Abstract
Morphological and immunohistochemical examinations were carried out on the pancreas of a hyperglycemic 5-year-old male cynomolgus monkey. Body weight gradually decreased from 6 months before termination, accompanying a slight reduction in food consumption and anorexia for the last 2 days. The blood glucose level was markedly elevated when examined at termination. Histopathologically, in the exocrine pancreas, diffuse hyperplasia of centroacinar and intercalated duct cells and diffuse atrophy of acinar cells with sporadic apoptosis were observed, although most centroacinar and intercalated duct cells were proliferating cell nuclear antigen (PCNA)-positive in both the present case and age-matched control animals. In the endocrine pancreas, the islets tended to be hypertrophic, with an increase in insulin-positive cells in comparison with the age-matched control animals. PCNA-positive cells also tended to increase in the islets, although positive cells for phospho-histone H3, a marker for mitotic cells, were not detected in the endocrine and exocrine pancreas. Moreover, neither inflammation nor amyloidosis was noted in the islets. In conclusion, the present case probably suffered from early-stage type 2 diabetes mellitus, and it provides fundamental information concerning pancreatic histopathology under insulin-related derangement in monkeys.
Keywords: pontaneous diabetes mellitus, pancreas, β-cell hyperplasia, centroacinar and intercalated duct cell hyperplasia, cynomolgus monkey
Diabetes mellitus (DM) is characterized by persistent hyperglycemia due to defects in insulin production, secretion or action and is roughly divided into type 1 and type 2 DM. Type 1 DM is primarily brought about by destruction of β-cells due to a polygenic autoimmune response1, resulting in a decrease in the number and size of islets. Type 1 DM is likely to occur at a young age, while type 2 DM, which is caused by insulin resistance in target tissues, commonly develops at an adult age. In the early stage of type 2 DM, proliferation of β-cells is one of the characteristic findings, and this seems to be a compensatory response to hyperglycemia in order to maintain euglycemia. Such proliferation of β-cells leads not only to islet hypertrophy but also to amyloid deposition, since β-cells can produce an amyloid peptide, amylin2,3,4,5. However, the cellularity decreases along with abundant amyloid deposition in the islets of advanced type 2 DM. In a survey of nonhuman primates with DM6, all animals examined had type 2 DM with amyloidosis in the islets. The clinicopathologic characterization of spontaneous DM in vervet monkeys was well documented by Cann et al.7. In cynomolgus monkeys, the natural occurrence of type 2 DM is higher than that of type 1 DM8. The present paper describes the histopathological and immunohistochemical features of the pancreas in a young cynomolgus monkey that probably suffered from early-stage type 2 DM.
The animal was a 5-year-old male cynomolgus monkey and was a spare animal for toxicological studies (Hainan Jingang Laboratory Animal Co., Ltd., Hainan Province, China). Behavioral and clinical tests had not been done on the animal except for measurement of body weight and food consumption at a several time points before termination. This animal was housed alone in a stainless steel cage (W730 × D720 × H800 mm) in an animal room maintained under controlled conditions (temperature, 21 ± 5°C; relative humidity, 55 ± 15%; air ventilation 8 to 10 times per hour; artificial lighting, 12-hour light/12-hour dark cycle), was supplied 150 g of pellet diet for monkeys (carbohydrate, protein and fat concentration: 52, 23 and 8%, SLACOM® SLAC-MK01, SLAC Laboratory Animal Co., Ltd., Shanghai, China) in the afternoon and also 50 g of fruits or vegetable in the morning and was allowed free access to tap water. The animal was cared for according to the principles outlined in the Regulations for the Administration of Affairs Concerning Experimental Animals, Decree No.2, approved by the State Council of the People’s Republic of China, 1988 and the Regulations for the Administration of Affairs Concerning Experimental Animals Approved by the Zhejiang Provincial Government in 2009.
