Abstract
Pro-inflammatory cytokines, such as interleukin-6 (IL-6), have been implicated in the underlying processes contributing to sleep regulation and fatigue. Despite evidence for sleep difficulties, fatigue, and elevations in IL-6 among women with ovarian cancer, the association between these symptoms and IL-6 has not been investigated. To address this knowledge gap, we examined relationships between sleep disturbance, fatigue, and plasma IL-6 in 136 women with ovarian cancer prior to surgery. These relationships were also examined in 63 of these women who were disease-free and not receiving chemotherapy one year post-diagnosis. At both time-points, higher levels of IL-6 were significantly associated with sleep disturbances (p < .05), controlling for potentially confounding biological and psychosocial covariates. Higher IL-6 was significantly associated with fatigue prior to surgery (p < .05); however, when sleep disturbance was included in the model, the relationship was no longer significant. IL-6 was not significantly associated with fatigue at one year. Changes in sleep over time were significantly associated with percent change in IL-6 from pre-surgery to one year, adjusting for covariates (p < .05). These findings support a direct association of IL-6 with sleep disturbances in this population, whereas the relationship between IL-6 and fatigue prior to surgery may be mediated by poor sleep. As this study is the first to examine cytokine contributions to sleep and fatigue in ovarian cancer, further research is warranted to clarify the role of biological correlates of sleep and fatigue in this population.
Keywords: Sleep, Ovarian Cancer, Interleukin-6, Cancer, Fatigue, Circadian rhythm, Sickness Behaviors
1. Introduction
High levels of both sleep disturbances and fatigue have been documented in women with ovarian cancer (Clevenger et al., under review; Anderson and Hacker, 2008; Sandadi et al., 2011). The co-occurrence of these symptoms in oncology patients has been well established (Roscoe et al., 2007) and both of these symptoms have been associated with poor quality of life in ovarian (Sandadi et al., 2011; Clevenger et al, under review; Holzner et al., 2003) and other cancer populations (Fiorentino and Ancoli-Israel, 2007; Ancoli-Israel et al., 2001).
Although inflammatory cytokines have been associated with both fatigue and sleep disturbances, the role of inflammatory cytokines with respect to these burdensome symptoms has not been investigated in ovarian cancer, a disease characterized by high levels of systemic pro-inflammatory cytokines that are thought to be tumor-derived (Tempfer et al., 1997). Pro-inflammatory cytokines such as interleukin-1 (IL-1), interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) are implicated in the regulation of sleep (Opp and Toth, 2003), modulation of sleep architecture (Kapas et. al, 1992; Opp et al., 1991), and appear to be involved in circadian regulation of sleep as well (Guess et al., 2009; Vgontzas et al., 2005). Sleep deprivation increases monocyte production of IL-6 (Irwin et al., 2006), and a daytime nap decreases IL-6 in individuals with nighttime sleep loss (Vgontzas et al., 2007), leading to suggestions of a bi-directional feedback loop between sleep and cytokine expression (Irwin, 2002).
Presence of fatigue has been described as a “pre-diagnostic symptom” for ovarian cancer, as it is one of the most commonly reported recurring symptoms prior to diagnosis of ovarian cancer (Goff et al., 2004; Goff et al., 2000). Though fatigue may be a consequence of poor sleep, cancer-related fatigue has been described as unique as it is persistent, intense, longer in duration, and not alleviated by rest as compared to more traditional fatigue (Bower, 2007). Tumor- and/or treatment-associated cytokines have a proposed role in cancer-related fatigue via effects on central nervous system pathways that elicit vegetative behaviors (Collado-Hidalgo et al., 2006; Bower et al., 2002; Scott et al., 2002; Dantzer, 2001). Supporting such hypotheses are findings that fatigued breast cancer survivors demonstrated significantly higher elevations of cytokines including IL-1ra, TNF-α, sTNF-RII, IL-6 and neopterin than non-fatigued survivors (Bower et al., 2002; Collado-Hidalgo et al., 2006; Bower, 2007), and circulating levels of IL-6, IL-1ra, and neopterin have been associated with fatigue in a quantitative review of cancer patients (Schubert et al., 2007).
Although the presence of sleep disturbances and fatigue has been well documented in women with ovarian cancer, biological and psychological mechanisms which may contribute to fatigue and sleep problems in ovarian cancer are poorly understood. To better understand these mechanisms, this study examined associations between circulating levels of IL-6, and self-reported symptoms of fatigue and disturbed sleep in ovarian cancer patients. We hypothesized that higher levels of IL-6 would be associated with greater fatigue and greater sleep disturbance, and that IL-6 would be associated with fatigue, independent of sleep disturbance. These hypotheses were examined prior to surgery, when effects of tumor-derived cytokines would not be confounded with the effects of chemotherapy, and at one year following surgery when participating patients had completed adjuvant treatment and showed no evidence of disease. We also investigated whether changes over time in IL-6 would be associated with changes over time in sleep and fatigue.
2. Methods
2.1 Participants
Women with a pelvic mass suspicious for ovarian cancer were recruited at a pre-surgical clinic visit as part of a larger study examining psychosocial factors, pro-angiogenic biomarkers and cancer progression. Exclusion criteria included use of systemic corticosteroid medication in the previous month, history of previous cancer, current pregnancy, inability to accurately answer questions (dementia), presence of a comorbid condition with known effects on the immune system, age less than 18 years, and non-ovarian primary site or metastases to the ovary from another site. Women completed psychosocial questionnaires between recruitment and surgery. Participants had a blood draw the morning of surgery between approximately 5:30 am and 12:00 pm; blood draws were typically completed two hours prior to surgery. Approximately 25 mL of blood was collected into heparin-containing vacutainer tubes (Becton Dickinson, Rutherford, NJ). Blood draws were obtained prior to administration of anesthetics or pre-surgical medications. Clinical information was extracted from medical records. Participants were included if their date of surgery would have permitted them to have a one year assessment by the time of data analysis, and if they had a valid IL-6 value at one year.
