MJ, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376(9741):615–23. doi: 10.1016/S0140-6736(10)60755-6. [DOI] [PubMed] [Google Scholar]
- 9.Patel M, Pedreira C, De Oliveira LH, Tate J, Orozco M, Mercado J, et al. Association between pentavalent rotavirus vaccine and severe rotavirus diarrhea among children in Nicaragua. JAMA. 2009;301(21):2243–51. doi: 10.1001/jama.2009.756. [DOI] [PubMed] [Google Scholar]
- 10.Rotavirus vaccine and intussusception: report from an expert consultation. Wkly Epidemiol Rec. 2011;86(30):317–21. [PubMed] [Google Scholar]
- 11.Victoria JG, Wang C, Jones MS, Jaing C, McLoughlin K, Gardner S, et al. Viral nucleic acids in live-attenuated vaccines: detection of minority variants and an adventitious virus. J Virol. 2010;84(12):6033–40. doi: 10.1128/JVI.02690-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McClenahan SD, Krause PR, Uhlenhaut C. Molecular and infectivity studies of porcine circovirus in vaccines. Vaccine. 2011;29(29–30):4745–53. doi: 10.1016/j.vaccine.2011.04.087. [DOI] [PubMed] [Google Scholar]
- 13.Beach NM, Cordoba L, Kenney SP, Meng XJ. Productive infection of human hepatocellular carcinoma cells by porcine circovirus type 1. Vaccine. 2011;29(43):7303–6. doi: 10.1016/j.vaccine.2011.06.097. [DOI] [PubMed] [Google Scholar]
- 14.Patel NC, Hertel PM, Estes MK, de la Morena M, Petru AM, Noroski LM, et al. Vaccine-acquired rotavirus in infants with severe combined immunodeficiency. N Engl J Med. 2010;362(4):314–9. doi: 10.1056/NEJMoa0904485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bakare N, Menschik D, Tiernan R, Hua W, Martin D. Severe combined immunodeficiency (SCID) and rotavirus vaccination: reports to the Vaccine Adverse Events Reporting System (VAERS) Vaccine. 2010;28(40):6609–12. doi: 10.1016/j.vaccine.2010.07.039. [DOI] [PubMed] [Google Scholar]
- 16.Addition of severe combined immunodeficiency as a contraindication for administration of rotavirus vaccine. MMWR Morb Mortal Wkly Rep. 2010;59(22):687–8. [PubMed] [Google Scholar]
- 17.Payne DC, Edwards KM, Bowen MD, Keckley E, Peters J, Esona MD, et al. Sibling Transmission of Vaccine-Derived Rotavirus (RotaTeq) Associated With Rotavirus Gastroenteritis. Pediatrics. 2010;125:438–41. doi: 10.1542/peds.2009-1901. [DOI] [PubMed] [Google Scholar]
- 18.Bellinzoni RC, Blackhall J, Baro N, Auza N, Mattion N, Casaro A, et al. Efficacy of an inactivated oil-adjuvanted rotavirus vaccine in the control of calf diarrhoea in beef herds in Argentina. Vaccine. 1989;7(3):263–8. doi: 10.1016/0264-410X(89)90241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang B, Gentsch JR, Glass RI. Inactivated rotavirus vaccines: a priority for accelerated vaccine development. Vaccine. 2008;26(52):6754–8. doi: 10.1016/j.vaccine.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Conner ME, Zarley CD, Hu B, Parsons S, Drabinski D, Greiner S, et al. Virus-like particles as a rotavirus subunit vaccine. J Infect Dis. 1996;174 (Suppl 1):S88–92. doi: 10.1093/infdis/174.supplement_1.s88. [DOI] [PubMed] [Google Scholar]
- 21.Vieira HL, Estevao C, Roldao A, Peixoto CC, Sousa MF, Cruz PE, et al. Triple layered rotavirus VLP production: kinetics of vector replication, mRNA stability and recombinant protein production. J Biotechnol. 2005;120(1):72–82. doi: 10.1016/j.jbiotec.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 22.Ward RL, McNeal MM. VP6: A candidate rotavirus vaccine. J Infect Dis. 2010;202 (Suppl):S101–7. doi: 10.1086/653556. [DOI] [PubMed] [Google Scholar]
