Abstract
Chronic kidney disease (CKD) is a public health epidemic that affects millions of people worldwide. Presence of CKD predisposes individuals to high risks of end-stage renal disease, cardiovascular disease and premature death. Disordered phosphate homeostasis with elevated circulating levels of fibroblast growth factor 23 (FGF23) is an early and pervasive complication of CKD. CKD is likely the most common cause of chronically elevated FGF23 levels, and the clinical condition in which levels are most markedly elevated. Although increases in FGF23 levels help maintain serum phosphate in the normal range in CKD, prospective studies in populations of pre-dialysis CKD, incident and prevalent end-stage renal disease, and kidney transplant recipients demonstrate that elevated FGF23 levels are independently associated with progression of CKD and development of cardiovascular events and mortality. It was originally thought that these observations were driven by elevated FGF23 acting as a highly sensitive biomarker of toxicity due to phosphate. However, FGF23 itself has now been shown to mediate “off-target,” direct, end-organ toxicity in the heart, which suggests that elevated FGF23 may be a novel mechanism of adverse outcomes in CKD. This report reviews recent advances in FGF23 biology relevant to CKD, the classical effects of FGF23 on mineral homeostasis, and the studies that established FGF23 excess as a biomarker and novel mechanism of cardiovascular disease. The report concludes with a critical review of the effects of different therapeutic strategies targeting FGF23 reduction and how these might be leveraged in a future randomized trial aimed at improving outcomes in CKD.
BRIEF OVERVIEW OF FGF23 AND ITS EFFECTS ON MINERAL METABOLISM
FGF23 is an endocrine hormone that is secreted by osteocytes and osteoblasts.1–3 The classical effects of FGF23 in the kidney and parathyroid glands are mediated by its binding to FGF receptors (FGFR) complexed to the co-receptor klotho, which increases the binding affinity of FGF23 for FGFR.4 The primary physiological actions of FGF23 are to stimulate phosphaturia by down-regulating luminal expression of sodium-phosphate co-transporters in the proximal tubule;5 to reduce systemic levels of 1,25-dihydroxyvitamin D by directly inhibiting the renal 1-α hydroxylase and stimulating the catabolic 24-hydroxylase;6 and to inhibit parathyroid hormone (PTH) secretion.7 For a more detailed review of the molecular biology of FGF23 and klotho, the reader should refer to recent comprehensive reviews.8–15
REGULATION OF FGF23 SECRETION IN HEALTH
Role of Dietary and Serum Phosphate
Healthy individuals are able to maintain their serum phosphate in a relatively narrow range regardless of dietary phosphate intake, in part, because FGF23 levels rise and fall in parallel with the amount of dietary phosphate. High FGF23 levels in response to high phosphate intake induce greater urinary fractional excretion of phosphate, and, by lowering 1,25-dihydroxyvitamin D levels, reduce the efficiency of phosphate absorption in the gut. When phosphate intake is low, FGF23 levels fall, renal phosphate reabsorption increases and the efficiency of phosphate absorption in the gut is enhanced by the resulting increase in 1,25-dihydroxyvitamin D levels. These observations were largely derived from small interventional feeding studies in healthy humans.16–18 A recent study of 1,261 physicians in the Health Professionals Follow-up Study was the first to confirm a direct correlation between phosphate intake and FGF23 levels at the population level.19 Although the absolute effect size was modest, this finding is noteworthy given the imprecise ascertainment of dietary phosphate in nutritional epidemiology studies.20
Although it is often assumed that higher serum phosphate stimulates FGF23 secretion directly, clear corroborating evidence is lacking. Indeed, changes in serum phosphate did not precede changes in FGF23 in the phosphate feeding studies,16–18 and when serum phosphate was raised through non-dietary approaches, FGF23 levels did not change.21,22 Furthermore, cultured osteoblasts increase FGF23 expression in response to 1,25-dihydroxyvitamin D and PTH23 but not phosphate.24 Thus, a critical question remains unanswered: Exactly what is FGF23 actually regulating? If it is primarily phosphate balance, how is this sensed if not via changes in serum phosphate levels? It is important to note that feeding studies found that the responsiveness of FGF23 to dietary phosphate is sluggish (hours to days) compared to the exquisite sensitivity of PTH to subtle changes in calcium homeostasis (seconds to minutes).25–27 Deciphering the physiological and molecular basis of this difference could shed light on the search for the highly sought after phosphate-sensing apparatus.
Role of Vitamin D
In a classic negative endocrine feedback loop, 1,25-dihydroxyvitamin D stimulates FGF23 secretion, and FGF23 lowers levels of 1,25-dihydroxyvitamin D.8 Regulation of FGF23 transcription is controlled by a vitamin D response element in the FGF23 promoter,24 such that some vitamin D activity is essential for FGF23 production. This is supported by the finding of undetectable FGF23 levels in vitamin D receptor-ablated mice, even after dietary phosphate loading,28 and in 1-α hydroxylase-ablated mice.29 Further support for a gatekeeper effect of vitamin D on FGF23 expression can be inferred from the finding that FGF23 levels were also undetectable when 1-α hydroxylase ablation was superimposed on klotho-ablation,29 which alone causes markedly elevated FGF23.4 Although the biochemical picture in the double mutants is somewhat clouded by hypophosphatemia and hypocalcemia, which may lower FGF23, these changes are unlikely to fully account for undetectable FGF23 in this model.29
Role of PTH
FGF23 and PTH also share a negative endocrine feedback loop. FGF23 inhibits PTH secretion via an FGFR-klotho-dependent pathway,7 and PTH stimulates FGF23 both directly and indirectly via PTH-mediated increases in 1,25-dihydroxyvitamin D.23,30–32 In vitro, animal and human data demonstrated the direct effect of PTH: FGF23 expression increased in an osteoblast cell line in response to PTH23 (although not in all studies24); expression of a constitutively active PTH receptor in osteocytes increased FGF23 production;32 parathyroidectomy prevented and rescued elevated FGF23 levels in an adenine model of CKD independent of 1,25-dihydroxyvitamin D and calcium;23 and, in hemodialysis patients administered intravenous PTH, FGF23 levels rose in the setting of no increase in 1,25-dihydroxyvitamin D.31 Although results from these models are convincing, biochemical analysis of patients with primary hypoparathyroidism and their response to therapy reveals the complexity of the integrated physiology. In the absence of PTH, FGF23 levels are elevated33 rather than severely depressed, as would be expected if PTH was essential for increased FGF23 secretion in humans. However, persistent hyperphosphatemia in untreated hypoparathyroidism suggests that FGF23 levels are either ineffective or inappropriately low, perhaps due to lack of PTH or the low levels of 1,25-dihydroxyvitamin D and serum calcium it causes.33 Indeed, after treatment with calcitriol, FGF23 increases and, despite the tendency of vitamin D to augment phosphate absorption in the gut, serum phosphate decreases rapidly towards normal, albeit incompletely.34,35 This emphasizes that dynamic changes in FGF23 expression can occur in the absence of PTH, that FGF23 can induce phosphaturia in the absence of PTH, but that both FGF23 and PTH are needed to maintain completely normal serum phosphate levels.
Role of Calcium
Although less well appreciated than other regulators, higher serum calcium also stimulates FGF23 secretion.28,36 When wild-type mice were treated with a high calcium diet that led to increased serum calcium, FGF23 levels rose.28 PTH and 1,25-dihydroxyvitamin D levels were not reported but undoubtedly decreased due to hypercalcemia. This supports a direct effect of calcium given that the other main stimuli of FGF23 decreased or were unchanged. In the same classic study by Shimada et al,28 a similar response was observed in vitamin D receptor-ablated mice, which is especially noteworthy because hypercalcemia induced an increase in otherwise undetectable FGF23 levels, but a high phosphate diet – the classic FGF23 stimulus – did not. Finally, elevated FGF23 has been reported in hypercalcemia of malignancy when PTH is suppressed.37,38 Although proof of a putative endocrine feedback loop awaits data on the effects of hypocalcemia, the finding that calcium loading directly stimulates FGF23 secretion may be highly relevant to the therapeutic management of phosphate homeostasis in CKD (reviewed below).
Roles of Iron and Iron Deficiency
Intravenous iron can induce an acute osteomalacia syndrome in non-CKD patients due to increased FGF23 secretion.39,40 FGF23 levels also increased in response to intravenous iron in patients with ESRD undergoing hemodialysis, but the magnitude of change was small.41 More recent data suggest that iron deficiency may stimulate FGF23 transcription.42,43 In healthy individuals and wild-type mice, lower serum iron concentrations correlated with elevated FGF23 levels measured with a C-terminal (cFGF23) but not an intact (iFGF23) assay.42,43 This suggests that iron deficiency stimulates FGF23 synthesis but may not lead to increased circulating levels of biologically active hormone, perhaps because it is cleaved by furin within osteocytes into fragments,44 which are released and can be detected with the C-terminal assay. In the setting of autosomal dominant hypophosphatemic rickets, in which an FGF23 mutation renders it resistant to cleavage, increased FGF23 transcription due to iron deficiency induces an increase in intact FGF23 levels, which results in hypophosphatemia.42,43 Further studies are needed to determine whether iron stores between the extremes of severe deficiency and high-dose infusion contribute to basal FGF23 levels in CKD.
