Abstract
Primary organ failure after transplantation (TX) remains a serious complication and leads to a high percentage of lethality. It is known, however, that the speed of rejection and tissue destruction depends on 3 main factors: antibody titer, the ability of the tissue to repair itself, and immunosuppressive measures. Especially with evidence for antibodies against human leukocyte antigen (HLA-ab), the immunological risk of persistent and acute episodes of rejection increases. The role of non-HLA-ab in rejection episodes is often underestimated and should be studied further. Antibody-mediated rejection (AMR) is still an unsolved problem in thoracic organ TX. An essential pillar of antihumoral therapy are the extracorporeal procedures like plasmapheresis (PP), therapeutic plasma exchange (TPE), and immunoadsorption (IA), because only they have the ability to remove preformed or de novo developed antibodies quickly and effectively. The quick removal of antibodies and other plasma factors through TPE or IA remains an effective and supportive method for treating AMR and allows the TX despite preformed antibodies. The pertinent literature does not disclose, however, how often and for how long treatment should be administered. It is known, that repeated treatment cycles with adequately processed plasma volume must be used to overcome redistribution of pathological antibodies. Based on our experience in heart transplant recipients with compromised graft function due to non-HLA-ab and HLA-ab, IA seems to be more effective.
Keywords: Antibody mediated rejection, Heart transplantation, Immunoadsorption, Lung transplantation, Plasma exchange
Abstract
Das primäre Organversagen nach Transplantation (TX) ist eine schwerwiegende Komplikation und mit einer hohen Letalität verbunden. Man weiß, dass die Geschwindigkeit der Abstoßung bzw. Gewebedestruktion vom Antikörpertiter, von der Möglichkeit zur Gewebereparatur und von den immunsuppressiven Maßnahmen beeinflusst wird. Das immunologische Risiko, persistierende oder akute Abstoßungen zu erleiden, erhöht sich vorzugsweise bei positivem Nachweis von HLA-Antikörpern (HLA-AK). Die Rolle von non-HLA-AK in der Pathogenese der antikörpervermittelten Abstoßung (AMR) ist möglicherweise unterbewertet und sollte weiter untersucht werden. Die AMR spricht typischerweise nicht auf konventionelle Therapien an, und es gibt keine standardisierten Schemata zur Behandlung; somit ist sie ein ungelöstes Problem in der TX thorakaler Organe. Die therapeutische Lücke schließen die extrakorporalen Therapieverfahren wie Plasmapherese (PP), therapeutischer Plasmaaustausch (TPA) und Immunadsorption (IA). Mit diesen Verfahren gelingt es, die präformierten Non-HLA-AK und HLA-AK schnell und wirksam zu entfernen. Die TX mit positiven Antikörpernachweis wird ermöglicht, und ein positiver Cross-Match in einen negativen konvertiert. Zurzeit gibt es in der Literatur keine Hinweise darauf, wie oft und wie lange die Antikörperelimi-nierung erfolgen soll, aber man weiß, dass wiederholte Behandlungszyklen mit einem adäquat prozessierten Plasmavolumen nötig sind, um das antikörpervermittelte Geschehen zu beherrschen. Basierend auf unseren Erfahrungen sollten herztransplantierte Patienten mit AMR eher mit IA behandelt werden, lungentransplantierte Patienten hingegen eher mit TPA.
Introduction
Primary organ failure after transplantation (TX) remains a serious complication and leads to a high percentage of lethality. Immunological problems like preformed donor-specific antibodies (DSA) or high degree of immunization complicate the TX and can limit the therapeutic success.
The immunological risk of persistent and acute episodes of rejection increases especially with retransplantations and with evidence for human leukocyte antigen antibodies (HLA-ab) with panel reactive antibodies (PRA) of >25%. An elevated pre-TX PRA is the only factor that has a significant impact on patient survival within the first 30 days after heart transplantation (HTX) and/or lung transplantation (LuTX) [2, 3]. The risk for early graft failure within the first 48 h is significantly higher in the presence of a positive cross-match (CM) with donor T lymphocytes, which, in the absence of activation, express only major histocompatibility complex (MHC) class I antigens, than with donor B lymphocytes, which strongly express both MHC class I und II antigens. In addition, the real risk for early graft failure after a positive CM appears to reside in the immunoglobulin (Ig) G fraction of DSA. Patients with HLA-ab waiting for a HTX or LuTX have to be identified prior to TX.