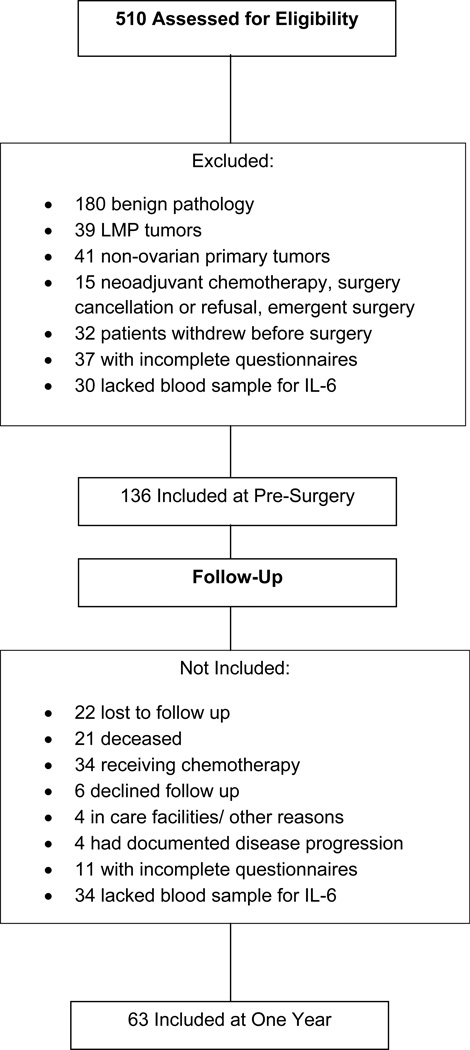
The final baseline sample included 136 participants. (See Figure 1 for patient flow). Three patients had fatigue data at one year but not at baseline; thus one year analyses covarying for baseline data included 63 patients for sleep analyses and 60 patients for fatigue analyses. As some patients were missing IL-6 data at baseline, analyses examining change scores included 50 for global sleep (13 missing IL-6 at baseline) analyses and 53 for fatigue (7 missing IL-6 at baseline) analyses. This research was approved by the institutional review boards at The University of Iowa and The University of Miami.
Figure 1.
Patient inclusion chart for participants at pre-surgery and one year follow up.
2.2 Psychosocial Measures
2.2.1. Sleep Quality
The Pittsburgh Sleep Quality Index (PSQI) is a 19-item self-report measure assessing types and frequency of sleep disturbances over a one-month interval (Buysse et al., 1989). A global score is obtained as the sum of the seven component scores: subjective sleep quality, sleep latency, habitual sleep efficiency, nighttime disturbances, sleep duration, use of sleep medications, and daytime dysfunction. A global score greater than 5 is considered to characterize poor sleep (Buysee et al., 1989). The scale has a diagnostic sensitivity of 89.6% and specificity of 86.5% (Carpenter and Andrykowski, 1998). The scale is psychometrically sound in cancer patients (Beck et al., 2004) and correlates with sleep log data in primary insomnia (Backhaus et al., 2002). As the global score includes a “daytime dysfunction” component that has some conceptual overlap with fatigue, we also calculated a modified sleep index eliminating the “daytime dysfunction” component to enable examination of effects of sleep on fatigue without this overlap. Use of sleep medications was also excluded from the modified sleep index as this was included as a covariate in regressions.
2.2.2. Fatigue
The Profile of Mood States - Short Form (POMS-SF) is a 37 item inventory of mood (Shacham, 1983) often used in research with cancer patients (Mendoza et al., 1999; Bradley et al., 2006). Six dimensions of mood are assessed: anxiety, depression, anger, vigor, fatigue, and confusion as well as a total mood disturbance scale. Participants rated statements describing fatigue (i.e. “Bushed,” “Fatigued”) cued to the previous week on a scale of 0 (“not at all”) to 4 (“extremely”). The POMS-SF has been psychometrically evaluated in cancer patients (Baker et al., 2002) and has demonstrated convergent validity with a variety of measures of fatigue in cancer patients, including the Multidimensional Fatigue Symptom Inventory and the Fatigue Symptom Inventory (Schwartz, 2002). The POMS-SF fatigue subscale was used as the measure of fatigue in this study.
2.2.3. Mood
The Center for Epidemiological Studies Depression Scale (CES-D) (Radloff, 1977) is a 20-item self-report measure rating mood during the previous week. Higher scores indicate greater depressive symptomatology (Radloff, 1977). The CES-D has been shown to be a valid and reliable measure for depressive symptoms in cancer populations (Hann et al., 1999; Schroevers et al., 2000). Four subscales have been described and validated by factor analysis (Sheehan et al., 1995). In this study, the CESD depressive mood subscale was utilized to obtain a measure of depressive symptoms independent of vegetative depression items.
Anxiety/Intrusive Thought
Thought intrusions were assessed with the Impact of Event Scale (IES) intrusion subscale, which served as an indicator of anxiety as intrusive thoughts are closely associated with anxiety in cancer patients (Baider and Kaplan De Nour, 1997). Participants indicated the extent to which unwelcome thoughts relevant to their ovarian cancer intruded into their awareness over the past week on a scale of “not at all” to “often” (Horowitz et al., 1979). The IES has been frequently used to examine distress and PTSD in cancer patients (Golden-Kreutz et al., 2005; Epping-Jordan et al., 1994).
As CESD depressive mood and thought intrusions were each significantly associated with global sleep and fatigue (all p<.001) and highly correlated with each other (pre-surgery: r=.53, p<.001; one year: r=.63, p<.001), a mood composite was created from the sum of the z-scores for the CES-D depressive mood and IES intrusion subscales. This was used as a covariate for all analyses.
2.2.4. Demographics and Health Behaviors
Self-reported demographic information on age, education, race, ethnicity, and marital status was collected prior to surgery as was information on caffeine and alcohol intake.
2.3 Cytokines
Detection of IL-6 in plasma was performed by enzyme-linked immunosorbent assay (R&D Diagnostics, Minneapolis, MN), with results interpolated from the standard curve provided with the kit. The minimum detectable level is less than 0.7 pg/mL and interassay variability ranges from 3.3% to 6.4%. IL-6 samples below the sensitivity of the regular assay were quantitated with the R&D High-Sensitivity ELISA. IL-6 levels in plasma are highly correlated with tumor levels, and are thought to represent the amount produced by tumor (Burger et al., 1995; Stone et al., 2012). IL-6 levels were not correlated with sampling time (r=0.12, p=.33).
2.4 Clinical information
Information regarding tumor stage and grade, chronic medical conditions, weight, height, hemoglobin, and current use of relevant medications, such as antidepressants, anxiolytics, hypnotics and pain medications was extracted from medical records. A categorical variable was created describing patient medication use on a scale of 0 (no use of any of the four medications listed) to 4 (use of all 4 types of mediations listed). Body mass index (BMI) was calculated from the standard formula of BMI = weight (kg)/(height m)2.
2.5 Statistical Analysis
Analyses were conducted using the Statistical Package for the Social Sciences v. 19.0 (SPSS, Chicago, IL). Distributions of variables were examined for violations of normality and potential outliers. Log transformations were applied to IL-6 data in all analyses except percent change. Paired sample t-tests were calculated to determine changes in psychosocial measures and IL-6 between pre-surgery and one year.