FGF23 LEVELS IN CKD
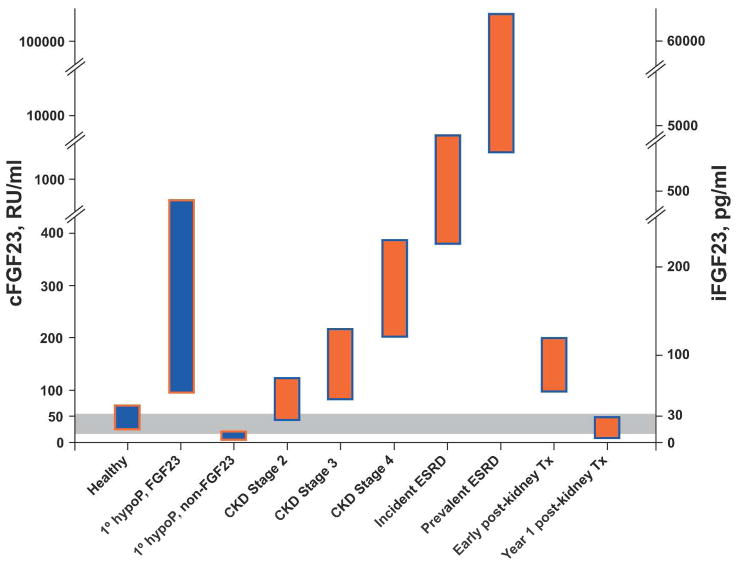
Several cross-sectional studies demonstrated that FGF23 levels are elevated in CKD compared with healthy individuals (Figure 1).25,45,46 Similar results were observed in studies that evaluated FGF23 in pediatric CKD populations.47,48 Although the exact CKD stage when FGF23 levels first became significantly elevated differed across studies, higher FGF23 on a continuous scale was consistently associated with higher serum phosphate, higher fractional excretion of phosphate, lower estimated glomerular filtration rate (eGFR), and lower levels of 1,25-dihydroxyvitamin D, independent of eGFR.25,45,46 The latter suggested that inhibition by FGF23, rather than insufficient renal mass, was the primary mechanism of reduced 1,25-dihydroxyvitamin D levels in progressive CKD. This was confirmed in animal studies, in which administration of neutralizing anti-FGF23 antibodies fully normalized 1,25-dihydroxyvitamin D levels without altering the severity of CKD (and despite lowering PTH).49 This elegant study suggested that secondary hyperparathyroidism in CKD results from increased FGF23 leading to reduced 1,25-dihydroxyvitamin D, which releases the parathyroid glands from feedback inhibition by decreasing vitamin D receptor activation and contributes to subtle hypocalcemia that chronically stimulates PTH.50
Figure 1. Representative levels of FGF23 in health, various states of CKD (orange bars), and in primary hypophosphatemic disorders (blue bars).
The dual y-axis presents FGF23 levels on the scales of the two commercially available assay platforms. The intact assay detects biologically intact FGF23 exclusively (iFGF23), whereas the C-terminus (cFGF23) assay is capable of detecting both the intact molecule and its C-terminal fragments.148 The grey area represents the presumed but incompletely defined normal ranges. “1° hypoP, FGF23” refers to hypophosphatemic disorders caused by primary FGF23 excess, for example, X-linked hypophosphatemia.149 “1° hypoP, non-FGF23” refers to hypophosphatemic disorders caused by mechanisms other than FGF23 excess, for example, hereditary hypophosphatemic rickets with hypercalciuria, in which FGF23 levels are secondarily suppressed.150,151
CKD, chronic kidney disease; ESRD, end-stage renal disease; Tx, transplantation
This pathophysiological sequence is supported by an analysis of mineral metabolites in 3,879 participants with CKD stages 2–4in the prospective Chronic Renal Insufficiency Cohort (CRIC) study.51 C-terminal, FGF23 levels were elevated in many stage 2 and most stage 3–4 patients, and an elevated FGF23 was more prevalent than elevated PTH or serum phosphate at all levels of eGFR.52 While the greater prevalence of elevated FGF23 versus PTH or phosphate in strata of more preserved eGFR was suggestive of “earlier” onset of FGF23 excess, this inference was based on a “pseudo-longitudinal” interpretation of cross-sectional data in which eGFR served as a surrogate of time. Conclusively defining the pathophysiological sequence of secondary hyperparathyroidism in human CKD will require serial evaluations over time within individual CKD patients, which has never been done for FGF23 or for other mineral metabolites, despite years of intense investigation. However, even those studies will need to be interpreted with caution because attempting to parse which mineral metabolite is deranged first as defined by being above a somewhat artificial cut point oversimplifies the intricacies underlying the integrated pathophysiology. Nevertheless, for the practicing clinician and the clinical trialist defining entry criteria, these data suggest that FGF23 is superior to existing markers as a sensitive screening test to identify which patients are developing disordered mineral metabolism in early CKD.
In patients with ESRD undergoing dialysis, FGF23 rises over time and often reaches levels that are more than 1000-fold above normal (Figure 1).53,54 This renders ESRD the clinical setting in which the highest levels of FGF23 have been reported. Although some studies suggest that inactive C-terminal fragments of FGF23 accumulate in ESRD,55 and others report that virtually all circulating FGF23 is biologically intact,56 analyses using intact assays confirm markedly elevated levels.54,56–58 This is likely due to a combination of increased bone production of FGF233 and decreased degradation, but clearance by the kidney or dialysis does not appear to contribute meaningfully to the circulating level.53 Indeed, the mechanisms of how FGF23 is removed from the circulation and where and how it is degraded remain unknown.
FGF23 levels decline rapidly following kidney transplantation in most patients with prompt allograft function, however, persistently elevated FGF23 levels in the very early post-transplant period contribute to post-transplant hypophosphatemia (Figure 1).59–62 By one year post-transplant, FGF23 levels are significantly reduced (Figure 1) but PTH often remains elevated.61 Thus, the modest but persistent reductions in serum phosphate levels in the late post-transplant period are more likely related to hyperparathyroidism. Perhaps PTH-mediated renal phosphate wasting in kidney transplant recipients,63 who are known to experience significant bone loss,64 induces total body phosphate depletion that causes FGF23 levels to decrease, akin to other FGF23-independent phosphate wasting syndromes (Figure 1).65,66 Phosphate balance studies in stable kidney transplant recipients with low phosphate and FGF23 levels would be particularly important.
REGULATION OF FGF23 SECRETION IN CKD
Among the least well understood yet critically important aspects of FGF23 biology is what drives levels up in early CKD. It has been proposed that FGF23 rises as an appropriate compensatory response to phosphate retention due to impaired renal excretion or due to reduced renal expression of klotho that induces resistance to FGF23. However, FGF23 levels are frequently elevated in early CKD, yet classic balance studies found no evidence of positive phosphate balance even in stage 5.67,68 Furthermore, among the 287 CRIC participants with eGFR 60–79 ml/min/1.73m2, median cFGF23 was already elevated and serum phosphate was actually significantly lower than in participants with higher eGFR.52 This modest but statistically significant reduction in serum phosphate was accompanied by elevated urinary fractional excretion of phosphate suggesting a mild degree of FGF23-mediated phosphate wasting. A similar phosphate leak was observed in young patients with CKD stage 1 due to autosomal dominant polycystic kidney disease, who had markedly higher FGF23 levels and lower serum phosphate than healthy controls.69 Phosphate leaking is incompatible with phosphate retention, and, since reduced klotho expression should impair phosphate excretion,70 the lack of even a subtle increase in serum phosphate in early CKD also argues against the primacy of klotho deficiency. Indeed, in an animal model of progressive CKD, significant increases in FGF23 levels preceded significant reductions in klotho expression.71
These data suggest that early CKD may be a state of primary FGF23 excess that reduces serum phosphate levels and klotho expression. The latter could occur via FGF23-mediated reductions in 1,25-dihydroxyvitamin D, which is known to stimulate klotho expression.72–74 A plausible hypothesis to explain primary FGF23 excess in early CKD is a defect in the bone that somehow stimulates FGF23 secretion directly.75,76 Although bone production of FGF23 was not increased in a mouse model of CKD with elevated serum levels,71 human bone biopsies demonstrated increased FGF23 production beginning in CKD stage 2 and correlation between osteocyte expression and circulating protein levels.3 If confirmed, this would focus further attention on the osteocyte as a likely site of phosphate sensing. Reduced degradation of FGF23 within osteocytes or after its secretion could also contribute to early increases in circulating levels in CKD.44 It is important to emphasize that even if reduced klotho expression does not account for the initial increase in FGF23 levels in CKD, progressive reduction of klotho expression clearly plays a critical role in CKD. In the parathyroid glands, reduced klotho expression along with reduced FGFR expression induces resistance to inhibition by FGF23 that contributes to the coexistence of secondary increases in both FGF23 and PTH in CKD.77–79 In addition, klotho deficiency has been implicated in the pathogenesis of vascular calcification,80 which is an important risk factor for mortality in CKD.
FGF23 AND CLINICAL OUTCOMES IN CKD
Numerous reports have linked elevated FGF23 to the main adverse clinical outcomes in CKD: progression to ESRD, cardiovascular disease and death.
FGF23 and Mortality
ESRD
Elevated levels of FGF23 were independently associated with greater risk of mortality in a 1:1, case-control study of 400 participants nested in a prospective cohort of patients with incident ESRD.58 In addition to the large, monotonic magnitude of effect, an unexpected finding was that adjusting for a large array of demographic characteristics and laboratory tests that are known to influence survival on hemodialysis had almost no impact compared with the unadjusted results. These observations helped spawn the concept that elevated FGF23 is not merely a sensitive biomarker of phosphate-mediated cardiovascular toxicity, but perhaps it is directly toxic itself.81 Although two studies revealed no association of FGF23 with mortality,82,83 a study of 219 prevalent hemodialysis patients confirmed that higher FGF23 levels were independently associated with greater risk of mortality in ESRD.84
Kidney Transplant Recipients
In a study of 984 prevalent kidney transplant recipients with a median transplant vintage at enrollment of 6 years, elevated FGF23 levels were independently associated with greater subsequent risks of mortality, allograft loss and their composite.85 The results were robust to whether FGF23 was modeled as a continuous variable or in quantiles, and regardless of whether the analysis focused on the entire population or the subgroup without extremely low or high baseline eGFR. Furthermore, when serum phosphate, PTH and hemoglobin were substituted as the primary exposure, neither was associated with outcomes. This suggests a specific effect of FGF23 on mortality rather than a general effect of impaired allograft function.