In 2011 in accordance with the Deutsche Stiftung Organtransplantation (DSO), 337 LuTX (435 announced patients) and 366 HTX (695 announced patients) were performed. 44% of all patients in Jena waiting for HTX and 33% of all patients waiting for LuTX are non-HLA-ab- and/or HLA-ab-positive. According to our risk assessment which was described previously [5], approximately 15% of all patients on the waiting list may have a benefit from apheresis procedures. Desensitization therapy should be considered when the calculated PRA is considered by the individual transplant center to be high enough to significantly decrease the likelihood for a compatible donor match or to decrease the likelihood of donor heart rejection where unavoidable mismatches occur [6]. The same should apply for LuTX.
Acute and chronic allograft rejection can occur in HLA-identical sibling transplants, implicating the importance of immune response against non-HLA targets. Non-HLA-ab are predominantly autoantibodies, may occur also as alloantibodies, and have the ability to trigger antibody-mediated rejection (AMR). Antigenic target of non-HLA-ab described so far include various minor MHC, vascular receptors, adhesion molecules, and intermediate filaments. Non-HLA-ab may function as complement- and non-complement-fixing antibodies, and they may induce a wide variety of allograft injuries [7, 8]. Pretransplant-detected non-HLA-ab often resulted in accelerated rejection of the allograft. Nevertheless, prospective transplant recipients are not examined for non-HLA-ab.
Assessment of the immunological risks associated with a transplant requires close co-operation between laboratory and the transplant team. The laboratory should be informed of all potential sensitizing events, including previous transplants, left ventricular assist device (LVAD), history of transfusions, pregnancies, miscarriages if known, recent infections, vaccinations, or Ig applications. Detection and differentiation of antibodies along with an assessment of the antibody titer and the specificity of the recipients’ most ‘positive’ blood sample (blood sample with the highest PRA ever) are therefore of great importance. Within the European Transplantation Association the complement-dependent lymphocytotoxicity method (CDC) is the standard procedure and also used for CM. Modern methods for detecting non-HLA-ab und HLA-ab like ELISA (enzyme-linked immunosorbent assay) and the Luminex© technology are considered highly sensitive and specific. However the clinical relevance of weak HLA-ab captured with these methods is debatable.
AMR is still an unsolved problem in thoracic organ TX. AMR typically does not respond to conventional therapies, and there are no standardized treatment schemes. Immunosuppressants generally affect only the cellular signal transfer [9, 10]. An essential pillar of antihumoral therapy are the extracorporeal procedures like plasmapheresis (PP), therapeutic plasma exchange (TPE), and immunoadsorption (IA) because only they have the ability to remove preformed DSA quickly and effectively. Antihumoral strategies will need to be tailored to the antibody specificity detected in any given patient and to the clinical situation.
Another option is to attempt to reduce HLA-ab titer with monthly administrations of high doses of Ig (2 g/kg body weight), daily administrations of 0.4 g/kg body weight over a period of 7 days and monoclonal antibodies like anti-CD20. However, immediate antibody reduction is not possible [11]. Anti-CD20 has little or no effect on the level of circulating antibodies.
The efficiency of the elimination procedures is measured by the reduction of the PRA and by the lowering of specific HLA-ab titers and subsequently confirmed by a negative CM. After TX, treatment success is measured by continued graft function.
Available Extracorporeal Procedures to Treat and Prevent Graft Rejection
PP or TPE
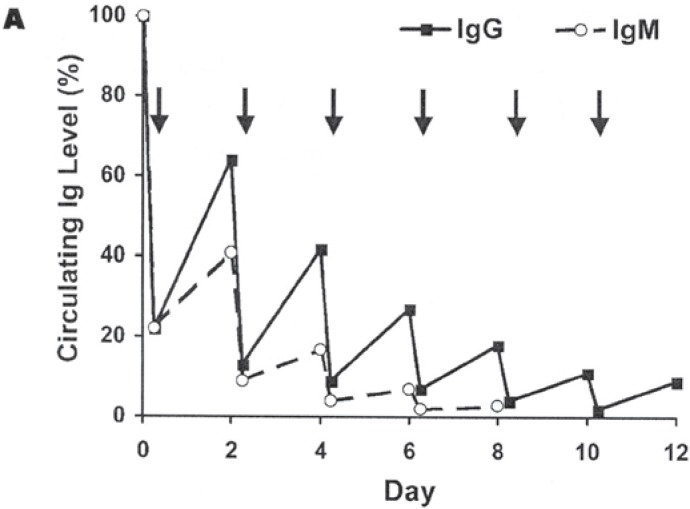
The efficiency of TPE depends on the volume exchanged. The amount of plasma to be exchanged during TPE must be determined in relation to the estimated plasma volume (ePV = 0.065 × kg body weight × (1 – hematocrit)) of the patient. A single exchange of 1.0 PV removes approximately 63% of all solutes in the plasma and an exchange of 1.5 PV removes about 78%. With an increasing exchange volume the elimination of pathogens increases but the efficiency decreases rapidly. The successful removal of harmful substances from the plasma depends on several factors: concentration, molecular weight, intravascular distribution, fractional turnover rate and half-life (table 1, fig. 1).