The animal showed no distinct abnormal clinical signs, except for a gradual decrease in body weight, from 4.9 kg at 6 months before sacrifice in moribund condition to 4.0 kg at termination (5.75 ± 1.11 kg with a range of 3.6 to 7.7 kg in our background data), and a slight loss of appetite and anorexia for the last 2 days. No data suggestive of obesity were recorded prior to development of the disease. Clinicopathological examinations done at termination revealed that the blood glucose level had markedly elevated to 565.5 mg/dL (82 ± 18 mg/dL with a range of 23 to 208 mg/dL in our background data), while the serum levels of triglycerides and total cholesterol were nearly within normal values (35 mg/dL and 168 mg/dL, respectively) (30 ± 17 mg/dL and 117 ± 26 mg/dL in our background data, respectively). No abnormal macroscopic changes were noted. After a complete necropsy, all tissues were preserved in 10% neutral-buffered formalin and then embedded in paraffin. As for the pancreas, the tissue was obtained from its tail part. Thin sections from all tissues were stained with hematoxylin and eosin (HE). To assess the islet size and number, morphometric analysis was conducted using a Luzex AP image processor (Nireko Corporation, Tokyo, Japan). In addition, additional sections of the pancreas were also subjected to Congo red staining for amyloid detection under polarized lens and immunohistochemistry for insulin (monoclonal, Z006, Nichirei Biosciences, Tokyo, Japan), proliferating cell nuclear antigen (PCNA) (monoclonal, PC10, 1:5000, DakoCytomation, Glostrup, Denmark), phospho-histone H3 (polyclonal, 1:150, Cell Signaling Technology, Beverly, MA, USA), a marker for cell division, and cleaved caspase-3 (CAS3) (polyclonal, 1:200, Cell Signaling Technology), a marker for apoptosis, using an Envision+ kit (DAKO Japan, Tokyo, Japan). For electron microscopic examination, small pieces of the formalin-fixed pancreatic tissues were refixed with 0.5% glutaraldehyde and 1.5% paraformaldehyde, postfixed with 1% osmium tetraoxide and embedded in epoxy resin (OkenShoji Co., Ltd., Tokyo, Japan). Ultrathin sections were stained with uranyl acetate and lead citrate and were examined under a transmission electron microscope. For the sake of comparison to the present case, the pancreases from 3 age-matched normal male cynomolgus monkeys were also examined in the same way as control animals.
Histopathologically, in addition to the pancreatic changes mentioned below, focal pneumonia, vacuolation of renal tubule epithelial cells, adrenocortical hypertrophy, lymphoid depletion in various lymphoid tissues and gastritis were observed minimally or mildly. These changes were thought to be secondary to the animal’s poor general condition and/or anorexia for 2 days before termination.
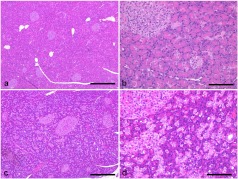
In the exocrine pancreas, diffuse proliferation of centroacinar and intercalated duct cells and diffuse atrophy of acinar cells (Figs. 1a–1d) with sporadic pyknotic and CAS3-positive acinar cells were noted (Fig. 2g), although most centroacinar and intercalated duct cells were PCNA positive in both the present case and the comparative animals (Figs. 2e and 2f).
Fig. 1.
Histological appearance of the pancreas. Normal pancreas from the comparative animals (a, b). Diffuse hyperplasia of the centroacinar and intercalated duct cells and diffuse atrophy of acinar cells in the present case (c, d). HE stain. Bar = 500 μm (a, c), 100 μm (b, d).
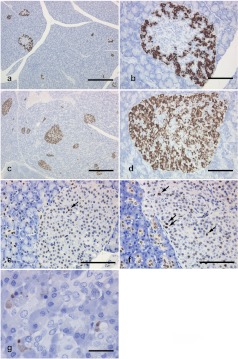
Fig. 2.
Immunohistochemistry of the pancreas. The number of insulin-positive cells in the present case (c, d) is greater than that in the comparative animals (a, b). In PCNA immunohistochemistry, the number of positive cells (arrows) in the islets from the present case (f) tends to increase in comparison with that in the comparative animals (e), whereas most centroacinar and intercalated duct cells are PCNA-positive in both the present case (f) and the comparative animals (e). In CAS3 immunohistochemistry, positive cells are sporadically seen in the exocrine pancreas from the present case (g). Bar = 500 μm (a, c), 100 μm (b, d, e, f) 25 μm (g).
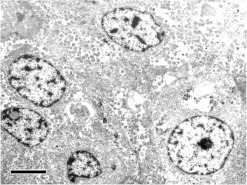
In the endocrine pancreas, the islets tended to be enlarged in comparison with the control animals, although there was no distinct difference in the number of islets (Figs. 1a and 1c, Table 1). Immunohistochemically, compared with control animals, the number of insulin-positive cells increased apparently in the central area of islets (Figs. 2a–2d), where glucagon-positive cells are usually the predominant component cells in normal monkeys. In addition, the number of PCNA-positive cells was slightly increased mainly in the peripheral area of islets as compared with the control animals. At least a part of such PCNA-positive cells may be β-cells. On the other hand, no phospho-histone H3-positive cells were detected in the endocrine and exocrine pancreas of any animals. In the islets, neither amyloid deposition nor inflammatory cell infiltration was noted in the islets, and there were no abnormal ultrastructural findings detected in the component cells (Fig. 3).