- 23.Blazevic V, Lappalainen S, Nurminen K, Huhti L, Vesikari T. Norovirus VLPs and rotavirus VP6 protein as combined vaccine for childhood gastroenteritis. Vaccine. 2011;29(45):8126–33. doi: 10.1016/j.vaccine.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 24.Ma Y, Qin M, Hu HQ, Ji G, Feng L, Gao N, et al. Immunogenicity of sabin inactivated poliovirus vaccine induced by diphtheria-tetanus-acellular pertussis and Sabin inactivated poliovirus combined vaccine. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2011;25(3):197–200. [PubMed] [Google Scholar]
- 25.Lee SY, Hwang HS, Kim JH, Kim HH, Lee HS, Chung EH, et al. Immunogenicity and safety of a combined diphtheria, tetanus, acellular pertussis, and inactivated poliovirus vaccine (DTaP-IPV) compared to separate administration of standalone DTaP and IPV vaccines: a randomized, controlled study in infants in the Republic of Korea. Vaccine. 2011;29(8):1551–7. doi: 10.1016/j.vaccine.2010.12.094. [DOI] [PubMed] [Google Scholar]
- 26.Kouiavskaia D, Collett MS, Dragunsky EM, Sarafanov A, Chumakov KM. Immunogenicity of inactivated polio vaccine with concurrent antiviral V-073 administration in mice. Clin Vaccine Immunol. 2011;18(8):1387–90. doi: 10.1128/CVI.05147-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frampton JE. DTaP(5)-IPV-Hib Vaccine (Pediacel(R)) Paediatr Drugs. 2011;13(6):401–15. doi: 10.2165/11207740-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 28.Hewlett AT. Combined hepatitis A and B vaccine: providing a bright future for preventing hepatitis. Expert Opin Biol Ther. 2009;9(9):1235–40. doi: 10.1517/14712590903160639. [DOI] [PubMed] [Google Scholar]
- 29.Larralde G, Li BG, Kapikian AZ, Gorziglia M. Serotype-specific epitope(s) present on the VP8 subunit of rotavirus VP4 protein. J Virol. 1991;65(6):3213–8. doi: 10.1128/jvi.65.6.3213-3218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arias CF, Lizano M, Lopez S. Synthesis in Escherichia coli and immunological characterization of a polypeptide containing the cleavage sites associated with trypsin enhancement of rotavirus SA11 infectivity. J Gen Virol. 1987;68 ( Pt 3):633–42. doi: 10.1099/0022-1317-68-3-633. [DOI] [PubMed] [Google Scholar]
- 31.Dunn SJ, Fiore L, Werner RL, Cross TL, Broome RL, Ruggeri FM, et al. Immunogenicity, antigenicity, and protection efficacy of baculovirus expressed VP4 trypsin cleavage products, VP5(1)* and VP8* from rhesus rotavirus. Arch Virol. 1995;140(11):1969–78. doi: 10.1007/BF01322686. [DOI] [PubMed] [Google Scholar]
- 32.Gil MT, de Souza CO, Asensi M, Buesa J. Homotypic protection against rotavirus-induced diarrhea in infant mice breast-fed by dams immunized with the recombinant VP8* subunit of the VP4 capsid protein. Viral Immunol. 2000;13(2):187–200. doi: 10.1089/vim.2000.13.187. [DOI] [PubMed] [Google Scholar]
- 33.Kovacs-Nolan J, Sasaki E, Yoo D, Mine Y. Cloning and expression of human rotavirus spike protein, VP8*, in Escherichia coli. Biochem Biophys Res Commun. 2001;282(5):1183–8. doi: 10.1006/bbrc.2001.4717. [DOI] [PubMed] [Google Scholar]
- 34.Perez Filgueira DM, Mozgovoj M, Wigdorovitz A, Dus Santos MJ, Parreno V, Trono K, et al. Passive protection to bovine rotavirus (BRV) infection induced by a BRV VP8* produced in plants using a TMV-based vector. Arch Virol. 2004;149(12):2337–48. doi: 10.1007/s00705-004-0379-7. [DOI] [PubMed] [Google Scholar]
- 35.Bellido D, Craig PO, Mozgovoj MV, Gonzalez DD, Wigdorovitz A, Goldbaum FA, et al. Brucella spp. lumazine synthase as a bovine rotavirus antigen delivery system. Vaccine. 2009;27(1):136–45. doi: 10.1016/j.vaccine.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 36.Marelli B, Perez AR, Banchio C, de Mendoza D, Magni C. Oral immunization with live Lactococcus lactis expressing rotavirus VP8 subunit induces specific immune response in mice. J Virol Methods. 2011;175(1):28–37. doi: 10.1016/j.jviromet.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 37.Tan M, Huang P, Xia M, Fang PA, Zhong W, McNeal M, et al. Norovirus P particle, a novel platform for vaccine development and antibody production. J Virol. 2011;85(2):753–64. doi: 10.1128/JVI.01835-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kapikian AZ, Hoshino Y, Chanock RM, In Knipe DM, Howley PM, Griffin DE, et al. Fields Virology. 4. Vol. 2. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. Rotaviruses; pp. 1787–833. [Google Scholar]
