Abstract
Sarcoidosis likely results from the exposure of a genetically susceptible subject to an environmental agent, possibly an infectious one. Mycobacterial and propionibacterial organisms are the most commonly implicated potential etiologic agents. Propionibacterium acnes is the only microorganism, however, found in sarcoid lesions by bacterial culture. To evaluate the pathogenic role of this indigenous bacterium, we screened for the bacterium in sarcoid and non-sarcoid tissues using immunohistochemical methods with novel P. acnes-specific monoclonal antibodies that react with cell-membrane-bound lipoteichoic acid (PAB antibody) and ribosome-bound trigger-factor protein (TIG antibody). We examined formalin-fixed and paraffin-embedded samples of lungs and lymph nodes from 196 patients with sarcoidosis, and corresponding control samples from 275 patients with non-sarcoidosis diseases. The samples were mostly from Japanese patients, with 64 lymph node samples from German patients. Immunohistochemistry with PAB antibody revealed small round bodies within sarcoid granulomas in 20/27 (74%) video-assisted thoracic surgery lung samples, 24/50 (48%) transbronchial lung biopsy samples, 71/81 (88%) Japanese lymph node samples, and 34/38 (89%) German lymph node samples. PAB antibody did not react with non-sarcoid granulomas in any of the 45 tuberculosis samples or the 34 samples with sarcoid reaction. In nongranulomatous areas, small round bodies detected by PAB antibody were found in alveolar macrophages of lungs and paracortical macrophages of lymph nodes from many sarcoid and some non-sarcoid patients. Large-spheroidal acid-fast bodies, Hamazaki–Wesenberg bodies, which were found in 50% of sarcoid and 15% of non-sarcoid lymph node samples, reacted with both PAB and TIG antibodies. Electron microscopy revealed that these Hamazaki–Wesenberg bodies had a single bacterial structure and lacked a cell wall with occasional protrusions from the body. The high frequency and specificity of P. acnes, detected by PAB antibody within sarcoid granulomas, indicates that this indigenous bacterium might be the cause of granuloma formation in many sarcoid patients.
Keywords: epithelioid cell granuloma, Hamazaki–Wesenberg body, lipoteichoic acid, trigger-factor protein
Sarcoidosis is a granulomatous disorder of unknown etiology that affects multiple organs. Sarcoidosis seems to result from the exposure of a genetically susceptible subject to an environmental agent, and microbial etiologies of sarcoidosis have long been considered based on the clinical similarities to infectious granulomatous diseases.1 Several epidemiological mechanisms may underlie the association of an infective agent or agents with the etiology of sarcoidosis, including spatial, seasonal, and occupational clustering.2 Data from the completed ACCESS (A Case Control Etiologic Study of Sarcoidosis) study support possible disease associations with selected microbially-rich environments.3
Mycobacterial and propionibacterial organisms are most commonly implicated as potential etiologic agents based on studies using polymerase chain reaction (PCR) methods that report the detection of microbial DNA from these organisms in tissues from sarcoid patients around the world.4, 5, 6 The results from different studies have varied considerably, however, with reports of microbial DNA in 0–80% of sarcoidosis tissues as well as in 0% to more than 30% of control tissues.7, 8 The failure to detect microbial DNA from these organisms in samples from some sarcoid patients suggests other causes of sarcoidosis in those patients, whereas the detection of microbial DNA in some control samples suggests latent infection of the bacterium.
Immune responses against microbial antigens from these organisms, such as ESAT-6 and KatG peptides from Mycobacterium tuberculosis and a recombinant trigger-factor protein from P.. acnes, have been examined in sarcoid patients and control subjects.9, 10 Although immune responses are frequently detected in sarcoid patients, such immune responses are also sometimes detected in non-sarcoid patients or healthy subjects. Latent infection of these organisms has complicated interpretation of the results from these immunological studies. Unless microbial antigens that cause a specific immune response found only in sarcoid patients can be used to stimulate an immune response, immunological approaches will not be sufficient to unequivocally confirm that these organisms are causative.
Granulomas usually form as a result of the persistence of a nondegradable product or as the result of hypersensitivity responses.11 An overlap of the two mechanisms occurs in most infectious diseases because microorganisms can serve both as foreign bodies and as antigens for immunological responses. Normally, granulomas are the result of protective mechanisms by which the invading agent is sequestered and further degraded. Given that an epithelioid cell granuloma is the pathological hallmark of sarcoidosis, any etiologic agent of sarcoidosis must be present or have been present within sarcoid granulomas. Therefore, histopathological studies to demonstrate mycobacterial or propionibacterial organisms or antigens within sarcoid granulomas are essential to prove an etiologic link between sarcoidosis and these organisms.
To date, P. acnes is the only microorganism that has been isolated from sarcoid lesions by bacterial culture.12 This indigenous bacterium was isolated from 78% of biopsied lymph nodes of Japanese patients with sarcoidosis.13 In the present study, we used immunohistochemical approaches to test the hypothesis that P. acnes is an etiologic agent of sarcoidosis. To locate P. acnes in routine histological sections, we developed P. acnes-specific monoclonal antibodies that react with this bacterium in formalin-fixed and paraffin-embedded tissue sections by immunizing mice with P. acnes or a recombinant trigger-factor protein from the bacterium. To elucidate an etiologic link between sarcoidosis and P. acnes, we examined the frequency of P. acnes found within sarcoid granulomas of the lungs and lymph nodes by immunohistochemistry with the novel antibodies. We also examined corresponding control samples from patients with diseases other than sarcoidosis to search for possible latent infection by the bacterium.
Materials and methods
Samples
We examined formalin-fixed and paraffin-embedded tissue sections of biopsy and surgical samples of the lungs (77 samples) and lymph nodes (119 samples) from 196 patients with sarcoidosis, together with corresponding control samples (110 lung and 165 lymph node samples) from 275 patients with diseases other than sarcoidosis. These tissue sections were obtained from the archives of the Tokyo Medical and Dental University, Japan Red Cross Medical Center, and Ruhrlandklinik in Essen, Germany. The diagnosis of sarcoidosis was based on the clinical picture, no evidence of current infection by M. tuberculosis or other organisms known to produce granulomatous diseases, and the presence of noncaseating granulomas in biopsy or surgical specimens of involved tissues, according to the statement published in 1999.1 Lung and lymph node samples from patients with tuberculosis or sarcoid reaction were used as non-sarcoid granulomatous lesions owing to the abundance of samples available for the study. Diagnosis of tuberculosis was based on the clinical picture, successful identification of M. tuberculosis by culture or Ziehl–Neelsen stain, and the presence of caseating epithelioid cell granulomas in the involved tissues. Patients with sarcoid reaction were defined as individuals without any symptoms or signs of systemic sarcoidosis who showed noncaseating epithelioid cell granulomas in lymph nodes draining a region housing a malignant tumor (lung, gastric, or colorectal cancer) or in the primary tumor itself.14 Lung samples from patients with idiopathic interstitial pneumonia or chronic hypersensitivity pneumonitis and lymph node samples from patients with reactive or necrotizing lymphadenitis were used as non-sarcoid inflammatory lesion samples. Cancer-free lung tissues from patients with lung cancer and cancer-free regional lymph nodes from patients with lung, gastric, or colorectal cancer were used as normal control tissues. The diagnosis of disease was established in each hospital and histologically reconfirmed by some of the authors before the study. This study was approved by the ethics committees of the three institutes from which the samples were obtained.