CKD Stages 2–4
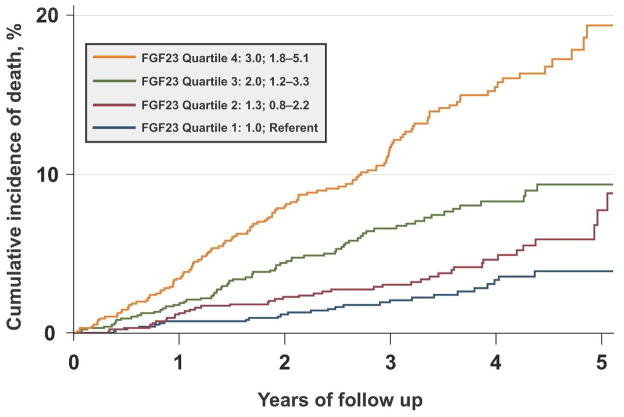
Two large studies analyzed FGF23 and mortality in pre-dialysis CKD.86,87 In an analysis of 3,879 participants in the CRIC study (mean eGFR 43 ± 14 ml/min/1.73m2) with median cFGF23 of 146 RU/ml (nearly 3 times the normal range), there were 266 deaths during a median follow-up of 3.5 years (20/1000 person-years).86 Elevated FGF23 levels were independently associated with greater risk of mortality in unadjusted and fully-adjusted analyses (Figure 2). The large magnitude of effect was homogenous across all strata of demographics, comorbidities, eGFR, and laboratory tests, and only mildly attenuated by multivariable adjustment. Among the mineral metabolites, the risk of mortality was specific to FGF23 as PTH, phosphate, fractional excretion of phosphate and vitamin D levels were not independently associated with mortality. Furthermore, FGF23 was the strongest predictor of mortality in the multivariable analyses, whereas the effects of proteinuria and low eGFR, which are powerful, CKD-specific risk factors, were abolished. These results suggest that elevated FGF23 mediates an important component of CKD-related risk of death.
Figure 2. FGF23 is an independent risk factor for mortality in CKD stages 2–4.
The cumulative incidence of death of CKD stage 2–4 patients increases significantly with ascending quartiles of baseline FGF23 levels in unadjusted analyses (plot) and after full multivariable adjustment (hazard ratios and 95% confidence intervals in the inset).86
The second study was an analysis of 1099 participants in the randomized Homocysteine in Kidney and End-Stage Renal Disease (HOST) study with mean creatinine clearance of 18 ± 6 ml/min/1.73m2 at enrollment.87 The median cFGF23 of 392 RU/ml was considerably higher than in the CRIC study but consistent with this cohort’s more advanced CKD. Similar to the results from CRIC, higher baseline FGF23 levels were independently associated with increased risk of mortality in a monotonic pattern with minimal attenuation in sequentially adjusted multivariable models, and FGF23 was the strongest predictor of mortality.
FGF23 and Progression of CKD
Several published studies indentified FGF23 as a risk factor for CKD progression. In 177 patients with non-diabetic CKD, higher levels of cFGF23 and iFGF23 were independently associated with incident ESRD.88 A subsequent study of 55 diabetic CKD patients reported similar results, although the small sample size with only 12 ESRD events precluded detailed multivariable analysis.89 In the CRIC study, ESRD developed in 410 participants (33/1000 person-years).86 Although the unadjusted association between elevated FGF23 and greater risk of ESRD was attenuated in multivariable analyses, the effect was significantly modified by baseline eGFR. In fully-adjusted stratified analyses, higher cFGF23 was associated with ESRD in participants with eGFR > 30 ml/min/1.73m2, and the magnitude of effect grew at more preserved levels of eGFR. In contrast to CRIC, elevated cFGF23 was independently associated with greater risk of ESRD in the HOST study of CKD stage 4–5 patients.87 In aggregate, the data suggest that elevated FGF23 is a potentially important risk factor for ESRD. Whether FGF23 is acting as a biomarker of cases of CKD that are destined to progress most rapidly or it is a direct mediator of disease progression requires further study.
FGF23 and Cardiovascular Disease Events
Data on FGF23 and risk of cardiovascular events are less developed. In 149 patients with mean eGFR of 36 ml/min/1.73m2, elevated cFGF23 was independently associated with greater risk of the composite of myocardial infarction, stroke, coronary, carotid or lower limb revascularization, lower extremity amputation or death.90 In the HOST Study, elevated cFGF23 was strongly associated with increased risk of the composite outcome of myocardial infarction, amputation or stroke.87 Similar to the analysis of mortality in the same study, there was minimal change in the point estimates of risk after multivariable analysis, and FGF23 superseded all classic cardiovascular risk factors to be the strongest predictor of developing a cardiovascular event. When the individual cardiovascular events were analyzed separately, the largest hazard ratios were observed for amputation, followed by myocardial infarction. FGF23 was not associated with stroke.
In an analysis of 833 participants in the Heart and Soul Study that recruited predominantly non-CKD patients (22% had eGFR <60 ml/min/1.73m2) with a history of coronary artery disease, the median cFGF23 was relatively normal (43 RU/ml), but those with higher levels had independently increased risk of death and risk of developing the composite of myocardial infarction, cerebrovascular event or hospitalization for congestive heart failure.91 The results were driven by significantly greater rates of congestive heart failure (n=119) and cerebrovascular events (n=36), since FGF23 was not associated with risk of myocardial infarction (n=88). Although it is possible that the entry criterion of established coronary artery disease could have biased the analysis of myocardial infarction to the null, another well-powered study of incident coronary artery disease in non-CKD patients (n=422 events) also revealed no link to FGF23.92
FGF23 and Intermediate Measures of Cardiovascular Risk
Several studies reported strong associations between FGF23 and cardiovascular risk factors. In 1261 participants in the Health Professionals Follow-up Study, the vast majority of whom had normal renal function, higher FGF23 levels were independently associated with older age, hypertension, obesity and smoking.19 Although not their primary focus, several studies of CKD patients similarly reported that higher FGF23 levels were associated with diabetes, smoking, prior cardiovascular disease, obesity and higher levels of inflammatory markers.19,86,87,91,93–96
The endothelium and vessel wall are targets of injury in CKD. Higher FGF23 levels were independently associated with endothelial dysfunction, marked by lower flow-mediated vasodilatation of the brachial artery in CKD stages 3–497 and in an older, predominantly non-CKD population.98 The data on FGF23 and vascular calcification are murky with some studies reporting an independent association99,100 and others reporting none.101,102 Small sample sizes, differential approaches to adjusting for confounding, imaging of different arterial beds, and lack of prospective data limit the conclusions that can be drawn from this body of work. When contrasted with the consistent data linking higher serum phosphate to more severe calcification,103–108 these data suggest lack of a true effect of FGF23.
In contrast to vascular calcification, higher FGF23 is consistently associated with LVH, which is an important mechanism of congestive heart failure and arrhythmia, and a potent risk factor for mortality in CKD.109,110 Thus, LVH is one plausible biological mechanism to explain the link between higher FGF23 and greater risk of mortality. Several cross-sectional studies in CKD, ESRD and non-CKD populations demonstrated that elevated FGF23 is independently associated with greater left ventricular mass index and greater prevalence of LVH.82,94,111–115 Elevated FGF23 was also associated with reduced ejection fraction and prevalent atrial fibrillation but not coronary artery disease in a cross-sectional study of 885 study participants undergoing elective coronary angiography, 19% of whom met criteria for CKD.94
The link between FGF23 and cardiac injury in CKD was solidified by an echocardiography study of 3,070 CRIC study participants.116 In the cross-sectional component of the analysis, higher cFGF23 levels were independently associated with reduced ejection fraction, greater LVMI and greater prevalence of concentric and eccentric LVH. In the first prospective analysis of FGF23 and risk of LVH, elevated cFGF23 predicted new-onset LVH in CRIC participants who had normal LV geometry at baseline and underwent repeat echocardiography three years later.116 The risk of incident LVH according to baseline FGF23 was magnified in the subgroup of participants without hypertension. Presumably, eliminating this major confounder allowed the independent effects of FGF23 to emerge more clearly.
“OFF-TARGET” TOXICITY OF FGF23
Lack of a consistently robust association between FGF23, coronary artery calcification and incident coronary events argue against occlusive atherosclerotic events as the primary link between FGF23 and death. Furthermore, progressive ventricular failure, arrhythmia and sudden death – components of the incompletely understood uremic cardiomyopathy117 – are more common causes of death in advanced CKD than acute myocardial infarction.118–120 Combined with the consistent association between FGF23 and LVH, these clinical clues focused the search for cardiovascular toxicity of FGF23 on the myocardium.
