Abstract
Objective
To compare the immunogenicity of one vs. two doses of meningococcal conjugate vaccine (MCV4) in youth infected with HIV.
Study design
P1065 was a Phase I/II immunogenicity and safety trial of MCV4 in 324 youth infected with HIV performed at 27 sites of the IMPAACT network in the U.S. At entry subjects received one dose of MCV4. At 24 weeks, those with screening CD4% ≥15 were randomized to receive a second dose or not, and all with screening CD4% <15 received a second dose. Immunogenicity was evaluated as the proportion of subjects with a ≥4-fold rise from entry in serum bactericidal antibody against each meningococcal serogroup at weeks 28 and 72. Logistic regression models adjusting for HIV disease severity were used to evaluate the effect of one vs. two MCV4 doses among those with screening CD4% ≥15.
Results
Subjects randomized to receive two vs. one MCV4 dose had significantly higher response rates to all serogroups at week 28 and to all except N meningitidis serogroupY at week 72, with adjusted ORs of 2.5–5.6. In 31 subjects with screening CD4% <15 who received two MCV4 doses, response rates ranged from 22–55% at week 28 and 6–28% at week 72.
Conclusion
In youth infected with HIV with a CD4% ≥15, a second dose of MCV4 given six months after the initial dose significantly improves response rates at 28 and 72 weeks. Subjects with CD4% <15 at entry had lower response rates despite 2 doses of MCV4.
In 2005, a quadrivalent (serogroups A, C, Y, and W-135) meningococcal polysaccharide conjugate vaccine (MCV4) was approved by the FDA for use in persons ages 11 to 55 years. Since May 2005, the Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention has recommended MCV4 for routine use in adolescents and others aged 11–55 years who are at increased risk of meningococcal disease, but there are limited data about its immunogenicity and safety in youth infected with HIV 1, 2.
Previous studies have demonstrated that the immunogenicity to vaccines is lower in children and adolescents infected with HIV than in healthy subjects3–16. This study (P1065) was a phase I/II study of immunogenicity and safety of MCV4 in youth infected with HIV. The purpose of the study was to determine if a single dose of MCV4, then the standard of care in healthy adolescents, yielded an adequate immune response in youth infected with HIV, or if two doses were necessary. Short-term immunogenicity and safety results after a single MCV4 dose in this clinical trial were reported previously17. The present report describes the long-term immunogenicity, at 28 and 72 weeks post-entry, and safety of one dose vs. two doses of MCV4 in youth infected with HIV.
METHODS
The study included youth infected with HIV aged 11 to 24 years, who were either on a stable antiretroviral therapy (ART) regimen for at least 90 days prior to vaccination, or were not receiving ART. Exclusion criteria included personal or family history (parent, sibling, or offspring) of Guillain-Barré syndrome (GBS), previous receipt of MCV4, or receipt of meningococcal polysaccharide vaccine (MPSV4) within 2 years prior to entry.
Subjects were enrolled into one of two groups based on entry CD4+ T-lymphocyte percentage (CD4%). Group 1 had CD4% ≥15 and were further stratified into two groups of similar size: CD4% 15 to <25 and CD4% ≥25, so that randomization to a 1-dose or 2-dose MCV4 regimen would be balanced by CD4%. Group 2 included subjects with screening CD4% <15. Subjects were enrolled at 27 sites in the U.S. and Puerto Rico of the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) network. All sites received approval for the study through their local Institutional Review Board (IRB). Subjects or their parents/legal guardians signed an informed consent prior to participation; subject assent was obtained as required by IRBs. This study was registered with ClinicalTrials.gov (NCT00459316).
At study entry, all subjects were administered MCV4 (Menactra, SanofiPasteur, Swiftwater, PA, 0.5 ml intramuscularly). At week 24, Group 1 subjects who remained eligible were randomized to receive or not to receive a second dose. Those randomized to the 2-dose arm received a second dose of MCV4 at that visit. Because prior studies of immunization in severely immunocompromised patients infected with HIV demonstrated poor response to vaccination, all eligible patients in Group 2 received a second dose of MCV4 at week 24. Subjects in both groups were followed for immunogenicity and safety for 72 weeks after entry.
Assessment Methods
Safety
Standardized questionnaires were administered during the study visits at weeks 0, 4, 24, 28 and 72 after initial immunization and were supplemented by observation for 30 minutes post-vaccination and telephone contact 3, 7 and 42 days after each vaccine administration. Safety of MCV4 was assessed by recording the development of GBS, death, and new grade 3 or higher adverse events (AEs) according to the December 2004 DAIDS AE Grading Table. AE assessments were based on laboratory evaluations of hematologic variables, signs and symptoms, injection site reactions, and AE reports. All subjects who received at least one dose of MCV4 were included in the safety analyses.
Immunogenicity Assays
Samples collected for immunogenicity were sent in batches to Sanofi Pasteur for analyses. Functional meningococcal antibody activity against serogroups (SG) A, C, Y, and W-135 was measured by a serum bactericidal assay using a baby rabbit complement source (rSBA), as previously described 18. The primary immunogenicity endpoint was the proportion of participants with a 4-fold or greater rise in rSBA titer for each SG 28 weeks post-vaccination compared with entry. Seroprotection was defined as rSBA titer ≥1:128. Protective antibody titer levels were used at 72 weeks representing the longest time period following immunization for which data were available.