2.5.1. Covariates
Stage, age, and body mass index (BMI) were included in all regressions a priori due to known relationships with the variables of interest. Tumor grade (high vs. low), education, racial and ethnic background, income, caffeine use before blood draw and in the week prior to surgery, history of conditions such as chronic obstructive pulmonary disease, sleep apnea, or coronary heart disease, the mood composite, medications, hemoglobin (pre-surgery only), and relationship status (married vs. not married) were tested as possible covariates, first in zero order correlations, and then in regressions. Hierarchical regressions were conducted with all variables that had significant associations with IL-6, sleep, fatigue, or their delta scores in zero-order correlations. After examining beta weights and significance values in regression models, reduced regression models were conducted including only those variables contributing significant variance to the models along with a priori covariates. At one year, patients were included in analyses if they were not currently on chemotherapy and did not have documented evidence of disease progression. Reduced regression models at both time-points thus included stage, BMI, age, mood, and medication use as covariates. Baseline models also included hemoglobin; one year models did not include hemoglobin as patients did not have routine hemoglobin assessment at follow up. As medication use was not significantly related to delta scores, it was not included in regressions involving delta scores to maximize subject/variable ratios,
A general linear model was used to test whether there were differences in means of pre-surgical IL-6, fatigue, and sleep between participants who were disease-free and eligible for inclusion in the one year sample as compared to those who were not included at one year (those who were deceased, had documented disease progression, were still receiving chemotherapy, were lost to follow up, or did not provide complete data). The model controlled for disease stage, age, and BMI.
2.5.2. Regression models at pre-surgery and one-year
To determine the associations of IL-6 with global sleep and fatigue, hierarchical regression models were constructed at the pre-surgery and one year time-points, adjusting for covariates as described above. One year analyses additionally included pre-surgical fatigue or sleep as covariates. Secondary analyses were conducted using the modified sleep index. Conditions for mediation were examined using hierarchical regression models to determine whether sleep disturbance mediated the relationship between IL-6 and fatigue (Baron and Kenny, 1986; Judd and Kenny, 1981). The modified sleep index was used for these analyses.
2.5.3. Models examining change
To examine associations between changes in fatigue and sleep over time, change scores were created for each of these variables. For fatigue and sleep, delta scores were obtained by subtracting baseline scores from one year scores. As higher scores on these measures indicate greater disturbance, a negative delta score would indicate a decrease in sleep score at one year (i.e., improvement in sleep) over time. Change in IL-6 over time was assessed by percent change ([one year IL-6 – baseline IL-6 / baseline IL-6] *100) where a negative value indicates percent decrease (greater normalization) in IL-6 over time. Non-transformed values were used in these calculations. Distributions of these change variables were assessed for normality and outliers. All delta scores were normally distributed except for IL-6 which had 2 outliers (values > 3 standard deviations from mean percent change). Therefore analyses were conducted with and without these outliers and both are reported.
3. Results
3.1. Participant Characteristics
At baseline, the 136 participants were primarily married, non-Hispanic Caucasians with a mean age of 60.4 years. The majority of participants had advanced stage and high grade disease (Table 1). As seen in Table 2, levels of IL-6 dropped substantially from pre-surgery to one year (p<.001). Mean levels of fatigue also decreased from pre-surgery to one year (p=.03). However, global sleep disturbance decreased only minimally over this time period (p=.41), with means at one year remaining above the cutoff of 5, indicating sleep disturbance. Means for the mood composite also were relatively stable over time (p=.56). There were no differences between levels of global sleep or fatigue at pre-surgery in individuals with vs. without ascites (fatigue: F(1,130)=.52, p=.47; sleep: F(1,130)=1.98, p=.16). At pre-surgery, no significant differences were seen between women included in the one year analyses and those not included with respect to IL-6 (p=.84), global sleep (p=.33), fatigue (p=.90), or the modified sleep index (p=.20), controlling for stage, age, and BMI.
Table 1.
Patient Characteristics
| Characteristic | Ovarian Cancer Patients | |
|---|---|---|
| Age, years | ||
| Mean (S.D) | 60.40 (12.39) | |
| Ethnicity | ||
| Non-Hispanic | 94.80% | |
| Hispanic | 5.20% | |
| Race | ||
| American Indian/Alaska native | 1.50% | |
| Asian | 0.00% | |
| Pacific Islander | 0.00% | |
| Black/African American | 1.50% | |
| White | 97.00% | |
| Education | ||
| Less than high school graduate | 3.70% | |
| High school graduate | 32.50% | |
| Trade school/some college | 35.60% | |
| College graduate | 19.30% | |
| Postgraduate | 8.90% | |
| Marital status | ||
| Single/Widowed/Divorced | 36.00% | |
| Married | 64.00% | |
| Stage | ||
| I | 21.30% | |
| II | 8.10% | |
| III | 61.00% | |
| IV | 9.60% | |
| Grade | ||
| Low | 15.40% | |
| High | 84.60% | |
| Tumor histology | ||
| Serous | 72.80% | |
| Endometroid | 13.20% | |
| Mucinous | 3.70% | |
| Clearcell | 3.70% | |
| Other/Unknown | 6.60% | |
| Surgical Debulking | ||
| Optimal | 74.30% | |
| Suboptimal | 25.70% | |
| Medication Use Pre-Surgery | ||
| Hypnotics | 14.70% | |
| Antidepressants | 23.50% | |
| Anxiolytics | 13.20% | |
| Pain Medications | 27.20% | |
| Medication Use at One Year | ||
| Hypnotics | 9.10% | |
| Antidepressants | 31.80% | |
| Anxiolytics | 10.60% | |
| Pain Medications | 24.20% | |
Table 2.
Means and standard deviations of biological and psychosocial measures at pre-surgery and one year.
| Baseline | One Year | ||||||
|---|---|---|---|---|---|---|---|
| Variable | N | M | SD | N | M | SD | t |
| Global Sleep | 133 | 7.72 | 3.55 | 63 | 7.23 | 4.06 | 0.83 |
| POMS Fatigue | 133 | 9.07 | 5.72 | 60 | 7.06 | 5.77 | 2.26* |
| Mood Composite | 136 | 0.09 | 1.71 | 63 | −0.06 | 1.92 | 0.58 |
| IL-6 pg/mL | 136 | 9.55 | 4.87 | 63 | 2.34 | 1.01 | 19.41*** |
Note: Significance levels for 2-sided tests.
p<.05,
p<.01,
p<.001;
IL-6 values are back-transformed values.
3.2. Pre-Surgical Sleep and Fatigue
Prior to surgery, higher IL-6 levels were significantly correlated with greater sleep disturbance, worse sleep index scores, and greater fatigue (all p values <.01), though IL-6 was not significantly associated with the mood composite (r=.04, p=.67). Regression models indicated that peripheral IL-6 was associated with significantly higher global sleep scores (β=0.21, p=.015) and greater fatigue (β=.18, p=.032), adjusting for covariates. Secondary analyses indicated that IL-6 significantly predicted modified sleep scores (β=0.19, p=.03). (Table 3A).
Table 3A.