In an in vitro experimental system that was previously used to demonstrate the hypertrophic effects of FGF2, escalating doses of FGF23 induced a dose-dependent increase in the surface area of neonatal rat ventricular cardiac myocytes and activated hypertrophic gene programs.116 Administration of a pan-FGFR inhibitor prevented FGF23-mediated hypertrophy, indicating that the effect was mediated by FGFRs, which are expressed on cardiac myocytes. These results were confirmed in a series of experiments in rodents.116 Injection of FGF23 into the left ventricular myocardium or intravenously in wild-type mice induced LVH. Most relevant to CKD, LVH in uremic rats that manifest markedly elevated FGF23 levels was prevented by treatment with a pan-FGFR inhibitor despite persistence of equally severe hypertension as in the rats that were untreated. These results confirmed the hypertension-independent effect of FGF23 that was reported in the prospective analysis of normotensive CRIC participants.116 An especially important finding was that klotho was not detected in cardiac myocytes, indicating that FGF23-mediated hypertrophy of cardiac myocytes occurs independent of membrane-bound klotho. The finding of LVH in klotho heterozygous mice that had moderately elevated FGF23 levels (3-fold above normal), and more severe LVH in klotho-ablated mice that had markedly elevated FGF23 levels (>15-fold above normal) excluded the possibility that the soluble form of klotho is needed for LVH to develop in states of elevated FGF23 levels. These results established the concept of klotho-independent, direct end-organ toxicity of FGF23 and a potentially prominent role of FGF23 in the pathogenesis of uremic cardiomyopathy. They also establish a precedent for future investigations to either confirm or refute whether direct effects of FGF23 underlie other epidemiological associations, for example, with arterial calcification, atherosclerosis, inflammation, and CKD progression.
THERAPEUTIC STRATEGIES TO LOWER FGF23 LEVELS
If elevated FGF23 is mechanistically linked to increased risk of death in CKD, therapeutic strategies to reduce levels could lead to improved survival. Several approaches have been proposed.
Diet
In non-CKD patients, reducing dietary phosphate intake lowers FGF23 levels.16–18 By comparison, data in CKD are scarce. Although the randomized assignment of a 750-mg phosphate diet did not lower FGF23 levels in a small pilot study, the 1500 mg diet raised FGF23 levels in several participants,121 suggesting that dietary phosphate intake does indeed contribute to dynamic changes in FGF23 levels in CKD. A cross-over study that compared the impact of a meat-based versus a vegetarian-based diet on FGF23 levels in 9 CKD patients with mean eGFR of 32 ml/min/1.73m2 confirmed an important effect of diet.122 The total phosphate contents of the diets were identical but due to lower bioavailability of phosphate in plants compared to meat,20,123 less phosphate was absorbed in the vegetarian phase as indicated by lower urinary phosphate excretion. Consistent with reduced phosphate absorption, consumption of the vegetarian diet significantly lowered serum phosphate and FGF23 levels. Similar results were observed in animals.124 Although these studies emphasized the role of phosphate bioavailability over absolute phosphate intake and did not analyze the effects of a phosphate additive-enriched diet that would have an even higher bioavailability of phosphate,20,123 they demonstrate that dietary manipulation can lower FGF23 levels significantly in CKD.
Phosphate binders
In addition to reducing total phosphate in the diet or manipulating its bioavailability profile, phosphate binders can be used to reduce absorption in the gut. Several studies reported that administration of the phosphate binders, lanthanum, sevelamer, and aluminum-magnesium, lowered FGF23 levels in healthy volunteers, hyperphosphatemic ESRD patients, and CKD patients with normal or elevated serum phosphate.17,125–128 Although another randomized study of CKD stage 3–4 patients found no reduction in FGF23 levels in participants treated with lanthanum versus placebo, the study duration was only 2 weeks,121 which may have been inadequate given that previous studies reported no change in FGF23 at 2 weeks but significant 22%–45% reductions after 4–6 weeks of treatment with lanthanum127 or sevelamer.126
Certain studies compared the effects on FGF23 of specific phosphate binders. In the first, sevelamer but not calcium acetate lowered FGF23 levels over a 6 week duration of intervention in CKD stage 3–4 patients with normal serum phosphate levels.126 The second study randomized 100 hyperphosphatemic stage 4 patients to sevelamer or calcium acetate.128 Although each intervention lowered serum phosphate, sevelamer but not calcium significantly lowered FGF23 and significantly improved endothelial function.
Although the results are provocative, several unusual biochemical findings characterized the study population. Despite a mean eGFR of 23 ml/min/1.73m2, participants had markedly elevated mean serum phosphate of 7.7 mg/dl, which is quite uncommon for a non-ESRD population. Even more unexpected was their mean iFGF23 of 40 pg/ml, which was disproportionately low for the degree of hyperphosphatemia and severity of CKD. Nevertheless, this study is important for two reasons. It established that a therapeutic intervention targeting phosphate homeostasis is capable of improving a meaningful intermediate measure of cardiovascular risk in CKD patients. Although the decrement in FGF23 but not serum phosphate was independently associated with improvement in endothelial function, it will be important to determine if improvements in endothelial function will also occur when FGF23 is reduced in CKD patients with normal serum phosphate.
Second, this study validated the previous finding that non-calcium-based phosphate binders reduce FGF23 more effectively than calcium-containing binders despite comparable effects on serum phosphate. Stimulation of FGF23 secretion by calcium likely offset the FGF23-lowering effect that would be expected by its phosphate binding. These results highlight the need for additional head-to-head trials of specific phosphate binders, with and without active vitamin D therapy, and utilizing different dietary strategies for prolonged durations of follow-up. Data from such studies are needed to further crystallize the optimal approach to FGF23 reduction that should be carried forward to a future placebo-controlled randomized trial of hard clinical outcomes in CKD stages 3–4.129
Cinacalcet
Animal and human studies suggest that cinacalcet lowers FGF23 levels in the setting of CKD.130–132 The incompletely elucidated mechanisms are likely multifactorial and may vary by stage. In models of pre-dialysis CKD, cinacalcet lowers FGF23 in addition to PTH leading to an increase in serum phosphate,132 but in ESRD, cinacalcet lowers FGF23 and PTH in association with a reduction in serum phosphate.131 This suggests that cinacalcet’s effect on FGF23 is not mediated by changes in serum phosphate. Reduced 1,25-dihydroxyvitamin D due to cinacalcet-mediated reductions in PTH could contribute to reduced FGF23 when there is residual renal function,132 but is probably not as important in ESRD, when 1,25-dihydroxyvitamin D levels are already severely depressed.133 A likely mechanism of how cinacalcet reduces FGF23 is via reduced PTH, which is a universal effect of cinacalcet across all stages of CKD.134,135 Although reduced serum calcium is another universal effect of cinacalcet that might theoretically contribute to reduced FGF23 levels, a putative effect of hypocalcemia seems less likely because the calcium sensing receptor actually “senses” hypercalcemia in the presence of calcimimetics.
Vitamin D
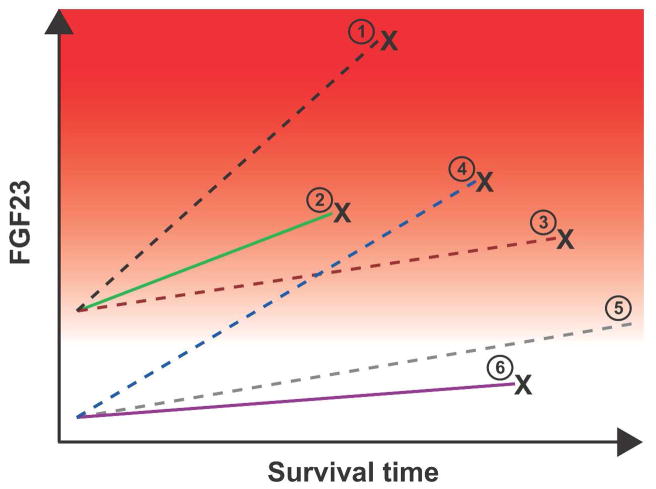
As expected based on the known physiology, animal and human studies demonstrate that active vitamin D raises FGF23 levels, and without significant differences between specific preparations.132,136,137 These findings present an apparent paradox at the intersection of FGF23 research and epidemiological studies of active vitamin D and clinical outcomes: Elevated FGF23 is associated with accelerated mortality, yet active vitamin D therapy, which raises FGF23, is associated with longer survival.138–143 The highly variable FGF23 response to active vitamin D therapy between individuals – from minimal to large increases– in all populations, including children with ESRD, adults with CKD stages 3–4, adults undergoing hemodialysis, and uremic rats provides important insight into this potential contradiction.132,136,137,144 Although a higher baseline FGF23 is a risk factor for mortality, vitamin D-treated patients who experience prolonged survival on dialysis may be those who exhibit a modest FGF23 response to treatment, whereas those who die early despite receiving therapy may be the ones who exhibit a greater increase in FGF23 (Figure 3). Studies that help clarify the interaction between FGF23 and active vitamin D will be crucial for designing integrated, evidence-based approaches to treating mineral metabolism in CKD and ESRD.
Figure 3. Hypothesis to reconcile the seemingly paradoxical effects of FGF23 and vitamin D on survival in CKD.
Baseline and change in FGF23 levels are plotted against time among 6 hypothetical patient groups. The spectrum of risk of mortality associated with FGF23 is demonstrated by the red background gradient (higher risk is darker red). Dashed lines represent active vitamin D treated-groups, and solid lines represent untreated groups. “X” connotes death. The known effect of elevated baseline FGF23 on risk of mortality58 is represented by the higher baseline FGF23 and earlier mortality among groups 1–3 vs. 4–6. The main effect of active vitamin D therapy on survival139 is represented by the longer survival of groups 1 and 5 vs. 2 and 6. In all groups, FGF23 levels increase with longer duration of ESRD,53 but the rate of increase is greater among those treated with active vitamin D (greater slopes of FGF23 in groups 1 and 4 vs. 2; 3 and 5 vs. 6). The hypothesized interaction between active vitamin D treatment and FGF23 is represented by the significantly greater slopes of increase in FGF23 among active vitamin D-treated groups who die sooner compared with those who survive longer (crossing lines of groups 4 vs. 3). Thus, it is hypothesized that survival is longest in group 5, which had low baseline FGF23, received active vitamin D therapy, but experienced only a modest increase in FGF23 in response.
