Summary
We performed a randomized pilot trial of PerMIT, a novel decision support tool for genotype-based warfarin initiation and maintenance dosing, to assess its efficacy for improving warfarin management. We prospectively studied 26 subjects to compare PerMIT-guided management with routine anticoagulation service management. CYP2C9 and VKORC1 genotype results for 13 subjects randomly assigned to the PerMIT arm were recorded within 24 h of enrollment. To aid in INR interpretation, PerMIT calculates estimated loading and maintenance doses based on a patient’s genetic and clinical characteristics and displays calculated S-warfarin plasma concentrations based on planned or administered dosages. In comparison to control subjects, patients in the PerMIT study arm demonstrated a 3.6-day decrease in the time to reach a stabilized INR within the target therapeutic range (4.7 vs. 8.3 days, p = 0.015); a 12.8% increase in time spent within the therapeutic interval over the first 25 days of therapy (64.3% vs. 55.3%, p = 0.180); and a 32.9% decrease in the frequency of warfarin dose adjustments per INR measurement (38.3% vs. 57.1%, p = 0.007). Serial measurements of plasma S-warfarin concentrations were also obtained to prospectively evaluate the accuracy of the pharmacokinetic model during induction therapy. The PerMIT S-warfarin plasma concentration model estimated 62.8% of concentrations within 0.15 mg/L. These pilot data suggest that the PerMIT method and its incorporation of genotype/phenotype information may help practitioners increase the safety, efficacy, and efficiency of warfarin therapeutic management.
Clinical Trials Registration
http://www.clinicaltrials.gov. Unique identifier: NCT00993200
Keywords: anticoagulation, warfarin, pharmacogenetics, algorithm, clinical trial
Introduction
Cytochrome P4502C9 (CYP2C9) and the vitamin K epoxide reductase complex subunit 1 (VKORC1) genotypes affect warfarin dose requirements (1–4) and influence thrombosis and bleeding risk (5, 6). The strength of the association between these genetic markers and warfarin therapy outcomes has generated interest in developing prospective genotype-based approaches to improve warfarin therapeutic management (7).
Warfarin is a racemic mixture of R- and S-enantiomers. Inherited differences in CYP2C9 and VKORC1 genes produce delayed metabolic clearance and a lower concentration threshold for response for S-warfarin, which accounts for the majority of the anticoagulation effect (8). These inherited characteristics have a profound effect on the clinical pharmacology of warfarin and compromise the utility of typical dosing and monitoring practices, which do not account for pharmacokinetic (PK) and pharmacodynamic (PD) differences between patients (9). These differences can be managed through prospective modeling techniques as previously described by our group (10). Ideal induction and maintenance dosage rates are dictated by the rate of drug metabolism (clearance, half-life) and target therapeutic concentration. Knowledge of these variables establishes the basis for calculating tailored induction and maintenance dosing regimens. CYP2C9 genotyping enables genotype-specific estimates of S-warfarin clearance and VKORC1 genotyping guides the selection of target therapeutic S-warfarin plasma concentrations (1, 11–13).
Therapeutic monitoring of chronically administered drugs is most informative when drug concentrations over the dosing interval remain consistent, a condition known as steady state. Approximately 40% of patients have diminished CYP2C9 capacity, which reduces S-warfarin clearance (8, 14, 15); these patients have not achieved steady state when standard therapeutic monitoring is performed during therapy initiation or after dose modifications. As a result, routine INR measurements for carriers of CYP2C9*2 or *3 alleles may not reflect the intended therapeutic endpoint and may thus reduce the fidelity of information on which clinical dosing decisions are based.
The application of pharmacogenetic diagnostics to warfarin therapy is hindered by the absence of clear guidance on whether and how genotyping may be used to improve patient therapy. Most trials have focused on the ability to estimate warfarin maintenance dosages based on statistical algorithms that associate dosage with the genetic and clinical characteristics of the patient. This approach fails to leverage the PK and PD information gained through genotyping, which can be applied for ongoing individualized therapeutic management. The Personalized Medicine Interface Tool (PerMIT) (10) extends the technique of calculating genotype-adjusted loading and maintenance dosages (3) by applying pharmacogenetic-based PK modeling to illustrate the influence of repeated dosing on plasma drug concentrations of S-warfarin. Plasma concentrations of R-warfarin are not influenced by genetic variation of CYP2C9 and thus are not considered in the model, which provides a visual representation of the relationship between INR measurements and S-warfarin concentration. This provides users with a proactive approach to INR interpretation and dose management. In preparation for appropriately powered prospective trials, we conducted a pilot trial to test the hypothesis that patients whose therapy is managed by practitioners using the PerMIT method will demonstrate a 10% increase in time within the therapeutic range, along with a 3-day advantage in time to first therapeutic INR and a 10-day advantage in time to reach stable maintenance dosing. In addition, we collected pre-dose plasma samples during the first 30 days of therapy in order to prospectively evaluate the accuracy of plasma concentration PK modeling in the context of loading and pre-maintenance dosing.
Materials and Methods
Study Design
We performed a prospective, randomized study of warfarin initiation and maintenance dosing by comparing PerMIT to standard-of-care (SOC, as defined by the University of Utah thrombosis service) methods of therapeutic management. Two separate and independent clinical teams, one for each study arm, were identified. Each team was comprised of 2 physicians and a pharmacist specializing in oral anticoagulation management. The care team responsible for the PerMIT arm was trained in a 2-hour online session that involved a basic review of pharmacology and the operation of the software using mock case studies.
Objectives
This pilot trial was designed to obtain preliminary data to inform the design of future multi-center studies and to identify weaknesses and/or limitations of the clinical protocol and/or study logistics.