Production of Monoclonal Antibodies
Novel monoclonal antibodies were developed to locate P. acnes in formalin-fixed and paraffin-embedded tissue sections. Antibodies were generated according to the protocol described in a laboratory manual15 with modifications. BALB/c mice (CLEA Japan, Tokyo, Japan) were immunized with sonicated whole bacterial lysate of P. acnes (ATCC 11828) or a recombinant trigger-factor protein from P. acnes that was constructed using the full sequence of the gene.10, 16 Hybridoma cell lines producing anti-P. acnes antibodies or anti-trigger-factor protein antibodies were checked by enzyme-linked immunosorbent assay with the bacterial antigens or the trigger-factor protein used as immunogens. Hybridoma cell lines with positive results were screened by immunohistochemistry with formalin-fixed and paraffin-embedded tissue sections of P. acnes-injected rat liver. P. acnes-injected rat liver was obtained by intravenous injection of 30 mg of heat-killed P. acnes into female Sprague–Dawley rats (CLEA Japan) 1 h before killing the rat. Similarly-prepared liver tissue sections of rats injected by each strain of other control bacteria were also examined to confirm the specificity to P. acnes. Hybridoma cell lines producing the antibody that generated a strong reaction specific to P. acnes on rat liver sections were selected and further screened by immunohistochemistry with formalin-fixed and paraffin-embedded tissue sections of sarcoid lymph nodes in which a large number of P. acnes genomes were detected in a previous study.17 The hybridoma producing the antibody that generated the most specific reaction product on human tissue sections was selected and cloned by two rounds of limiting dilution. A single hybridoma clone was then implanted into the intraperitoneal space of severe combined immunodeficiency mice (CLEA Japan). At 1 or 2 weeks after implantation, ascites was collected and used as an undiluted antibody without further purification. The antibody obtained by immunization of the bacterial lysate was named PAB antibody (IgM, κ) and the antibody obtained by immunization of the recombinant trigger-factor protein was named TIG antibody (IgG, λ) in the present study.
Immunohistochemistry
Histological sections (4 μm-thick) were cut from formalin-fixed and paraffin-embedded tissue samples and mounted on silane-coated slides (Muto Pure Chemicals, Tokyo, Japan). After the sections were de-paraffinized and rehydrated, they were microwaved (Microwave Processor H2850; Energy Beam Sciences, East Granby, CT, USA) in 10 mmol/l citrate buffer (pH 6.0) for 40 min at 97 °C. The sections were then treated with 3% hydrogen peroxide in methanol for 10 min. The sections were first incubated with normal horse serum (Vectastain Universal Elite ABC Kit; Vector Laboratories, Burlingame, CA, USA) and then incubated overnight at room temperature with one of the appropriately diluted antibodies (PAB antibody 1:15 000 and TIG antibody 1:8000) in a humidified chamber. The sections were then incubated for 30 min with biotinylated secondary antibody, followed by 30-min incubation with streptavidin–peroxidase complex (Vectastain Universal Elite ABC Kit), both at room temperature. Before and after each step, the sections were washed in phosphate-buffered saline containing 0.5% Tween-20. The signal was developed as a brown reaction product using peroxidase substrate diaminobenzidine (Histofine Simplestain DAB Solution; Nichirei Bioscience, Tokyo, Japan). All specimens were counterstained with Mayer's hematoxylin. Adjacent histological sections were also examined with hematoxylin and eosin staining as well as Fite staining for acid-fastness.18
Immunofluorescence Double-Staining
According to the methods described previously,19 formalin-fixed and paraffin-embedded tissue sections of sarcoid lymph nodes with many P. acnes-positive cells were used for immunofluorescence double-staining to phenotype the cells with intracellular P. acnes using the antibodies to phagocytes, anti-human CD68 monoclonal antibody (clone KP1; DAKO) for macrophages, and anti-human fascin monoclonal antibody (clone 55K-2; DAKO) for dendritic cells.
Immunoelectron Microscopy
Formalin-fixed paraffin-embedded tissue sections of sarcoid lymph nodes with many P. acnes-positive cells were used for immunoelectron microscopy with PAB and TIG antibodies according to the previously described methods.19
Western Blot
The specificity of PAB and TIG antibodies was examined by western blot with ATCC strains and clinical isolates of P. acnes, other cutaneous propionibacteria (P. granulosum, P. avidum, P. lymphophilum, and P. propionicum), dairy propionibacteria (P. freudenreichii, P. jensenii, P. thoenii, and P. acidipropionici), and other control bacteria, including mycobacteria (Table 1). Three strains of serotype I P. acnes (ATCC 6919, ATCC 11827, and clinical isolate S4) and two strains of serotype II P. acnes (ATCC 11828 and clinical isolate S7) were used in the study, based on the results of a previous serotyping analysis.20 Lipoteichoic acid was purified from P. acnes, according to the previously described method21 with modifications. Western blot was performed according to the previously described methods.19
Table 1. Specificity of the PAB and TIG antibodies examined by western blot.
| Bacteria | Strain |
Reactivity of |
|
|---|---|---|---|
| PAB antibody | TIG antibody | ||
| Propionibacterium acnes | ATCC 6919 | + | + |
| Propionibacterium acnes | ATCC 11827 | + | + |
| Propionibacterium acnes | ATCC 11828 | + | + |
| Propionibacterium acnes | Clinical isolate S4 | + | + |
| Propionibacterium acnes | Clinical isolate S7 | + | + |
| Propionibacterium granulosum | ATCC 25564 | − | − |
| Propionibacterium avidum | ATCC 25577 | − | − |
| Propionibacterium lymphophilum | ATCC 27520 | − | − |
| Propionibacterium propionicum | ATCC 14157 | − | − |
| Propionibacterium freudenreichii | ATCC 6207 | − | − |
| Propionibacterium jensenii | ATCC 4868 | − | − |
| Propionibacterium thoenii | ATCC 4874 | − | − |
| Propionibacterium acidipropionici | ATCC 25562 | − | − |
| Mycobacterium bovis | ATCC 19274 | − | − |
| Mycobacterium tuberculosis | ATCC 25177 | − | − |
| Mycobacterium avium | ATCC 25291 | − | − |
| Mycobacterium intracellulare | ATCC 13950 | − | − |
| Actinomyces pyogenes | ATCC 19411 | − | − |
| Bifidobacterium bifidum | ATCC 29521 | − | − |
| Lactobacillus acidophilus | ATCC 4356 | − | − |
| Bacteroides fragilis | ATCC 25285 | − | − |
| Fusobacterium nucleatum | ATCC 25586 | − | − |
| Listeria monocytogenes | ATCC 15313 | − | − |
| Nocardia asteroides | ATCC 19247 | − | − |
| Staphylococcus aureus | ATCC 25923 | − | − |
| Escherichia coli | ATCC 11775 | − | − |
| Pseudomonas aeruginosa | ATCC 27853 | − | − |
| Klebsiella pneumoniae | ATCC 13884 | − | − |
Statistical Analyses
The χ2 test for proportion with Yates' correction was used to evaluate differences in frequency between pairs of groups. Differences with P values of <0.05 were considered statistically significant. StatView software (version 5.0; SAS Institute, Cary, NC, USA) was used for the statistical calculations.