Table 1. Morphometric Analysis of Isletsa) in the Present Case and the Age-matched Control Animals.
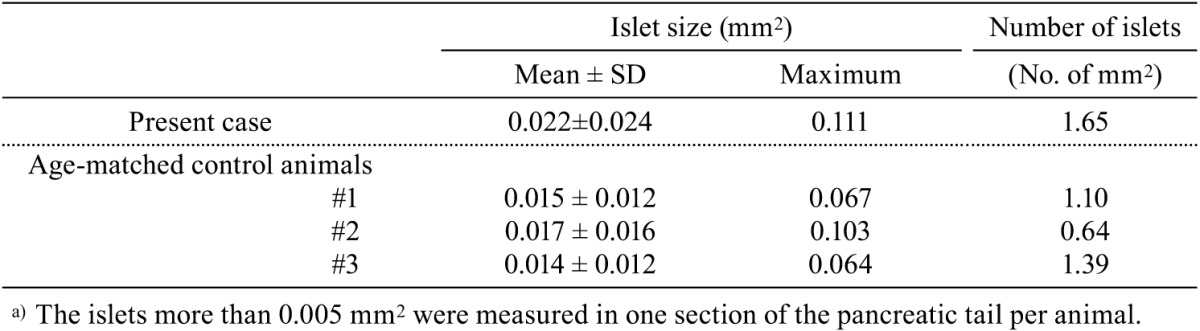
Fig. 3.
Ultrastructure of islets from the present case. No distinct abnormal changes are seen in the component cells. Bar = 5 μm.
Previous investigators demonstrated the differences in pancreatic histology and immunohistochemistry between type 1 DM and type 2 DM in monkeys4,8. In type 2 DM, the islets are hypertrophic and hyperplastic with intense immunoreactivity to insulin and deposition of minimal to moderate amounts of amyloid at the early stage, whereas at the advanced stage, the intensity of insulin immunoreactivity reduces and the cellularity in the islet decreases due to abundant amyloid deposition. On the other hand, in type 1 DM, β-cells are primarily damaged, resulting in a decrease in the number and size of islets with very little insulin immunostainability and no amyloid deposition in the islets. The above-mentioned histological and immunohistochemical observations in the present case were comparable to those of early-stage type 2 DM, except for the lack of amyloid deposition in the present case. In addition to such histological and immunohistochemical similarities, marked hyperglycemia and absence of DM-related complications further supported that the present case might be early-stage type 2 DM. On the other hand, the continuous decrease in body weight observed in the present case is not a general finding of early-stage type 2 DM and may not be related to the diabetic condition. Judging from the above-mentioned histological, ultrastructural and immunohistochemical findings of β-cells, the hyperglycemia observed in the present case might be related to a defect in insulin action or to insulin resistance in target tissues, not to a defect in insulin production, although blood insulin concentrations were not measured in the present study.
It is well known that islet cells have a potential to proliferate and regenerate themselves. Experimentally, proliferation of β-cells has been reported following such treatments as cellophane wrapping of the pancreas9, partial pancreatectomy10,11, ductal ligature12 and streptozotocin injection13. Kim et al.14 demonstrated that insulin-positive cells appeared in the acini and intercalated ducts in streptozotocin-induced diabetes in rats. Similarly, proliferation of ductuloendocrine cells was demonstrated in young dogs with spontaneous DM15. β-cell neogenesis is a matter of concern, especially in regeneration medicine for DM. To date, intra-islet precursor cells16, acinar and ductal cells17,18,19,20,21, stem cells in the ducts22,23 and transdifferentiated acinar and/or duct cells24,25,26,27,28,29,30 have been suggested as possible precursors of islets. In the present case, although there was no direct evidence of islet neogenesis, it was suggested that the preexisting β-cells in the islets might slowly proliferate.
As mentioned above, diffuse proliferation of centroacinar and intercalated duct cells was one of the discriminative findings in the present case. Pour31 demonstrated a highly proliferative activity in centroacinar cells in insular regeneration and speculated that the centroacinar cells and intercalated duct cells may have potential as stem cells. In the present case, diffuse proliferation of centroacinar and intercalated duct cells could be a slowly developing reactive change in response to exocrine and/or endocrine derangement that was probably initiated a long time ago, since no positive cells for phospho-histone H3, a marker for cell division, were detected. On the other hand, diffuse atrophy with sporadic appearance of apoptosis in acinar cells may be related to deterioration and/or anorexia, since it was associated with no degenerative changes.