Statistical Analyses
Response rates for a ≥4-fold response in rSBA titer from entry level and corresponding confidence intervals based on the binomial distribution were calculated for each SG. In addition, rates of seroprotection and geometric mean titers (GMTs) were calculated for each SG. The target sample size for Group 1 (CD4% ≥15 was 119 subjects/arm) was chosen to provide a power of 0.80 to detect a 2.5 odds ratio (OR) between 28-week response rates in recipients of two versus one dose of MCV4 with a two-sided significance level of 0.05, given an anticipated response to SG C in the one-dose arm of 70%. The sample size for Group 2 (CD4% <15) was limited to 40 subjects because the goal for this severely immune suppressed group was to describe immunogenicity and safety outcomes.
For Group 1, generalized estimating equation (GEE) models were used to evaluate the association of predictors on immunologic responses compared with entry titer for all four SGs simultaneously, taking into account the correlation among responses within the same subject using an unstructured correlation; predictors included demographic characteristics, HIV infection category (perinatal vs. non-perinatal), ART use, and HIV disease severity, with separate models for ≥4-fold response at week 28 and seroprotection (rSBA titer ≥ 1:128) at week 72. All models included a term indicating whether subjects were randomized to the two doses or one dose study arm without regard to statistical significance, due to the primary interest in evaluating the effects of a second MCV4 dose. All factors with p < 0.20 in univariate GEE models were included as candidate predictors; the final model retained only those covariates with p < 0.10. The GEE models for all four SGs were confirmed by separate adjusted logistic regression models.
For Group 1, subject characteristics and toxicity rates were compared between CD4% strata and between study arms using Fisher’s exact test or Wilcoxon tests as appropriate. Within-subject changes in titer and response rate at weeks 4 and 28 for subjects receiving two doses of MCV4 were examined using McNemar test to compare response rates and Wilcoxon rank-sum tests for titer data. Statistical analyses were conducted using SAS Version 9 (SAS Institute, Cary, NC). A two-tailed p-level ≤ 0.05 was considered statistically significant.
RESULTS
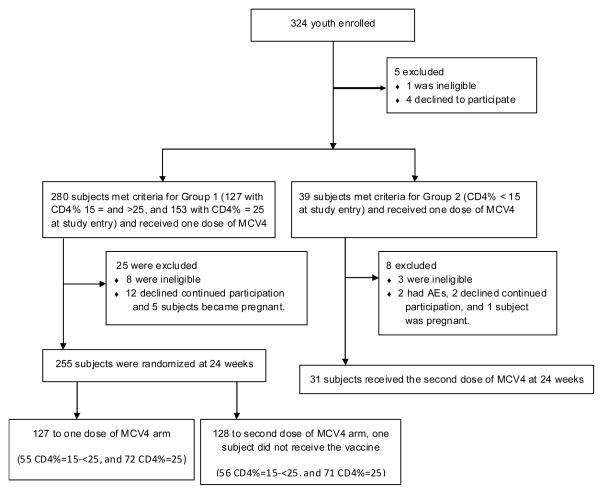
From July to October of 2007, 324 subjects were enrolled (Figure; available at www.jpeds.com). Baseline characteristics of subjects still on study at week 24 (255 with CD4% ≥ 15, 31 with CD4% < 15) are shown in Table I; characteristics of participants at study entry were reported previously17. Randomization at week 24 to study arms in Group 1 was well-balanced by CD4% strata, clinical and demographic characteristics (Table I). There was no significant change in subjects’ CD4 counts from the time of entry to the second dose (data not shown).
Figure 1.
Table 1.
Characteristics of subjects on study at week 24 (randomization for Group 1)
| Characteristic | Total (N=286) | CD4% at Study Entry | P-Value1 | |||
|---|---|---|---|---|---|---|
| Group2: CD4%<1 5 (N=31) | Group1: CD4%≥15: 1-Dose (N=127) | Group1: CD4%≥15 : 2-Dose (N=128) | ||||
| Screening CD4%≥25 | 0 | 72 (57%) | 72 (56%) | >0.99 | ||
| Median CD4%* | 27 | 7 | 29 | 28 | 0.81 | |
| Male | 168 (59%) | 19 (61%) | 75 (59%) | 74 (58%) | 0.90 | |
| Age* (years) | Median | 18 | 19.7 | 17.8 | 17.8 | 0.80 |
| 11–15 | 103 (36%) | 7 (23%) | 45 (35%) | 51 (40%) | ||
| 16–19 | 84 (29%) | 9 (29%) | 39 (31%) | 36 (28%) | ||
| 20–24 | 95 (33%) | 14 (45%) | 42 (33%) | 39 (30%) | ||
| 25+ | 4 (1%) | 1 (3%) | 1 (1%) | 2 (2%) | ||
| Black | 146 (51%) | 15 (48%) | 62 (49%) | 69 (54%) | 0.29 | |
| Latino | 107 (37%) | 15 (48%) | 47 (37%) | 45 (35%) | 0.89 | |
| HIV RNA<400 copies/mL* | 146 | 6 (19%) | 74 (58%) | 66 (52%) | 0.31 | |
| CDC Class C* | 69 (%) | 9 (29%) | 30 (24%) | 30 (23%) | 0.93 | |
| On HAART* | 208 | 19 (61%) | 95 (75%) | 94 (73%) | 0.89 | |
CDC= Centers for Disease Control and Prevention; HAART=highly active antiretroviral therapy.