Inclusion and Exclusion Criteria
The study included warfarin-naïve patients aged ≥ 18 years, for whom warfarin initiation was indicated with intent to treat for a minimum of 12 weeks with written informed consent. Patients were excluded from enrollment in both arms of the trial if they were currently enrolled in other investigational trials, pregnant or unwilling to use reliable contraception, or had significant co-morbidities that precluded standard dosing (e.g. terminal disease, hepatic insufficiency or transplantation, renal insufficiency/creatinine > 2.5 mg/dL).
Enrollment, Randomization, and Blinding
Consenting patients who met the enrollment criteria were initially stratified by age, gender, and target INR, then randomized to the PerMIT or SOC arms of the trial. At enrollment, whole blood was collected from all patients for CYP2C9 and VKORC1 genotyping. Genotyping results were immediately made available to the care team assigned to the PerMIT arm and remained blinded for the SOC arm. This protocol was approved by the University of Utah Institutional Review Board.
PerMIT
PerMIT (10) is a software-based method for clinical decision support. The method employs clinical and genetic information from each patient to calculate an estimate of the theoretical maintenance dose. PerMIT employs traditional pharmacokinetic modeling calculations where the values for S-warfarin oral clearance and target therapeutic S-warfarin plasma concentration are substituted for general population-based estimates according to the patient’s CYP2C9 and VKORC1 genotypes. The performance characteristics of PerMIT with respect to retrospective estimation of theoretical maintenance dose and PK modeling have been previously published (10). The software allows users to create longitudinal case files for each patient, thus building a temporal framework for evaluating INR monitoring results in light of previous and future dosing decisions (16).
Warfarin Dosing
Warfarin dosing was managed by the clinical team assigned to each arm of the trial. All patients in the SOC arm were generally initiated with a 5 mg dose, but clinicians were allowed to deviate at their discretion. During the first week of therapy, dose adjustments were made based on the warfarin induction algorithm as described by Kovacs et al. (17). INRs were routinely measured on days 0, 3, 5, twice during the second week of therapy, once in weeks 3 and 4, and monthly thereafter. Out-of-range INR responses were defined and managed per the American College of Chest Physician (ACCP) guidelines (18). Patients in the PerMIT arm of the trial were also initiated on a 5 mg dose until genotyping results were reported on day 2, at which time a transition/loading dosage was calculated by the PerMIT software based on the patient’s genotype and estimated eventual maintenance dosage. This calculated dosage was either accepted (12 subjects) or rejected (1 subject) by the dosing clinician. Subsequent dosing adjustments were made based on evaluation of INR measurements in light of the PerMIT-modeled plasma S-warfarin concentration-time profiles, patient history, and clinical judgment. Dosage adjustments were managed with the intent to limit broad fluctuations in the plasma S-warfarin concentration-time profile and to interpret INR measurements in the context of progress towards achieving steady state.
Study Duration
The study duration for each patient was 12 consecutive weeks. Patient enrollment began in January 2010 and concluded in September 2010.
DNA Extraction and Genotyping
All patient genotyping was performed by ARUP Laboratories (Salt Lake City, Utah), a CLIA-certified clinical testing facility. DNA was extracted from EDTA whole blood with Roche MagNa Pure reagents (Indianapolis, IN). The extracted DNA was amplified by polymerase chain reaction and genotyping was performed using Simple Probe reagents provided by Idaho Technology, Inc. (Salt Lake City, Utah) and a Roche LightCycler (Indianapolis, IN). The CYP2C9*2 (rs1799853) and *3 (rs1057910) and the VKORC1 -1639 G>A (rs9923231) polymorphisms were detected by high-resolution melting profile analysis. Genotyping was generally performed and reported within 24 h of enrollment.
Plasma S-Warfarin Measurement
Patients in each treatment arm consented to provide blood at each INR assessment to be used for S-warfarin analysis and quantification. These values were used to evaluate the accuracy of the PK modeling function of PerMIT following completion of enrollment. For each patient, approximately 10 samples covering the first 30 days of therapy were obtained during initiation phase and follow-up. All specimens were collected at least 16 h after the last dose to estimate trough concentrations. Blood (EDTA) was centrifuged and plasma promptly removed from the cells. Plasma was stored frozen until analysis. All samples (PerMIT and SOC arms) were analyzed after completion of the study. Briefly, samples were spiked with p-chlorowarfarin internal standard, purified by solid phase extraction and analyzed by chiral HPLC (19). Measured trough concentrations were compared for absolute and trend agreement to corresponding concentrations calculated by PerMIT. A goal of 55% of estimates within 0.15 mg/L of the measured was set based on previous retrospective analysis (10). Actual measurements were not used nor intended to be used in the trial or clinical application.
Endpoints
Time to First Stable Therapeutic INR
Time to first stable therapeutic INR is defined as the time interval in days from the first warfarin dosage to the first time interval where the INR remains within the predefined acceptable range (INR 1.8 to 3.2) for a minimum of 4 consecutive days.
Determination of Stable Maintenance Dose and Time to Stable Therapy
Stable maintenance dose was conservatively defined as the average daily dosage that consistently yielded a minimum of 2 consecutive INR results within acceptable limits (INR 1.8 to 3.2) and measured a minimum of 14 days apart. Time to stable therapy is the time interval from the first warfarin dosage to the first administration of the stable average daily dosage as described above.
Determination of Time in Range
To evaluate time in therapeutic range, the upper and lower limits of the acceptable INR range were established as the median of the target range ± 0.7 INR units. For all patients, the limits of acceptability fell between an INR of 1.8 to 3.2. Time-in-range was evaluated by linear interpolation (20) and reported as the percentage of days within the allowable limits for days 0 to 25 and days 25 to 60, which is the longest common endpoint.