- 39.Kraschnefski MJ, Scott SA, Holloway G, Coulson BS, von Itzstein M, Blanchard H. Cloning, expression, purification, crystallization and preliminary X-ray diffraction analysis of the VP8* carbohydrate-binding protein of the human rotavirus strain Wa. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2005;61(Pt 11):989–93. doi: 10.1107/S1744309105032999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wyatt RG, James WD, Bohl EH, Theil KW, Saif LJ, Kalica AR, et al. Human rotavirus type 2: cultivation in vitro. Science. 1980;207(4427):189–91. doi: 10.1126/science.6243190. [DOI] [PubMed] [Google Scholar]
- 41.Kalica AR, Sereno MM, Wyatt RG, Mebus CA, Chanock RM, Kapikian AZ. Comparison of human and animal rotavirus strains by gel electrophoresis of viral RNA. Virology. 1978;87(2):247–55. doi: 10.1016/0042-6822(78)90130-7. [DOI] [PubMed] [Google Scholar]
- 42.Hoshino Y, Wyatt RG, Flores J, Midthun K, Kapikian AZ. Serotypic characterization of rotaviruses derived from asymptomatic human neonatal infections. J Clin Microbiol. 1985;21(3):425–30. doi: 10.1128/jcm.21.3.425-430.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wyatt RG, Greenberg HB, James WD, Pittman AL, Kalica AR, Flores J, et al. Definition of human rotavirus serotypes by plaque reduction assay. Infect Immun. 1982;37(1):110–5. doi: 10.1128/iai.37.1.110-115.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sommerville RG. The production of fluorescent antibody reagents for virus diagnosis in the albino mouse. I. Hyperimmune anti-species serum. Archiv fur die gesamte Virusforschung. 1967;20(4):445–51. doi: 10.1007/BF01275225. [DOI] [PubMed] [Google Scholar]
- 45.Hoshino Y, Jones RW, Kapikian AZ. Serotypic characterization of outer capsid spike protein VP4 of vervet monkey rotavirus SA11 strain. Arch Virol. 1998;143(6):1233–44. doi: 10.1007/s007050050371. [DOI] [PubMed] [Google Scholar]
- 46.Brito LA, Singh M. Acceptable levels of endotoxin in vaccine formulations during preclinical research. J Pharm Sci. 2011;100(1):34–7. doi: 10.1002/jps.22267. [DOI] [PubMed] [Google Scholar]
- 47.Offit PA. Host factors associated with protection against rotavirus disease: the skies are clearing. J Infect Dis. 1996;174 (Suppl 1):S59–64. doi: 10.1093/infdis/174.supplement_1.s59. [DOI] [PubMed] [Google Scholar]
- 48.Matson DO. Protective immunity against group A rotavirus infection and illness in infants. Arch Virol Suppl. 1996;12:129–39. doi: 10.1007/978-3-7091-6553-9_15. [DOI] [PubMed] [Google Scholar]
- 49.Yuan L, Saif LJ. Induction of mucosal immune responses and protection against enteric viruses: rotavirus infection of gnotobiotic pigs as a model. Vet Immunol Immunopathol. 2002;87(3–4):147–60. doi: 10.1016/S0165-2427(02)00046-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Franco MA, Angel J, Greenberg HB. Immunity and correlates of protection for rotavirus vaccines. Vaccine. 2006;24(15):2718–31. doi: 10.1016/j.vaccine.2005.12.048. [DOI] [PubMed] [Google Scholar]