Regression Models predicting Global Sleep and Fatigue from IL-6 – Pre-surgery
| Outcome Variable | R final | Δ R2 | Beta | t | |
|---|---|---|---|---|---|
| Fatigue | |||||
| BMI | 0.06 | 0.68 | |||
| Stage | 0.03 | 0.34 | |||
| Medications | 0.12 | 1.49 | |||
| Mood | 0.33 | 3.97*** | |||
| Age | −0.02 | −0.23 | |||
| Hemoglobin | −0.09 | −1.08 | |||
| IL-6 | .46 | .03 | 0.18 | 2.17* | |
| Sleep | |||||
| BMI | 0.01 | 0.08 | |||
| Stage | −0.02 | −0.21 | |||
| Medications | 0.20 | 2.39* | |||
| Mood | 0.24 | 2.94** | |||
| Age | −0.13 | −1.49 | |||
| Hemoglobin | −0.13 | −1.54 | |||
| IL-6 | .46 | .04 | 0.21 | 2.47** | |
p<.05
p<.01
p<.001
Because of the significant positive associations between sleep, fatigue, and IL-6 and a significant positive association between the modified sleep index and fatigue (β=.29, p<.001) we assessed whether the relationship between IL-6 and fatigue was mediated by sleep, as lack of sleep might affect daytime fatigue. When the modified sleep index was included in the regression model for IL-6 and fatigue, the association between IL-6 and fatigue became non-significant (β=.13, p=.13), although the drop in standardized beta weights was not particularly large, suggesting that sleep partially mediated the association between IL-6 and fatigue. (Table 3B).
Table 3B.
Regression Model predicting Fatigue from IL-6 controlling for Revised Sleep - Pre-surgery
| Outcome Variable | R final | Δ R2 | Beta | t | |
|---|---|---|---|---|---|
| Fatigue | |||||
| BMI | 0.05 | 0.61 | |||
| Stage | 0.02 | 0.23 | |||
| Medications | 0.11 | 1.29 | |||
| Mood | 0.25 | 2.94** | |||
| Age | 0.04 | 0.43 | |||
| Hemoglobin | −0.07 | −0.83 | |||
| Revised Sleep | 0.26 | 3.03** | |||
| IL-6 | .50 | .02 | 0.13 | 1.52 | |
p<.05
p<.01
p<.001
3.3. Sleep and Fatigue at One-Year Follow-up
One year analyses were conducted on 63 participants who had completed adjuvant treatment, did not have documented disease progression, and were not on chemotherapy at the time of follow-up. IL-6 levels, global sleep, the sleep index score, and fatigue were all significantly associated at one year (all r ’s > .30, all p values < .05), whereas IL-6 was not significantly associated with the mood composite (r=.06, p=.70). All analyses controlled for pre-surgery values of the dependent variables (n=63 for sleep; n=60 for fatigue). At one year, regression models indicated that higher levels of IL-6 were significantly associated with greater sleep disturbance (β=.23, p=.02), adjusting for covariates. (Table 4). Secondary analyses with the revised sleep index showed similar results, though the relationship was non-significant (β=.18, p=.09). IL-6 was positively related to fatigue (β=.12, p=.20) adjusting for covariates including pre-surgical fatigue, but this relationship was not significant.
Table 4.
Regression Models Predicting Fatigue and Sleep from IL-6 – One Year
| Outcome Variable | R final | Δ R2 | Beta | t | |
|---|---|---|---|---|---|
| Fatigue | |||||
| BMI | 0.07 | 0.65 | |||
| Stage | −0.10 | −1.03 | |||
| Medications | −0.05 | −0.48 | |||
| Mood | 0.25 | 2.46* | |||
| Age | −0.12 | −1.29 | |||
| Pre-Surgical Fatigue | 0.56 | 5.77*** | |||
| IL-6 | .79 | .01 | 0.20 | 1.73 | |
| Sleep | |||||
| BMI | −0.01 | −0.05 | |||
| Stage | −0.03 | −0.31 | |||
| Medications | 0.20 | 1.95 | |||
| Mood | 0.46 | 4.38*** | |||
| Age | 0.06 | 0.61 | |||
| Pre-Surgical Sleep | 0.23 | 2.31* | |||
| IL-6 | .74 | .05 | 0.23 | 2.34* | |
p<.05
p<.01
p<.001
3.4. Associations of Changes in Sleep, Fatigue, and IL-6 Over Time
To examine the relationships between changes in IL-6, fatigue and sleep over time, analyses were conducted on the subset of patients who had completed measures of fatigue, sleep, and had levels of IL-6 at both time points. Controlling for stage, age, BMI, baseline sleep and one year mood, percent change in IL-6 over time was significantly associated with change in sleep over time (β=.27, p=.01). This indicates that, for example, a 10% decrease in IL-6 would correspond with a 0.13 point improvement in the global sleep change score, suggesting a minimal effect, although statistically significant. (Figure 2). Secondary analyses excluding 2 outliers were consistent with these findings (β= .21, p=.049). In parallel analyses, including similar covariates and pre-surgery fatigue, percent change in IL-6 over time was associated with change in fatigue over time but findings did not reach significance (β= .22, p=.06). (Table 5). Secondary analyses excluding the two IL-6 outliers were consistent with these findings (β= .22, p=.053).
Figure 2.
Graph of relationship between Percent Change in IL-6 and Change in Global Sleep from pre-surgery to one year. Y-axis values reflect unstandardized residuals controlling for stage, age, BMI, pre-surgical sleep, and mood at one year.
Table 5.
Regression Models Predicting Change in Sleep and Fatigue from Percentage Change in IL-6 Over Time
| Outcome Variable | R final | Δ R2 | Beta | t | |
|---|---|---|---|---|---|
| Fatigue Change | |||||
| BMI | 0.12 | 1.03 | |||
| Stage | −0.11 | −0.91 | |||
| Mood | 0.41 | 3.35** | |||
| Age | −0.12 | −1.02 | |||
| Pre-Surgical Fatigue | −0.60 | −5.30*** | |||
| IL-6 % Change | .72 | .04 | −0.23 | −2.34 | |
| Sleep Change | |||||
| BMI | 0.05 | 0.47 | |||
| Stage | 0.11 | 0.93 | |||
| Mood | 0.63 | 5.94*** | |||
| Age | 0.04 | 0.39 | |||
| Pre-Surgical Sleep | −0.64 | −6.18*** | |||
| IL-6 % Change | .77 | .06 | 0.27 | 2.64* | |
p<.05
p<.01
p<.001
4. Discussion
Ovarian cancer patients report sleep disturbances which do not significantly improve between pre-surgery and one year follow up, and elevated levels of IL-6 in peripheral blood were associated with poorer sleep at both time points. These relationships were independent of potentially confounding clinical covariates as well as depressive mood and thought intrusions. Fatigue symptoms significantly decreased from pre-surgery to one year. The relationship between IL-6 and fatigue was significant at pre-surgery and approached significance at one year; however, the relationship at pre-surgery was attenuated when sleep was included in the model, suggesting that poor sleep at the time of surgery may account for a substantial part of the fatigue experienced by these patients. Decreases in IL-6 between pre-surgery and one year were associated with significant, but small improvements in both fatigue and sleep disturbance. The current data are novel as they are the first to examine the contribution of cytokines to self-reported sleep disturbance and fatigue in ovarian cancer patients.