Table 1.
Characteristics of IgG and IgM [12]
| Protein | Concentration, mg/ml | MW, kDa | Intravascular, % | Fractional turn-over rate, %/day | Half-life, days |
|---|---|---|---|---|---|
| IgG (except IgG3 subclass) | 12 | 150 | 45 | 7 | 22 |
| IgM | 0.9 | 950 | 78 | 19 | 5 |
Fig. 1.
Hypothetical depletion of whole body Ig levels by TPE after 1.5 PV exchanges performed every 2 days [13].
In case of slowly forming antibodies, 5 separate treatments during a 7- to 10-day period will be required to remove 90% of the patients’ initial total-body burden. From Coopers et al. [14] point of view the TPE should be repeated daily for a minimum of 3 days and can be done for 5–7 days or until the circulating antibodies (usually multiple) are reduced to very low titer. This therapy may be life-saving, has very little associated morbidity, and should be initiated as soon as possible. The elimination of DSA appeared to be independent of antibody titer or specificity, the number of different antibody specificities, or whether or not the target antigen was a repeat mismatch. The effect appears to be long lasting, with no return of DSA observed in patients followed for an average of 13 months [15].
For the choice of the best substitution solution, possible risks and expected benefit of the treatment for the individual patient have to be balanced. Most commonly used are human serum albumin and therapeutic plasma. Octaplas® LG and pathogen-reduced fresh frozen plasma (‘Gefrorenes patho-genreduziertes Apheresefrischplasma Th-J’) should be used for TPE, because of their known advantages over untreated single donor plasma. It was important to use plasma that we knew to be virtually cell-free [16–18]. In the early postoperative phase the replacement with therapeutic plasma is necessary, especially regarding cardiac transplant patients. Later, the replacement fluid may consist of a 1:1 mix of therapeutic plasma and 5% human albumin.
Immunosuppressive drugs such as prednisolone or azathioprine are not significantly removed by TPE, but Tacrolimus is significantly eliminated [19].
IA
The principle of IA is based on affinity adsorption and chromatographic methods. IA is capable to eliminate huge amounts of Ig from the patients’ circulation with a minimum of side effects. Simple removal of Ig from the circulation does not necessarily result in stopping immunological processes. Repeated treatment cycles with adequately processed PV must be used to overcome redistribution of pathological antibodies.
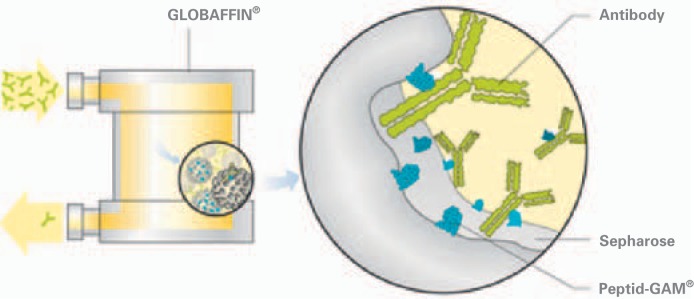
Regenerable adsorbers were designed to repeatedly process high volumes of plasma [20]. The following adsorbers for high-volume IA, used in TX medicine, are available: Staphylococcus protein A (SPA) adsorber – Immunosorba®, the sheep anti-human IgG adsorber – Therasorb®, and the synthetic broad band adsorber – Globaffin® (fig. 2).
Fig. 2.
Broad band adsorber Globaffin®.
IA allows nearly complete clearance of circulating Ig of all types and subtypes without the concomitant substitution of fresh frozen plasma or albumin solutions. For this reason, IA has high therapeutic efficacy even in the treatment of diseases in which TPE had failed to achieve improvement. Treatment in general is performed by a combination of two IA sessions within 48 h, reducing the serum IgG level by >95% by treating at least twice the PV [21]. After that patients are treated every 2–3 days until all antibodies have been eliminated or until clinical situation has improved.
Following the initial course of 4–6 IA, most patients have been monitored with respect to changes in PRA and HLA-ab titers. If titers of predominating HLA-ab are > 1:256, no marked effects on PRA have to be expected, although exceptions are reported [22].
Concomitant administration of intravenous immunoglobulin (IVIG) seems to attenuate the effect of IA in certain circumstances, although both treatments have been shown to be effective when used by themselves.
Application of Extracorporeal Procedures
It is known, however, that the speed of rejection and tissue destruction depends on 3 main factors: antibody titer, the ability of the tissue to repair itself, and immunosuppressive measures [23].