In conclusion, the present case was considered to be young-onset type 2 DM in a monkey with diffuse hyperplasia of centroacinar and intercalated duct cells, which has not been described in the previous reports of DM. Further studies are needed to clarify the meaning of the hyperplastic changes observed in the present case. DM is one of the highly prevalent human diseases, and antidiabetic drugs are now being actively developed. The present report may contribute to assessment of histopathological changes of the pancreas under insulin or blood glucose derangement induced by chemical treatments.
Acknowledgments
The authors would like to thank Dr. Kunio Doi, Professor Emeritus of the University of Tokyo, for critical review of the manuscript and Mr. Pete Aughton, D.A.B.T., ITR Laboratories Canada Inc., for language editing of this paper. The expert technical assistance for electron microscopic examination of Dr. Rie Andoh is kindly appreciated.
References
- 1.Pinkse GG, Tysma OH, Bergen CA, Kester MG, Ossendrop F, van Veelen PA, Keymeulen B, Pipeleers D, Drijfhout JW, Roep BO. Autoreactive CD8 T cells associated with beta cell destruction in type 1 diabetes. Proc Natl Acad Sci USA. 102: 18425–18430 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson KH, O’Brien TD, Hayden DH, Jordan K, Ghobrial HKG, Mahoney WC, Westermark P. Immunolocalization of islet amyloid polypeptide (IAPP) in pancreatic beta cells by means of peroxidase-antiperoxidase (PAP) and protein A-gold techniques. Am J Pathol. 130: 1–8 1988. [PMC free article] [PubMed] [Google Scholar]
- 3.Butler PC, Chou J, Carter WB, Wang Y, Bu B, Chang D, Chang J, Rizza RA. Effects of meal ingestion on plasma amylin concentration in NIDDM and nondiabetic humans. Diabetes. 39: 752–756 1990. [DOI] [PubMed] [Google Scholar]
- 4.O’Brien TD, Wagner JD, Litwak KN, Carlson CS, Cefalu WT, Jordan K, Johnson KH, Bulter PC. Islet amyloid and islet amyloid polypeptide in Cynomolgus Macaques (Macaca fascicularis): An animal model of human non-insulin-dependent diabetes mellitus. Vet Pathol. 33: 479–485 1996. [DOI] [PubMed] [Google Scholar]
- 5.Wagner JD, Carlson CS, O’Brien TD. Diabetes mellitus and islet amyloidosis in cynomolgus monkey. Lab Anim Sci. 46: 36–41 1996. [PubMed] [Google Scholar]
- 6.Palotay JL, Howerd CF., JrInsular amyloidosis in spontaneously diabetic nonhuman primates. Vet Pathol. 19(Supple): 181–192 1982. [PubMed] [Google Scholar]
- 7.Cann JA, Kavanagh K, Jorgensen MJ, Mohanan S, Howard TD, Gray SB, Hawkins GA, Fairbanks LA, Wagner JD. Clinicopathologic characterization of naturally occurring diabetes mellitus in verbet monkeys. Vet Pathol. 47: 713–718 2010. [DOI] [PubMed] [Google Scholar]
- 8.Wagner JD, Cline JM, Shadoan MK, Bullock BC, Rankin SE, Cefalu WT. Naturally occurring and experimental diabetes in cynomolgus monkeys: a comparison of carbohydrate and lipid metabolism and islet pathology. Toxicol Pathol. 29: 142–148 2001. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg L, Brown RA, Duguid WP. A new approach to the induction of duct epithelial hyperplasia and nesidioblastosis by cellophane wrapping of the hamster pancreas. J Surg Res. 35: 63–72 1983. [DOI] [PubMed] [Google Scholar]
- 10.Brockenbrough JS, Weir GC, Bonner-Weir S. Discordance of exocrine and endocrine growth aftr 90% pancreatectomy in rats. Diabetes. 37: 232–236 1988. [DOI] [PubMed] [Google Scholar]
- 11.Bonner-Weir S, Baxter LA, Schuppin GT, Smith FE. A second pathway for regeneration of adult exocrine and endocrine pancreas. A possible recapitulation of embryonic development. Diabetes. 42: 1715–1720 1993. [DOI] [PubMed] [Google Scholar]
- 12.Wang RN, Kliöppel G, Bouwens L. Duct- to islet-cell differentiation and islet growth in the pancreas of duct-ligated adult rats. Diabetologia. 38: 1405–1411 1995. [DOI] [PubMed] [Google Scholar]