Frequency of Dosage Adjustments
Frequency of dosage modifications was measured as the number of patient interactions at which a dosage change occurred, excluding the very first dosage and pre-planned multi-day dosing of unequal amounts.
Safety and Clinical Events Committees
Weekly conference calls were conducted and included a quorum of the authors to discuss patient recruitment, study logistics, compliance, and potential safety issues. No patient safety concerns introduced by use of the software or the trial design were identified.
Statistical Analysis
Differences in clinical characteristics between groups were evaluated using Fisher’s exact test. Differences in means were evaluated using the t-test. Differences in time to first therapeutic INR, time in therapeutic range, and frequency of dosage adjustments are not normally distributed and were thus compared using Wilcoxon’s rank-sum test. Differences in time to stable maintenance dosing were evaluated by the log-rank test. All calculations were performed using SAS software (Version 9.1, SAS Institute Inc., Cary, NC, USA).
Results
Patient Enrollment and Demographics
Thirty four patients were enrolled with 26 patients included in the final analysis. Attrition was attributed to warfarin discontinuation, patient noncompliance, unreported comorbidities, and insufficient INR data. Clinical characteristics (Table 1) were balanced between study arms. The mean age in the PerMIT arm was 14 years older (p = 0.117). The distribution of CYP2C9 and VKORC1 genotypes were similar between groups and fell within the expected distribution as calculated using the Hardy-Weinberg law and the allele frequencies observed in this study. The observed allele frequencies were similar to previous literature reports (21, 22).
TABLE 1.
Patient Characteristics
| Characteristic | SOC Arm | PerMIT Arm |
|---|---|---|
| Number of patients | 13 | 13 |
| Age (y), mean (range) | 45 (21–90) | 59 (24–73) |
| Females, % | 46 | 46 |
| Atrial Fibrillation, % | 31 | 38 |
| Deep Vein Thrombosis, % | 38 | 54 |
| Stroke, % | 7 | 7 |
| Other, % | 24 | 0 |
| Ethnicity/Race, % White | 85 | 100 |
| CYP2C9 variant, % | 38 | 31 |
| VKORC1 variant, % | 62 | 46 |
| Any variant, % | 84 | 61 |
| Average Maintenance Dose (mg/d), mean ± SD | 5.1 ± 2.2 | 5.6 ± 1.7 |
No statistical difference in M/F ratio, p = 1.0; median age, p = 0.117; variant allele proportions, p = 0.626; or mean maintenance dose, p = 0.523
Time to First Stable Therapeutic INR
The results of this pilot study revealed a 3.6-day advantage in time to first stable therapeutic INR for patients managed by the PerMIT method (Table 2). Stable therapeutic INR was achieved for 38.5% of SOC versus 69.2% of PerMIT subjects within the first 5 days (p = 0.1156) and for 61.5% of SOC versus 100% of PerMIT subjects within the first 8 days (p = 0.0128) (Table 2). Time to first above-range INR (>3.3) did not significantly differ between the SOC and PerMIT arms (p = 0.228). Nine of the SOC subjects experienced an INR > 3.2 with the first occurrence averaging 10.4 days following initiation. Eight of the PerMIT subjects experienced an INR > 3.2 with the first occurrence averaging 13.6 days following initiation.
Table 2.
End point results
| Characteristic | SOC Arm | PerMIT Arm | P |
|---|---|---|---|
| Time to first stable therapeutic INR (days) | 8.3 (3.5–17.5) | 4.7 (3.5–8) | 0.0152 |
| Therapeutic INR by day 5, % | 38.5 | 69.2 | 0.1156 |
| Therapeutic INR by day 8, % | 61.5 | 100 | 0.0128 |
| Time within therapeutic range, first 25 days, % | 55.3 ± 16.6 | 63.4 ± 15.8 | 0.1805 |
| Time within therapeutic range, first 60 days, % | 70.3 ± 17.9 | 77.7 ± 11.3 | 0.441 |
| Dose adjustments per INR measurement, % | 57.1 ± 10.9 | 38.3 ± 8.50 | 0.007 |
| INR > 3.2, % | 18.5 | 18.5 | 1 |
Time to Stable Maintenance Dose
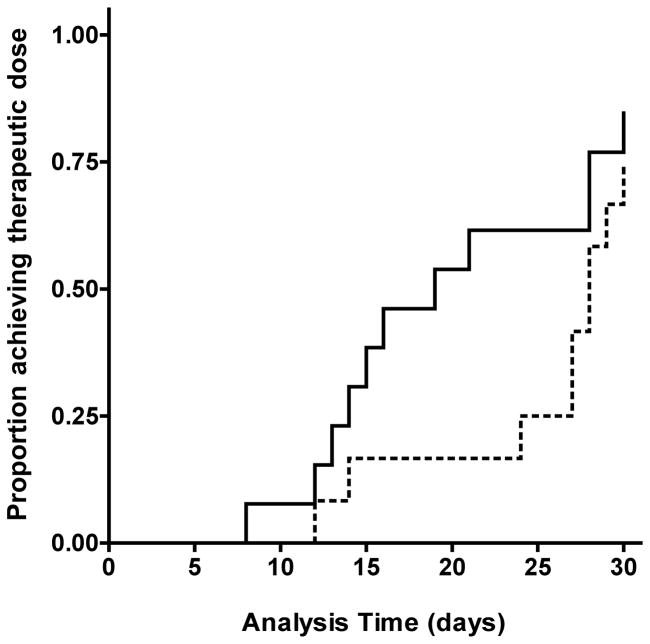
Within the first 25 days of therapy, 8 of 13 (61.5%) PerMIT subjects achieved stable maintenance dosing, in contrast to 3 of 13 (23%) SOC subjects (Log-rank test p = 0.0553). This data is depicted in the form of a Kaplan-Meier survival plot in Figure 1. Beyond the initial 25-day period, there were no significant differences in time to achieve stable maintenance dosing.
