Dear Editor,
Histone H2B lysine 120 monoubiquitination (H2BK120ub) is a key histone modification that plays critical roles in gene transcriptional regulation and higher order chromatin organization in many species1. H2BK120ub has been reported to be associated with highly expressed active genes in human cells2. In yeast, H2B lysine 123 monoubiquitination (the homolog of mammalian H2BK120ub) is a determinant of two important histone H3 methylations, H3K4 methylation and H3K79 methylation1,3. Histone H3K4me3 is a well-defined gene activation marker. Both H3K4 and H3K79 methylation significantly affect chromatin structure and gene transcription. In a recent study, the presence of H2B monoubiquitination was also found to stimulate H3K4me3 during gene transcription in mammals4, which further supports a conserved role for H2B monoubiquitination in transcription.
RAD6 is a well-characterized E2 ubiquitin-conjugating enzyme for H2B ubiquitination, and BRE1 (RNF20) is its corresponding E3 ligase4,5. In our recent studies, we found that Rad6 also targets the degradation of p53 in both Drosophila and mammals6,7. The molecular regulation of RAD6/BRE1 and H2BK120ub has been thoroughly investigated5,8. However, the biological functions of H2BK120ub and its related enzymes in stem cell differentiation are poorly understood.
Embryonic stem cells (ESCs) have two important characteristics: self-renewal and pluripotency9. An increasing number of studies indicate that epigenetic regulation plays a key role in the control of these two stem cell properties10. The balance between histone H3K4 and H3K9 methylation is believed to be critical in determining stem cell differentiation or reprogramming11. Several other histone modifications, such as H3K27 methylation, also play important roles in stem cell differentiation11. However, are there other histone modifications that regulate stem cell differentiation? Here, we report for the first time that H2B lysine 120 monoubiquitination (H2BK120ub) is required for embryonic stem cell differentiation.
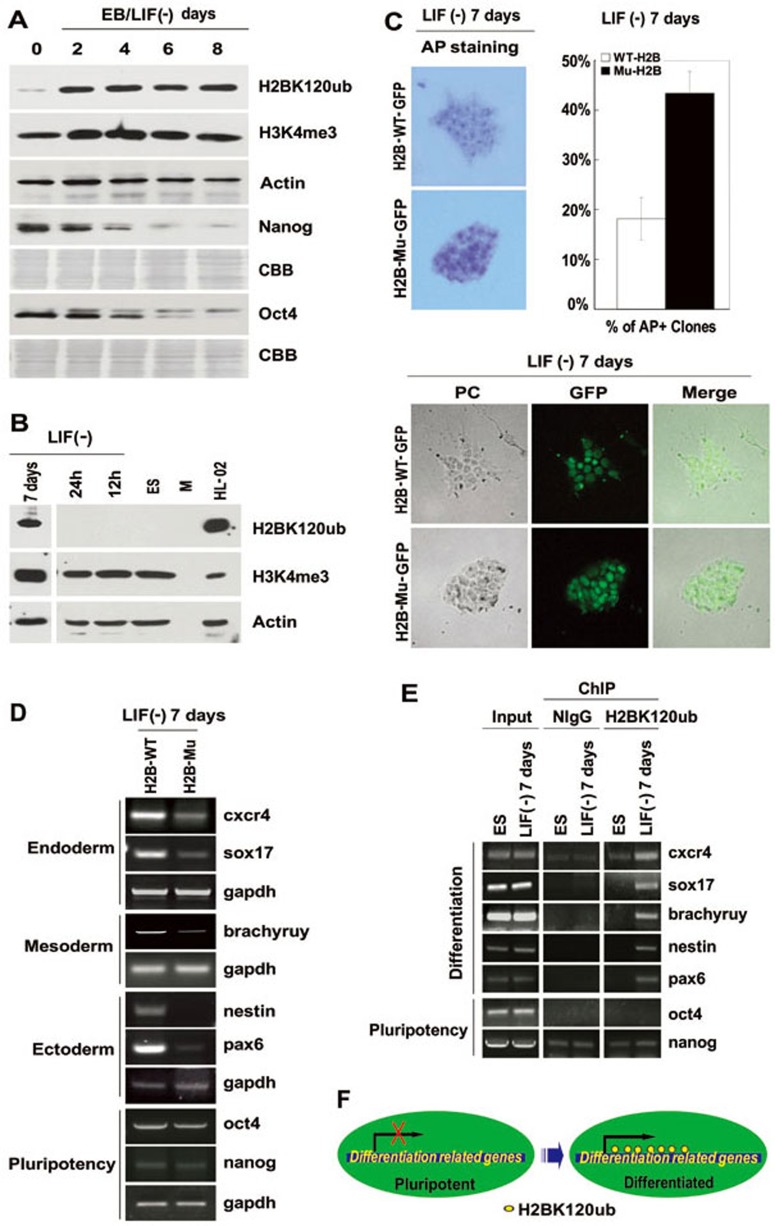
Known to be associated with the transcription of Nanog, p53 is a critical regulator of stem cell self-renewal and reprogramming12. The fact that RAD6 regulates both H2BK120ub and the degradation of p536,7 raised the question of whether RAD6 also plays a role in stem cell differentiation. To test this possibility, we first performed western blot analysis to detect changes in the expression of RAD6 and several histone modifiers, including H2BK120ub. Surprisingly, we found that H2BK120ub increases substantially in the early stages of embryoid body (EB) and ESC differentiation (Figure 1A), although no or very weak RAD6 signal was detected at the same stage (Supplementary information, Figure S1 and data not shown). As shown in Figure 1A and 1B, there was no or very