HTX: Preformed Antibodies, de novo Post-Transplant Antibodies, AMR without Detectable Antibodies
AMR (prevalence 15%) has been shown to be associated with a significantly worse survival and to predispose patients to coronary vasculopathy [24]. This form of rejection primarily is caused by IgG donor-specific class I HLA-ab and presents during the first month after TX or as early as 2–7 days after TX, if the patient has a history of pre-sensitization to donor HLA. The presence of IgG class II HLA-ab frequently detected in patients with LVAD or undergoing re-transplantation at the time of TX was found to be a major risk factor both for early high-grade cellular rejection and for accelerated vasculopathy in HTX recipients [4]. LVAD recipients develop prominent B-cell activation as evidenced by increased production of HLA-ab class I and II.
The development of DSA after TX is also associated with AMR. In a series of studies, investigators at the Columbia University demonstrated the relationship between the development of HLA-ab and both acute and chronic rejection of heart allografts [25, 26]. In contrast, the production of antibodies not specific to donor antigens was not correlated with the incidence or severity of acute rejections [27]. Post-transplant development of both IgG and IgM class II HLA-ab, especially anti-HLA DQ, has been shown to be associated with onset of AMR [28, 29].
Morphological findings of AMR with clinical graft dysfunction may have occurred in the absence of detectable DSA and have been shown to respond to PP [30]. The explanation for the failure to detect circulating HLA-ab is that the antibodies were bound to the graft. In addition to HLA-ab, there are considerable data indicating that antibodies against non-HLA structures can contribute to AMR.
LuTX: Preformed Antibodies, de novo Post-Transplant Antibodies, AMR without Detectable Antibodies
Early respiratory failure and death after LuTX can result from airway complications, hyperacute rejection, acute rejection, infection, or ischemia/reperfusion lung injury (IRLI). More than 50% of the episodes of acute respiratory failure were attributable to IRLI, and <10% were attributed to infectious etiologies. Allograft rejection and airway complications contributed to 5 and 11% of the episodes of respiratory failure, respectively [31].
Both early and late graft survivals in LuTX are lower than in kidney, heart or liver TX. Clinically there is no difference between IRLI and AMR: both lead to inflammation, complement activation, and endothelial damage. AMR of the lung has been associated with hyperacute rejection clinically manifested by primary graft failure within minutes, hours or days of TX in the setting of preformed antibodies, even at low levels, to either donor HLA or endothelial cells [9]. The presence of preformed DSA and/or development of de novo DSA after TX have been documented in patients with high-grade and steroid-refractory rejections. Furthermore, several investigators have shown that class I and II HLA-ab play an important role in the pathogenesis of bronchiolitis obliterans (BO) and demonstrated that de novo alloantibody formation is associated with BO [32, 33].
Treatment Protocols
Two treatment protocols have been established for reducing HLA-ab to overcome a positive CM or rescue organs undergoing AMR: high-dose IVIG and PP combined with low-dose cytomegalovirus (CMV) hyperimmune globulin or IVIG. These protocols, developed to desensitize patients in preparation for TX, are currently being used to treat AMR [9, 34]. PP, one PV exchange replaced with albumin or FFP, should be performed every other day followed by a low-dose (100 mg/kg) CMV Ig infusion until DSA is eliminated. Patients with an increased risk of AMR additional receive anti-CD20. As shown in table 2, in various transplant centers different desensitization therapies are available [6].
Table 2.
Desensitization therapies [6]a
| Therapy | Dose | Frequency |
|---|---|---|
| PP | (A, F) 1.5 volume exchanges | (A) 5 consecutive days |
| (B) 5 times, every other day | ||
| (C) 2–3 times/week until transplant | ||
| (D) 5 times, every other day, every 2–4 weeks | ||
| IVIG | (A, B) 2 g/kg IV divided over 2 days | (A) every 2–4 weeks |
| (C) 2–3 g/kg IV divided over 4 days | (D) every 2–4 weeks | |
| (D) 0.1 mg/kg IV | (G) every 4 weeks | |
| (E) 100 mg/kg IV | ||
| (f) 20 g (of 10% IVIG) | ||
| (G) 150 g (of 10% IVIG) divided over 3 rounds | ||
| Rituximab | (A) 1 g IV | (A) weekly × 4 |
| (C) 375 mg/m2 | (C) × 2 doses | |
| (G) 500 mg | (G) every 2 weeks | |
| Cyclophosphamide (used in the past) | (A) 1 mg/kg orally | (A) daily |
| (c) 0.5 µg/m2 | ||
| (D) 1 mg/kg orally |
A = UCLA; B = Stanford University; C = University of Maryland; D = University of Toronto; E = University of Wisconsin; F = Loyola University Chicago; G = University of Berlin.