4.1 Effects of inflammatory cytokines
There are several mechanisms by which cytokine-associated changes may affect sleep, including modulation of sleep architecture, changes in circadian patterns, and a general increase in vegetative symptoms.
4.1.1 Effects of inflammatory cytokine signaling on sleep architecture
The present findings are consistent with data indicating that inflammatory cytokines such as IL-6 are associated with changes from slow wave to increased REM sleep and with longer sleep latency (Opp, 2005; Thomas et al., 2011; Redwine et al., 2003; Motivala et al., 2005; Vgontzas et al., 1999). Modulation of sleep architecture by IL-6 has also been observed in experimental settings (Späth-Schwalbe et al., 1998; Motivala and Irwin, 2007). Although specific effects on sleep architecture may vary across different experiments and species, inflammatory processes appear to be able to induce sleep disturbances via alterations in sleep architecture; conversely, sleep disturbances have been shown to induce inflammatory cytokines (Irwin, 2002; Kapsimalis et al., 2008).
Although decreased IL-6 was related to changes towards normalized sleep patterns over time, sustained sleep disturbances were still observed at one year, even though IL-6 levels dropped to a relatively normal level. Interestingly, the sleep literature describes changes in sleep architecture accompanying relatively modest IL-6 inductions in healthy individuals with normal IL-6 levels (Späth-Schwalbe et al., 1998). Moreover, mean IL-6 levels approximating those in the range of our patients at one year were associated with longer sleep latency and increased REM density in patients with major depressive disorder (Motivala et al., 2005). Taken together, such findings are consistent with the interpretation that IL-6 fluctuations could be associated with sleep disturbances at one year despite marked decreases in IL-6 compared to pre-surgical levels. It is also possible that IL-6 may have been implicated in the initial sleep disruption, but that over time, sleep disturbances may have become sustained due to maintenance of dysfunctional sleep behaviors and beliefs (Fiorentino and Ancoli-Israel, 2007), leading to a long-term change in sleep regulation.
4.1.2 Effects of inflammatory cytokine signaling on circadian patterns
IL-6 is thought to be a primary mediator of sleepiness and the homeostatic sleep drive (Vgontzas et al., 2005; Vgontzas et al., 1999; Shearer et al., 2001). Elevations in serum IL-6 have been associated with two specific variations in Per3, a clock gene associated with circadian preference and sleep in a sample of male veterans (Guess et al., 2009). Data supports both a direct relationship between IL-6 and sleep as well as indirect influences on sleep via effects on the circadian pacemaker (Guess et al., 2009; Redwine et al., 2000). It is also possible that sleep-wake regulation may be influenced by interactions between neuroendocrine hormones, cytokines, and the circadian pacemaker (VanItallie, 2006; Born et al., 1997).
4.1.3 Effects of inflammatory cytokines on vegetative symptoms
Proinflammatory cytokines, such as IL-6, are known to stimulate a cluster of central “sickness behaviors” such as anhedonia, lethargy, anorexia, hypersomnia, withdrawal, and loss of interest (Dantzer and Kelley, 2007). In the face of a challenge to the organism resulting in inflammation, sleep is an important part of the constellation of behaviors to support an adequate host defense (Opp, 2005; Guan et al., 2005). There may thus be direct central cytokine effects on sleep and fatigue.
4.2. Mood, Fatigue, and Sleep Disturbances
Fatigue and sleep were both significantly influenced by mood, in the absence of a direct association between mood and IL-6, suggesting that there may be direct influences of mood disturbance on sleep and fatigue. Symptoms such as lack of interest and fatigue are frequently displayed in depressed individuals (Dantzer, 2001) and depression has been well-established as a correlate of fatigue in cancer patients (Campos et al., 2011; Holzner et al., 2003; Andryowski et al., 2005). Other results support the observed association; fatigue has been associated with depressed mood as well as sleep disturbance in women with breast cancer (Bower et al., 2000; Koopman et al., 2002) and other populations of oncology patients (Fiorentino and Ancoli-Israel, 2007; Akechi et al., 2007). Fatigue has also been associated with anxiety in patients receiving chemotherapy (Redeker et al., 2000).
4.2.1. Fatigue and Inflammation in Ovarian and Breast Cancer Patients
Despite the association between fatigue and IL-6 observed pre-surgery in the present study, the finding that this relationship was partially mediated by effects of IL-6 on sleep did not support our initial hypotheses and is not consistent with the direct associations between inflammatory cytokines and fatigue previously reported among breast cancer patients (Bower et al, 2002; 2007). Differences in findings in these two populations may be secondary to differences in treatment regimens (Howard and Bland, in press; Hennessy et al., 2009), sample composition, cytokines studied, and length of follow-up. For example, Bower and colleagues (2002; 2005) studied breast cancer survivors reporting sustained elevations in fatigue whereas the ovarian cancer patients in the present study represented a full spectrum of clinic patients without self-selection for fatigue.
4.3 Clinical Implications
Given the documented sleep impairments in ovarian cancer patients (Sandadi et al., 2011; Clevenger et al., under review), present findings that inflammatory cytokines may be related to both sleep and fatigue suggest the importance of screening for sleep disturbance in this population, and development of interventions targeting inflammatory cytokines. Substantial support has accumulated for non-pharmacologic treatment of fatigue and sleep in cancer patients, including cognitive-behavioral therapy, mindfulness based stress reduction, exercise, hypnosis, relaxation, sleep interventions, and psycho-education (Mock et al., 1997; Sprod et al., 2010; Lotfi-Jam et al., 2008; Berger et al., 2002; Shapiro et al., 2003; Dirksen and Epstein, 2008; Savard et al., 2005). It is possible that pharmacological treatments that interfere with inflammatory cytokine activity may also be useful in this regard.
4.4 Limitations
The absence of premorbid data on sleep patterns and fatigue limits our ability to determine how assessed patterns may have been influenced by long-term disturbances. Additionally, self-report measures are subject to reporting biases and retrospective recall. Polysomnography and actigraphy provide objective assessments of sleep disturbances; however, these technologies were deemed too intrusive for this patient population, particularly when facing major surgery for a life-threatening disease. As the study design was correlational, definitive causal relationships cannot be determined. Given the attrition between pre-surgery and one year, the women included in one year analyses reflect a group of surviving women who were healthy and motivated to participate in contrast with a larger, more heterogeneous pre-surgical group. Though we statistically tested for differences in women at pre-surgery who went on to have known worse disease related outcomes, the possible differences in samples should be considered when interpreting the results. Analyses involving change scores required women to have complete information at both time points, which restricted the sample size, potentially limiting the power to detect significant associations between changes in IL-6 and fatigue. As the follow-up period was limited to one year post-surgery, we were unable to see associations that might be observed in a longer follow-up period. As IL-6 was the only inflammatory cytokine assessed, our ability to make more general conclusions regarding the role of pro-inflammatory cytokines is limited. Additionally, other influences such as HPA patterns could also be contributing to poor sleep, fatigue, and affective disorders. Although there was no relationship observed between IL-6 levels and time of blood sampling, there is a known diurnal cycle of IL-6 (Vgontzas et al., 2005) and thus it is possible that blood sampling time, necessitated by surgical scheduling, may have in some way influenced results.