weak H2BK120ub signal in the lysates of stem cells and early differentiated ESCs, as detected by H2BK120ub-specific antibodies (data not shown, EB 0 day in Figure 1A and ES in Figure 1B). However, a dramatic increase in H2BK120ub can be detected 2 days after EB differentiation (LIF withdrawal) (LIF (–); Figure 1A) and 7 days after ESC differentiation (LIF (–); Figure 1B). H2BK120ub was stably maintained during EB differentiation (compare day 2 to 8 in LIF (–); Figure 1A), and the increase in H2BK120ub seemed to occur much earlier than the decreases in Oct4 and Nanog, indicating that the presence of H2BK120ub may be an earlier event in the initiation of stem cell differentiation. We also tested changes in H3K4me3 levels in both differentiated EBs and ESCs, and found that H3K4me3 increased one- to twofold after 2 days of EB differentiation, although there was already a relatively high level of H3K4me3 in ESCs (EB 0 in Figure 1A and ES in Figure 1B).
Figure 1.
H2BK120ub determines mouse embryonic stem cell differentiation. (A) The level of H2BK120ub increases significantly after embryoid body (EB) differentiation. EBs were prepared and differentiated for the indicated number of days in medium without LIF (LIF(–)). EBs were lysed and western blots were performed using the indicated antibodies. CBB indicates Coomassie Brilliant Blue staining. (B) H2BK120ub was undetectable in ES cells, but a striking increase was observed after ESC differentiation. ESCs were cultured without LIF (LIF(–)) for varying times and lysed for western blot assays with the indicated antibodies. H3K4 trimethylation (H3K4me3) showed no obvious changes during the first 24 h of differentiation. M indicates marker lane; HL-02 was used as a positive control for antibody activity. (C) H2BK120 mutation inhibits the efficiency of ESC differentiation. AP staining of H2B-WT and H2B-Mu mutant ESCs was performed after ESCs were cultured without LIF (LIF(–)) for 7 days (upper panel). The percentage of AP stained clones is shown on the bar diagram. The morphology of H2B-WT and H2B-Mu mutant ESCs after being cultured without LIF (LIF(–)) for 7 days is shown (lower panel). (D) The H2BK120R mutant inhibits the transcription of differentiation-related genes during ESC differentiation. RT-PCR assays were performed after H2B-WT and H2B-Mu mutant ESCs were cultured without LIF for 7 days. (E) H2BK120ub is preferentially associated with differentiation-related genes. ChIP analysis was performed using anti-H2BK120ub antibodies in H2B-WT and H2B-Mu mutant ESCs cultured without LIF for 7 days. NlgG was used as a negative control for the antibody. (F) A working model for H2BK120ub in the regulation of ESC differentiation. The presence of H2BK120ub in the regulatory elements of differentiation-related genes triggers changes in transcription and subsequent ESC differentiation.