Choices to consider as desensitization therapies include IVIG infusion, PP, either alone or combined, Rituximab, and in very selected cases, splenectomy.
In this context Kobashigawa et al. [35] have retrospectively shown in 523 HTX patients that the successful treatment of highly sensitized patients (4%) pre-transplant enabled these patients to safely undergo HTX with comparable post-transplant outcomes compared to lowly and non-sensitized patients. The physicians treated sensitized patients with 1–6 courses of combination therapy, including PP (daily for 5 days) and/or IVIG at 2 g/kg divided dose over 2 days and/or rituximab at 375 mg/m2 to reduce antibodies.
Nevertheless a large randomized controlled clinical trial is missing but needed to assess the effectiveness of desensitization strategies.
HTX
Wang et al. [36] showed the effect of TPE on graft survival in 12 patients with AMR after HTX. The TPE was conducted on 5 consecutive days, and twice the PV was replaced with FFP. Two of the described patients who died 3 days and 1 month after TPE, respectively, required LVAD for maintaining cardiac functionality. The results of Rummler et al. [5] are comparable with those of Wang et al. [36] although they did not exchange twice the PV. Treatment was typically administered on 3 consecutive days and every 2nd or 3rd day after that until graft functionality was established or the graft was lost.
Grauhan et al. [37] altogether observed 29 humoral rejections with hemodynamic compromise (HRHC) episodes. 18 HRHC (7 patients) episodes were treated without PP, but only 2 patients survived, whereas in 11 HRHC episodes (6 patients) therapy included PP and all patients survived.
Treatment protocols must be flexible and adapted to the pertinent clinical situation. If it is impossible to improve the clinical situation with TPE, IA may be a treatment option. As a rule, this strategy succeeded in completely eliminating DSA. In 3 cardiac transplant recipients with HRHC, refractory to standard therapy, IA was performed using a protein A column (PA-IA). Concomitant with a decrease in PRA after PA-IA treatments, histological findings and ventricular function improved and normalized. One patient died from infection 2 months after resolution of AMR; the other 2 patients survived [38].
Ruiz et al. [39] treated 1 patient with PRA > 80% prior to HTX with PA-IA. 11 sessions were performed during a period of 35 days. Concomitantly with the reduction of Ig levels, the PRA was completely abolished. CM converted from positive to negative. The TX and immediate postoperative period were uneventful, but the patient died of an incurable infection.
Kaczmarek et al. [10] described 1 patient who developed HRHC 2 years after HTX after a switch of immunosuppressants. He presented with a PRA of 100%. The patient was treated with 3 cycles of IA using sheep antihuman IgG adsorber, followed by a single administration of anti-CD20. After IA, HLA-ab were completely eliminated and no longer detectable.
LuTX
Astor et al. [40] performed PP/TPE on 5 consecutive days in patients with rejection capillaritis, exchanging 1.0 to 1.5 times the PV for equal parts of human albumin and FFP. The authors reported a response rate of 67%. There are 5 reported cases of AMR after LuTX, and only 1 patient who received TPE survived [41]. It is conclusive that in cases of AMR caused by DSA the additional use of PP can give patients a chance for survival.
Rummler et al. [5] described 5 LuTX recipients with compromised graft function. Four of the 5 treated patients showed adequate graft functionality 1 year after TX. This was achieved with an average of 5 (range 2–11) TPEs. One patient received 3 TPEs before TX, because of evidence of DSA (HLA-ab class II). The patients with second LuTX received additional 0.4 g/kg body weight IVIG for 3 consecutive days after TPE.
Appel et al. [42] treated LuTX recipients that developed de novo DSA with a weekly dose of 500 mg/kg body weight IVIG, and in cases where lung functionality was heavily compromised IA was administered every 3rd and 7th day as well. Patients with de novo non-DSA received only IVIG. Forced expiratory volume in 1 s (FEV1) improved in both groups in 75% of the cases.
TX with Existing Non-HLA-ab
Unlike bone marrow or kidney TX, proven to be successful in patients with systemic lupus erythematodes (SLE), HTX or LuTX/HTX have only rarely been reported with a variable outcome. Data on HTX in SLE are extremely limited and found to be previously reported in 7 SLE patients. In 5 of these 7 patients, the TX was indicated for disease secondary to pulmonary disorders. LuTX/HTX was successful in 5 patients followed up for 14 months to 4 years. Two patients died after TX due to mesenteric occlusion. These 2 patients had SLE with secondary antiphospholipid syndrome (APS) and anti-cardiolipin antibodies (ACLA) [43]. Rummler et al. [5] described 1 patient with SLE and ACLA undergoing LuTX/HTX. The hyperacute rejection due to non-HLA-ab was controlled with 4 TPEs once a day. Because of persisting and considerably elevated ACLA over the 1st year after TX, additional extracorporeal treatments were performed in spite of good graft functionality. The 3-year follow-up is uneventful regarding the transplanted organs.