4.5 Conclusions
At both pre-surgery and one year after diagnosis, higher levels of IL-6 were related to poorer sleep, adjusting for clinical covariates. IL-6 was also associated with greater fatigue prior to surgery, although this relationship appeared to be secondary to sleep disturbance at the time of surgery. Further research aimed at identifying additional underlying candidate biological and psychological contributors to sleep disturbance and fatigue is warranted and can assist in identifying potential interventions to help remediate these troublesome symptoms in ovarian cancer patients.
Highlights.
High levels of interleukin-6 in ovarian cancer patients are associated with sleep disturbances at the time of surgery and at one year post-diagnosis.
Acknowledgements
We gratefully acknowledge Bridget Zimmerman, Ph.D. for statistical assistance, and Katherine Collins, B.A. for assistance in data collection.
This research was funded in part by support from NIH grants CA104825 and CA140933 to SL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Akechi T, Okuyama T, Akizuki N, Shimizu K, Inagaki M, Fujimori M, Shima Y, Furukawa TA, Uchitomi Y. Associated and predictive factors of sleep disturbance in advanced cancer patients. Psychooncology. 2007;16:888–894. doi: 10.1002/pon.1122. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, Moore PJ, Jones V. The relationship between fatigue and sleep in cancer patients: a review. Eur. J. Cancer. 2001;10:245–255. doi: 10.1046/j.1365-2354.2001.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson NJ, Hacker ED. Fatigue in women receiving intraperitoneal chemotherapy for ovarian cancer. A review of contributing factors. Clin. J. Oncol. Nurs. 2008;12:445–454. doi: 10.1188/08.CJON.445-454. [DOI] [PubMed] [Google Scholar]
- Andrykowski MA, Schmidt JE, Salsman JM, Beacham AO, Jacobsen PB. Use of a case definition approach to identifying cancer-related fatigue in women undergoing adjuvant therapy for breast cancer. J. Clin. Oncol. 2005;27:6613–6622. doi: 10.1200/JCO.2005.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhaus J, Junghanns K, Broocks A, Riemann D, Hohagen F. Test retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J. Psychosom. Res. 2002;53:737–740. doi: 10.1016/s0022-3999(02)00330-6. [DOI] [PubMed] [Google Scholar]
- Baider L, Kaplan De-Nour A. Psychological distress and intrusive thoughts in cancer patients. J. Nerv. Ment. Dis. 1997;185:346–348. doi: 10.1097/00005053-199705000-00010. [DOI] [PubMed] [Google Scholar]
- Baker F, Denniston M, Zabora J, Polland A, Dudley WN. A POMS short form for cancer patients: psychometric and structural evaluation. Psychooncology. 2002;11:273–281. doi: 10.1002/pon.564. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Beck SL, Schwartz AL, Towsley G, Dudley W, Barsevick A. Psychometric evaluation of the Pittsburgh Sleep Quality Index in cancer patients. J. Pain Symptom. Manage. 2004;27:140–148. doi: 10.1016/j.jpainsymman.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Berger AM, VonEssen S, Khun BR, Piper BF, Farr L, Agrawal S, Lynch JC, Higginbotham P. Feasibility of a sleep intervention during adjuvant breast cancer chemotherapy. Oncol. Nurs. Forum. 2002;29:1431–1441. doi: 10.1188/02.ONF.1431-1441. [DOI] [PubMed] [Google Scholar]
- Born J, Lange T, Hansen K, Mölle M, Fehm HL. Effects of sleep and circadian rhythm on human circulating immune cells. J. Immunol. 1997;158:4454–4464. [PubMed] [Google Scholar]
- Bower JE. Cancer-related fatigue: Links with inflammation in cancer patients and survivors. Brain Behav. Immun. 2007;21:863–871. doi: 10.1016/j.bbi.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Aziz N, Fahey JL. Fatigue and pro-inflammatory cytokine activity in breast cancer survivors. Psychosom. Med. 2002;64:604–611. doi: 10.1097/00006842-200207000-00010. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: Occurrence, correlates, and impact on quality of life. J. Clin. Oncol. 2000;18:743–753. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Dickerson SS, Petersen L, Aziz N, Fahey JL. Diurnal cortisol rhythm and fatigue in breast cancer survivors. Psychoneuroendocrinology. 2005;30:92–100. doi: 10.1016/j.psyneuen.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Bradley S, Rose S, Lutgendorf S, Costanzo E, Anderson B. Quality of life and mental health in cervical and endometrial cancer survivors. Gynecol. Oncol. 2006;100:479–486. doi: 10.1016/j.ygyno.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Burger RA, Grosen EA, Ioli GR, Van Eden ME, Park M, Berman ML, Manetta A, Disaia PJ, Granger GA, Gatanaga T. Spontaneous release of interleukin-6 by primary cultures of lymphoid and tumor cell populations purified from human ovarian carcinoma. J. Interferon Cytokine Res. 1995;15:255–260. doi: 10.1089/jir.1995.15.255. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Campos MP, Hassan BJ, Riechelmann R, Del Giglio A. Cancer-related fatigue: a review. Rev. Assoc. Med. Bras. 2011;57:211–219. doi: 10.1590/s0104-42302011000200021. [DOI] [PubMed] [Google Scholar]
- Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh Sleep Quality Index. J. Psychosom. Res. 1998;45:5–13. doi: 10.1016/s0022-3999(97)00298-5. [DOI] [PubMed] [Google Scholar]
- Clevenger L, DeGeest K, Bender D, Goodheart M, Ahmed A, Dahmoush L, Penedo F, Lucci J, III, Thaker PH, Mendez L, Sood AK, Lutgendorf SK. Sleep disturbances and quality of life in ovarian cancer patients during the first year post diagnosis. doi: 10.1002/cncr.28188. (under review). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado-Hidalgo A, Bower JE, Ganz PA, Cole SW, Irwin MR. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin. Cancer Res. 2006;12:2759–2766. doi: 10.1158/1078-0432.CCR-05-2398. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behavior: mechanisms and implications. Ann. N.Y. Acad. Sci. 2001;933:222–234. doi: 10.1111/j.1749-6632.2001.tb05827.x. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav. Immun. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirksen SR, Epstein DR. Efficacy of an insomnia intervention on fatigue, mood, and quality of life in breast cancer survivors. J. Adv. Nurs. 2008;61:664–675. doi: 10.1111/j.1365-2648.2007.04560.x. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan JE, Compas BE, Howell DC. Predictors of cancer progression in young adult men and women: avoidance, intrusive thoughts, and psychological symptoms. Health Psychol. 1994;13:539–547. doi: 10.1037//0278-6133.13.6.539. [DOI] [PubMed] [Google Scholar]