Results
Specificity of Monoclonal Antibodies
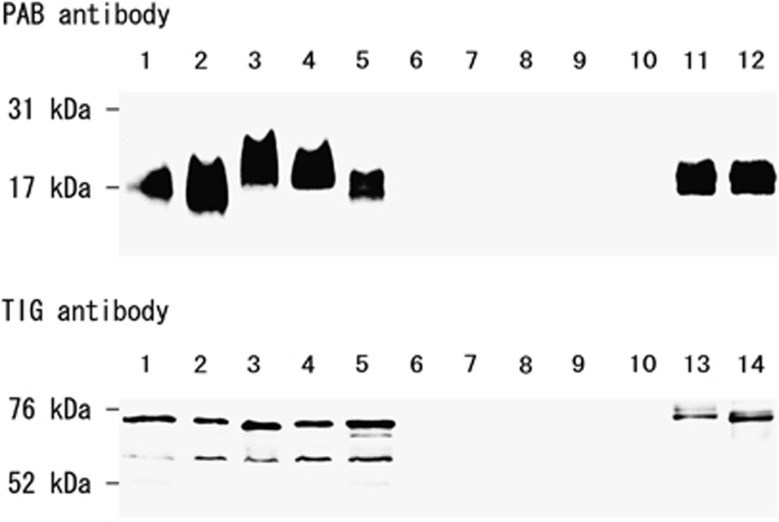
The specificity of PAB and TIG antibodies is shown in Table 1. These monoclonal antibodies reacted with all strains of P. acnes, irrespective of the serotype (I or II), strain type, or clinical isolate, and there was no cross-reactivity with other propionibacteria or the control bacteria. PAB antibody recognized a P. acnes-specific epitope of the lipoteichoic acid commonly shared by all strains of this bacterium (Figure 1). TIG antibody reacted with the recombinant trigger-factor protein from P. acnes and two or three protein bands were observed on the membrane with the whole bacterial lysate of this bacterium.
Figure 1.
Western blot analysis for reactivity of the anti-P. acnes antibodies. Western blot analysis with PAB and TIG antibodies was performed using sonicated whole-cell lysate (lanes 1–10) with the appropriate positive control samples. Lane 1, serotype I P. acnes (ATCC 6919); lane 2, serotype I P. acnes (ATCC 11827); lane 3, serotype II P. acnes (ATCC 11828); lane 4, serotype I P. acnes (clinical isolate S4); lane 5, serotype II P. acnes (clinical isolate S7); lane 6, P. granulosum; lane 7, P. avidum; lane 8, M. tuberculosis; lane 9, M. avium; and lane 10, M. intracellulare. Positive control samples for the PAB antibody were lipoteichoic acid purified from serotype I (lane 11) and serotype II (lane 12) of P. acnes. Positive control samples for the TIG antibody were recombinant trigger-factor protein from the whole-genome sequence of P. acnes serotype I (lane 13) and serotype II (lane 14).
Localization of P. acnes Within Sarcoid Granulomas
In the lungs and lymph nodes, PAB antibody reacted with small round bodies in some of the immature and mature epithelioid cells and multinucleated giant cells of sarcoid granulomas (Figures 2 and 3). The appearance of the small round bodies detected by PAB antibody within sarcoid granulomas did not differ between lungs and lymph nodes. The amount of these small round bodies varied from each granuloma in identical sarcoid samples as well as from each sarcoid tissue sample. In sarcoid granulomas with many small round bodies, the cytoplasm of some granuloma cells was filled with small round bodies, just like intracellular proliferation of the bacterium (Figures 2b,d and 3b,d). In many sarcoid granulomas, a few small round bodies were scattered in some of the granuloma cells with occasionally degraded or large-sized features of the bodies (Figures 2f and 3f).
Figure 2.
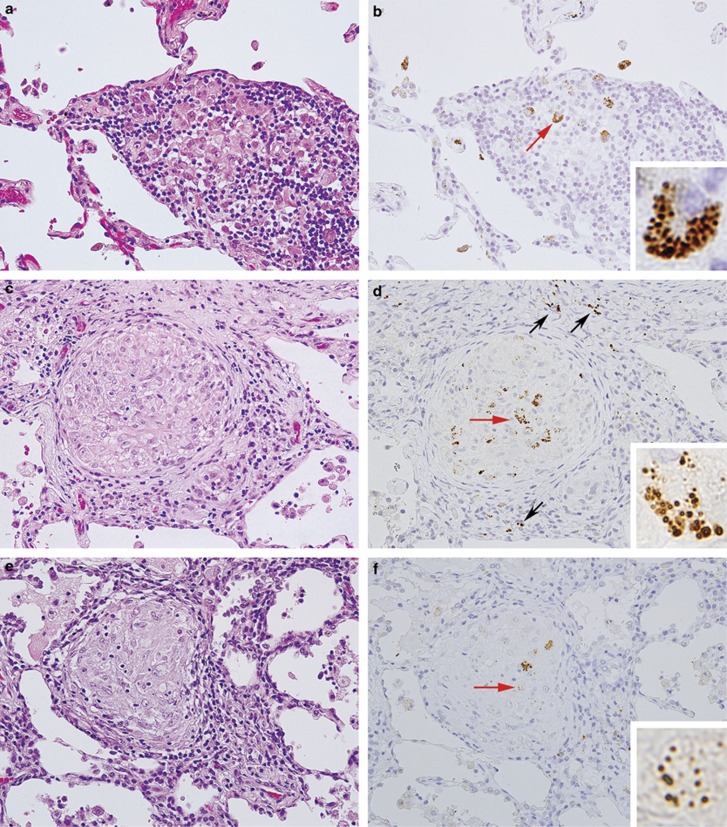
P. acnes within sarcoid granulomas of the lungs. Hematoxylin and eosin staining (left) and immunostaining with PAB antibody (right) are shown pairwise for three different video-assisted thoracic surgery samples of the lungs from sarcoid patients (original magnification, × 400). A higher magnification of the positive reaction products indicated by the red arrows is shown in each inset. In the granulomatous alveolitis lesion (a, b), some swollen macrophages of the immature granuloma were filled with many small round bodies detected by PAB antibody. In the granuloma lesion surrounded by prominent inflammatory cell infiltration (c, d), such small round bodies were detected not only in the granuloma cells but also in some of the inflammatory cells (black arrows). In a mature granuloma (e, f), a few granuloma cells with degraded forms of the small round bodies were sparsely distributed within the granulomas.
Figure 3.
P. acnes within sarcoid granulomas of the lymph nodes. Hematoxylin and eosin staining (left) and immunostaining with PAB antibody (right) are shown pairwise for two Japanese samples (a, b, and c, d) and one German sample (e, f) of lymph nodes from patients with sarcoidosis (original magnification, × 1000). A higher magnification of the positive reaction products indicated by the red arrows is shown in each inset. Many small round bodies were detected within an immature granuloma (a, b) and a few small round bodies were detected within a mature granuloma (c, d). Granuloma cells filled with such small round bodies were observed at the rim of a granuloma (b, d). In many sarcoid granulomas, including in the German samples shown here (e, f), large spheroidal bodies detected by PAB antibody were sparsely distributed within a mature granuloma.
Detection Frequency of P. acnes Within Granulomas
In the lungs, small round bodies detected by PAB antibody within granulomas were found in 44 (57%) of 77 lung samples from sarcoid patients (Table 2). The frequency was significantly higher in samples obtained by video-assisted thoracic surgery than in samples obtained by transbronchial lung biopsy (74% vs 48%, P=0.049). No reactivity was detected within granulomas in any of the lung samples of the 17 patients with tuberculosis or the 10 patients with sarcoid reaction. A few cells with small round bodies were scattered in interstitial inflammatory cells around the sarcoid granulomas of the lungs (Figure 2d) in 22 (81%) of the surgically-resected samples and 18 (36%) of the biopsy samples from sarcoid patients.