ABO-Incompatible LuTX
There are two reports about unintentional, successful ABO-incompatible pulmonary transplants due to clerical error. Only one case of successful intentional LuTX (donor AB, recipient O) is described. One PP initiated immediately before surgery, in combination with IVIG treatment during TX was sufficient for a marked reduction of blood group antibodies (initially from 1:128 for both to 1:2 anti-A and 1:1 anti-B). Since direct evidence for a specific antibody titer threshold allowing for successful ABO-incompatible LuTX is not available, the authors aimed at keeping the antibody titer below 1:16 [44].
Disclosure Statement
The authors declare that there are no conflicts of interest regarding this publication.
References
- 1.Teschner S, Kurschat C, Burst V. Therapeutic apheresis in transplantation: Overview and critical evaluation of available modalities in respect to indications, evidence and costs. Tx Med. 2010;22:266–272. [Google Scholar]
- 2.Nwakanma LU, Williams JA, Weiss ES, Russell SD, Baumgartner WA, Conte JV. Influence of pre-transplant panel-reactive antibody on outcomes in 8,160 heart transplant recipients in recent era. Ann Thorac Surg. 2007;84:1556–1563. doi: 10.1016/j.athoracsur.2007.05.095. [DOI] [PubMed] [Google Scholar]
- 3.Shah AS, Nwakanma LU Simpkins C, Williams J, Chang DC, Conte JV. Pretransplant panel reactive antibodies in human lung transplantation: an analysis of over 10,000 patients. Ann Thorac Surg. 2008;85:1919–1924. doi: 10.1016/j.athoracsur.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Itescu S, Tung TC, Burke EM, Weinberg A, Moazami N, Artrip JH, Suciu-Foca N, Rose EA, Oz MC, Michler RE. Performed IgG antibodies against major histocompatibility complex class II antigens are major risk factors for high-grade cellular rejection in recipients of heart transplantation. Circulation. 1998;98:786–793. doi: 10.1161/01.cir.98.8.786. [DOI] [PubMed] [Google Scholar]
- 5.Rummler S, Breuer M, Maier K, Sandhaus T, Steinke T, Pauli T, Hekmat K, Barz D. Extracorporeal immune modulation of sensitized recipients of thoracic organ transplants. Tx Med. 2010;22:369–378. [Google Scholar]
- 6.Costanzo MR. (Chair): The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J Heart Lung Transplant. 2010;29:914–956. doi: 10.1016/j.healun.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 7.Magro CM, Klinger DM, Adams PW, Orosz CG, Pop-Harman AL, Waldman WJ, Knight D, Ross P., Jr Evidence that humoral allograft rejection in lung transplant patients is not histocompatibility antigen-related. Am J Transpl. 2003;3:1264–1272. doi: 10.1046/j.1600-6143.2003.00229.x. [DOI] [PubMed] [Google Scholar]
- 8.Dragun D, Hegner B. Non-HLA antibodies post-transplantation: clinical relevance and treatment in solid organ transplantation. Contrib Nephrol. 2009;162:129–139. doi: 10.1159/000170845. [DOI] [PubMed] [Google Scholar]
- 9.Takemoto SK, Zeevi A, Feng S, Colvin RB, Jordan S, Kobashigawa J, Kupiec-Weglinski J, Matas A, Montegomery A, Nickerson P, Platt JL, Rabb H, Thistlethwaite R, Tyan D, Delmonico FL. National conference to assess antibody-mediated rejection in solid organ transplantation. Am J Transpl. 2004;4:1033–1041. doi: 10.1111/j.1600-6143.2004.00500.x. [DOI] [PubMed] [Google Scholar]
- 10.Kaczmarek I, Deutsch MA, Sadoni S, Brenner P, Schmauss D, Daebritz SH, Weiss M, Meiser B, Reichart B. Successful management of antibody-mediated cardiac allograft rejection with combined immunoadsorption and anti-CD20 monoclonal antibody treatment: case report and literature report. J Heart Lung Transplant. 2007;26:511–515. doi: 10.1016/j.healun.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 11.Jordan SC, Vo A, Tyan D, Toyota M. Desensitization therapy with intravenous gammaglobulin (IVIG): applications in solid organ transplantation. Trans Clin Climatol Assoc. 2006;117:199–211. [PMC free article] [PubMed] [Google Scholar]