- 13.Fernandes A, King LC, Guz Y, Stein R, Wright CV, Teitelman G. Differentiation of new insulin-producing cells is induced by injury in adult pancreatic islets. Endocrinology. 138: 1750–1762 1997. [DOI] [PubMed] [Google Scholar]
- 14.Kim JN, Chang IY, Kim HI, Yoon SP. Long-term effects of chitosan oligosaccharide in streptozotocin-induced diabetic rats. Islets. 1: 111–116 2009. [DOI] [PubMed] [Google Scholar]
- 15.Minkus G, Breuer W, Arun S, Kirsch M, Müller D, Mueller J, Hermanns W. Ductuloendocrine cell proliferation in the pancreas of two young dogs with diabetes mellitus. Vet Pathol. 34: 164–167 1997. [DOI] [PubMed] [Google Scholar]
- 16.Banerjee M, Bhonde RR. Islet generation from intra islet precursor cells of diabetic pancreas: In vitro studies depicting in vivo differentiation. JOP. 4: 137–145 2003. [PubMed] [Google Scholar]
- 17.Bensley RR. Studies on the pancreas of the guinea pig. Am J Anat. 12: 297–388 1911. [Google Scholar]
- 18.Sétáló G. Electron microscopic investigation of acinoinsular transformation in the rat. Acta Biol Acad Sci Hung. 18: 323–333 1967. [PubMed] [Google Scholar]
- 19.Sétáló G. Light microscopic demonstration of acino-insular transformation. Acta Morphol Acad Sci Hung. 18: 359–367 1970. [PubMed] [Google Scholar]
- 20.Leduc EH, Jones EE. Acinar-islet cell transformation in mouse pancreas. J Ultrastruct Res. 24: 165–169 1968. [DOI] [PubMed] [Google Scholar]
- 21.Granger A, Kushner JA. Cellular origins of beta-cell regeneration: a legacy view of historical controversies. J Intern Med. 266: 325–338 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonner-Weir S. Perspective: Postnatal pancreatic beta cell growth. Endocrinology. 141: 1926–1929 2000. [DOI] [PubMed] [Google Scholar]
- 23.Hayashi KY, Tamaki H, Handa K, Takahashi T, Kakita A, Yamashina S. Differentiation and proliferation of endocrine cells in the regenerating rat pancreas after 90% pancreatectomy. Arch Histol Cytol. 66: 163–174 2003. [DOI] [PubMed] [Google Scholar]
- 24.Mashima H, Ohnishi H, Wakabayashi K, Mine T, Miyagawa JI, Hanafusa T, Seno M, Yamada H, Kojima I. Betacellulin and activin A coordinately convert amylase-secreting pancreatic AR42J cells into insulin-secreting cells. J Clin Invest. 97: 1647–1654 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouwens L. Trans differentiation versus stem cell hypothesis for the regeneration of islet beta-cells in the pancreas. Microsc Res Tech. 43: 332–336 1998. [DOI] [PubMed] [Google Scholar]
- 26.Zhou J, Wang X, Pineyro MA, Egan JM. Glucagon-like peptide 1 and extendin-4 convert pancreatic AR42J cells into glucagon- and insulin-producing cells. Diabetes. 48: 2358–2366 1999. [DOI] [PubMed] [Google Scholar]
- 27.Lardon J, Huyens N, Rooman I, Bouwens I. Exocrine cell transdifferentiation in dexamethasone-treated rat pancreas. Virchows Arch. 444: 61–65 2004. [DOI] [PubMed] [Google Scholar]
- 28.Baeyens L, De Breuck S, Lardon J, Mfopou JK, Rooman I, Bouwens L. In vitro generation of insulin-producing cells from adult exocrine pancreatic cells. Diabetologia. 48: 49–57 2005. [DOI] [PubMed] [Google Scholar]
- 29.Lipsett MA, Castellarin ML, Rosenberg I. Acinar plasticity; development of a novel in vitro model to study human acinar-to-duct-to-islet differentiation. Pancreas. 34: 452–457 2007. [DOI] [PubMed] [Google Scholar]
- 30.Fanjul M, Alvarez L, Salvador C, Gymr V, Kerr-Conte J, Pattou F, Carter N, Hollande E. Evidence for a membrane carbonic anhydrase IV anchored by its C-terminal peptide in normal human pancreatic ductal cells. Histochem Cell Biol. 121: 91–99 2004. [DOI] [PubMed] [Google Scholar]
- 31.Pour PM. Pancreatic centroacinar cells. The regulator of both exocrine and endocrine function. Int J Pancreatol. 15: 51–64 1994. [PubMed] [Google Scholar]