Figure 1. Time to Stable Maintenance Dose.
Kaplan-Meier survival analysis of time to first continuous administration of the average daily maintenance dose required to achieve a sustained INR response with the therapeutic range for a minimum of 14 consecutive days. Solid line, SOC arm; dashed line, PerMIT-guided arm. PerMIT-guided therapy yielded a greater proportion of patients achieving stable maintenance therapy within the first 25 days of therapy (log-rank, p = 0.0553).
Time within Therapeutic Range
Overall time within the therapeutic range was evaluated over the first 25 days and over the entire trial duration of 60 days. Over the initial 25-day time interval, SOC patients maintained a therapeutic INR 55.3% of the time, approximately 14 of 25 days, and PerMIT patients remained within the therapeutic range 63.4% of the time or 15.8 of 25 days (p = 0.181) (Table 2). Over the 60-day period, SOC patients remained within the therapeutic range 70.3% of the time or 42 days, PerMIT patients for 77.7% of the time or 46.6 days (p = 0.441) (Table 2).
Frequency of Dose Adjustments
Previous studies have demonstrated that pharmacogenetics (PGx)-guided therapy required fewer dosage adjustments than SOC (23). Over the 60-day evaluation period, SOC patients had their dosage modified 57.1% of the time (average 8.5 of 15 encounters) when an INR measurement was taken. In contrast, the dosage was modified 38.3% of the time (average 5.7 of 15 encounters) in the PerMIT arm (p = 0.007) (Table 2).
Safety Observations
There were no serious adverse events reported for any of the subjects. A previous trial of PGx-guided warfarin therapy defined an INR ≥ 4.0 as an adverse event. In the SOC and PerMIT arms, 40% of subjects experienced an INR ≥ 4.0 over the term of the trial. In the SOC arm, all INRs ≥ 4.0 occurred within the first 25 days of therapy (range 7–23); in the PerMIT arm, 4 of 6 events occurred within the first 25 days.
Accuracy of S-warfarin Concentration Estimates
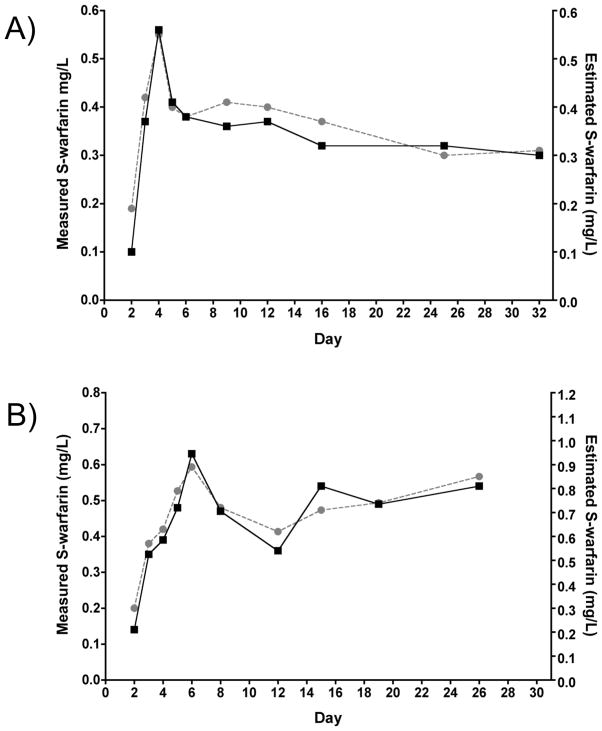
S-warfarin plasma concentration was measured in 255 samples collected from 26 patients with an average of 10 samples per patient over the first 30 days. For each collection, the measured value was compared to the concentration calculated by the PerMIT PK model. Overall, 62.8% of the PerMIT-estimated plasma trough S-warfarin concentrations were within 0.15 mg/L of the measured concentrations (Table 3). This exceeds the goal of 55% based on retrospective analysis (10). Concentration estimates for each genotype are given in Table 3. The highest level of agreement between measured and calculated S-warfarin concentrations was 73.0% for the CYP2C9*1/*3 genotype. For 8 patients in our study (including 5 CYP2C9*1/*1 and 3 CYP2C9*1/*2 patients), the level of quantitative agreement between the measured and calculated concentrations was below goal; however, the estimated concentration-time profiles demonstrated parallelism (trend agreement) with the measured concentration-time profiles. Figure 2 depicts a comparison of 2 patients with profile trend agreement and varying degrees of quantitative agreement. The level of agreement where the PK modeling predicts the correct profile trend was further analyzed, based on a point-to-point comparison between the measured and estimated concentrations, as no change, a concentration increase, or a concentration decrease. Based on these criteria, the level of agreement between calculated and measured S-warfarin concentration-time profiles was 78.2% (Table 3). R-warfarin concentrations were also obtained. The S:R ratio for each genotype was similar to previous reports (Table 3).
Table 3.
Accuracy of estimated S-warfarin concentrations
| Genotype | % within 0.15 mg/L | % Trend agreement | Average (Expected) S:R ratio(14) |
|---|---|---|---|
| CYP2C9*1/*1 | 66.5 | 75.4 | 0.54 (0.44) |
| CYP2C9*1/*2 | 46.7 | 79.6 | 0.69 (0.61) |
| CYP2C9*1/*3 | 73.0 | 78.2 | 1.07 (0.71) |
| Overall | 62.8 | 78.2 | -- |
Figure 2. Measured and PerMIT-estimated S-warfarin concentration versus time profiles.