– P-value for comparison between 1-dose and 2-dose arms, using Wilcoxon test for age, and Fisher exact test for all other characteristics.
at Step 2 entry
Safety
Descriptions of AEs following the first MCV4 dose were published previously17. Within 42 days of receiving the second MCV4 dose, Group 1 had no AEs, and two subjects in Group 2 reported grade 3 or higher signs/symptoms (yielding AE rates of 6.5% for Group 2 vs. 0 for Group 1, Fisher exact test p=0.01). One subject reported migraine and ocular pain (judged possibly treatment related) and one reported a lip lesion (judged not related). Similar to the safety evaluation after the first dose, there were no serious hematological AEs. The most common post-vaccination site injection grade was “mild” (primarily pain and tenderness), and was reported by fewer than 5% of the subjects. No subjects developed GBS and there were no cases of invasive meningococcal infections or meningitis reported during the study period. Two subjects died while in the study, neither of which was judged to be treatment related (one methamphetamine overdose, one HIV-related complications.
Group 1 (CD4%≥15)
Immunogenicity at Weeks 28 and 72 after 1 versus 2 doses of MCV4
Of the 255 subjects in Group 1 eligible for randomization, 232 (83%) had both baseline and week 28 meningococcal serology data, and there were no significant differences in subject characteristics between treatment arms.
GMTs were higher at week 28 for subjects receiving two versus one dose (Table II). However, by week 72 these differences persisted but had diminished.
Table 2.
GMT rSBA Titers by Serogroup for Subjects with Serology Data at Weeks 0, 4, 24, 28 and 72 by Treatment Arm
| SG | Group | Geometric Mean Titer* (95% Confidence Limits) | ||||
|---|---|---|---|---|---|---|
| Week 0 | Week 4 | Week 24 | Week 28 | Week 72 | ||
| A | Group 1: 1-Dose | 42 (25, 70) | 1911 (1135, 3216) | 287 (166, 497) | 205 (119, 355) | 127 (67, 240) |
| Group 1: 2-Doses | 32 (20, 52) | 1901 (1203, 3004) | 218 (127, 374) | 969 (633, 1483) | 281 (159, 497) | |
| Group 2 | 31 (9, 108) | 137 (32, 587) | 27 (8, 91) | 84 (23, 308) | 14 (4, 43) | |
| C | Group 1: 1-Dose | 8 (6, 10) | 196 (107, 356) | 30 (18, 49) | 24 (15, 37) | 14 (9, 23) |
| Group 1: 2-Doses | 6 (5, 8) | 131 (74, 234) | 21 (13, 32) | 174 (105, 290) | 38 (22, 64) | |
| Group 2 | 6 (3, 12) | 15 (4, 59) | 8 (4, 18) | 12 (4, 35) | 5 (3, 9) | |
| W-135 | Group 1: 1-Dose | 11 (8, 15) | 468 (307, 715) | 306 (193, 485) | 251 (156, 405) | 113 (67, 189) |
| Group 1: 2-Doses | 9 (7, 13) | 333 (215, 517) | 215 (129, 359) | 754 (488, 1165) | 167 (102, 275) | |
| Group 2 | 4 (n.a.) | 12 (5, 30) | 10 (5, 21) | 20 (7, 55) | 7 (4, 14) | |
| Y | Group 1: 1-Dose | 36 (23, 56) | 574 (368, 895) | 266 (174, 407) | 269 (172, 422) | 174 (107, 284) |
| Group 1: 2-Doses | 27 (18, 40) | 314 (201, 490) | 175 (114, 268) | 557 (380, 816) | 206 (133, 319) | |
| Group 2 | 5 (3, 9) | 24 (8, 77) | 17 (6, 46) | 32 (9, 108) | 13 (5, 33) | |
Due to assay limitations, exact titers could not be provided for results <8 or >131,072. For purposes of calculating medians and geometric mean titers, results <8 were set equal to 4 and those >131,072 were set equal to 262,144.
Sample sizes, range depending on SG:
Group 1, 1 dose: week 0 to 28: N=108–110, week 72: N=92–95
Group 1, 2-doses: week 0 to 28: N=112–115, week 72: N=95–98
Group 2: week 0 to 28: N=18–20, week 72: N=16–18
At weeks 24, 28 and 72, more subjects in the 1-dose arm had seroprotective antibody levels (rSBA ≥ 1:128) than at entry, though fewer than at 4 weeks post-vaccination (Table III). For those receiving 2 doses, rates of seroprotection were higher at week 28 than at week 4 for SGs C, W-135, and Y. The findings were similar when seroprotection was analyzed as rSBA ≥ 1:8 Group 1 subjects who received two doses had higher week 28 response rates and rates of seroprotection than those who received one dose; the differences, although diminished, persisted through week 72.
Table 3.