- Fiorentino MS, Ancoli-Israel S. Sleep dysfunction in patients with cancer. Curr. Treat. Options Neurol. 2007;9:337–346. [PMC free article] [PubMed] [Google Scholar]
- Goff BA, Mandel LS, Melancon CH, Muntz HG. Frequency of symptoms of ovarian cancer in women presenting to primary care clinics. JAMA. 2004;291:2705–2712. doi: 10.1001/jama.291.22.2705. [DOI] [PubMed] [Google Scholar]
- Goff BA, Mandel L, Muntz HG, Melancon CH. Ovarian carcinoma diagnosis. Cancer. 2000;89:2086–2075. doi: 10.1002/1097-0142(20001115)89:10<2068::aid-cncr6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Golden-Kreutz DM, Thornton LM, Wells-DiGregorio S, Frierson GM, Jim HS, Carpenter KM, Shelby RA, Andersen BL. Traumatic stress, perceived global stress, and life events: prospectively predicting quality of life in breast cancer patients. Health Psychol. 2005;24:288–296. doi: 10.1037/0278-6133.24.3.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Z, Vgontzas AN, Omori T, Peng X, Bixler EO, Fang J. Interleukin-6 levels fluctuate with the light-dark cycle in the brain and peripheral tissues in rats. Brain Behav. Immun. 2005;19:526–529. doi: 10.1016/j.bbi.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Guess J, Burch JB, Ogoussan K, Armstead CA, Zhang H, Wagner S, Hebert JR, Wood P, Youngstedt SD, Hofseth LJ, Singh UP, Xie D, Hrushesky WJ. Circadian disruption, Per3, and human cytokine secretion. Integr. Cancer Ther. 2009;8:329–336. doi: 10.1177/1534735409352029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hann D, Winter K, Jacobsen P. Measurement of depressive symptoms in cancer patients: evaluation of the Center for Epidemiological Studies Depression scale (CES-D) J. Psychosom. Res. 1999;46:437–443. doi: 10.1016/s0022-3999(99)00004-5. [DOI] [PubMed] [Google Scholar]
- Hennessy BT, Coleman RL, Markman M. Ovarian cancer. Lancet. 2009;374:1371–1382. doi: 10.1016/S0140-6736(09)61338-6. [DOI] [PubMed] [Google Scholar]
- Holzner B, Kemmler G, Meraner V, Maislinger A, Kopp M, Bodner T, Nguyen Van Tam D, Zeimet AG, Fleischhacker WW, Sperner-Unterweger B. Fatigue in ovarian carcinoma patients. A neglected issue? Cancer. 2003;97:1564–1572. doi: 10.1002/cncr.11253. [DOI] [PubMed] [Google Scholar]
- Horowitz M, Wilner N, Alvarez W. Impact of events scale: a measure of subjective stress. Psychosom. Med. 1979;41:209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- Howard JH, Bland KI. Current management and treatment strategies for breast cancer. Curr. Opin. Obstet. Gynecol. doi: 10.1097/GCO.0b013e32834da4b1. In press. [DOI] [PubMed] [Google Scholar]
- Irwin M. Effects of sleep and sleep loss on immunity and cytokines. Brain Behav. Immun. 2002;16:503–512. doi: 10.1016/s0889-1591(02)00003-x. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch. Intern. Med. 2006;166:1756–1762. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- Judd CM, Kenny DA. Process analysis: Estimating mediation in treatment evaluations. Eval. Rev. 1981;5:602–619. [Google Scholar]
- Kapás L, Hong L, Cady AB, Opp MR, Postelthwaite AE, Seyer JM, Krueger JM. Somnogenic, pyrogenic, and anorectic activities of tumor necrosis factoralpha and TNF-alpha fragments. Am. J. Physiol. 1992;263:R708–R715. doi: 10.1152/ajpregu.1992.263.3.R708. [DOI] [PubMed] [Google Scholar]
- Kapsimalis F, Basta M, Varouchakis G, Gourgoulianis K, Vgontzas A, Kryger M. Cytokines and pathological sleep. Sleep Med. 2008;9:603–614. doi: 10.1016/j.sleep.2007.08.019. [DOI] [PubMed] [Google Scholar]
- Koopman C, Nouriani B, Erickson V, Anupindi R, Butler LD, Bachmann MH, Sephton SE, Spiegel D. Sleep disturbances in women with metastatic breast cancer. Breast J. 2002;8:362–370. doi: 10.1046/j.1524-4741.2002.08606.x. [DOI] [PubMed] [Google Scholar]
- Lofti-Jam K, Carey M, Jefford M, Schofield P, Charleson C, Aranda S. Nonpharmacologic strategies for managing common chemotherapy adverse effects: a systematic review. J. Clin. Oncol. 2008;26:5618–5629. doi: 10.1200/JCO.2007.15.9053. [DOI] [PubMed] [Google Scholar]
- Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, Huber SL. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85:1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Mock V, Dow KH, Meares CJ, Grimm PM, Dienemann JA, Haisfield-Wolfe ME, Quitasol W, Mitchel S, Chakravarthy A, Gage I. Effects of exercise on fatigue, physical functioning, and emotional distress during radiation therapy for breast cancer. Oncol. Nurs. Forum. 1997;24:991–1000. [PubMed] [Google Scholar]
- Motivala SJ, Irwin MR. Sleep and immunity. Cytokine pathways linking sleep and health outcomes. Curr. Dir. Psychol. Sci. 2007;16:21–25. [Google Scholar]
- Motivala SJ, Sarfatti A, Olmos L, Irwin MR. Inflammatory markers and sleep disturbance in major depression. Psychosom. Med. 2005;67:187–194. doi: 10.1097/01.psy.0000149259.72488.09. [DOI] [PubMed] [Google Scholar]
- Opp MR. Cytokines and sleep. Sleep Med Rev. 2005;9:355–364. doi: 10.1016/j.smrv.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Opp MR, Obal F, Jr, Krueger JM. Interleukin 1 alters rat sleep: temporal and dose-related effects. Am. J. Physiol. 1991;260:R52–R58. doi: 10.1152/ajpregu.1991.260.1.R52. [DOI] [PubMed] [Google Scholar]
- Opp MR, Toth LA. Neural-immune interactions in the regulation of sleep. Front. Biosci. 2003;8:d768–d779. doi: 10.2741/1061. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977;1:385–401. [Google Scholar]
- Redeker NS, Lev EL, Ruggiero J. Insomnia, fatigue, anxiety, depression and quality of life of cancer patients undergoing chemotherapy. Sch. Inq. Nurs. Pract. 2000;14:275–290. [PubMed] [Google Scholar]