Measured concentrations (black squares) and PerMIT-estimated concentrations (gray circles) are shown for 2 PerMIT-managed patients. A) CYP2C9*1/*1 patient, for whom 100% of the estimated concentrations were within 0.15 mg/L of the measured concentrations and 78% of estimated concentrations followed the measured concentrations in terms of no change, increase, or decrease from prior measurements (Trend); B) CYP2C9*1/*2 patient, for whom none of the estimated concentrations were within 0.15 mg/L of the measured concentrations; however, 90% of estimated concentrations follow the changes in measured concentrations. Despite below-goal quantitative agreement, this patient is an example of a correctly predicted concentration-time profile that allows for the interpretation of INR in the context of steady-state S-warfarin concentrations.
Discussion
We conducted a randomized control pilot trial in preparation for larger studies and to substantiate our hypotheses regarding clinical outcomes improvement. The pilot trial was conducted within the context of a specialized anti-coagulation service. This service routinely manages anticoagulation for inpatients and outpatients. The trial was designed to be an “add-on” trial, where the introduction of genotyping and use of the PerMIT method were incorporated into an existing SOC environment. Each arm of the trial involved separate clinical investigators to avoid learning bias.
The strengths of this pilot trial include the prospective randomized control design, the ability to apply genotype-enabled decision support within the first 2 warfarin dosages without postponing therapy, representation of variant genotypes, and observational data that support the hypotheses to be tested in our formal multi-site trials. The limitations of the trial include the small sample size and the fact that more than 90% of subjects are of white European-American descent.
The outcomes of interest included time to first stable therapeutic INR, time to stable maintenance dosing, time within the therapeutic range, and frequency of dose adjustments. Our randomized control trial design was powered to test the hypothesis that patients whose therapy is managed with the PerMIT method will demonstrate a 10% increase in time within the therapeutic range. This power level also allows detection of a 3-day advantage in time to first therapeutic INR and a 10-day advantage in time to reach stable maintenance dosing.
At the outset, we defined the time to first stable therapeutic INR as the time from the first warfarin dose until the time of the first INR that exceeded the lower limit of the acceptable range (e.g. > 1.8) (7, 24). However, analysis of the first few patients revealed a flaw in this definition, as certain patients demonstrated an INR response that rapidly surpassed the lower threshold and then exceeded the upper limit of the acceptable INR interval. This does not represent our definition of “therapeutic”. In order to control for this type of response, we increased the stringency of the definition to require that the INR be maintained within the acceptable interval for a minimum of 4 days in order to meet a minimal definition of therapeutic. This definition was employed to avoid defining therapeutic status based on a single INR measurement and fit within the INR monitoring schedule defined by the trial protocol. This change in criteria likely accounts for the fact that the time to first therapeutic INR in the SOC arm is longer than has been reported in earlier trials (23, 24). After being subjected to the same level of stringency in both arms of this trial, the results revealed a 3.6-day advantage in time to first stable therapeutic INR for those patients managed by the PerMIT method, thus supporting the original hypothesis (Table 2).
Time to reach stable maintenance dosing was defined as the time from initiation until the first administration of the average daily maintenance dose that yielded a minimum of 2 consecutive INR results within acceptable limits and measured a minimum of 14 days apart without an intervening dosing change. The median time to stable maintenance dose was 20 days in the PerMIT arm versus 28.5 days in the SOC arm, an 8.5 day advantage. The results are presented as a Kaplan-Meier survival analysis in Figure 1, which shows that within the first 25 days, 25% of SOC patients had achieved stable maintenance dosing versus 58% of patients managed with PerMIT.
Several reports have clearly documented the fact that the period immediately following warfarin induction represents the greatest risk of above-range INRs and the lowest proportion of time spent within the therapeutic range (5, 24–27). Following the precedent study of Caraco et al. (24), we evaluated time in range over 2 discrete intervals: the first 25 days and from day 26 to the end of the trial. In order to avoid bias introduced by different durations of therapy, we limited the term to day 60, which is the follow-up period for all subjects included in the analysis. Within the first 25 days, time within therapeutic range was improved by 12.8% in the PerMIT arm. Over the entire trial term, time within therapeutic range was improved by 9.5% (70.3% vs. 77.7%). These data support a hypothesis of approximately 10% improvement of time in range with a higher likelihood and potentially greater improvement of time in range during the initiation period. Interestingly, we found that the overall time in range of 77.7% was achieved with less than 40% of INR measurements provoking a dosage modification among the PerMIT-managed subjects, whereas, to achieve an overall time in range of 70% in the SOC arm, dose modification was required for 57% of INR measurements.
Determination of a patient’s CYP2C9 and VKORC1 genotypes can be applied to categorical assessment of warfarin sensitivity, calculated estimation of maintenance dosage, adjustments to loading dosages, and adjustments to INR-based criteria for dose titration. Methods of applying genotype information include: i) a categorical approach where patients are categorized on a scale from very high to less than normal warfarin sensitivity based on genotype; ii) a statistical approach, where a mathematical algorithm, derived from retrospective data of stabilized patients during the maintenance phase of therapy, is used to estimate an individual’s maintenance dose based on the net effect of a sub-set of clinical and genetic characteristics; and/or iii) a pharmacological approach where the influence of CYP2C9 genotype on S-warfarin clearance and VKORC1 genotype on S-warfarin PD response (target CSS or IC50) are considered using standard PK methods. Various examples of each of these approaches have been reported in the literature. Epstein et al. performed a prospective comparative effectiveness study based on reporting warfarin sensitivity categories and demonstrated a 43% lower risk of hospitalization due to bleeding events or thrombosis for those patients who received CYP2C9 and VKORC1 genotyping (HR 0.57 95%CI:0.39 to 0.83, p = 0.003) (28). However, this trial design did not provide insight into how or if this information was used in patient care.