Response Rates and % Subjects with rSBA Titers ≥ 128 by Serogroup for Subjects with Data at Weeks 0, 4, 24, 28 and 72
| SG | Group | ≥4-fold increase in rSBA titer at Week 4* | ≥4-fold increase in rSBA titer at Week 28* | % (n/N) Subjects with rSBA titer ≥ 128 | % (n/N) Subjects with rSBA titer ≥ 8 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Baseline | Week 4 | Week 24 | Week 28 | Week 72 | Week 28 | Week 72 | ||||
|
| ||||||||||
| A | Group 1: 1-Dose | 75% (83/110) | 45% (49/110) | 43% (47/110) | 87% (96/110) | 70% (77/110) | 66% (73/110) | 57% (54/95) | 68% (75/110) | 59% (56/95) |
| Group 1: 2-Doses | 76% (85/112) | 72% (81/112) | 38% (43/112) | 91% (102/112) | 68% (76/111) | 88% (99/112) | 71% (67/95) | 88% (99/112) | 76% (72/95) | |
| Group 2 | 25% (5/20) | 40% (8/20) | 35% (7/20) | 60% (12/20) | 35% (7/20) | 55% (11/20) | 22% (4/18) | 60% (12/20) | 28% (5/18) | |
|
| ||||||||||
| C | Group 1: 1-Dose | 62% (67/108) | 34% (37/108) | 14% (15/108) | 65% (70/108) | 35% (37/107) | 31% (33/108) | 21% (19/92) | 42% (45/108) | 24% (22/92) |
| Group 1: 2-Doses | 59% (66/112) | 69% (77/112) | 9% (10/112) | 59% (66/112) | 30% (33/111) | 64% (72/112) | 35% (34/96) | 72% (81/112) | 49% (47/96) | |
| Group 2 | 17% (3/18) | 17% (3/18) | 6% (1/18) | 22% (4/18) | 17% (3/18) | 22% (4/18) | 6% (1/16) | 22% (4/18) | 13% (2/16) | |
|
| ||||||||||
| W- 135 | Group 1: 1-Dose | 83% (91/109) | 72% (79/109) | 17% (19/109) | 83% (91/109) | 76% (83/109) | 72% (79/109) | 60% (56/94) | 79% (86/109) | 69% (65/94) |
| Group 1: 2-Doses | 82% (93/113) | 86% (97/113) | 16% (18/113) | 78% (88/113) | 67% (75/112) | 86% (97/113) | 66% (63/96) | 89% (100/113) | 77% (74/96) | |
| Group 2 | 32% (6/19) | 42% (8/19) | 0% (0/19) | 21% (4/19) | 16% (3/19) | 26% (5/19) | 6% (1/17) | 42% (8/19) | 24% (4/17) | |
|
| ||||||||||
| Y | Group 1: 1-Dose | 69% (75/109) | 59% (64/109) | 38% (41/109) | 83% (90/109) | 79% (86/109) | 74% (81/109) | 63% (59/94) | 82% (89/109) | 80% (75/94) |
| Group 1: 2-Doses | 64% (74/115) | 75% (86/115) | 33% (38/115) | 73% (84/115) | 71% (81/114) | 83% (96/115) | 71% (70/98) | 91% (105/115) | 84% (82/98) | |
| Group 2 | 40% (8/20) | 40% (8/20) | 5% (1/20) | 30% (6/20) | 30% (6/20) | 30% (6/20) | 28% (5/18) | 45% (9/20) | 28% (5/18) | |
Compared with entry
Generalized Estimating Equations (GEE) Models for Evaluation of 2 doses versus 1 dose of MCV4
Week 28 Response (defined as 4-fold increase over baseline)
A repeated measures GEE model fit to 4-fold responses to each SG at week 28, adjusting for the correlation among SGs within the same subject, showed a significant benefit of two versus one dose of MCV4 (Table IV; available at www.jpeds.com). Group 1 subjects randomized to a second dose were more than three times more likely to respond (AOR=3.35, p<0.001). Separate logistic regression models fit for each serogroup also found a consistently significant benefit of two versus one MCV4 dose, with estimated adjusted ORs ranging from 1.97 for SG Y to 5.55 for SG C (AORs SG A 3.58, p<0.001; SG C 5.55, p<0.001; SG W-135 2.34, p=0.02; SG Y 1.97, p=0.03) In the pooled GEE model, there was a significant association between response rates and both screening CD4% ≥ 25 (AOR= 1.89, p=0.001) and Black race (AOR 1.46, p=0.04). Response rates were lower in subjects with entry HIV viral loads (VL) >10,000 copies/ml (AOR 0.44, p=0.001) and subjects older than 15 years at entry (AOR 0.49 for 16–19 years, p=0.003; AOR 0.61 for 20–24 years p=0.03). In addition, there was a significant association with serogroup compared with SG Y (AOR 0.67 for SG A, p=0.04; AOR 0.45 for SG C, p<0.001; AOR 1.94 for SG W-135, p<0.001).
Table 4.