Other approaches
The finding that elevated FGF23 directly induces cardiac injury might suggest a potential utility in CKD of neutralizing FGF23 with antibodies that are under development for the treatment of syndromes of primary FGF23 excess, such as X-linked hypophosphatemia.145 However, use of anti-FGF23 antibodies that were specifically designed to prevent phosphate wasting caused by FGF23 could be dangerous in CKD because they would induce or exacerbate hyperphosphatemia49 and perhaps hypercalcemia due to increases in 1,25-dihydroxyvitamin D, as shown in FGF23-ablated mice.146 Alternatively, novel antibodies could be raised against the FGFR that mediates the cardiac toxicity of FGF23 but do not interfere with binding to renal FGFR-klotho, thereby retaining the phosphaturic effects of FGF23 that are desirable in CKD. Assessing the feasibility of this approach will require detailed understanding of the cardiac FGFRs that underlie FGF23-mediated LVH and the physical-chemical aspects of their interaction with FGF23 relative to FGFR in the kidney.
CONCLUSIONS
Recurring themes in research of FGF23 and mortality include consistency of results within specific study populations (one CKD study versus another), consistency between study populations (CKD, dialysis, post-transplant), large monotonic magnitudes of effect in prospective studies, minimal confounding, and independence from and specificity relative to other mineral metabolites. Enumerated on this list are several of the Hill criteria that suggest causality.147 Translational data support a causal role for FGF23 in the pathogenesis of LVH, which is a leading pattern of cardiovascular injury in CKD that is strongly associated with death. Although in its infancy, an emerging body of work suggests promising approaches to reduce FGF23 levels. At the end of the long and winding scientific tunnel ahead lies hope that a randomized trial will leverage the last decade’s data on FGF23 and culminate in a meaningful improvement in clinical outcomes for CKD patients of the future.
Acknowledgments
Dr. Wolf is supported by grants R01DK076116 and R01DK081374 from the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST
Dr. Wolf has received honoraria or research support from Abbott, Amgen, Genzyme, Luitpold, Sanofi and Shire.
References
- 1.Riminucci M, Collins MT, Fedarko NS, et al. FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J Clin Invest. 2003;112:683–92. doi: 10.1172/JCI18399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu S, Zhou J, Tang W, et al. Pathogenic role of Fgf23 in Hyp mice. Am J Physiol Endocrinol Metab. 2006;291:E38–49. doi: 10.1152/ajpendo.00008.2006. [DOI] [PubMed] [Google Scholar]
- 3.Pereira RC, Juppner H, Azucena-Serrano CE, et al. Patterns of FGF-23, DMP1, and MEPE expression in patients with chronic kidney disease. Bone. 2009;45:1161–8. doi: 10.1016/j.bone.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Urakawa I, Yamazaki Y, Shimada T, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–4. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 5.Shimada T, Urakawa I, Yamazaki Y, et al. FGF-23 transgenic mice demonstrate hypophosphatemic rickets with reduced expression of sodium phosphate cotransporter type IIa. Biochem Biophys Res Commun. 2004;314:409–14. doi: 10.1016/j.bbrc.2003.12.102. [DOI] [PubMed] [Google Scholar]
- 6.Shimada T, Hasegawa H, Yamazaki Y, et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19:429–35. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- 7.Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, et al. The parathyroid is a target organ for FGF23 in rats. J Clin Invest. 2007;117:4003–8. doi: 10.1172/JCI32409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolf M. Forging forward with 10 burning questions on FGF23 in kidney disease. J Am Soc Nephrol. 2010;21:1427–35. doi: 10.1681/ASN.2009121293. [DOI] [PubMed] [Google Scholar]
- 9.Komaba H, Fukagawa M. FGF23-parathyroid interaction: implications in chronic kidney disease. Kidney Int. 2010;77:292–8. doi: 10.1038/ki.2009.466. [DOI] [PubMed] [Google Scholar]
- 10.Kuro OM. Phosphate and Klotho. Kidney Int Suppl. 2011:S20–3. doi: 10.1038/ki.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quarles LD. Endocrine functions of bone in mineral metabolism regulation. J Clin Invest. 2008;118:3820–8. doi: 10.1172/JCI36479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silver J, Naveh-Many T. FGF23 and the parathyroid glands. Pediatr Nephrol. 2010;25:2241–5. doi: 10.1007/s00467-010-1565-3. [DOI] [PubMed] [Google Scholar]
- 13.Juppner H. Phosphate and FGF-23. Kidney Int Suppl. 2011:S24–7. doi: 10.1038/ki.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prie D, Urena Torres P, Friedlander G. Latest findings in phosphate homeostasis. Kidney Int. 2009;75:882–9. doi: 10.1038/ki.2008.643. [DOI] [PubMed] [Google Scholar]
- 15.Quarles LD. The bone and beyond: ‘Dem bones’ are made for more than walking. Nat Med. 2011;17:428–30. doi: 10.1038/nm0411-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrari SL, Bonjour JP, Rizzoli R. Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J Clin Endocrinol Metab. 2005;90:1519–24. doi: 10.1210/jc.2004-1039. [DOI] [PubMed] [Google Scholar]
- 17.Burnett SM, Gunawardene SC, Bringhurst FR, et al. Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J Bone Miner Res. 2006;21:1187–96. doi: 10.1359/jbmr.060507. [DOI] [PubMed] [Google Scholar]
- 18.Antoniucci DM, Yamashita T, Portale AA. Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J Clin Endocrinol Metab. 2006;91:3144–9. doi: 10.1210/jc.2006-0021. [DOI] [PubMed] [Google Scholar]
- 19.Gutierrez OM, Wolf M, Taylor EN. Fibroblast growth factor 23, cardiovascular disease risk factors, and phosphorus intake in the Health Professionals Follow-up Study. Clin J Am Soc Nephrol. 2011;6:2871–8. doi: 10.2215/CJN.02740311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gutierrez OM, Wolf M. Dietary phosphorus restriction in advanced chronic kidney disease: merits, challenges, and emerging strategies. Semin Dial. 2010;23:401–6. doi: 10.1111/j.1525-139X.2010.00750.x. [DOI] [PubMed] [Google Scholar]
- 21.Burnett-Bowie SM, Mendoza N, Leder BZ. Effects of gonadal steroid withdrawal on serum phosphate and FGF-23 levels in men. Bone. 2007;40:913–8. doi: 10.1016/j.bone.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ito N, Fukumoto S, Takeuchi Y, et al. Effect of acute changes of serum phosphate on fibroblast growth factor (FGF)23 levels in humans. J Bone Miner Metab. 2007;25:419–22. doi: 10.1007/s00774-007-0779-3. [DOI] [PubMed] [Google Scholar]
- 23.Lavi-Moshayoff V, Wasserman G, Meir T, et al. PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: a bone parathyroid feedback loop. Am J Physiol Renal Physiol. 2010;299:F882–9. doi: 10.1152/ajprenal.00360.2010. [DOI] [PubMed] [Google Scholar]
- 24.Liu S, Tang W, Zhou J, et al. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol. 2006;17:1305–15. doi: 10.1681/ASN.2005111185. [DOI] [PubMed] [Google Scholar]
- 25.Larsson T, Nisbeth U, Ljunggren O, et al. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 2003;64:2272–9. doi: 10.1046/j.1523-1755.2003.00328.x. [DOI] [PubMed] [Google Scholar]
- 26.Isakova T, Gutierrez O, Shah A, et al. Postprandial mineral metabolism and secondary hyperparathyroidism in early CKD. J Am Soc Nephrol. 2008;19:615–23. doi: 10.1681/ASN.2007060673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishida Y, Taketani Y, Yamanaka-Okumura H, et al. Acute effect of oral phosphate loading on serum fibroblast growth factor 23 levels in healthy men. Kidney Int. 2006;70:2141–7. doi: 10.1038/sj.ki.5002000. [DOI] [PubMed] [Google Scholar]
- 28.Shimada T, Yamazaki Y, Takahashi M, et al. Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am J Physiol Renal Physiol. 2005;289:F1088–95. doi: 10.1152/ajprenal.00474.2004. [DOI] [PubMed] [Google Scholar]
- 29.Ohnishi M, Nakatani T, Lanske B, et al. Reversal of mineral ion homeostasis and soft-tissue calcification of klotho knockout mice by deletion of vitamin D 1alpha-hydroxylase. Kidney Int. 2009;75:1166–72. doi: 10.1038/ki.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopez I, Rodriguez-Ortiz ME, Almaden Y, et al. Direct and indirect effects of parathyroid hormone on circulating levels of fibroblast growth factor 23 in vivo. Kidney Int. 2011;80:475–82. doi: 10.1038/ki.2011.107. [DOI] [PubMed] [Google Scholar]
- 31.Wesseling-Perry K, Harkins GC, Wang HJ, et al. The calcemic response to continuous parathyroid hormone (PTH)(1–34) infusion in end-stage kidney disease varies according to bone turnover: a potential role for PTH(7–84) J Clin Endocrinol Metab. 2010;95:2772–80. doi: 10.1210/jc.2009-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rhee Y, Bivi N, Farrow E, et al. Parathyroid hormone receptor signaling in osteocytes increases the expression of fibroblast growth factor-23 in vitro and in vivo. Bone. 2011;49:636–43. doi: 10.1016/j.bone.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta A, Winer K, Econs MJ, et al. FGF-23 is elevated by chronic hyperphosphatemia. J Clin Endocrinol Metab. 2004;89:4489–92. doi: 10.1210/jc.2004-0724. [DOI] [PubMed] [Google Scholar]