Next, we wondered whether the increase in H2BK120ub was due to changes in the expression of BRE1 (RNF20) and RAD6A/B, the corresponding E3 ligase and E2 conjugating enzyme for catalyzing the monoubiquitination of H2B5. However, we found that no or very weak RAD6A/B signal can be detected during EB differentiation, indicating that RAD6A/B protein levels are quite low (Supplementary information, Figure S1A and data not shown), and BRE1 protein levels decreased during EB differentiation, which is in contrast to the increase observed for H2BK120ub (Supplementary information, Figure S1A). We next tested the effect of BRE1 on the regulation of H2BK120ub in ESCs. Our results showed that knockdown of BRE1 with shRNA indeed decreases the differentiation-induced H2BK120ub level (Supplementary information, Figure S2). These data showed that BRE1 (RNF20) is the corresponding E3 ligase for H2BK120ub in ESCs, although no obvious correlation at the protein level could be found between H2BK120ub and its related enzymes (RAD6 and BRE1) during ESC differentiation. Notably, a recent report showed that the maintenance of stem cells in Drosophila seems to require a deubiquitinase, Scrawny, to maintain H2B monoubiquitination at a very low level13, arguing the possibility that a H2BK120ub-specific deubiquitinase functions in the control of low levels of H2BK120ub in ESCs.
We next aimed to determine whether H2BK120ub directly participates in the regulation of ESC differentiation. Transfection of an H2BK120R mutant was reported to efficiently inhibit endogenous H2BK120ub levels in HEK-293T cells2. Therefore, we produced an H2BK120R-GFP mutant construct to test whether a reduction in H2BK120ub affects the differentiation of ESCs. H2BK120ub levels in differentiated ES cells after transfection with H2B-WT-GFP (H2B-WT) or H2BK120R-GFP (H2B-Mu) were determined. The results showed that the H2BK120R mutant construct indeed resulted in efficient downregulation of H2BK120ub in differentiated ESCs (7 days after LIF withdrawal) (Supplementary information, Figure S3). ES cells transfected with H2B-WT or H2B-Mu were further cultured under LIF withdrawal (LIF (–)) conditions for 7 days to induce differentiation followed by alkaline phosphatase (AP) staining to examine the self-renewal property of ES cells. Alkaline phosphatase is a stem cell membrane marker, and elevated expression of this enzyme is associated with undifferentiated pluripotent stem cells. All primate pluripotent stem cells, such as embryonic stem (ES), embryonic germ (EG) and embryonal carcinoma (EC) cells, show alkaline phosphatase activity, while differentiated cells have lower AP activity. The results showed that H2B-Mu ES cells have much more intense AP staining compared to that in H2B-WT ES cells (43% AP-positive colonies in H2B-Mu and 18% in H2B-WT) (Figure 1C, upper panel), indicating less cell differentiation. In addition, H2B-WT and H2B-Mu ES cell colonies have quite different morphology. H2B-Mu clones seem to have a better pluripotency morphology than H2B-WT (Figure 1C, lower panel). We also tested the effect of BRE1 (RNF20) on ESC differentiation using a similar method (AP staining). Our results showed that knockdown of BRE1 with shRNA inhibits ESC differentiation ability (37% AP-positive colonies in shRNF20 and 19% in shCont) (Supplementary information, Figure S4A), and decreases the expression of differentiation-related genes (Supplementary information, Figure S4B).
To further elucidate the role of H2BK120ub in regulating stem cell differentiation, we next performed RT-PCR using the H2B-WT and H2B-Mu strains differentiated for 7 days (LIF (–)) to detect changes in expression of marker genes associated with ESCs and differentiated ESCs. From the results shown in Figure 1D, we observe that the presence of the H2BK120R mutant inhibits the transcription of differentiation-related genes after LIF withdrawal for 7 days (differentiation status), while the mutant has little effect on the expression of pluripotency-related genes. This indicated that the presence of H2BK120ub is essential for the transcription of differentiation-related genes but not pluripotency-related genes.
Next, we performed chromatin immunoprecipitation (ChIP) analysis to determine the direct role of H2BK120ub in the transcription of the tested genes during ESC differentiation. As shown in Figure 1E, we found that H2BK120ub is preferentially enriched in the coding region of differentiation-related genes but not pluripotency-related genes after ESC differentiation. These results confirmed that the presence of H2BK120ub is associated with differentiation-related genes, and the reduction in H2BK120ub affected their transcription, triggering further alterations in ESC differentiation.
In summary, we report a novel role for H2BK120ub in ESC differentiation. We further propose that H2BK120ub may be a key histone modification in triggering ESC differentiation. Further studies are necessary to elucidate its regulatory mechanism and the enzymes responsible for this modification. Materials and Methods are described in the Supplementary information, Data S1. Primers used in this study are shown in Supplementary information, Table S1.
During the revision of our manuscript, two reports were published in Mol Cell14,15. They show similar results to our data and support our conclusion significantly.
Acknowledgments
This work was supported by the National Basic Research Program of China (973 Program) from the Ministry of Science and Technology (2011CB965300, 2009CB825603).