Table 2. Frequency and localization of P. acnes detected by PAB antibody in sarcoid and non-sarcoid lung samples.
| Disease | Sampling method | Total number of samples |
Number (%) of samples with P. acnes detected in: |
||
|---|---|---|---|---|---|
| Granulomas | Interstitial inflammatory cells | Alveolar macrophages | |||
| Sarcoidosis | VATS | 27 | 20 (74) | 22 (81) | 14 (52) |
| TBLB | 50 | 24 (48) | 18 (36) | 14 (28) | |
| Tuberculosis | VATS | 12 | 0 | 0 | 3 (25) |
| TBLB | 5 | 0 | 0 | 0 | |
| Lung cancer with sarcoid reactiona | Operation | 10 | 0 | 0 | 4 (40) |
| Lung cancerb | Operation | 18 | — | — | 7 (39) |
| TBLB | 26 | — | — | 0 | |
| IIPsc | VATS | 5 | — | 0 | 1 (20) |
| TBLB | 11 | — | 0 | 2 (18) | |
| Chronic HP | VATS | 8 | — | 0 | 1 (13) |
| TBLB | 15 | — | 0 | 0 | |
IIPs: idiopathic interstitial pneumonia; HP: hypersensitivity pneumonitis; VATS: video-assisted thoracic surgery; TBLB: transbronchial lung biopsy.
Tissue samples with non-caseating epithelioid-cell granulomas around the cancer lesion were examined. These lung cancer patients had no symptoms or signs of systemic sarcoidosis.
Cancer-free lung tissue sections were selected from operation materials for the study. All pieces of TBLB samples, including cancer lesions, were examined for the study.
IIPs include usual interstitial pneumonia, non-specific interstitial pneumonia, and organizing pneumonia.
In the lymph nodes, small round bodies detected by PAB antibody within sarcoid granulomas were found in 105 (88%) of 119 lymph node samples (Table 3). The frequency was not significantly different between samples from Japanese and German patients (88% vs 89%, respectively) or between samples of mediastinal and superficial lymph nodes from Japanese patients (86% vs 89%, respectively). No reactivity was detected within granulomas in any of the lymph node samples from 28 patients with tuberculosis and 24 patients with sarcoid reaction.
Table 3. Frequency and localization of P. acnes detected by PAB antibody in sarcoid and non-sarcoid lymph node samples.
| Disease | Location | Total number of samples |
Number (%) of samples with P. acnes detected in: |
||
|---|---|---|---|---|---|
| Granulomas |
Macrophages located at |
||||
| Paracortex | Sinus | ||||
| Sarcoidosis | Mediastinal | 44 | 38 (86) | 13 (30) | 26 (59) |
| Mediastinala | 38 | 34 (89) | 8 (21) | 19 (50) | |
| Superficial | 37 | 33 (89) | 5 (14) | 15 (41) | |
| Tuberculosis | Mediastinal | 3 | 0 | 0 | 0 |
| Mediastinala | 8 | 0 | 0 | 1 (13) | |
| Superficial | 17 | 0 | 2 (12) | 1 (6) | |
| Sarcoid reactionb | Mediastinal | 8 | 0 | 1 (13) | 5 (63) |
| Perigastric | 16 | 0 | 4 (25) | 3 (19) | |
| Lung cancerc | Mediastinal | 18 | — | 0 | 1 (6) |
| Mediastinala | 18 | — | 0 | 5 (28) | |
| Gastric cancerc | Perigastric | 20 | — | 3 (15) | 0 |
| Colorectal cancerc | Mesenteric | 20 | — | 6 (30) | 6 (30) |
| Reactive lymphadenitis | Superficial | 26 | — | 2 (8) | 2 (8) |
| Necrotizing lymphadenitis | Superficial | 11 | — | 0 | 0 |
Samples from German patients.
All samples with sarcoid reaction were obtained from cancer-free regional lymph nodes from 8 patients with lung cancer and 16 patients with gastric cancer. None of these cancer patients had symptoms or signs of systemic sarcoidosis.
Cancer-free regional lymph nodes from cancer patients were used for the study.
Intracellular P. acnes in Nongranulomatous Areas
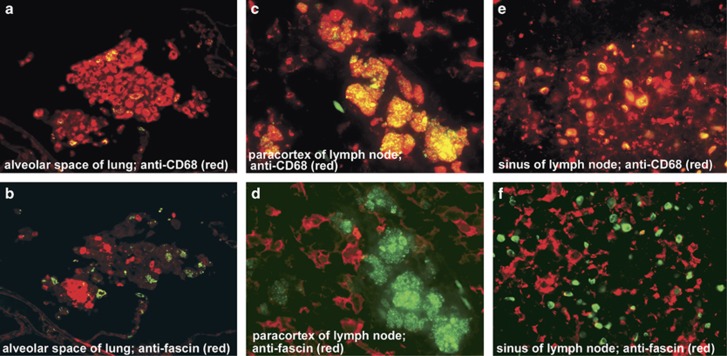
Cells with many small round bodies detected by PAB antibody were observed in alveolar spaces of the sarcoid lungs (Figure 4a) as well as in granuloma-free paracortical areas of sarcoid lymph nodes (Figure 4c). Most alveolar and paracortical cells with such small round bodies were stained with the anti-CD68 antibody, but not with the anti-fascin antibody (Figure 5a–d).
Figure 4.
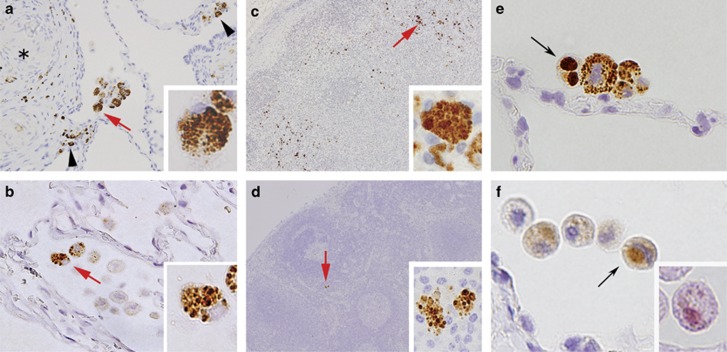
P. acnes in non-granulomatous areas of the sarcoid and non-sarcoid lungs and lymph nodes. Many macrophages with many small round bodies detected by PAB antibody were found in alveolar spaces adjacent to granulomas (*) and areas of inflammation (indicated by the arrow heads) of many video-assisted thoracic surgery samples from sarcoid patients (a). A few such alveolar macrophages were also detected in some lung samples from non-sarcoid patients (b). Clusters of macrophages with many small round bodies detected by PAB antibody were found in granuloma-free paracortical areas of the lymph nodes with sarcoid granulomas (c). A few paracortical macrophages with many small round bodies detected by PAB antibody were also found in some samples from non-sarcoid patients (d). Higher magnification of the positive reaction products indicated by the red arrows is shown in each inset (a–d). A few large spheroidal bodies detected by PAB antibody, indicated by the black arrow, were occasionally observed in alveolar macrophages of the sarcoid lungs (e). Such a large spheroidal body also reacted with TIG antibody (f), as indicated by the black arrow and was acid-fast with Fite staining (f, inset). Original magnification: a–b, × 400; c–d, × 200; e–f, × 1000.
Figure 5.
Immunofluorescence double-staining to phenotype the P. acnes-positive cells in non-granulomatous areas of sarcoid lungs and lymph nodes. Green signal (FITC): PAB antibody; red signals (TRITC): anti-CD68 antibody (a, c, e), anti-fascin antibody (b, d, f). Results of double-staining are merged in all pictures. In the sarcoid lung, all of the P. acnes signals overlapped (yellow) with CD68-positive alveolar macrophages (a), whereas the fascin-positive alveolar dendritic cells did not contain small round bodies detected by PAB antibody (b). In the paracortical area of the sarcoid lymph node, small round body detected by PAB antibody were found in the CD68-positive macrophages (c), but not in the fascin-positive dendritic cells (d). In the sinus of the sarcoid lymph node, Hamazaki-Wesenberg bodies detected by PAB antibody overlapped with CD68-positive macrophages (e), but not with the fascin-positive dendritic cells (f). Original magnification: a–b, × 400; c–f, × 1000.