- 34.Collins MT, Lindsay JR, Jain A, et al. Fibroblast growth factor-23 is regulated by 1alpha,25-dihydroxyvitamin D. J Bone Miner Res. 2005;20:1944–50. doi: 10.1359/JBMR.050718. [DOI] [PubMed] [Google Scholar]
- 35.Hill LF, Davies M, Taylor CM, et al. Treatment of hypoparathyroidism with 1,25-dihydroxycholecalciferol. Clin Endocrinol (Oxf) 1976;5 (Suppl):167S–73S. doi: 10.1111/j.1365-2265.1976.tb03824.x. [DOI] [PubMed] [Google Scholar]
- 36.Evenepoel P, Viaene L, Meijers B. PTH, FGF23, and calcium: it takes three to tango? Kidney Int. 2011;80:1377. doi: 10.1038/ki.2011.350. [DOI] [PubMed] [Google Scholar]
- 37.Singh RJ, Kumar R. Fibroblast growth factor 23 concentrations in humoral hypercalcemia of malignancy and hyperparathyroidism. Mayo Clin Proc. 2003;78:826–9. doi: 10.4065/78.7.826. [DOI] [PubMed] [Google Scholar]
- 38.Tebben PJ, Kalli KR, Cliby WA, et al. Elevated fibroblast growth factor 23 in women with malignant ovarian tumors. Mayo Clin Proc. 2005;80:745–51. doi: 10.1016/S0025-6196(11)61528-0. [DOI] [PubMed] [Google Scholar]
- 39.Schouten BJ, Hunt PJ, Livesey JH, et al. FGF23 elevation and hypophosphatemia after intravenous iron polymaltose: a prospective study. J Clin Endocrinol Metab. 2009;94:2332–7. doi: 10.1210/jc.2008-2396. [DOI] [PubMed] [Google Scholar]
- 40.Shimizu Y, Tada Y, Yamauchi M, et al. Hypophosphatemia induced by intravenous administration of saccharated ferric oxide: another form of FGF23-related hypophosphatemia. Bone. 2009;45:814–6. doi: 10.1016/j.bone.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 41.Takeda Y, Komaba H, Goto S, et al. Effect of intravenous saccharated ferric oxide on serum FGF23 and mineral metabolism in hemodialysis patients. Am J Nephrol. 2011;33:421–6. doi: 10.1159/000327019. [DOI] [PubMed] [Google Scholar]
- 42.Imel EA, Peacock M, Gray AK, et al. Iron Modifies Plasma FGF23 Differently in Autosomal Dominant Hypophosphatemic Rickets and Healthy Humans. J Clin Endocrinol Metab. 2011;96:3541–9. doi: 10.1210/jc.2011-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farrow EG, Yu X, Summers LJ, et al. Iron deficiency drives an autosomal dominant hypophosphatemic rickets (ADHR) phenotype in fibroblast growth factor-23 (Fgf23) knock-in mice. Proc Natl Acad Sci U S A. 2011;108:E1146–55. doi: 10.1073/pnas.1110905108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhattacharyya N, Wiench M, Dumitrescu C, et al. Mechanism of FGF23 processing in fibrous dysplasia. J Bone Miner Res. 2012 doi: 10.1002/jbmr.1546. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shigematsu T, Kazama JJ, Yamashita T, et al. Possible involvement of circulating fibroblast growth factor 23 in the development of secondary hyperparathyroidism associated with renal insufficiency. Am J Kidney Dis. 2004;44:250–6. doi: 10.1053/j.ajkd.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 46.Gutierrez O, Isakova T, Rhee E, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16:2205–15. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 47.van Husen M, Fischer AK, Lehnhardt A, et al. Fibroblast growth factor 23 and bone metabolism in children with chronic kidney disease. Kidney Int. 2010;78:200–6. doi: 10.1038/ki.2010.107. [DOI] [PubMed] [Google Scholar]
- 48.Bacchetta J, Dubourg L, Harambat J, et al. The influence of glomerular filtration rate and age on fibroblast growth factor 23 serum levels in pediatric chronic kidney disease. J Clin Endocrinol Metab. 2010;95:1741–8. doi: 10.1210/jc.2009-1576. [DOI] [PubMed] [Google Scholar]
- 49.Hasegawa H, Nagano N, Urakawa I, et al. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int. 2010;78:975–80. doi: 10.1038/ki.2010.313. [DOI] [PubMed] [Google Scholar]
- 50.Isakova T, Wolf MS. FGF23 or PTH: which comes first in CKD ? Kidney Int. 2010;78:947–9. doi: 10.1038/ki.2010.281. [DOI] [PubMed] [Google Scholar]
- 51.Feldman HI, Appel LJ, Chertow GM, et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. J Am Soc Nephrol. 2003;14:S148–53. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 52.Isakova T, Wahl P, Vargas GS, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79:1370–8. doi: 10.1038/ki.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Isakova T, Xie H, Barchi-Chung A, et al. Fibroblast growth factor 23 in patients undergoing peritoneal dialysis. Clin J Am Soc Nephrol. 2011;6:2688–95. doi: 10.2215/CJN.04290511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Viaene L, Bammens B, Meijers BK, et al. Residual renal function is an independent determinant of serum FGF-23 levels in dialysis patients. Nephrol Dial Transplant. 2011 doi: 10.1093/ndt/gfr596. In Press. [DOI] [PubMed]
- 55.Weber TJ, Liu S, Indridason OS, et al. Serum FGF23 levels in normal and disordered phosphorus homeostasis. J Bone Miner Res. 2003;18:1227–34. doi: 10.1359/jbmr.2003.18.7.1227. [DOI] [PubMed] [Google Scholar]
- 56.Shimada T, Urakawa I, Isakova T, et al. Circulating fibroblast growth factor 23 in patients with end-stage renal disease treated by peritoneal dialysis is intact and biologically active. J Clin Endocrinol Metab. 2010;95:578–85. doi: 10.1210/jc.2009-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakanishi S, Kazama JJ, Nii-Kono T, et al. Serum fibroblast growth factor-23 levels predict the future refractory hyperparathyroidism in dialysis patients. Kidney Int. 2005;67:1171–8. doi: 10.1111/j.1523-1755.2005.00184.x. [DOI] [PubMed] [Google Scholar]
- 58.Gutierrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–92. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pande S, Ritter CS, Rothstein M, et al. FGF-23 and sFRP-4 in chronic kidney disease and post-renal transplantation. Nephron Physiol. 2006;104:p23–32. doi: 10.1159/000093277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bhan I, Shah A, Holmes J, et al. Post-transplant hypophosphatemia: Tertiary ‘Hyper-Phosphatoninism’? Kidney Int. 2006;70:1486–94. doi: 10.1038/sj.ki.5001788. [DOI] [PubMed] [Google Scholar]
- 61.Evenepoel P, Meijers BK, de Jonge H, et al. Recovery of hyperphosphatoninism and renal phosphorus wasting one year after successful renal transplantation. Clin J Am Soc Nephrol. 2008;3:1829–36. doi: 10.2215/CJN.01310308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Economidou D, Dovas S, Papagianni A, et al. FGF-23 levels before and after renal transplantation. J Transplant. 2009;2009:379082. doi: 10.1155/2009/379082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sirilak S, Chatsrisak K, Ingsathit A, et al. Renal phosphate loss in long-term kidney transplantation. Clin J Am Soc Nephrol. 2011 doi: 10.2215/CJN.06380611. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Julian BA, Laskow DA, Dubovsky J, et al. Rapid loss of vertebral mineral density after renal transplantation. N Engl J Med. 1991;325:544–50. doi: 10.1056/NEJM199108223250804. [DOI] [PubMed] [Google Scholar]
- 65.Saidenberg-Kermanac’h N, Souabni L, Prendki V, et al. Normal plasma FGF23 levels kinetic in tenofovir-related hypophosphatemic osteomalacia in an HIV-infected patient with von Recklinghausen disease. Joint Bone Spine. 2011;78:306–8. doi: 10.1016/j.jbspin.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 66.Tencza AL, Ichikawa S, Dang A, et al. Hypophosphatemic rickets with hypercalciuria due to mutation in SLC34A3/type IIc sodium-phosphate cotransporter: presentation as hypercalciuria and nephrolithiasis. J Clin Endocrinol Metab. 2009;94:4433–8. doi: 10.1210/jc.2009-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu SH, Chu HI. Studies on calcium and phosphorus metabolism with special reference to pathogenesis and effect of dihydrotachysterol (A.T. 10) and iron. Medicine. 1943;22:103–61. [Google Scholar]
- 68.Litzow JR, Lemann J, Jr, Lennon EJ. The effect of treatment of acidosis on calcium balance in patients with chronic azotemic renal disease. J Clin Invest. 1967;46:280–6. doi: 10.1172/JCI105530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pavik I, Jaeger P, Kistler AD, et al. Patients with autosomal dominant polycystic kidney disease have elevated fibroblast growth factor 23 levels and a renal leak of phosphate. Kidney Int. 2011;79:234–40. doi: 10.1038/ki.2010.375. [DOI] [PubMed] [Google Scholar]
- 70.Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 71.Stubbs JR, He N, Idiculla A, et al. Longitudinal evaluation of FGF23 changes and mineral metabolism abnormalities in a mouse model of chronic kidney disease. J Bone Miner Res. 2011 doi: 10.1002/jbmr.516. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tsujikawa H, Kurotaki Y, Fujimori T, et al. Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol Endocrinol. 2003;17:2393–403. doi: 10.1210/me.2003-0048. [DOI] [PubMed] [Google Scholar]