Most clinical trials have used statistical algorithms (23, 28–31). The advantage of these algorithms is that they provide a convenient quantitative adjustment to standard maintenance dosages based on factors that influence warfarin dose requirements such as age, weight, smoking, target INR, and amiodarone, for which a clear quantitative adjustment to standard pharmacologic parameters such as clearance (Cl), volume of distribution (Vd), or target therapeutic concentration (Cmin or IC50) have not been derived. This basic approach lacks methodological rationale for loading strategies and does not provide guidance for interpreting ongoing INR results. Using this approach, Hillman et al. demonstrated the feasibility of prospectively applying CYP2C9 genotyping at the onset of warfarin therapy to predict maintenance dose; however, they failed to demonstrate improvement in the primary outcome of time within therapeutic range (32). Anderson et al. (23) extended the approach by doubling the calculated maintenance dose for the first one or two dosages as a refined loading strategy in a PGx-guided arm versus a standard 10 mg warfarin dosing nomogram (17). Anderson demonstrated fewer dosage modifications in the PGx-guided arm but failed to demonstrate improvements in the percentage of out-of-range INRs (primary outcome) or in the time within therapeutic range (69.7% vs. 68.6%). In a recent, larger follow-up trial, Anderson et al. employed this same strategy as well as a multi-step dose-revision algorithm where the initial PGx algorithm-calculated dosage was revised based on INR measurements after the first 3 or 4 dosages. This trial did demonstrate an improvement in time in therapeutic range over parallel controls (~72% vs. 59%) with no additional improvements gained with the multi-step dose revision algorithm. In both studies, there was no apparent change to standard INR monitoring strategy (29).
In a study including 229 subjects, McMillin et al. used a basic PGx algorithm to estimate maintenance dose in combination with a modified standard initial INR-based dosing algorithm for patients with at least one CYP2C9 variant to delay dose increases when INR results were sub-therapeutic following the 4th or 5th dosage. However, it is not clear whether this modification had any impact on the trial outcomes and the trial failed to demonstrate significant differences from standard practice (31). The impact of the genotype-guided dose estimation may have been reduced in this study due to the skilled management of warfarin therapy in the SOC arm, similar to our current study.
The study by Caraco et al. (24) was among the first examples of a pharmacological approach to leverage the change in S-warfarin clearance associated with various CYP2C9 genotypes. In a study of 185 patients, Caraco developed CYP2C9-adjusted loading and maintenance dosing calculations and demonstrated significantly faster time to first INR > 2 (4.8 vs. 7.53 days), shorter time from induction to stable maintenance (14.1 to 32.2 days), and increased time in therapeutic range (80.4 vs. 63.4 days) compared to standard warfarin induction (24). This trial preceded current knowledge of the influence of VKORC1 genotype, so this information was not included in the treatment methodology. Additionally, the authors did not describe changes to INR monitoring or interpretation to account for CYP2C9-dependent changes in warfarin pharmacokinetics. Finally, this purely pharmacokinetic approach does not allow for a priori dosing corrections based on clinical criteria such as the factors represented in statistical algorithms for estimating maintenance dose. Aside from our PerMIT-based method, the most comprehensive methodology for PGx-based warfarin therapy was described by Gong et al. (30). In this prospective trial, the PK effects of CYP2C9 and PD effects of VKORC1 were considered in order to adjust loading and maintenance dosing and to facilitate interpretation of INR measurements up to day 9 of therapy. This trial was designed to demonstrate that an anticipated quality of care disparity attributed to genetic variation in CYP2C9 and VKORC1 could be eliminated with PGx-guided therapy. Although the authors successfully demonstrate there were no differences (e.g. anticipated disparity averted) in quality outcomes between patients of different genotypes when PGx-guidance was employed, the cohort trial design did not provide any measure of overall improvement in quality outcomes over standard of care (30).
The PerMIT method employs a statistical algorithm to capture the influence of clinical factors and genetics on maintenance dose, employs basic PK/PD theory to calculate loading and transition dosages, and provides a graphical representation of INR measurements in PK context throughout the duration of therapy. Thus, to our knowledge, this is the first application of CYP2C9 and VKORC1 genotyping to fully leverage the potential of the statistical and pharmacological methods to address each of the 3 aspects (i.e. loading, maintenance dosing, and INR interpretation) of warfarin therapy that can be informed by a patient’s CYP2C9 and VKORC1 genotype. Further, we introduce a new definition for time to first therapeutic INR to better capture a sustained response within the therapeutic range.
We have previously reported on the ability of the PerMIT PK model to calculate trough plasma concentrations of S-warfarin under maintenance dosing conditions (10). Retrospectively analyzed PerMIT-calculated S-warfarin concentrations were within 0.15 mg/L of the measured concentrations for > 55% of samples. We took advantage of the present trial design to prospectively evaluate the ability of the PerMIT PK model to calculate serial plasma S-warfarin concentrations under loading and initial dosing conditions and appropriately track changes in plasma concentration after dose modifications. The overall agreement between calculated and measured values exceeded expectations, as 62.8% of calculated values fell within 0.15 mg/L. Interestingly, CYP2C9*1/*3 individuals showed the highest level of predictive accuracy (73% of calculated values within 0.15mg/L of measured), consistent with the lower inter-individual variation of S-warfarin elimination for this genotype (14).