In the lungs, alveolar macrophages with many small round bodies detected by PAB antibody were found in 28 (36%) of 77 sarcoid samples and 18 (16%) of 110 non-sarcoid samples (Figure 4b; Table 2). The frequency was significantly higher in sarcoid samples (P=0.0031). Such alveolar macrophages occasionally contained one or a few large spheroidal bodies detected by PAB antibody (Figure 4e) that were acid-fast with Fite staining and also reacted with the TIG antibody (Figure 4f).
In the lymph nodes, paracortical macrophages with many small round bodies detected by PAB antibody were observed in 26 (22%) of 119 sarcoid samples and 18 (11%) of 165 non-sarcoid samples (Figure 4d; Table 3). The frequency was significantly higher in the sarcoid samples (P=0.019).
Hamazaki–Wesenberg Bodies in the Lymph Nodes
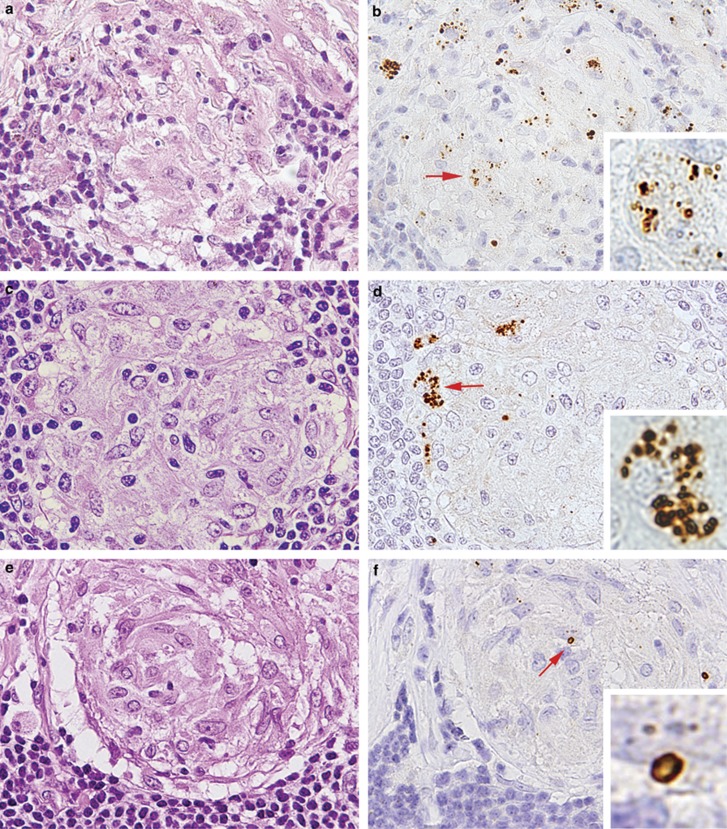
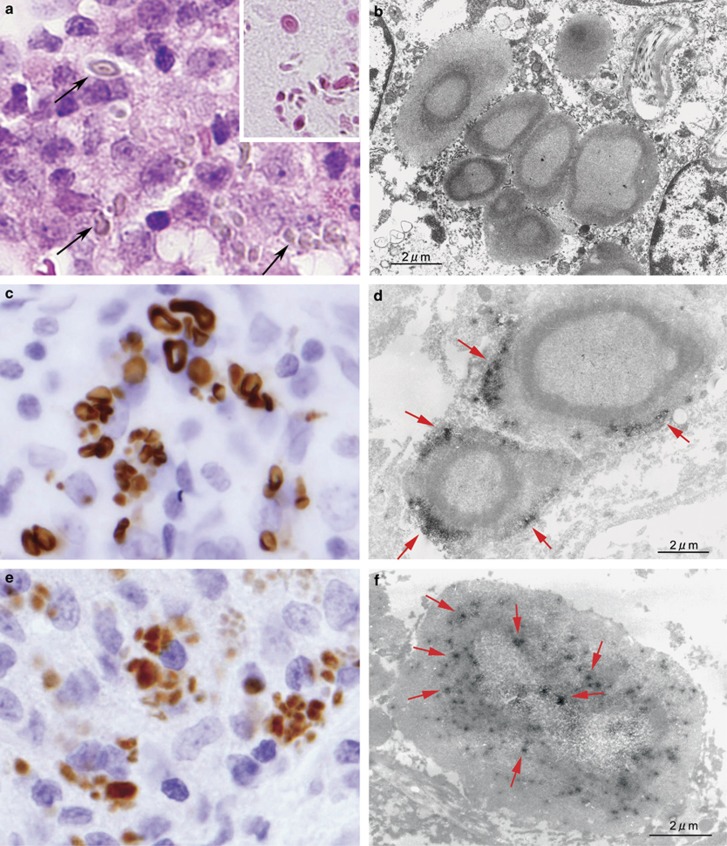
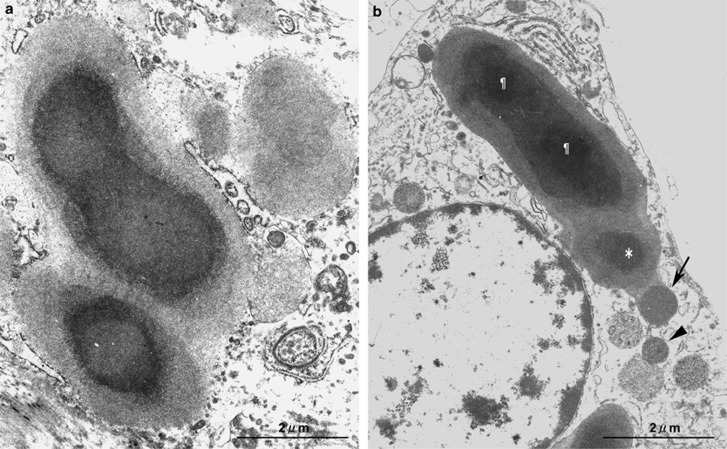
In the lymph nodes, large spheroidal bodies detected by PAB antibody were observed in sinus macrophages stained with the anti-CD68 antibody but not in dendritic cells stained with the anti-fascin antibody (Figure 5e–f), in 60 (50%) of 119 sarcoid samples and in 24 (15%) of 165 non-sarcoid samples (Figure 6c; Table 3). The frequency was significantly higher in sarcoid samples (P<0.001). The large spheroidal bodies detected by PAB antibody in sinus macrophages were yellow–brown in color with hematoxylin and eosin staining and strongly acid-fast with Fite staining (Figure 6a), consistent with so-called Hamazaki–Wesenberg bodies (Figure 6b). Most of the Hamazaki–Wesenberg bodies detected by PAB antibody also reacted with the TIG antibody (Figure 6e), although the TIG antibody did not react with small round bodies detected by PAB antibody in granuloma cells or paracortical macrophages. Immunoelectron microscopy revealed that cell membrane-bound lipoteichoic acid detected by PAB antibody was confined to the outer margins of the Hamazaki–Wesenberg bodies (Figure 6d), and the ribosome-bound trigger factor detected by TIG antibody was scattered throughout the inner areas of the bodies (Figure 6f). Electron microscopic analysis revealed that Hamazaki–Wesenberg bodies in the sarcoid lymph nodes lacked a cell-wall structure with occasional protrusions from the body (Figure 7).