- 73.Forster RE, Jurutka PW, Hsieh JC, et al. Vitamin D receptor controls expression of the anti-aging klotho gene in mouse and human renal cells. Biochem Biophys Res Commun. 2011;414:557–62. doi: 10.1016/j.bbrc.2011.09.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marsell R, Krajisnik T, Goransson H, et al. Gene expression analysis of kidneys from transgenic mice expressing fibroblast growth factor-23. Nephrol Dial Transplant. 2008;23:827–33. doi: 10.1093/ndt/gfm672. [DOI] [PubMed] [Google Scholar]
- 75.Samadfam R, Richard C, Nguyen-Yamamoto L, et al. Bone formation regulates circulating concentrations of fibroblast growth factor 23. Endocrinology. 2009;150:4835–45. doi: 10.1210/en.2009-0472. [DOI] [PubMed] [Google Scholar]
- 76.Schiavi SC. Bone talk. Nat Genet. 2006;38:1230–1. doi: 10.1038/ng1106-1230. [DOI] [PubMed] [Google Scholar]
- 77.Galitzer H, Ben-Dov IZ, Silver J, et al. Parathyroid cell resistance to fibroblast growth factor 23 in secondary hyperparathyroidism of chronic kidney disease. Kidney Int. 2010;77:211–8. doi: 10.1038/ki.2009.464. [DOI] [PubMed] [Google Scholar]
- 78.Komaba H, Goto S, Fujii H, et al. Depressed expression of Klotho and FGF receptor 1 in hyperplastic parathyroid glands from uremic patients. Kidney Int. 2010;77:232–8. doi: 10.1038/ki.2009.414. [DOI] [PubMed] [Google Scholar]
- 79.Canalejo R, Canalejo A, Martinez-Moreno JM, et al. FGF23 fails to inhibit uremic parathyroid glands. J Am Soc Nephrol. 2010;21:1125–35. doi: 10.1681/ASN.2009040427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hu MC, Shi M, Zhang J, et al. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2011;22:124–36. doi: 10.1681/ASN.2009121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Juppner H, Wolf M, Salusky IB. FGF-23: More than a regulator of renal phosphate handling? J Bone Miner Res. 2010;25:2091–7. doi: 10.1002/jbmr.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hsu HJ, Wu MS. Fibroblast growth factor 23: a possible cause of left ventricular hypertrophy in hemodialysis patients. Am J Med Sci. 2009;337:116–22. doi: 10.1097/MAJ.0b013e3181815498. [DOI] [PubMed] [Google Scholar]
- 83.Olauson H, Qureshi AR, Miyamoto T, et al. Relation between serum fibroblast growth factor-23 level and mortality in incident dialysis patients: are gender and cardiovascular disease confounding the relationship? Nephrol Dial Transplant. 2010;25:3033–8. doi: 10.1093/ndt/gfq191. [DOI] [PubMed] [Google Scholar]
- 84.Jean G, Terrat JC, Vanel T, et al. High levels of serum fibroblast growth factor (FGF)-23 are associated with increased mortality in long haemodialysis patients. Nephrol Dial Transplant. 2009;24:2792–6. doi: 10.1093/ndt/gfp191. [DOI] [PubMed] [Google Scholar]
- 85.Wolf M, Molnar MZ, Amaral AP, et al. Elevated fibroblast growth factor 23 is a risk factor for kidney transplant loss and mortality. J Am Soc Nephrol. 2011;22:956–66. doi: 10.1681/ASN.2010080894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Isakova T, Xie H, Yang W, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305:2432–9. doi: 10.1001/jama.2011.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kendrick J, Cheung AK, Kaufman JS, et al. FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol. 2011;22:1913–22. doi: 10.1681/ASN.2010121224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fliser D, Kollerits B, Neyer U, et al. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol. 2007;18:2600–8. doi: 10.1681/ASN.2006080936. [DOI] [PubMed] [Google Scholar]
- 89.Titan SM, Zatz R, Graciolli FG, et al. FGF-23 as a predictor of renal outcome in diabetic nephropathy. Clin J Am Soc Nephrol. 2011;6:241–7. doi: 10.2215/CJN.04250510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Seiler S, Reichart B, Roth D, et al. FGF-23 and future cardiovascular events in patients with chronic kidney disease before initiation of dialysis treatment. Nephrol Dial Transplant. 2010;25:3983–9. doi: 10.1093/ndt/gfq309. [DOI] [PubMed] [Google Scholar]
- 91.Parker BD, Schurgers LJ, Brandenburg VM, et al. The associations of fibroblast growth factor 23 and uncarboxylated matrix Gla protein with mortality in coronary artery disease: the Heart and Soul Study. Ann Intern Med. 2010;152:640–8. doi: 10.1059/0003-4819-152-10-201005180-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Taylor EN, Rimm EB, Stampfer MJ, et al. Plasma fibroblast growth factor 23, parathyroid hormone, phosphorus, and risk of coronary heart disease. Am Heart J. 2011;161:956–62. doi: 10.1016/j.ahj.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Manghat P, Fraser WD, Wierzbicki AS, et al. Fibroblast growth factor-23 is associated with C-reactive protein, serum phosphate and bone mineral density in chronic kidney disease. Osteoporos Int. 2010;21:1853–61. doi: 10.1007/s00198-009-1142-4. [DOI] [PubMed] [Google Scholar]
- 94.Seiler S, Cremers B, Rebling NM, et al. The phosphatonin fibroblast growth factor 23 links calcium-phosphate metabolism with left-ventricular dysfunction and atrial fibrillation. Eur Heart J. 2011 doi: 10.1093/eurheartj/ehr215. In Press. [DOI] [PubMed] [Google Scholar]
- 95.Tanaka H, Hamano T, Fujii N, et al. The impact of diabetes mellitus on vitamin D metabolism in predialysis patients. Bone. 2009;45:949–55. doi: 10.1016/j.bone.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 96.Wahl P, Xie H, Scialla J, et al. Earlier onset and greater severity of disordered mineral metabolism in diabetic patients with chronic kidney disease. Diabetes Care. 2012 doi: 10.2337/dc11-2235. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yilmaz MI, Sonmez A, Saglam M, et al. FGF-23 and vascular dysfunction in patients with stage 3 and 4 chronic kidney disease. Kidney Int. 2010;78:679–85. doi: 10.1038/ki.2010.194. [DOI] [PubMed] [Google Scholar]
- 98.Mirza MA, Larsson A, Lind L, et al. Circulating fibroblast growth factor-23 is associated with vascular dysfunction in the community. Atherosclerosis. 2009;205:385–90. doi: 10.1016/j.atherosclerosis.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 99.Desjardins L, Liabeuf S, Renard C, et al. FGF23 is independently associated with vascular calcification but not bone mineral density in patients at various CKD stages. Osteoporos Int. 2011 doi: 10.1007/s00198-011-1838-0. In Press. [DOI] [PubMed] [Google Scholar]
- 100.Srivaths PR, Goldstein SL, Silverstein DM, et al. Elevated FGF 23 and phosphorus are associated with coronary calcification in hemodialysis patients. Pediatr Nephrol. 2011;26:945–51. doi: 10.1007/s00467-011-1822-0. [DOI] [PubMed] [Google Scholar]
- 101.Roos M, Lutz J, Salmhofer H, et al. Relation between plasma fibroblast growth factor-23, serum fetuin-A levels and coronary artery calcification evaluated by multislice computed tomography in patients with normal kidney function. Clin Endocrinol (Oxf) 2008;68:660–5. doi: 10.1111/j.1365-2265.2007.03074.x. [DOI] [PubMed] [Google Scholar]
- 102.Inaba M, Okuno S, Imanishi Y, et al. Role of fibroblast growth factor-23 in peripheral vascular calcification in non-diabetic and diabetic hemodialysis patients. Osteoporos Int. 2006;17:1506–13. doi: 10.1007/s00198-006-0154-6. [DOI] [PubMed] [Google Scholar]
- 103.Goodman WG, Goldin J, Kuizon BD, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342:1478–83. doi: 10.1056/NEJM200005183422003. [DOI] [PubMed] [Google Scholar]
- 104.Blacher J, Guerin AP, Pannier B, et al. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension. 2001;38:938–42. doi: 10.1161/hy1001.096358. [DOI] [PubMed] [Google Scholar]
- 105.Cozzolino M, Brancaccio D, Gallieni M, et al. Pathogenesis of vascular calcification in chronic kidney disease. Kidney Int. 2005;68:429–36. doi: 10.1111/j.1523-1755.2005.00421.x. [DOI] [PubMed] [Google Scholar]
- 106.Jono S, McKee MD, Murry CE, et al. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. 2000;87:E10–7. doi: 10.1161/01.res.87.7.e10. [DOI] [PubMed] [Google Scholar]
- 107.Reynolds JL, Joannides AJ, Skepper JN, et al. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: a potential mechanism for accelerated vascular calcification in ESRD. J Am Soc Nephrol. 2004;15:2857–67. doi: 10.1097/01.ASN.0000141960.01035.28. [DOI] [PubMed] [Google Scholar]
- 108.Shroff RC, Donald AE, Hiorns MP, et al. Mineral metabolism and vascular damage in children on dialysis. J Am Soc Nephrol. 2007;18:2996–3003. doi: 10.1681/ASN.2006121397. [DOI] [PubMed] [Google Scholar]
- 109.Silberberg JS, Barre PE, Prichard SS, et al. Impact of left ventricular hypertrophy on survival in end-stage renal disease. Kidney Int. 1989;36:286–90. doi: 10.1038/ki.1989.192. [DOI] [PubMed] [Google Scholar]
- 110.Glassock RJ, Pecoits-Filho R, Barberato SH. Left ventricular mass in chronic kidney disease and ESRD. Clin J Am Soc Nephrol. 2009;4 (Suppl 1):S79–91. doi: 10.2215/CJN.04860709. [DOI] [PubMed] [Google Scholar]