Week 28 4-fold response Generalized Estimating Equations (GEE) results
| Parameter | Adjusted OR | p-value | |
|---|---|---|---|
| 2-Dose Arm | 3.35 | <0.001 | |
| Serogroup | A | 0.67* | <0.001 |
| C | 0.45** | ||
| W-135 | 1.94** | ||
| Y | reference | ||
| CD4%≥25 | 1.89 | 0.001 | |
| HIV VL>10,000 copies/ml | 0.44 | 0.001 | |
| Entry age (years) | 11–15 | reference | 0.01 |
| 16–19 | 0.49*** | ||
| 20–24 | 0.61† | ||
| Black | 1.46 | 0.04 | |
p=0.04,
p<0.001,
p=0.003,
p=0.03 compared to reference
Week 72 Immunogenicity
A repeated measures GEE model fit for seroprotection (rSBA titer > 1:128) to each SG at week 72 showed that the benefit of a second MCV4 dose remained significant, but with lower magnitude than the week 28 response levels. In the final multivariable GEE model, the odds of having seroprotection at week 72 were modestly higher in the 2-dose arm (AOR 1.52, p=0.05). Screening CD4% ≥25 (AOR 1.64, p=0.03) and having VL <400 copies/mL (AOR 0.52, p=0.0004) were also statistically significant predictors. As was true for week 28 response, SG C was associated with lower response rates than the other serogroups (AOR 0.22 compared with SG Y, p<0.001).
Group 2 (CD4% <15)
GMTs, median titers, and response rates were lower for subjects with CD4% <15. Response rates for Group 2 subjects ranged from 22–55% at week 28 to 6–28% at week 72 (Table III).
Groups 1 and 2: Comparison of 4-fold Response and Titers at Week 4 and 28 after 2 doses
For SGs C and Y, a large proportion of Group 1 subjects who did not have 4-fold responses at week 4 responded at week 28 (SG C 35%, p=0.02; SG Y 51%, p=0.03, McNemar test of agreement); there was no significant change in response for SGs A or W-135. Of 107 subjects with data for all four SGs at weeks 4 and 28 who received two MCV4 doses, 34 (32%) responded at week 28 to at least one SG to which they had not responded at week 4. Similar results were observed when comparing the proportion with antibody titers ≥1:128 at week 4 to those at week 28, with significant changes for SGs Y and W-135 (SG Y 55%, p=0.01; SG W-135 52%, p=0.03, McNemar test of agreement). For Group 2 subjects, there were no significant changes in response rates between weeks 4 and 28.
Within subject comparisons of log base 2 titers at weeks 4 and 28 for each SG found that median SG A titers were 1 dilution lower at week 28 than at week 4, but still higher than baseline (p<0.001). There were no significant decreases for the other SGs.
DISCUSSION
Youth infected with HIV with CD4% ≥ 15 benefited from MCV4 vaccination, with the majority having 4-fold responses to each SG after the first dose; results for those with CD4% <15 were more equivocal. Most participants with CD4%≥15 maintained titers ≥ 1:128 for all SGs except C after their first vaccination throughout the 72 weeks of the study, whether they received one or 2 doses of MCV4. At all time points (Weeks 4, 24, 28 and 72), immune response and seroprotection were associated with a higher CD4% and lower viral load at entry as has been described with response to other vaccines in children infected with HIV 5, 16. There were substantial differences in responses among the four SGs, with antibody titers and response rates lowest for SG C throughout the study. Based on the GEE model, the independent effects of 2-doses, low VL and high CD4 on vaccine response mean that the 2-dose benefit is important even for those who have their HIV infection well controlled.
Although participants with CD4%≥15 who received two doses of MCV4 showed robust responses to the second MCV4 dose and significantly higher seroprotection rates through week 72, the magnitude of the differences in antibody concentrations between those who received one versus two doses diminished over time. The duration of additional protection afforded by a second dose may be limited, particularly for SG C. The persistence of these differences over a longer time period and evaluation of the immunologic memory response to an additional dose of MCV4 should be investigated.
A significant number of subjects who failed to respond to SG C and SG Y after one dose responded after a second vaccination. It is noteworthy that after the second dose, a large proportion of subjects showed responses to at least one SG they had not responded to after the first dose. These findings provide a rationale for a routine 2-dose MCV4 series in youth infected with HIV, who are both at higher risk of poorer response to one MCV4 dose and likely have higher risk of contracting meningococcal disease than uninfected youth19.
Although there have been reports of hyporesponsiveness to repeated vaccinations with the MPSV4, that is, lower titers after the second dose than after the first 20–22, there is no published evidence of hyporesponsiveness after repeated doses of MCV4 or other conjugate vaccines 18. There was no evidence of lower titers after the second versus the first MCV4 dose for SG C, Y and W-135. However, titers for SG A were lower after dose two versus dose one, raising some concern for hyporesponsiveness. The additional finding that subjects in the 2-dose arm were more likely to have seroprotection at 72 weeks to all SGs, including SG A, is reassuring evidence that a second dose improves rather than impairs the level of seroprotection.
Two doses of MCV4 vaccination appear to be safe in youth infected with HIV across the full range of CD4 values. In subjects with CD4% ≥ 15, receiving a second dose of MCV4 increased the proportion with a 4-fold response and increased the rates of seroprotection over 72 weeks for all SGs. However, subjects with CD4% <15 at entry had low response rates despite 2 doses of MCV4, raising questions about the value of MCV4 vaccination in this group. These results suggest that youth infected with HIV with a CD4% >15, who account for the great majority of youth infected with HIV in the US population would benefit from a routine two-dose initial MCV4 series and support the current ACIP recommendation for a 2-dose primary MCV4 series for youth infected with HIV23, 24.