- Redwine L, Dang J, Hall M, Irwin M. Disordered sleep, nocturnal cytokines, and immunity in alcoholics. Psychosom Med. 2003;65:75–85. doi: 10.1097/01.psy.0000038943.33335.d2. [DOI] [PubMed] [Google Scholar]
- Redwine L, Hauger RL, Gillin JC, Irwin M. Effects of sleep and sleep deprivation on interleukin-6, growth hormone, cortisol, and melatonin levels in humans. J. Clin. Endocrinol. Metab. 2000;85:3597–3603. doi: 10.1210/jcem.85.10.6871. [DOI] [PubMed] [Google Scholar]
- Roscoe JA, Kaufman ME, Matteson-Rusby SE, Palesh OG, Ryan JL, Kohli S, Perlis ML, Morrow GR. Cancer-related fatigue and sleep disorders. Oncologist. 2007;12(suppl 1):35–42. doi: 10.1634/theoncologist.12-S1-35. [DOI] [PubMed] [Google Scholar]
- Sandadi S, Frasure HE, Broderick MJ, Waggoner SE, Miller JA, von Gruenigen VE. The effect of sleep disturbance on quality of life in women with ovarian cancer. Gynecol. Oncol. 2011;123:351–355. doi: 10.1016/j.ygyno.2011.07.028. [DOI] [PubMed] [Google Scholar]
- Savard J, Simard S, Ivers H, Morin CM. Randomized study on the efficacy of cognitive behavioral therapy for insomnia secondary to breast cancer, part I: sleep and psychological effects. J. Clin. Oncol. 2005;23:6083–6096. doi: 10.1200/JCO.2005.09.548. [DOI] [PubMed] [Google Scholar]
- Schroevers MJ, Sanderman R, van Sonderen E, Ranchor AV. The evaluation of the Center for Epidemiologic Studies Depression (CES-D) scale: depressed and positive affect in cancer patients and healthy reference subjects. Qual. Life Res. 2000;9:1015–1029. doi: 10.1023/a:1016673003237. [DOI] [PubMed] [Google Scholar]
- Schubert C, Hong S, Natarajan L, Mills PJ, Dimsdale JE. The association between fatigue and inflammatory marker levels in cancer patients: a quantitative review. Brain Behav. Immun. 2007;21:413–427. doi: 10.1016/j.bbi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Schwartz AH. Validity of cancer-related fatigue instruments. Pharmacotherapy. 2002;22:1433–1441. doi: 10.1592/phco.22.16.1433.33690. [DOI] [PubMed] [Google Scholar]
- Scott HR, McMillan DC, Forrest LM, Brown DJ, McArdle CS, Milroy R. The systemic inflammatory response, weight loss, performance status, and survival in patients with inoperable non-small cell lung cancer. Br. J. Cancer. 2002;87:264–267. doi: 10.1038/sj.bjc.6600466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shacham S. A shortened version of the Profile of Mood States. J. Pers. Assess. 1983;47:305–306. doi: 10.1207/s15327752jpa4703_14. [DOI] [PubMed] [Google Scholar]
- Shapiro SL, Bootzin RR, Figueredo AJ, Lopez AM, Schwartz GE. The efficacy of mindfulness-based stress reduction in the treatment of sleep disturbances in women with breast cancer: an exploratory study. J. Psychosom. Res. 2003;54:85–91. doi: 10.1016/s0022-3999(02)00546-9. [DOI] [PubMed] [Google Scholar]
- Shearer WT, Reuben JM, Mullington JM, Price NJ, Lee BN, Smith EO, Szuba MP, Van Dongen HP, Dinges DF. Soluble TNF-alpha receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J. Allergy Clin. Immunol. 2001;107:165–170. doi: 10.1067/mai.2001.112270. [DOI] [PubMed] [Google Scholar]
- Sheehan TJ, Fifield J, Reisine S, Tennen H. The measurement structure of the Center for Epidemiologic Studies Depression scale. J. Pers. Assess. 1995;64:507–521. doi: 10.1207/s15327752jpa6403_9. [DOI] [PubMed] [Google Scholar]
- Späth-Schwalbe E, Hansen K, Schmidt F, Schrezenmeier H, Marshall L, Burger K, Fehm HL, Born J. Acute effects of recombinant human interleukin-6 on endocrine and central nervous sleep functions in healthy men. J. Clin. Endocrinol. Metab. 1998;83:1573–1579. doi: 10.1210/jcem.83.5.4795. [DOI] [PubMed] [Google Scholar]
- Sprod LK, Palesh OG, Janelsins MC, Peppone LJ, Heckler CE, Adams MJ, Morrow GR, Mustian KM. Exercise, sleep quality, and mediators of sleep in breast and prostate cancer patients receiving radiation therapy. Community Oncol. 2010;7:463–471. doi: 10.1016/s1548-5315(11)70427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone RL, Nick AM, Armaiz-Pena N, Han HD, Lopez G, Afshar-Kharghan V, Vasquez HG, Urbauer D, Bottsford-Miller J, Landen CN, Gershenson H, Matsuo K, Shahzad MMK, King ER, Ahn EH, Bond VK, Chiu W, Wang R, Drew AF, Collins K, DeGeest K, Lutgendorf S, Sood AK. Bad Blood: The clinical implications, underlying mechanism, and biological significance of paraneoplastic thrombocytosis. N. Engl. J. Med. 2012;366:610–618. doi: 10.1056/NEJMoa1110352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempfer C, Zeisler H, Sliutz G, Haeusler G, Hanzal E, Kainz C. Serum evaluation of interleukin 6 in ovarian cancer patients. Gynecol. Oncol. 1997;66:27–30. doi: 10.1006/gyno.1997.4726. [DOI] [PubMed] [Google Scholar]
- Thomas KS, Motivala S, Olmstead R, Irwin MR. Sleep depth and fatigue: Role of cellular inflammatory system. Brain Behav. Immun. 2011;25:53–58. doi: 10.1016/j.bbi.2010.07.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanItallie TB. Sleep and energy balance: interactive homeostatic systems. Metabolism. 2006;55(suppl 2)(10):S30–S35. doi: 10.1016/j.metabol.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Vgontzas ZN, Bixler EO, Lin HM, Prolo P, Trakada G, Chrousos GP. IL-6 and its circadian secretion in humans. Neuroimmunomodulation. 2005;12:131–140. doi: 10.1159/000084844. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Papanicolaou DA, Bixler EO, Lotsikas A, Zachman K, Kales A, Prolo P, Wong ML, Licino J, Gold PW, Hermida RC, Mastorakos G, Chrousos GP. Circadian interleukin-6 secretion and quantity and depth of sleep. J. Clin. Endocrinol. Metab. 1999;84:2603–2607. doi: 10.1210/jcem.84.8.5894. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Pejovic S, Zoumakis E, Lin HM, Bixler EO, Basta M, Fang J, Sarrigiannidis A, Chrousos GP. Daytime napping after a night of sleep loss decreases sleepiness, improves performance, and causes beneficial changes in cortisol and interleukin-6 secretion. Am. J. Physiol. Endocrinol. Metab. 2007;292:E253–E261. doi: 10.1152/ajpendo.00651.2005. [DOI] [PubMed] [Google Scholar]