- 12.Kaplan AA. Therapeutic plasma exchange: Core Curriculum 2008. Am J Kidney Dis. 2008;52:1180–1196. doi: 10.1053/j.ajkd.2008.02.360. [DOI] [PubMed] [Google Scholar]
- 13.Linenberger ML, Price TH. Use of cellular and plasma apheresis in the critically ill patient: part 1: technical and physiological considerations. J Intensive Care Med. 2005;20:18–27. doi: 10.1177/0885066604271394. [DOI] [PubMed] [Google Scholar]
- 14.Cooper DKC. Miller LW. Patterson A. 2nd. Heidelberg: Springer; 1997. The Transplantation and Replacement of Thoracic Organs: The Present Status of Biological and Mechanical Replacement of the Heart and Lungs. [Google Scholar]
- 15.Zachary AA, Montgomery RA, Ratner LE, Samaniego-Picota M, Haas M, Kopchaliiska D, Leffell MS. Specific and durable elimination of antibody to donor HLA antigens in renal-transplant patients. Transplantation. 2003;76:1519–1525. doi: 10.1097/01.TP.0000090868.88895.E0. [DOI] [PubMed] [Google Scholar]
- 16.Barz D. Detection of antigen structures in blood cells in various prepared plasma transfusions. Anaesthesiol Reanim. 1994;19:155–158. [PubMed] [Google Scholar]
- 17.Barz D, Budde U. Hellstern P. Therapeutic plasma exchange and plasma infusion in thrombotic microvascular syndromes. Thromb Res. 2002;107:23–27. doi: 10.1016/s0049-3848(02)00148-2. [DOI] [PubMed] [Google Scholar]
- 18.Wurm K, Rummler S, Barz D. How free of residual cells and cell antigens is human blood plasma? A comparison of different production methods of human plasma and the risk of the products for patients. Transfus Apher Sci. 2008;38:149–157. doi: 10.1016/j.transci.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Piekoszewski W, Jusko WJ. Plasma protein binding of tacrolimus in humans. J Pharm Sci. 1993;82:341. doi: 10.1002/jps.2600820325. [DOI] [PubMed] [Google Scholar]
- 20.Braun N, Bosch T. Immunoadsorption, current status and future developments. Exp Opin Invest Drugs. 2000;9:2017–2038. doi: 10.1517/13543784.9.9.2017. [DOI] [PubMed] [Google Scholar]
- 21.Schmaldienst S, Müllner M, Goldammer A, Spitzauer S, Banyai S, Hörl WH, Derfler K. Intravenous immunoglobulin application following immunoadsorption: benefit or risk in patients with autoimmune diseases? Rheumatology. 2001;40:513–521. doi: 10.1093/rheumatology/40.5.513. [DOI] [PubMed] [Google Scholar]
- 22.Gjörstrup P, Watt RM. Therapeutic protein A immunoadsorption. A review. Transfus Sci. 1990;11:281–302. [Google Scholar]
- 23.Cai J, Terasaki PI. Humoral theory of transplantation: mechanism, prevention, and treatment. Hum Immunol. 2005;66:334–342. doi: 10.1016/j.humimm.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 24.Reed EF, Demetris AJ, Hammond E, Itescu S, Kobashigawa JA, Reinsmoen NL, Rodriguez ER, Rose M, Stewart S, Sucius-Foca N, Zeevi A, Fish-bein MC. Acute antibody-mediated rejection of cardiac transplants. J Heart Lung Transplant. 2006;25:153–159. doi: 10.1016/j.healun.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Cherry R, Nielsen H, Reed E, Reemtsma K, Suciu-Foca N, Marboe CC. Vasular rejection in human cardiac allograft biopsies: relation to circulation anti-HLA antibodies. J Heart Lung Transplant. 1992;11:24–30. [PubMed] [Google Scholar]
- 26.Rose EA, Pepino P, Barr ML. Relation of HLA antibodies and graft atherosclerosis in human cardiac allograft recipients. J Heart Lung Transplant. 1992;11:S120–123. [PubMed] [Google Scholar]
- 27.Smith JD, Danskine AJ, Rose ML, Yacoub MH. Specificity of lymphocytotoxic antibodies formed after cardiac transplantation and correlation with rejection episodes. Transplantation. 1992;53:1358–1362. doi: 10.1097/00007890-199206000-00034. [DOI] [PubMed] [Google Scholar]
- 28.Leech SH, Mather PJ, Eisen HJ. Donor-specific HLA antibodies after transplantation are associated with deterioration in cardiac function. Clin Transplant. 1996;10:639–645. [PubMed] [Google Scholar]
- 29.Nikaein A, Alivizatos PA, Monahan K, Stone MJ. The role of anti-class II HLA antibodies in heart transplantation. Transplantation. 1995;59:439–442. [PubMed] [Google Scholar]