Figure 6.
Large-sized acid-fast bodies detected by PAB and TIG monoclonal antibodies in sarcoid lymph nodes. In this lymph node sample from a Japanese patient with sarcoidosis, many large-sized yellow-brown bodies, as indicated by the arrows, were observed with hematoxylin and eosin staining in hyperplastic sinuses (a). These bodies were strongly acid-fast with Fite staining (a, inset). Electron-microscopy of the same sample revealed typical features of Hamazaki-Wesenberg bodies (b). This sarcoid lymph node sample was examined by immunohistochemistry using PAB antibody (c, d) and TIG antibody (e, f) with light microscopy and electron microscopy. The Hamazaki-Wesenberg bodies reacted with PAB antibody (c) and abundant reaction products of the antibody, as indicated by the arrows, located along the outer margin of the body (d). The Hamazaki-Wesenberg bodies also reacted with TIG antibody (e) and spotty reaction products of the antibody, as indicated by the arrows, located throughout the inner areas of the body (f). Original magnification: a, c, and e, × 1000. Scale bars indicate 2 μm in b, d, and f.
Figure 7.
Protrusions from Hamazaki-Wesenberg bodies in sarcoid lymph nodes. Electron-microscopic analysis revealed Hamazaki-Wesenberg bodies with protrusions in sinus macrophages of two different sarcoid lymph nodes. Multiple protrusions (a) are represented by fused bodies, with or without a central osmiophilic core, surrounded by a discontinuous limiting membrane. One-by-one protrusions (b) are represented by a large spheroidal body with two fused central cores (¶) and a separated core (*) linked together with a small round body (indicated by the black arrow). Adjacent to and separated from this small round body, another similar-shaped small round body (indicated by the arrowhead) was observed, which might reveal how these small round bodies are derived from the Hamazaki-Wesenberg body. Scale bars indicate 2 μm.
Correlation between Detection of P. acnes in Granulomas and in Nongranulomatous Areas
In all seven sarcoid lung samples obtained by video-assisted thoracic surgery in which no PAB antibody reactivity was observed within the granulomas, PAB antibody reactivity was also totally absent in nongranulomatous areas, including alveolar macrophages (Table 4). Similarly, PAB antibody reactivity was not observed in nongranulomatous areas in 23 (88%) of the 26 sarcoid samples obtained by transbronchial lung biopsy with no PAB antibody reactivity within the granulomas.
Table 4. Correlation between P. acnes detected in granulomas and non-granulomatous areas in sarcoid samples.
| Organ | Sample category | Localization of P. acnes in non-granulomatous area |
Detection status of P. acnes within granulomas |
|
|---|---|---|---|---|
| Positive | Negative | |||
| Lung | VATS | Total number of samples | 20 | 7 |
| Number (%) of samples with P. acnes in alveolar macrophages | 13 (65%) | 0 | ||
| TBLB | Total number of samples | 24 | 26 | |
| Number (%) of samples with P. acnes in alveolar macrophages | 11 (46%) | 3 (12%) | ||
| Lymph node | Japan | Total number of samples | 71 | 10 |
| Number (%) of samples with P. acnes in paracortical macrophages | 18 (25%) | 0 | ||
| Number (%) of samples with Hamazaki-Wesenberg bodies detected by PAB antibody | 38 (54%) | 2 (20%) | ||
| Germany | Total number of samples | 34 | 4 | |
| Number (%) of samples with P. acnes in paracortical macrophages | 8 (24%) | 0 | ||
| Number (%) of samples with Hamazaki–Wesenberg bodies detected by PAB antibody | 19 (56%) | 0 | ||
VATS: video-assisted thoracic surgery; TBLB: transbronchial lung biopsy.
The same association as in the lung samples was found in sarcoid lymph node samples from Japanese and German patients (Table 4). PAB antibody reactivity in paracortical macrophages was not observed in any of the 4 German or 10 Japanese lymph node samples with no PAB antibody reactivity within the granulomas. Hamazaki–Wesenberg bodies detected by PAB antibody were not found in any of the German samples or in 8 (80%) of the Japanese samples with no PAB antibody reactivity within the granulomas.
Discussion
Granuloma formation is a critical step in the delayed immune response that stops the spread of noxious and infectious microorganisms.22 Sarcoid granulomas are thought to be formed by a hypersensitive Th1 immune response to certain causative agents.1 To establish a causative link, agents that putatively cause sarcoidosis must be demonstrated within sarcoid granulomas. In the present study, PAB antibody specific to P. acnes that recognize lipoteichoic acid of the plasmalemma reacted with small round bodies within sarcoid granulomas in 88% of lymph node samples and 57% of lung samples. Reactivity to the antibody was not observed in non-sarcoid granulomas, including those from patients with tuberculosis and so-called sarcoid reaction. The high frequency and specificity of P. acnes detected within sarcoid granulomas indicates that this indigenous bacterium might be the cause of granuloma formation in many patients with sarcoidosis. P. acnes was not detected within sarcoid granulomas in samples from some patients with sarcoidosis, however, suggesting that sarcoidosis has various causes, including noninfectious causes.
In many sarcoid samples in which P. acnes was not detected within granulomas, P. acnes was also not detected in nongranulomatous areas. It is likely that in some such sarcoid samples, other bacteria, such as P. granulosum or M. tuberculosis, are the cause of the granuloma formation, because the PAB antibody was specific to P. acnes and did not cross-react with these bacteria. M. tuberculosis is a suspected cause of sarcoidosis in Western countries.8, 9, 23, 24, 25 In a previous international collaboration study using real-time quantitative PCR, many P. acnes genomes were detected in many samples from European patients with sarcoidosis, whereas a few M. tuberculosis genomes were detected in samples from some of these patients.7 In the present study, P. acnes was detected within sarcoid granulomas by the PAB antibody in 89% of lymph node samples from German patients. P. acnes may cause sarcoidosis more frequently than does M. tuberculosis, even in European patients. Ishige et al26 found many P. granulosum genomes in 3 of 15 Japanese patients with sarcoidosis. None of the three patients with P. granulosum had P. acnes genomes in their biopsy samples. They also found a few M. tuberculosis genomes in 3 of 15 patients with sarcoidosis. The three patients with M. tuberculosis also had many P. acnes genomes in their biopsy samples. Thus, P. granulosum is more likely than M. tuberculosis to be the cause of sarcoidosis in patients without P. acnes detected within granulomas by the PAB antibody.
Another possible explanation for the failure to detect P. acnes within granulomas of some sarcoid samples is that the P. acnes antigens were completely degraded by the granuloma cells, which possess a greater capacity for intracellular digestion than macrophages.27, 28 In the present study, PAB antibody reactivity was observed more frequently in immature granulomas than in mature granulomas. The P. acnes antigen may have thus been degraded and abolished in the granuloma cells as the granuloma matured. Indeed, even in the same samples, reactivity was found in some granulomas but was totally absent in other granulomas. Such an inhomogeneous distribution of granulomas with PAB antibody reactivity may have contributed to the failure to detect P. acnes within granulomas, especially in small samples with few granulomas. The inhomogeneous distribution might explain the finding that the frequency (48%) of granulomas with PAB antibody reactivity in samples obtained by transbronchial lung biopsy was significantly lower than that (74%) in samples obtained by video-assisted thoracic surgery. Surgically-resected samples are larger and include more granulomas than the biopsy samples. Differences in the size between samples obtained by surgery and biopsy may also explain why PAB antibody reactivity was observed in alveolar macrophages in some (12%) of the sarcoid biopsy samples with no PAB antibody reactivity within the granulomas.