Acknowledgments
The authors would like to thank Dr. Jane Carver, from the University of South Florida’s Clinical and Translational Science Institute, for editing this manuscript. We appreciate the participation of patients and families in this study and the assistance of research personnel at the study sites.
Appendix 1
Additional members of the IMPAACT P1065 Protocol Team include: Kenneth D Braun Jr., B.A., Frontier Science & Technology Research Foundation, Amherst, NY; Donna Picard, R.N., Department of Pediatrics, Lawrence Family Health Center/UMASS, Lawrence, MA; Paul Tran, R.Ph., Center for Drug Evaluation and Research, U.S. Food and Drug Administration, Rockville, MD; Scott Watson, R.N., B.S., Clinical Research Associate, Westat Inc., Rockville, MD.
Participating sites and site personnel include: 2802 NJ Medical School CRS (James Oleske,M.D.; Arlene Bardeguez, M.D.; Arry Dieudonne, M.D.; Linda Bettica, B.S.N.); 3601 UCLA-Los Angeles/Brazil AIDS Consortium (LABAC) CRS (Nicole Falgout, R.N., Joseph Geffen, Karin Nielsen, M.D., M.P.H., Jaime Deville, M.D.); 3801 Texas Children’s Hospital CRS (Chivon Jackson, R.N., B.S.N.; Shelley Buscher, R.N., CMW; Faith Mingala, R.N., B.S.N.; Mary E. Paul, M.D.); 4101 Columbia IMPAACT CRS (Alice Higgins, R.N., M.P.A.; Andrea Jurgrau, R.N., C.P.N.P.; Marc Foca, M.D.; Seydi Vasquez, R.N.); 4201 University of Miami Miller School of Medicine – Jackson Memorial Hospital (Gwendolyn B. Scott, M.D.; Charles D. Mitchell, M.D.; Patricia Bryan, R.N.; Claudia Florez, M.D.); 4601 UCSD Maternal Child and Adolescent HIV CRS (Rolando M. Viani, M.D.,MTP; Jeanne Manning, R.N.; Kim Norris, R.N.); 4701 DUMC Ped. CRS (Margaret Donnelly, P.A.; Joan Wilson, R.N.; Mary Jo Hassett, R.N.; Kathleen McGann, M.D.); 5012 NYU NY Site (Aditya Kaul, M.D.; Sulachni Chandwani, M.D.; William Borkowsky, M.D.; Nagamah Deygoo, M.S.); 5013 Jacobi Medical Center Bronx Site (Andrew Wiznia, M.D.; Marlene Burey, R.N.; Jorge Sansary, M.D.; Francisco Reinoso, R.N.); 5017 Seattle Children’s Hospital Site (Ann J. Melvin M.D., M.P.H.; Lisa M. Frenkel M.D.; Joycelyn Thomas, R.N., B.S.N.); 5018 USF – Tampa Site (Denise Casey, R.N.; Carolyn Graisbery, R.N.; Gail Lewis, R.N.; Carina Rodriguez, M.D.); 5031 San Juan City Hospital PR Site (Midnela Acevedo-Flores, M.D.; Milagros González-Díaz, M.D.; Lizbeth Fábregas, B.S., M.S.; Orlando Alonso, M.D.); 5040 SUNY Stony Brook Site (Denise Ferraro, R.N., C.C.R.C., B.S.; Erin Infanzon; Michele Kelly, R.N., P.N.P); 5041 Children’s Hospital of Michigan Site (Ellen C. Moore M.D.; Chokechai Rongkavilit M.D.; Elizabeth Secord M.D.; Ulyssa Hancock R.N., B.S.N.); 5044 Howard University Washington DC Site (Sohail Rana, M.D.; Helga Finke-Castro, M.D.; Meseret Deressa, M.D.; Connie Nguyen, R.Ph.); 5048 USC LA Site (James Homans, M.D.; Andrea Kovacs, M.D.; LaShonda Spencer, M.D.; Michael Neely, M.D); 5051 University of Florida Jacksonville Site (Mobeen H. Rathore, M.D.; Nizar Maraqa, M.D.; Kathy Thoma., M.A.; Chas Griggs, M.Ed, C.C.R.P.); 5052 University of Colorado Denver Site (Myron J. Levin, M.D.; Emily Barr, C.P.N.P., C.N.M., M.S.N; Megan Canon; Jason Child, Pharm.D.); 5055 South Florida CDC Ft. Lauderdale Site (Ana M. Puga, M.D.; Kathleen Graham, Pharm.D.; Kevin Brown, R.N.; Carol Owens, R.N. ); 5057 Strong Memorial Hospital Rochester NY Site (Geoffrey A. Weinberg, M.D.; Barbra Murante, R.N., M.S., P.N.P.; Susan Laverty, R.N.; Francis Gigliotti, M.D.); 5090 Children’s Hospital of Los Angeles Site (Marvin Belzer M.D.; Johanna Olson, M.D.; Diane Tucker, M.S.N; Nancy Flores); 6501 St. Jude/UTHSC CRS (Jill Utech R.N., M.S.N, C.C.R.C.; Nehali Patel, M.D.; Lennie Lott, R.N.; Aditya H. Gaur, M.D.); 6601 Univ. of Puerto Rico Ped. HIV/AIDS Research Program CRS (Irma Febo, M.D.; Licette Lugo, M.D., Ruth Santos, R.N.; Maritza Cruz); 6701 The Children’s Hospital of Philadelphia IMPAACT CRS (Steven D. Douglas, M.D.; Richard M. Rutstein, M.D.; Carol A. Vincent, C.R.N.P, M.S.N; Patricia C. Coburn., R.N., B.S.N.); 6901 Bronx-Lebanon Hospital IMPAACT CRS (Aida Matias, R.N.; Mavis Dummitt, R.N.; Stefan Hagmann, M.D.; Murli Purswani, M.D.); 7301 WNE Maternal Pediatric Adolescent AIDS CRS (Richard Moriarty, M.D.; Katherine Luzuriaga, M.D.; Jessica Pagano-Therrien, R.N.); 4001 Chicago Children’s Memorial Hospital (Ram Yogev, M.D.).