Footnotes
(Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary Material
(A) No correlation was observed between the protein levels of RAD6 and BRE1 (RNF20) and H2BK120ub during embryoid body (EB) differentiation.
BRE1 affects the differentiation-induced H2BK120ub level.
The H2BK120R mutant inhibits endogenous H2BK120ub levels compared with H2B-WT.
BRE1 affects ESC differentiation ability and the transcription of differentiation-related genes.
Materials and Methods
Primer sequences used in this work
References
- Sun ZW, Allis CD. Ubiquitination of histone H2B regulates H3 methylation and gene silencing in yeast. Nature. 2002;418:104–108. doi: 10.1038/nature00883. [DOI] [PubMed] [Google Scholar]
- Minsky N, Shema E, Field Y, et al. Monoubiquitinated H2B is associated with the transcribed region of highly expressed genes in human cells. Nat Cell Biol. 2008;10:483–488. doi: 10.1038/ncb1712. [DOI] [PubMed] [Google Scholar]
- Nakanishi S, Lee JS, Gardner KE, et al. Histone H2BK123 monoubiquitination is the critical determinant for H3K4 and H3K79 trimethylation by COMPASS and Dot1. J Cell Biol. 2009;186:371–377. doi: 10.1083/jcb.200906005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Guermah M, McGinty RK, et al. RAD6-Mediated transcription-coupled H2B ubiquitylation directly stimulates H3K4 methylation in human cells. Cell. 2009;137:459–471. doi: 10.1016/j.cell.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Hake SB, Roeder RG. The human homolog of yeast BRE1 functions as a transcriptional coactivator through direct activator interactions. Mol Cell. 2005;20:759–770. doi: 10.1016/j.molcel.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Chen S, Wei HM, Lv WW, Wang DL, Sun FL. E2 ligase dRad6 regulates DMP53 turnover in Drosophila. J Biol Chem. 2011;286:9020–9030. doi: 10.1074/jbc.M110.190314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Wang DL, Liu Y, Zhao L, Sun FL. RAD6 regulates the dosage of p53 by a combination of transcriptional and posttranscriptional mechanisms. Mol Cell Biol. 2012;32:576–587. doi: 10.1128/MCB.05966-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Roeder RG. Direct Bre1-Paf1 complex interactions and RING finger-independent Bre1-Rad6 interactions mediate histone H2B ubiquitylation in yeast. J Biol Chem. 2009;284:20582–20592. doi: 10.1074/jbc.M109.017442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipori D. The stem state: plasticity is essential, whereas self-renewal and hierarchy are optional. Stem Cells. 2005;23:719–726. doi: 10.1634/stemcells.2005-0030. [DOI] [PubMed] [Google Scholar]
- Orkin SH, Hochedlinger K. Chromatin connections to pluripotency and cellular reprogramming. Cell. 2011;145:835–850. doi: 10.1016/j.cell.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Shukla A, Wang X, et al. Role for Dpy-30 in ES cell-fate specification by regulation of H3K4 methylation within bivalent domains. Cell. 2011;144:513–525. doi: 10.1016/j.cell.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T, Chao C, Saito S, et al. p53 induces differentiation of mouse embryonic stem cells by suppressing Nanog expression. Nat Cell Biol. 2005;7:165–171. doi: 10.1038/ncb1211. [DOI] [PubMed] [Google Scholar]
- Buszczak M, Paterno S, Spradling AC. Drosophila stem cells share a common requirement for the histone H2B ubiquitin protease scrawny. Science. 2009;323:248–251. doi: 10.1126/science.1165678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs G, Shema E, Vesterman R, et al. RNF20 and USP44 regulate stem cell differentiation by modulating H2B monoubiquitylation. Mol Cell. 2012;46:662–673. doi: 10.1016/j.molcel.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpiuk O, Najafova Z, Kramer F, et al. The histone H2B monoubiquitination regulatory pathway is required for differentiation of multipotent stem cells. Mol Cell. 2012;46:705–713. doi: 10.1016/j.molcel.2012.05.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) No correlation was observed between the protein levels of RAD6 and BRE1 (RNF20) and H2BK120ub during embryoid body (EB) differentiation.
BRE1 affects the differentiation-induced H2BK120ub level.
The H2BK120R mutant inhibits endogenous H2BK120ub levels compared with H2B-WT.
BRE1 affects ESC differentiation ability and the transcription of differentiation-related genes.
Materials and Methods
Primer sequences used in this work