- 51.Desselberger U, Huppertz HI. Immune responses to rotavirus infection and vaccination and associated correlates of protection. J Infect Dis. 2011;203(2):188–95. doi: 10.1093/infdis/jiq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Conner ME, Crawford SE, Barone C, Estes MK. Rotavirus vaccine administered parenterally induces protective immunity. J Virol. 1993;67(11):6633–41. doi: 10.1128/jvi.67.11.6633-6641.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McNeal MM, Rae MN, Conner ME, Ward RL. Stimulation of local immunity and protection in mice by intramuscular immunization with triple- or double-layered rotavirus particles and QS-21. Virology. 1998;243(1):158–66. doi: 10.1006/viro.1998.9060. [DOI] [PubMed] [Google Scholar]
- 54.Wang Y, Azevedo M, Saif LJ, Gentsch JR, Glass RI, Jiang B. Inactivated rotavirus vaccine induces protective immunity in gnotobiotic piglets. Vaccine. 2010;28(33):5432–6. doi: 10.1016/j.vaccine.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 55.Westerman LE, McClure HM, Jiang B, Almond JW, Glass RI. Serum IgG mediates mucosal immunity against rotavirus infection. Proc Natl Acad Sci U S A. 2005;102(20):7268–73. doi: 10.1073/pnas.0502437102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Santos N, Hoshino Y. Global distribution of rotavirus serotypes/genotypes and its implication for the development and implementation of an effective rotavirus vaccine. Rev Med Virol. 2005;15(1):29–56. doi: 10.1002/rmv.448. [DOI] [PubMed] [Google Scholar]
- 57.Kapikian AZ, Hoshino Y. To serotype or not to serotype: that is still the question. J Infect Dis. 2007;195(5):611–4. doi: 10.1086/510862. [DOI] [PubMed] [Google Scholar]
- 58.Linhares AC, Velazquez FR, Perez-Schael I, Saez-Llorens X, Abate H, Espinoza F, et al. Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomised, double-blind, placebo-controlled phase III study. Lancet. 2008;371(9619):1181–9. doi: 10.1016/S0140-6736(08)60524-3. [DOI] [PubMed] [Google Scholar]
- 59.Holmes JL, Kirkwood CD, Gerna G, Clemens JD, Rao MR, Naficy AB, et al. Characterization of unusual G8 rotavirus strains isolated from Egyptian children. Arch Virol. 1999;144(7):1381–96. doi: 10.1007/s007050050594. [DOI] [PubMed] [Google Scholar]
- 60.Sanz JC, Barbas JF, Lasheras MD, Jimenez M, Ramos B, Sanchez-Fauquier A. Detection of a rotavirus G9P[8] outbreak causing gastroenteritis in a geriatric nursing home. Enferm Infecc Microbiol Clin. 2009;27(4):219–21. doi: 10.1016/j.eimc.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 61.Mukherjee A, Dutta D, Ghosh S, Bagchi P, Chattopadhyay S, Nagashima S, et al. Full genomic analysis of a human group A rotavirus G9P[6] strain from Eastern India provides evidence for porcine-to-human interspecies transmission. Arch Virol. 2009;154(5):733–46. doi: 10.1007/s00705-009-0363-3. [DOI] [PubMed] [Google Scholar]
- 62.Cunliffe NA, Ngwira BM, Dove W, Nakagomi O, Nakagomi T, Perez A, et al. Serotype g12 rotaviruses, Lilongwe, Malawi. Emerg Infect Dis. 2009;15(1):87–90. doi: 10.3201/eid1501.080427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Castello AA, Nakagomi T, Nakagomi O, Jiang B, Kang JO, Glass RI, et al. Characterization of genotype P[9]G12 rotavirus strains from Argentina: high similarity with Japanese and Korean G12 strains. J Med Virol. 2009;81(2):371–81. doi: 10.1002/jmv.21384. [DOI] [PubMed] [Google Scholar]
- 64.Matthijnssens J, Rahman M, Van Ranst M. Two out of the 11 genes of an unusual human G6P[6] rotavirus isolate are of bovine origin. J Gen Virol. 2008;89(Pt 10):2630–5. doi: 10.1099/vir.0.2008/003780-0. [DOI] [PubMed] [Google Scholar]
- 65.Rahman M, Sultana R, Ahmed G, Nahar S, Hassan ZM, Saiada F, et al. Prevalence of G2P[4] and G12P[6] rotavirus, Bangladesh. Emerg Infect Dis. 2007;13(1):18–24. doi: 10.3201/eid1301.060910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Steele AD, Ivanoff B. Rotavirus strains circulating in Africa during 1996–1999: emergence of G9 strains and P[6] strains. Vaccine. 2003;21(5–6):361–7. doi: 10.1016/s0264-410x(02)00616-3. [DOI] [PubMed] [Google Scholar]