- 111.Gutierrez OM, Januzzi JL, Isakova T, et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119:2545–52. doi: 10.1161/CIRCULATIONAHA.108.844506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mirza MA, Larsson A, Melhus H, et al. Serum intact FGF23 associate with left ventricular mass, hypertrophy and geometry in an elderly population. Atherosclerosis. 2009;207:546–51. doi: 10.1016/j.atherosclerosis.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 113.Kirkpantur A, Balci M, Gurbuz OA, et al. Serum fibroblast growth factor-23 (FGF-23) levels are independently associated with left ventricular mass and myocardial performance index in maintenance haemodialysis patients. Nephrol Dial Transplant. 2011;26:1346–54. doi: 10.1093/ndt/gfq539. [DOI] [PubMed] [Google Scholar]
- 114.Canziani ME, Tomiyama C, Higa A, et al. Fibroblast growth factor 23 in chronic kidney disease: bridging the gap between bone mineral metabolism and left ventricular hypertrophy. Blood Purif. 2011;31:26–32. doi: 10.1159/000321368. [DOI] [PubMed] [Google Scholar]
- 115.Stevens KK, McQuarrie EP, Sands W, et al. Fibroblast growth factor 23 predicts left ventricular mass and induces cell adhesion molecule formation. Int J Nephrol. 2011;2011:297070. doi: 10.4061/2011/297070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Faul C, Amaral AP, Oskouei B, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393–408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gross ML, Ritz E. Hypertrophy and fibrosis in the cardiomyopathy of uremia--beyond coronary heart disease. Semin Dial. 2008;21:308–18. doi: 10.1111/j.1525-139X.2008.00454.x. [DOI] [PubMed] [Google Scholar]
- 118.Wanner C, Krane V, Marz W, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238–48. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 119.US Renal Data System, USRDS. Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2010. [Google Scholar]
- 120.Shastri S, Tangri N, Tighiouart H, et al. Predictors of sudden cardiac death: a competing risk approach in the hemodialysis study. Clin J Am Soc Nephrol. 2011 doi: 10.2215/CJN.06320611. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Isakova T, Gutierrez OM, Smith K, et al. Pilot study of dietary phosphorus restriction and phosphorus binders to target fibroblast growth factor 23 in patients with chronic kidney disease. Nephrol Dial Transplant. 2011;26:584–91. doi: 10.1093/ndt/gfq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Moe SM, Zidehsarai MP, Chambers MA, et al. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:257–64. doi: 10.2215/CJN.05040610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Uribarri J, Calvo MS. Hidden sources of phosphorus in the typical American diet: does it matter in nephrology? Semin Dial. 2003;16:186–8. doi: 10.1046/j.1525-139x.2003.16037.x. [DOI] [PubMed] [Google Scholar]
- 124.Moe SM, Chen NX, Seifert MF, et al. A rat model of chronic kidney disease-mineral bone disorder. Kidney Int. 2009;75:176–84. doi: 10.1038/ki.2008.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Koiwa F, Kazama JJ, Tokumoto A, et al. Sevelamer hydrochloride and calcium bicarbonate reduce serum fibroblast growth factor 23 levels in dialysis patients. Ther Apher Dial. 2005;9:336–9. doi: 10.1111/j.1744-9987.2005.00293.x. [DOI] [PubMed] [Google Scholar]
- 126.Oliveira RB, Cancela AL, Graciolli FG, et al. Early control of PTH and FGF23 in normophosphatemic CKD patients: a new target in CKD-MBD therapy? Clin J Am Soc Nephrol. 2010;5:286–91. doi: 10.2215/CJN.05420709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gonzalez-Parra E, Gonzalez-Casaus ML, Galan A, et al. Lanthanum carbonate reduces FGF23 in chronic kidney disease Stage 3 patients. Nephrol Dial Transplant. 2011;26:2567–71. doi: 10.1093/ndt/gfr144. [DOI] [PubMed] [Google Scholar]
- 128.Yilmaz MI, Sonmez A, Saglam M, et al. Comparison of calcium acetate and sevelamer on vascular function and fibroblast growth factor 23 in CKD patients: a randomized clinical trial. Am J Kidney Dis. 2011 doi: 10.1053/j.ajkd.2011.11.007. In Press. [DOI] [PubMed] [Google Scholar]
- 129.Isakova T, Gutierrez OM, Wolf M. A blueprint for randomized trials targeting phosphorus metabolism in chronic kidney disease. Kidney Int. 2009;76:705–16. doi: 10.1038/ki.2009.246. [DOI] [PubMed] [Google Scholar]
- 130.Wetmore JB, Liu S, Krebill R, et al. Effects of cinacalcet and concurrent low-dose vitamin D on FGF23 levels in ESRD. Clin J Am Soc Nephrol. 2010;5:110–6. doi: 10.2215/CJN.03630509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Koizumi M, Komaba H, Nakanishi S, et al. Cinacalcet treatment and serum FGF23 levels in haemodialysis patients with secondary hyperparathyroidism. Nephrol Dial Transplant. 2011 doi: 10.1093/ndt/gfr384. In Press. [DOI] [PubMed] [Google Scholar]
- 132.Finch JL, Tokumoto M, Nakamura H, et al. Effect of paricalcitol and cinacalcet on serum phosphate, FGF-23, and bone in rats with chronic kidney disease. Am J Physiol Renal Physiol. 2010;298:F1315–22. doi: 10.1152/ajprenal.00552.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wolf M, Shah A, Gutierrez O, et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2007;72:1004–13. doi: 10.1038/sj.ki.5002451. [DOI] [PubMed] [Google Scholar]
- 134.Block GA, Martin KJ, de Francisco AL, et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med. 2004;350:1516–25. doi: 10.1056/NEJMoa031633. [DOI] [PubMed] [Google Scholar]
- 135.Charytan C, Coburn JW, Chonchol M, et al. Cinacalcet hydrochloride is an effective treatment for secondary hyperparathyroidism in patients with CKD not receiving dialysis. Am J Kidney Dis. 2005;46:58–67. doi: 10.1053/j.ajkd.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 136.Wesseling-Perry K, Pereira RC, Sahney S, et al. Calcitriol and doxercalciferol are equivalent in controlling bone turnover, suppressing parathyroid hormone, and increasing fibroblast growth factor-23 in secondary hyperparathyroidism. Kidney Int. 2011;79:112–9. doi: 10.1038/ki.2010.352. [DOI] [PubMed] [Google Scholar]
- 137.Hansen D, Rasmussen K, Pedersen SM, et al. Changes in fibroblast growth factor 23 during treatment of secondary hyperparathyroidism with alfacalcidol or paricalcitol. Nephrol Dial Transplant. 2011 doi: 10.1093/ndt/gfr668. In Press. [DOI] [PubMed] [Google Scholar]
- 138.Teng M, Wolf M, Lowrie E, et al. Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med. 2003;349:446–56. doi: 10.1056/NEJMoa022536. [DOI] [PubMed] [Google Scholar]
- 139.Teng M, Wolf M, Ofsthun MN, et al. Activated injectable vitamin D and hemodialysis survival: a historical cohort study. J Am Soc Nephrol. 2005;16:1115–25. doi: 10.1681/ASN.2004070573. [DOI] [PubMed] [Google Scholar]
- 140.Shoben AB, Rudser KD, de Boer IH, et al. Association of oral calcitriol with improved survival in nondialyzed CKD. J Am Soc Nephrol. 2008;19:1613–9. doi: 10.1681/ASN.2007111164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kovesdy CP, Ahmadzadeh S, Anderson JE, et al. Association of activated vitamin D treatment and mortality in chronic kidney disease. Arch Intern Med. 2008;168:397–403. doi: 10.1001/archinternmed.2007.110. [DOI] [PubMed] [Google Scholar]
- 142.Naves-Diaz M, Alvarez-Hernandez D, Passlick-Deetjen J, et al. Oral active vitamin D is associated with improved survival in hemodialysis patients. Kidney Int. 2008;74:1070–8. doi: 10.1038/ki.2008.343. [DOI] [PubMed] [Google Scholar]
- 143.Kalantar-Zadeh K, Kuwae N, Regidor DL, et al. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 2006;70:771–80. doi: 10.1038/sj.ki.5001514. [DOI] [PubMed] [Google Scholar]
- 144.Isakova T, Xie H, Barchi-Chung A, et al. Daily variability in mineral metabolites in CKD and effects of dietary calcium and calcitriol. Clin J Am Soc Nephrol. 2012 doi: 10.2215/CJN.11721111. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Aono Y, Yamazaki Y, Yasutake J, et al. Therapeutic effects of anti-FGF23 antibodies in hypophosphatemic rickets/osteomalacia. J Bone Miner Res. 2009;24:1879–88. doi: 10.1359/jbmr.090509. [DOI] [PubMed] [Google Scholar]
- 146.Shimada T, Kakitani M, Yamazaki Y, et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004;113:561–68. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hill AB. The Environment and Disease: Association or Causation? Proc R Soc Med. 1965;58:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zisman AL, Wolf M. Recent advances in the rapidly evolving field of fibroblast growth factor 23 in chronic kidney disease. Curr Opin Nephrol Hypertens. 2010;19:335–42. doi: 10.1097/mnh.0b013e328338f536. [DOI] [PubMed] [Google Scholar]
- 149.Jonsson KB, Zahradnik R, Larsson T, et al. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med. 2003;348:1656–63. doi: 10.1056/NEJMoa020881. [DOI] [PubMed] [Google Scholar]
- 150.Lorenz-Depiereux B, Benet-Pages A, Eckstein G, et al. Hereditary hypophosphatemic rickets with hypercalciuria is caused by mutations in the sodium-phosphate cotransporter gene SLC34A3. Am J Hum Genet. 2006;78:193–201. doi: 10.1086/499410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yu Y, Sanderson SR, Reyes M, et al. Novel NaPi-IIc mutations causing HHRH and idiopathic hypercalciuria in several unrelated families: Long-term follow-up in one kindred. Bone. 2012 doi: 10.1016/j.bone.2012.02.015. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]