Conclusions
The PerMIT method is a hybrid approach to the application of CYP2C9 and VKORC1 genotypes to warfarin management. This pilot investigation suggests that the trial design will not introduce safety issues and will not be burdensome to the patients or trial investigators. The pilot trial also provided prospective evidence to support the hypotheses that will be tested in adequately powered multi-center investigations. In light of previous reports, this data strengthens the case for prospective utilization of genotype information for warfarin therapy management. What remains to be determined is which approach yields the greatest, most sustainable and reproducible improvements to warfarin therapy management.
Acknowledgments
We gratefully acknowledge the assistance of Bronwyn Ramey-Hartung, Brittany Patterson, Pamela Proctor, and Tom Edwards.
Financial support: The study was funded by grants from National Heart, Lung and Blood Institute R44HL090055 (ML, RV, KR) and ARUP Laboratories, Salt Lake City Utah.
Footnotes
Disclosures
PerMIT and the PerMIT technology are products of PGXL Laboratories. Roland Valdes Jr. and Mark W. Linder hold equity interest in PGXL.
References
- 1.D’Andrea G, D’Ambrosio RL, Di Perna P, et al. A polymorphism in the VKORC1 gene is associated with an interindividual variability in the dose-anticoagulant effect of warfarin. Blood. 2005 Jan 15;105(2):645–9. doi: 10.1182/blood-2004-06-2111. [DOI] [PubMed] [Google Scholar]
- 2.Rieder MJ, Reiner AP, Gage BF, et al. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N Engl J Med. 2005 Jun 2;352(22):2285–93. doi: 10.1056/NEJMoa044503. [DOI] [PubMed] [Google Scholar]
- 3.Zhu Y, Shennan M, Reynolds KK, et al. Estimation of warfarin maintenance dose based on VKORC1 (−1639 G>A) and CYP2C9 genotypes. Clin Chem. 2007 Jul;53(7):1199–205. doi: 10.1373/clinchem.2006.078139. [DOI] [PubMed] [Google Scholar]
- 4.Voora D, Eby C, Linder MW, et al. Prospective dosing of warfarin based on cytochrome P-450 2C9 genotype. Thrombosis and haemostasis. 2005 Apr;93(4):700–5. doi: 10.1160/TH04-08-0542. [DOI] [PubMed] [Google Scholar]
- 5.Aithal GP, Day CP, Kesteven PJ, et al. Association of polymorphisms in the cytochrome P450 CYP2C9 with warfarin dose requirement and risk of bleeding complications. Lancet. 1999 Feb 27;353(9154):717–9. doi: 10.1016/S0140-6736(98)04474-2. [DOI] [PubMed] [Google Scholar]
- 6.Margaglione M, Colaizzo D, D’Andrea G, et al. Genetic modulation of oral anticoagulation with warfarin. Thrombosis and haemostasis. 2000 Nov;84(5):775–8. [PubMed] [Google Scholar]
- 7.Higashi MK, Veenstra DL, Kondo LM, et al. Association between CYP2C9 genetic variants and anticoagulation-related outcomes during warfarin therapy. JAMA. 2002 Apr 3;287(13):1690–8. doi: 10.1001/jama.287.13.1690. [DOI] [PubMed] [Google Scholar]
- 8.Linder MW, Looney S, Adams JE, 3rd, et al. Warfarin dose adjustments based on CYP2C9 genetic polymorphisms. J Thromb Thrombolysis. 2002 Dec;14(3):227–32. doi: 10.1023/a:1025052827305. [DOI] [PubMed] [Google Scholar]
- 9.Bon Homme M, Reynolds KK, Valdes R, Jr, et al. Dynamic pharmacogenetic models in anticoagulation therapy. Clin Lab Med. 2008 Dec;28(4):539–52. doi: 10.1016/j.cll.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Linder MW, Bon Homme M, Reynolds KK, et al. Interactive modeling for ongoing utility of pharmacogenetic diagnostic testing: application for warfarin therapy. Clin Chem. 2009 Oct;55(10):1861–8. doi: 10.1373/clinchem.2009.125898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bodin L, Verstuyft C, Tregouet DA, et al. Cytochrome P450 2C9 (CYP2C9) and vitamin K epoxide reductase (VKORC1) genotypes as determinants of acenocoumarol sensitivity. Blood. 2005 Jul 1;106(1):135–40. doi: 10.1182/blood-2005-01-0341. [DOI] [PubMed] [Google Scholar]
- 12.Sconce EA, Khan TI, Wynne HA, et al. The impact of CYP2C9 and VKORC1 genetic polymorphism and patient characteristics upon warfarin dose requirements: proposal for a new dosing regimen. Blood. 2005 Oct 1;106(7):2329–33. doi: 10.1182/blood-2005-03-1108. [DOI] [PubMed] [Google Scholar]
- 13.Yuan HY, Chen JJ, Lee MT, et al. A novel functional VKORC1 promoter polymorphism is associated with inter-individual and inter-ethnic differences in warfarin sensitivity. Human molecular genetics. 2005 Jul 1;14(13):1745–51. doi: 10.1093/hmg/ddi180. [DOI] [PubMed] [Google Scholar]