Appendix 2
Supported by the National Institute of Allergy and Infectious Diseases (NIAID) (U01 AI068632), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Institute of Mental Health (NIMH) (AI068632), and the Statistical and Data Analysis Center at Harvard School of Public Health (under the National Institute of Allergy and Infectious Diseases cooperative agreement #5 [U01 AI41110] with the Pediatric AIDS Clinical Trials Group and #1 [U01 AI068616] with the IMPAACT Group). Support of the sites was provided by NIAID, NICHD International and Domestic Pediatric and Maternal HIV Clinical Trials Network (funded by NICHD [contract N01-DK-9-001/HHSN267200800001C]). Additional support was provided with federal funds from the NIAID/NIH Department of Health and Human Services (under contract HHSN272200800014C). Sanofi-Pasteur Inc. provided study vaccine and performed meningococcal serum bactericidal antibody activity assays. M.D. is an employee and stock holder of Sanofi-Pasteur, the manufacturer of the vaccine evaluated in this study. S.S. holds stock in Sanofi-Pasteur, manufacturer of the vaccine evaluated in this study. The other authors declare no conflicts of interest.
Footnotes
No reprints available.
Registered with ClinicalTrials.gov: NCT00459316.
Financial support and conflict of interest information is available at www.jpeds.com (Appendix 2). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Revised recommendations of the Advisory Committee on Immunization Practices to Vaccinate all Persons Aged 11–18 Years with Meningococcal Conjugate Vaccine. MMWR Morb Mortal Wkly Rep. 2007;56:794–5. [PubMed] [Google Scholar]
- 2.Bilukha OO, Rosenstein N. Prevention and control of meningococcal disease. Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2005;54:1–21. [PubMed] [Google Scholar]
- 3.Tasker SA, Wallace MR, Rubins JB, Paxton WB, O’Brien J, Janoff EN. Reimmunization with 23-valent pneumococcal vaccine for patients infected with human immunodeficiency virus type 1: clinical, immunologic, and virologic responses. Clin Infect Dis. 2002;34:813–21. doi: 10.1086/339044. [DOI] [PubMed] [Google Scholar]
- 4.Abzug MJ, Pelton SI, Song LY, Fenton T, Levin MJ, Nachman SA, et al. Immunogenicity, safety, and predictors of response after a pneumococcal conjugate and pneumococcal polysaccharide vaccine series in human immunodeficiency virus-infected children receiving highly active antiretroviral therapy. Pediatr Infect Dis J. 2006;25:920–9. doi: 10.1097/01.inf.0000237830.33228.c3. [DOI] [PubMed] [Google Scholar]
- 5.Abzug MJ, Warshaw M, Rosenblatt HM, Levin MJ, Nachman SA, Pelton SI, et al. Immunogenicity and immunologic memory after hepatitis B virus booster vaccination in HIV-infected children receiving highly active antiretroviral therapy. J Infect Dis. 2009;200:935–46. doi: 10.1086/605448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibb D, Giacomelli A, Masters J, Spoulou V, Ruga E, Griffiths H, et al. Persistence of antibody responses to Haemophilus influenzae type b polysaccharide conjugate vaccine in children with vertically acquired human immunodeficiency virus infection. Pediatr Infect Dis J. 1996;15:1097–101. doi: 10.1097/00006454-199612000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Klugman KP, Madhi SA, Huebner RE, Kohberger R, Mbelle N, Pierce N. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med. 2003;349:1341–8. doi: 10.1056/NEJMoa035060. [DOI] [PubMed] [Google Scholar]
- 8.Levin MJ, Gershon AA, Weinberg A, Blanchard S, Nowak B, Palumbo P, et al. Immunization of HIV-infected children with varicella vaccine. J Pediatr. 2001;139:305–10. doi: 10.1067/mpd.2001.115972. [DOI] [PubMed] [Google Scholar]