The PAB antibody obtained by immunization with a serotype II strain (ATCC 11828) reacted with lipoteichoic acid from both serotype I and II strains of P. acnes. We confirmed that the PAB antibody reacted with both serotype I and II P. acnes on formalin-fixed and paraffin-embedded liver tissues of rats injected intravenously by either serotype I or II P. acnes (data not shown). Furukawa et al20 reported that clinical isolates from sarcoid tissues are either serotype I (70%) or serotype II (30%), and only serotype I isolates invade cells. Although the present study does not provide information about P. acnes serotypes within sarcoid granulomas, we conclude that the failure to detect P. acnes in some sarcoid samples is not caused by potential differences in the P. acnes serotype found in sarcoid granulomas.
The large-spheroidal acid-fast bodies that reacted with both PAB and TIG antibodies in sinus macrophages of the lymph nodes were consistent with so-called Hamazaki–Wesenberg bodies. Hamazaki–Wesenberg bodies frequently appear in sarcoid lymph nodes although these bodies are not specific to sarcoidosis.29, 30, 31 In the present study, Hamazaki–Wesenberg bodies were found in 50% of sarcoid samples and 15% of non-sarcoid samples. Moscovic29 suggested that Hamazaki–Wesenberg bodies are mycobacterial L-forms based on morphological studies. Alavi and Moscovic32 reported that Hamazaki–Wesenberg bodies reacted with World Health Organization monoclonal antibodies (TB68 and TB71) to M. tuberculosis complex, although they did not examine the specificities of the antibodies used in the study. In the present study, the PAB and TIG antibodies that reacted with Hamazaki–Wesenberg bodies found in both sarcoid and non-sarcoid samples were specific to P. acnes and did not react with the M. tuberculosis complex. Moreover, immunoelectron microscopy revealed that immunoreactive products with PAB antibody and TIG antibody were differentially distributed in the outer and inner areas of the Hamazaki–Wesenberg bodies, respectively. The different localization of the reaction products of the two P. acnes-specific antibodies suggests that the reactivity of these antibodies with Hamazaki–Wesenberg bodies was not caused by a nonspecific reaction. Thus, we conclude that Hamazaki–Wesenberg bodies are structures derived from P. acnes.
Cell membrane-bound lipoteichoic acid detected by the PAB antibody and ribosome-bound trigger factor detected by the TIG antibody were both found in the Hamazaki–Wesenberg bodies, preserving the original distribution pattern (plasmalemmal and protoplasmic localization, respectively) of these bacterial components in terms of the morphological structure of the bacterium. A mixed distribution of these differentially-distributed bacterial components in identical areas was not observed in the bodies. These immunoelectron-microscopic findings suggest that Hamazaki–Wesenberg bodies might not be phagolysosomally-degraded products of P. acnes, but rather intact forms of intracellular bacteria. Furthermore, conventional electron microscopy revealed that these bodies lack a cell-wall structure and occasionally exhibit protrusions from the body. Further studies are needed to elucidate the nature of Hamazaki–Wesenberg bodies in connection with P. acnes.
Intracellular small round bodies detected by PAB antibody were also observed in nongranulomatous areas of sarcoid and non-sarcoid samples. In sarcoid lungs, alveolar macrophages filled with many small round bodies might contribute to granuloma formation in the lung parenchyma because such macrophages were occasionally found in granulomatous inflammation or within immature granulomas of the lungs. In sarcoid lymph nodes, a cluster of granuloma-free paracortical macrophages filled with many small round bodies might be an early focal site of sarcoid granuloma because such macrophages were occasionally found within sarcoid granulomas of the lymph nodes. Although intracellular P. acnes was not specific to sarcoid samples, the frequency was higher and the extent more prominent in sarcoid samples than in non-sarcoid samples.
P. acnes is the most common commensal bacterium in the lungs and lymph nodes of subjects without sarcoidosis.33 P. acnes is found in 21% of non-sarcoid lymph nodes by bacterial culture13 and 15% of non-sarcoid lymph nodes by PCR.26 In the non-sarcoid samples examined in the present study, we found P. acnes in 18% of lung samples and 22% of lymph node samples. Occasional detection of P. acnes in nongranulomatous areas of the lungs and lymph nodes from non-sarcoid patients suggests that host factors may be more critical than agent factors in the etiology of sarcoidosis.
A particular protein, referred to as a trigger factor, from P. acnes causes a cellular immune response in some sarcoid patients, but not in subjects without sarcoidosis.10 The P. acnes trigger-factor protein induces pulmonary granulomas in mice sensitized with the protein and adjuvant, but only in mice with latent P. acnes infection in their lungs.16 This pathogenesis was confirmed by further experiments using mice sensitized with P. acnes and adjuvant, in which the eradication of P. acnes by antibiotics prevented granulomas in this experimental model.34 The results of this experimental model of sarcoidosis might explain the recently reported effectiveness of tetracyclines for treating sarcoidosis.35, 36, 37, 38
In the present study, we developed novel P. acnes-specific monoclonal antibodies that can detect P. acnes in formalin-fixed paraffin-embedded tissue sections. The high frequency and specificity of P. acnes detected by PAB antibody within sarcoid granulomas suggests an etiologic link between sarcoidosis and this indigenous bacterium. The PAB antibody may be useful for diagnosing sarcoidosis caused by P. acnes, when the reactivity is detected in idiopathic granulomas.
Acknowledgments
The present work was supported by the Japanese Society for the Promotion of Science Grant-in-Aid for Scientific Research 18390112 (YE) and 22659287 (YE).