- 14.Scordo MG, Pengo V, Spina E, et al. Influence of CYP2C9 and CYP2C19 genetic polymorphisms on warfarin maintenance dose and metabolic clearance. Clin Pharmacol Ther. 2002 Dec;72(6):702–10. doi: 10.1067/mcp.2002.129321. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi H, Echizen H. Pharmacogenetics of warfarin elimination and its clinical implications. Clin Pharmacokinet. 2001;40(8):587–603. doi: 10.2165/00003088-200140080-00003. [DOI] [PubMed] [Google Scholar]
- 16.Linder MW, Moyer T, Reynolds KK, Tucker WW, O’Kane D, Valdes R., Jr Pharmacogenetic modeling to predict and avoid above range INR measurements. Clin Chem. 2009;55:A224. Abstract. [Google Scholar]
- 17.Kovacs MJ, Rodger M, Anderson DR, et al. Comparison of 10-mg and 5-mg warfarin initiation nomograms together with low-molecular-weight heparin for outpatient treatment of acute venous thromboembolism. A randomized, double-blind, controlled trial. Ann Intern Med. 2003 May 6;138(9):714–9. doi: 10.7326/0003-4819-138-9-200305060-00007. [DOI] [PubMed] [Google Scholar]
- 18.Ansell J, Hirsh J, Hylek E, et al. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008 Jun;133(6 Suppl):160S–98S. doi: 10.1378/chest.08-0670. [DOI] [PubMed] [Google Scholar]
- 19.Henne KR, Gaedigk A, Gupta G, et al. Chiral phase analysis of warfarin enantiomers in patient plasma in relation to CYP2C9 genotype. Journal of chromatography B, Biomedical sciences and applications. 1998 Jun 12;710(1–2):143–8. doi: 10.1016/s0378-4347(98)00099-1. [DOI] [PubMed] [Google Scholar]
- 20.Rosendaal FR, Cannegieter SC, van der Meer FJ, et al. A method to determine the optimal intensity of oral anticoagulant therapy. Thrombosis and haemostasis. 1993 Mar 1;69(3):236–9. [PubMed] [Google Scholar]
- 21.Gage BF, Eby CS. Pharmacogenetics and anticoagulant therapy. J Thromb Thrombolysis. 2003 Aug-Oct;16(1–2):73–8. doi: 10.1023/B:THRO.0000014598.24114.62. [DOI] [PubMed] [Google Scholar]
- 22.Reynolds K, Valdes R, Jr, Hartung B, et al. Individualizing warfarin therapy. Personalized Medicine. 2007;4(1):11–31. doi: 10.2217/17410541.4.1.11. [DOI] [PubMed] [Google Scholar]
- 23.Anderson JL, Horne BD, Stevens SM, et al. Randomized trial of genotype-guided versus standard warfarin dosing in patients initiating oral anticoagulation. Circulation. 2007 Nov 27;116(22):2563–70. doi: 10.1161/CIRCULATIONAHA.107.737312. [DOI] [PubMed] [Google Scholar]
- 24.Caraco Y, Blotnick S, Muszkat M. CYP2C9 genotype-guided warfarin prescribing enhances the efficacy and safety of anticoagulation: a prospective randomized controlled study. Clin Pharmacol Ther. 2008 Mar;83(3):460–70. doi: 10.1038/sj.clpt.6100316. [DOI] [PubMed] [Google Scholar]
- 25.Fihn SD, McDonell M, Martin D, et al. Risk factors for complications of chronic anticoagulation. A multicenter study. Warfarin Optimized Outpatient Follow-up Study Group. Ann Intern Med. 1993 Apr 1;118(7):511–20. doi: 10.7326/0003-4819-118-7-199304010-00005. [DOI] [PubMed] [Google Scholar]
- 26.Kamali F, Pirmohamed M. The future prospects of pharmacogenetics in oral anticoagulation therapy. Br J Clin Pharmacol. 2006 Jun;61(6):746–51. doi: 10.1111/j.1365-2125.2006.02679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beyth RJ, Milligan PE, Gage BF. Risk factors for bleeding in patients taking coumarins. Current hematology reports. 2002 Sep;1(1):41–9. [PubMed] [Google Scholar]
- 28.Epstein RS, Moyer TP, Aubert RE, et al. Warfarin genotyping reduces hospitalization rates results from the MM-WES (Medco-Mayo Warfarin Effectiveness study) Journal of the American College of Cardiology. 2010 Jun 22;55(25):2804–12. doi: 10.1016/j.jacc.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 29.Anderson JL, Horne BD, Stevens SM, et al. A Randomized and Clinical Effectiveness Trial Comparing Two Pharmacogenetic Algorithms and Standard Care for Individualizing Warfarin Dosing (CoumaGen-II) Circulation. 2012 Apr 24;125(16):1997–2005. doi: 10.1161/CIRCULATIONAHA.111.070920. [DOI] [PubMed] [Google Scholar]
- 30.Gong IY, Tirona RG, Schwarz UI, et al. Prospective evaluation of a pharmacogenetics-guided warfarin loading and maintenance dose regimen for initiation of therapy. Blood. 2011 Sep 15;118(11):3163–71. doi: 10.1182/blood-2011-03-345173. [DOI] [PubMed] [Google Scholar]
- 31.McMillin GA, Melis R, Wilson A, et al. Gene-based warfarin dosing compared with standard of care practices in an orthopedic surgery population: a prospective, parallel cohort study. Ther Drug Monit. 2010 Jun;32(3):338–45. doi: 10.1097/FTD.0b013e3181d925bb. [DOI] [PubMed] [Google Scholar]
- 32.Hillman MA, Wilke RA, Yale SH, et al. A prospective, randomized pilot trial of model-based warfarin dose initiation using CYP2C9 genotype and clinical data. Clinical medicine & research. 2005 Aug;3(3):137–45. doi: 10.3121/cmr.3.3.137. [DOI] [PMC free article] [PubMed] [Google Scholar]