- 9.Madhi SA, Adrian P, Cotton MF, McIntyre JA, Jean-Philippe P, Meadows S, et al. Effect of HIV infection status and anti-retroviral treatment on quantitative and qualitative antibody responses to pneumococcal conjugate vaccine in infants. J Infect Dis. 2010;202:355–61. doi: 10.1086/653704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madhi SA, Kuwanda L, Saarinen L, Cutland C, Mothupi R, Kayhty H, et al. Immunogenicity and effectiveness of Haemophilus influenzae type b conjugate vaccine in HIV infected and uninfected African children. Vaccine. 2005;23:5517–25. doi: 10.1016/j.vaccine.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 11.Madhi SA, Petersen K, Khoosal M, Huebner RE, Mbelle N, Mothupi R, et al. Reduced effectiveness of Haemophilus influenzae type b conjugate vaccine in children with a high prevalence of human immunodeficiency virus type 1 infection. Pediatr Infect Dis J. 2002;21:315–21. doi: 10.1097/00006454-200204000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Mehta N, Cunningham CK, Flynn P, Pepe J, Obaro S, Kapogiannis BG, et al. Impaired generation of hepatitis B virus-specific memory B cells in HIV infected individuals following vaccination. Vaccine. 2010;28:3672–8. doi: 10.1016/j.vaccine.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nachman S, Kim S, King J, Abrams EJ, Margolis D, Petru A, et al. Safety and immunogenicity of a heptavalent pneumococcal conjugate vaccine in infants with human immunodeficiency virus type 1 infection. Pediatrics. 2003;112:66–73. doi: 10.1542/peds.112.1.66. [DOI] [PubMed] [Google Scholar]
- 14.Obaro SK, Pugatch D, Luzuriaga K. Immunogenicity and efficacy of childhood vaccines in HIV-1-infected children. Lancet Infect Dis. 2004;4:510–8. doi: 10.1016/S1473-3099(04)01106-5. [DOI] [PubMed] [Google Scholar]
- 15.Read JS, Frasch CE, Rich K, Fitzgerald GA, Clemens JD, Pitt J, et al. The immunogenicity of Haemophilus influenzae type b conjugate vaccines in children born to human immunodeficiency virus-infected women. Women and Infants Transmission Study Group. Pediatr Infect Dis J. 1998;17:391–7. doi: 10.1097/00006454-199805000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Weinberg A, Gona P, Nachman SA, Defechereux P, Yogev R, Hughes W, et al. Antibody responses to hepatitis A virus vaccine in HIV-infected children with evidence of immunologic reconstitution while receiving highly active antiretroviral therapy. J Infect Dis. 2006;193:302–11. doi: 10.1086/498979. [DOI] [PubMed] [Google Scholar]
- 17.Siberry GK, Williams PL, Lujan-Zilbermann J, Warshaw MG, Spector SA, Decker MD, et al. Phase I/II, open-label trial of safety and immunogenicity of meningococcal (groups A, C, Y, and W-135) polysaccharide diphtheria toxoid conjugate vaccine in human immunodeficiency virus-infected adolescents. Pediatr Infect Dis J. 2010;29:391–6. doi: 10.1097/INF.0b013e3181c38f3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keyserling H, Papa T, Koranyi K, Ryall R, Bassily E, Bybel MJ, et al. Safety, immunogenicity, and immune memory of a novel meningococcal (groups A, C, Y, and W-135) polysaccharide diphtheria toxoid conjugate vaccine (MCV-4) in healthy adolescents. Arch Pediatr Adolesc Med. 2005;159:907–13. doi: 10.1001/archpedi.159.10.907. [DOI] [PubMed] [Google Scholar]
- 19.Cohen C, Singh E, Wu HM, Martin S, de Gouveia L, Klugman KP, et al. Increased incidence of meningococcal disease in HIV-infected individuals associated with higher case-fatality ratios in South Africa. AIDS. 2010;24:1351–60. doi: 10.1097/QAD.0b013e32833a2520. [DOI] [PubMed] [Google Scholar]
- 20.Borrow R, Joseph H, Andrews N, Acuna M, Longworth E, Martin S, et al. Reduced antibody response to revaccination with meningococcal serogroup A polysaccharide vaccine in adults. Vaccine. 2000;19:1129–32. doi: 10.1016/s0264-410x(00)00317-0. [DOI] [PubMed] [Google Scholar]
- 21.Granoff DM, Pollard AJ. Reconsideration of the use of meningococcal polysaccharide vaccine. Pediatr Infect Dis J. 2007;26:716–22. doi: 10.1097/INF.0b013e3180cc2c25. [DOI] [PubMed] [Google Scholar]
- 22.Richmond P, Kaczmarski E, Borrow R, Findlow J, Clark S, McCann R, et al. Meningococcal C polysaccharide vaccine induces immunologic hyporesponsiveness in adults that is overcome by meningococcal C conjugate vaccine. J Infect Dis. 2000;181:761–4. doi: 10.1086/315284. [DOI] [PubMed] [Google Scholar]
- 23.Updated recommendations for use of meningococcal conjugate vaccines --- Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2011;60:72–6. [PubMed] [Google Scholar]
- 24.Van Dyke RB, Patel K, Siberry GK, Burchett SK, Spector SA, Chernoff MC, et al. Antiretroviral treatment of US children with perinatally acquired HIV infection: temporal changes in therapy between 1991 and 2009 and predictors of immunologic and virologic outcomes. J Acquir Immune Defic Syndr. 2011;57:165–73. doi: 10.1097/QAI.0b013e318215c7b1. [DOI] [PMC free article] [PubMed] [Google Scholar]