The authors declare no conflict of interest
References
- Hunninghake GW, Costabel U, Ando M, et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:149–173. [PubMed] [Google Scholar]
- Baughman RP, Lower EE, du Bois RM. Sarcoidosis. Lancet. 2003;361:1111–1118. doi: 10.1016/S0140-6736(03)12888-7. [DOI] [PubMed] [Google Scholar]
- Newman LS, Rose CS, Bresnitz EA, et al. A case control etiologic study of sarcoidosis: environmental and occupational risk factors. Am J Respir Crit Care Med. 2004;170:1324–1330. doi: 10.1164/rccm.200402-249OC. [DOI] [PubMed] [Google Scholar]
- McGrath DS, Goh N, Foley PJ, et al. Sarcoidosis: genes and microbes—soil or seed. Sarcoidosis Vasc Diffuse Lung Dis. 2001;18:149–164. [PubMed] [Google Scholar]
- du Bois RM, Goh N, McGrath D, et al. Is there a role for microorganisms in the pathogenesis of sarcoidosis. J Intern Med. 2003;253:4–17. doi: 10.1046/j.1365-2796.2003.01073.x. [DOI] [PubMed] [Google Scholar]
- Drake WP, Newman LS. Mycobacterial antigens may be important in sarcoidosis pathogenesis. Curr Opin Pulm Med. 2006;12:359–363. doi: 10.1097/01.mcp.0000239554.01068.94. [DOI] [PubMed] [Google Scholar]
- Eishi Y, Suga M, Ishige I, et al. Quantitative analysis of mycobacterial and propionibacterial DNA in lymph nodes of Japanese and European patients with sarcoidosis. J Clin Microbiol. 2002;40:198–204. doi: 10.1128/JCM.40.1.198-204.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell I, Ramírez-Valle F, Sanchez M, et al. Evidence for mycobacteria in sarcoidosis. Am J Respir Cell Mol Biol. 2011;45:899–905. doi: 10.1165/rcmb.2010-0433TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake WP, Dhason MS, Nadaf M, et al. Cellular recognition of Mycobacterium tuberculosis ESAT-6 and KatG peptides in systemic sarcoidosis. Infect Immun. 2007;75:527–530. doi: 10.1128/IAI.00732-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebe Y, Ikushima S, Yamaguchi T, et al. Proliferative response of peripheral blood mononuclear cells and levels of antibody to recombinant protein from Propionibacterium acnes DNA expression library in Japanese patients with sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2000;17:256–265. [PubMed] [Google Scholar]
- Zumla A, James DG. Granulomatous infections: etiology and classification. Clin Infect Dis. 1996;23:146–158. doi: 10.1093/clinids/23.1.146. [DOI] [PubMed] [Google Scholar]
- Homma JY, Abe C, Chosa H, et al. Bacteriological investigation on biopsy specimens from patients with sarcoidosis. Jpn J Exp Med. 1978;48:251–255. [PubMed] [Google Scholar]
- Abe C, Iwai K, Mikami R, et al. Frequent isolation of Propionibacterium acnes from sarcoidosis lymph nodes. Zentralbl Bakteriol Mikrobiol Hyg A. 1984;256:541–547. doi: 10.1016/s0174-3031(84)80032-3. [DOI] [PubMed] [Google Scholar]
- Brincker H. Sarcoid reactions in malignant tumours. Cancer Treat Rev. 1986;13:147–156. doi: 10.1016/0305-7372(86)90002-2. [DOI] [PubMed] [Google Scholar]
- Harlow E, Lane D. Antibodies: A Laboratory Manual. Cold Spring Harbor Laboratory: New York; 1988. Monoclonal antibodies; pp. 139–281. [Google Scholar]
- Minami J, Eishi Y, Ishige Y, et al. Pulmonary granulomas caused experimentally in mice by a recombinant trigger-factor protein of Propionibacterium acnes. J Med Dent Sci. 2003;50:265–274. [PubMed] [Google Scholar]
- Yamada T, Eishi Y, Ikeda S, et al. In situ localization of Propionibacterium acnes DNA in lymph nodes from sarcoidosis patients by signal amplification with catalysed reporter deposition. J Pathol. 2002;198:541–547. doi: 10.1002/path.1243. [DOI] [PubMed] [Google Scholar]
- WADE HW. A modification of the Fite formaldehyde (Fite I) method for staining acid-fast bacilli in paraffin sections. Stain Technol. 1957;32:287–292. doi: 10.3109/10520295709111642. [DOI] [PubMed] [Google Scholar]
- Ito T, Kobayashi D, Uchida K, et al. Helicobacter pylori invades the gastric mucosa and translocates to the gastric lymph nodes. Lab Invest. 2008;88:664–681. doi: 10.1038/labinvest.2008.33. [DOI] [PubMed] [Google Scholar]
- Furukawa A, Uchida K, Ishige Y, et al. Characterization of Propionibacterium acnes isolates from sarcoid and non-sarcoid tissues with special reference to cell invasiveness, serotype, and trigger factor gene polymorphism. Microb Pathog. 2009;46:80–87. doi: 10.1016/j.micpath.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Fischer W, Koch HU, Haas R. Improved preparation of lipoteichoic acids. Eur J Biochem. 1983;133:523–530. doi: 10.1111/j.1432-1033.1983.tb07495.x. [DOI] [PubMed] [Google Scholar]
- Kumar V, Abbas A, Fausto N, et al. Granulomatous inflammation, In: Robbins and Cotran's pathologic basis of disease: 8th edn. Saunders: London. 2010;pp:73–74. [Google Scholar]
- Moller DR. Potential etiologic agents in sarcoidosis. Proc Am Thorac Soc. 2007;4:465–468. doi: 10.1513/pats.200608-155MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta D, Agarwal R, Aggarwal AN, et al. Molecular evidence for the role of mycobacteria in sarcoidosis: a meta-analysis. Eur Respir J. 2007;30:508–516. doi: 10.1183/09031936.00002607. [DOI] [PubMed] [Google Scholar]
- Swaisgood CM, Oswald-Richter K, Moeller SD, et al. Development of a sarcoidosis murine lung granuloma model using Mycobacterium superoxide dismutase A peptide. Am J Respir Cell Mol Biol. 2011;44:166–174. doi: 10.1165/rcmb.2009-0350OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishige I, Usui Y, Takemura T, et al. Quantitative PCR of mycobacterial and propionibacterial DNA in lymph nodes of Japanese patients with sarcoidosis. Lancet. 1999;354:120–123. doi: 10.1016/S0140-6736(98)12310-3. [DOI] [PubMed] [Google Scholar]
- Carr I. Sarcoid macrophage giant cells. Ultrastructure and lysozyme content. Virchows Arch B Cell Pathol Incl Mol Pathol. 1980;32:147–155. doi: 10.1007/BF02889023. [DOI] [PubMed] [Google Scholar]
- Okabe T, Suzuki A, Ishikawa H, et al. Cells originating from sarcoid granulomas in vitro. Am Rev Respir Dis. 1981;124:608–612. doi: 10.1164/arrd.1981.124.5.608. [DOI] [PubMed] [Google Scholar]
- Moscovic EA. Sarcoidosis and mycobacterial L-forms. A critical reappraisal of pleomorphic chromogenic bodies (Hamazaki corpuscles) in lymph nodes. Pathol Annu. 1978;13 (Pt 2:69–164. [PubMed] [Google Scholar]
- Boyd JF, Valentine JC. Unidentified yellow bodies in human lymph-nodes. J Pathol. 1970;102:58–60. doi: 10.1002/path.1711020112. [DOI] [PubMed] [Google Scholar]
- Doyle WF, Brahman HD, Burgess JH. The nature of yellow-brown bodies in peritoneal lymph nodes. Arch Pathol. 1973;96:320–326. [PubMed] [Google Scholar]
- Alavi HA, Moscovic EA. Immunolocalization of cell-wall-deficient forms of Mycobacterium tuberculosis complex in sarcoidosis and in sinus histiocytosis of lymph nodes draining carcinoma. Histol Histopathol. 1996;11:683–694. [PubMed] [Google Scholar]
- Ishige I, Eishi Y, Takemura T, et al. Propionibacterium acnes is the most common bacterium commensal in peripheral lung tissue and mediastinal lymph nodes from subjects without sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2005;22:33–42. [PubMed] [Google Scholar]
- Nishiwaki T, Yoneyama H, Eishi Y, et al. Indigenous pulmonary Propionibacterium acnes primes the host in the development of sarcoid-like pulmonary granulomatosis in mice. Am J Pathol. 2004;165:631–639. doi: 10.1016/S0002-9440(10)63327-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki E, Ando M, Fukami T, et al. Minocycline for the treatment of sarcoidosis: is the mechanism of action immunomodulating or antimicrobial effect. Clin Rheumatol. 2008;27:1195–1197. doi: 10.1007/s10067-008-0903-3. [DOI] [PubMed] [Google Scholar]
- Park DJ, Woog JJ, Pulido JS, et al. Minocycline for the treatment of ocular and ocular adnexal sarcoidosis. Arch Ophthalmol. 2007;125:705–709. doi: 10.1001/archopht.125.5.705. [DOI] [PubMed] [Google Scholar]
- Baba K, Yamaguchi E, Matsui S, et al. A case of sarcoidosis with multiple endobronchial mass lesions that disappeared with antibiotics. Sarcoidosis Vasc Diffuse Lung Dis. 2006;23:78–79. [PubMed] [Google Scholar]
- Bachelez H, Senet P, Cadranel J, et al. The use of tetracyclines for the treatment of sarcoidosis. Arch Dermatol. 2001;137:69–73. doi: 10.1001/archderm.137.1.69. [DOI] [PubMed] [Google Scholar]