- 30.Hammond MEH. Renlund DG. Cardiac allograft vascular (microvascular) rejection. Curr Opin Organ Tranplant. 2002;7:233–239. [Google Scholar]
- 31.Zaas D, Palmer SM. Respiratory failure early after lung transplantation. Chest. 2003;123:14–16. doi: 10.1378/chest.123.1.14. [DOI] [PubMed] [Google Scholar]
- 32.Girnita AL, McCurry KR, Iacono AT, Duquesnoy R, Corcoran TE, Awad M, Spichty KJ, Yousem SA, Burckart G, Dauber JH, Griffith BP, Zeevi A. HLA-specific antibodies are associated with high-grade and persistent-recurrent lung allograft acute rejection. J Heart Lung Transplant. 2004;23:1135–1141. doi: 10.1016/j.healun.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 33.Sundaresan S, Mohanakumar T, Smith MA. HLA-A locus mismatches and development of antibodies to HLA after lung transplantation correlate with the development of bronchiolitis obliterans syndrome. Transplantation. 1998;65:648–653. doi: 10.1097/00007890-199803150-00008. [DOI] [PubMed] [Google Scholar]
- 34.Zachary AA, Montgomery RA, Leffell MS. Desensitization protocols improving access and outcome in transplantation. Clin Applied Immunol. Rev. 2005;5:373–395. [Google Scholar]
- 35.Kobashigawa JA, Patel JA, Kittleson MM, Kawano MA, Kiyosaki KK, Davis SN, Moriguchi JD, Reed EF, Ardehali AA. The long-term outcome of treated sensitized patients who undergo heart transplantation. Clin Transplant. 2011;25:E61–67. doi: 10.1111/j.1399-0012.2010.01334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang SS, Chou NK, Ko WJ, Hung SC, Hsu RB, Yu HY, Chen YS, Chu SH, Tsao CI, Shun CT. Effect of plasmapheresis for acute humoral rejection after heart transplantation. Transplant Proc. 2006;38:3692–3694. doi: 10.1016/j.transproceed.2006.10.060. [DOI] [PubMed] [Google Scholar]
- 37.Grauhan O, Knosalla C, Ewert R, Hummel M, Loebe M, Weng YG, Hetzer R. Plasmapheresis and cyclophosphamide in the treatment of humoral rejection after heart transplantation. J Heart Lung Transplant. 2001;20:316–321. doi: 10.1016/s1053-2498(00)00211-4. [DOI] [PubMed] [Google Scholar]
- 38.Olivari MT, May CB, Johnson NA, Ring WS, Stephens MK. Treatment of acute vascular rejection with immunoadsorption. Circulation. 1994;90:1170–1173. [PubMed] [Google Scholar]
- 39.Ruiz JC, de Francisco ALM. Vazquez de Prada JA. Ruano J. Pastor JM. Alcade G. Arias M. Successful heart transplantation after anti-HLA antibody removal with protein A immunoadsorption in a hyperimmunized patient. J Thorac Cardiovasc Surg. 1994;107:1366. [PubMed] [Google Scholar]
- 40.Astor TL, Weill D, Cool C, Teitelbaum I, Schwarz MI, Zamora MR. Pulmonary capillaritis in lung transplant recipients: treatment and effect on allograft function. J Heart Lung Transplant. 2005;24:2091–2097. doi: 10.1016/j.healun.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 41.Camargo JP, Camargo SM, Schio SM, Machuca TN, Perin FA. Hyperacute rejection after single lung transplantation: a case report. Transplant Proc. 2008;40:867–869. doi: 10.1016/j.transproceed.2008.02.052. [DOI] [PubMed] [Google Scholar]
- 42.Appel JZ, Hartwig MG, Duana Davis L. Reinsmoen NL. Utility of peritransplant and rescue intravenous immunoglobulin and extracorporeal immunoadsorption in lung transplant recipients sensitized to HLA antigens. Hum Immunol. 2005;66:378–386. doi: 10.1016/j.humimm.2005.01.025. [DOI] [PubMed] [Google Scholar]
- 43.Tweezer-Zaks N, Zandman-Goddard G. Lidar M. Har-Zahav Y. Livneh A. Langevitz P. A long-term follow-up after cardiac transplantation in a lupus patient. Ann N Y Acad Sci. 2007;1110:539–543. doi: 10.1196/annals.1423.057. [DOI] [PubMed] [Google Scholar]
- 44.Strüber M, Warnecke G, Hafer C, Goudeva L, Fegbeutel C, Fischer S, Gottlieb J, Avsar M, Simon AR, Haverich A. Intentional ABO-incompatible lung transplantation. Am J Transplant. 2008;8:2476–2478. doi: 10.1111/j.1600-6143.2008.02405.x. [DOI] [PubMed] [Google Scholar]