Direct seeding is replacing transplanting in rice. Early flooding suppresses weeds but selective action is compromised by the sharing of flood-tolerance traits. Understanding adaptive traits in both species is therefore a prerequisite for developing direct seeding systems that control weeds while leaving rice seedlings relatively unharmed.
Abstract
Background and aims
Direct seeding of rice is being adopted in rainfed and irrigated lowland ecosystems because it reduces labour costs in addition to other benefits. However, early flooding due to uneven fields or rainfall slows down seed germination and hinders crop establishment. Conversely, early flooding helps suppress weeds and reduces the costs of manual weeding and/or dependence on herbicides; however, numerous weed species are adapted to lowlands and present challenges for the use of flooding to control weeds. Advancing knowledge on the mechanisms of tolerance of flooding during germination and early growth in rice and weeds could facilitate the development of improved rice varieties and effective weed management practices for direct-seeded rice.
Principal results
Rice genotypes with a greater ability to germinate and establish in flooded soils were identified, providing opportunities to develop varieties suitable for direct seeding in flooded soils. Tolerance of flooding in these genotypes was mostly attributed to traits associated with better ability to mobilize stored carbohydrates and anaerobic metabolism. Limited studies were undertaken in weeds associated with lowland rice systems. Remaining studies compared rice and weeds and related weed species such as Echinochloa crus-galli and E. colona or compared ecotypes of the same species of Cyperus rotundus adapted to either aerobic or flooded soils.
Conclusions
Tolerant weeds and rice genotypes mostly developed similar adaptive traits that allow them to establish in flooded fields, including the ability to germinate and elongate faster under hypoxia, mobilize stored starch reserves and generate energy through fermentation pathways. Remarkably, some weeds developed additional traits such as larger storage tubers that enlarge further in deeper flooded soils (C. rotundus). Unravelling the mechanisms involved in adaptation to flooding will help design management options that will allow tolerant rice genotypes to adequately establish in flooded soils while simultaneously suppressing weeds.
Introduction
Rice is cultivated in a wide range of environments, from tropical to temperate climates, including in aerobic soils in uplands to wet lowlands with uncontrolled flooding, e.g. flood-prone and deepwater rice areas. Water plays a pivotal role in the management of rice systems and the different rice agroecosystems are mostly classified based on their hydrology and the extent of water availability (Khush 1984). Irrigated lowland systems comprise ∼55 % of the total rice area and provide 70 % of global rice production, while rainfed lowland and flood-prone areas constitute ∼35 % of the total rice area, covering 47 million ha in Asia but providing only ∼25 % of global rice production because of various abiotic challenges associated with rainfed ecosystems (International Rice Research Institute 1997).
Farmers establish rice crops by either transplanting or direct seeding. Transplanting of rice seedlings from nurseries into ‘well-puddled’ waterlogged or flooded soils gives an advantage to rice over weeds due to seedling size and the flooded soil in which weed species must initially germinate and establish (Rao et al. 2007). In many areas, transplanting of rice and subsequent manual weeding have enabled the sustainability of this system as it provides good weed management, but it is labour intensive and requires considerable water for land preparation. In some rainfed areas, however, such as in Bangladesh and eastern India, where water accumulates in the field to depths exceeding 30 cm within a few days of the start of rainfall, transplanting taller and older seedlings still remains the only viable option.
Numerous variations in direct seeding are being practised based on water availability and field hydrology (Chin and Mortimer 2002; Rao et al. 2007). Rice can be dry seeded by broadcasting, dibble sowing or drilling dry seeds on dry or unsaturated soils. This technique is mostly used in areas where water availability is limited or uncertain during crop establishment, as in rainfed areas. Wet seeding uses pre-germinated rice seeds for sowing in saturated puddled soils, as commonly practised in irrigated areas. It is sometimes used in flood-prone areas after flood recession, when it is possible to drain additional standing water from the field, followed by soil puddling to suppress weeds. Water is then re-introduced into the fields 7–10 days after sowing, when seedlings are established, and the water depth gradually increased as the rice seedlings grow. Water seeding involves pre-germinated rice seeds broadcast in standing water, and is practised in some cooler areas, as in California, Central Asia and Australia. The main advantage of this method is that the majority of weed species are suppressed by the standing water. This is common in temperate irrigated areas but could potentially be used in flood-prone rainfed lowlands in the tropics where farmers can practise early sowing without waiting for complete floodwater recession, to minimize the risk of delayed maturity and late-season drought (Pandey et al. 2002). Once the rice crop has established, in most direct-seeded systems and based on water availability and control, the field is flooded to suppress weed growth and water depth is then maintained at 5–10 cm through most of the season before water is gradually drained prior to harvest.
Rice farmers in rainfed and irrigated areas are shifting to direct seeding from transplanted rice as it provides opportunities to reduce costs and can result in earlier harvest (Balasubramanian and Hill 2002). There are constraints, however, that limit its large-scale adoption, the most important of them being (i) poor germination and uneven stand establishment in areas where the land is not well levelled or water is not well controlled as in rainfed areas, and (ii) high weed infestation (Du and Tuong 2002). Commonly, lowland fields are not well levelled, which means that they can neither be completely drained nor flooded to an even depth to control weeds. With rainfall being unpredictable, flooding of low-lying areas can result in a severe reduction in rice establishment. This is likely to be a particular problem with monsoon-season rice crops.
Weeds constitute a major problem for the large-scale adoption of direct-seeded rice, with yield losses of ∼20 % of attainable yield or even total loss if not controlled (Rao et al. 2007). Although direct seeding provides opportunities for labour and water savings, current management systems for dry- and wet-seeded rice in the tropics do not usually allow standing water to be used effectively to completely suppress weed growth. Direct-seeded rice therefore faces severe challenges from competition if weeds are not adequately managed and many direct-seeded systems are reliant on herbicide use to control weeds. The use of herbicides has attendant problems such as cost, concerns related to health and the environment, and the evolution of herbicide resistance in weeds. With good water management, though, weeds can be controlled effectively by flooding the soil after direct seeding (Tuong et al. 2000).
The development of rice varieties that can germinate and emerge in flooded soils will therefore help reduce the hazards of early floods, to which rice is very sensitive (Ismail et al. 2009), and this also provides an efficient means for weed control through early flooding. This can be achieved through effectively exploiting the genetic variation in flooding tolerance during seed germination and early establishment. Moreover, the effectiveness of flooding for weed management will depend on the responses of various weeds associated with rice to early flooding, an area that has not been studied sufficiently.
Aquatic weeds and those well adapted to flooded soils are major problems in lowland rice fields. In the Philippines, the cultivation of lowland rice in rotation with upland crops and vegetables in the same fields has resulted in the selection of ecotypes of ‘upland’ weeds such as Cyperus rotundus that can tolerate flooded soils (Peña-Fronteras et al. 2009; Fuentes et al. 2010). Adaptation in these ecotypes suggests changes in metabolic and/or morphological growth processes that make these weeds more adapted to the flooded conditions of paddy fields.
Water management has long been recognized as an effective cultural weed control practice in lowland rice (Rao et al. 2007), as flooding the soil could affect the density, vigour and uniformity of rice stands, as well as the severity of weed competition and the effectiveness of herbicides (Kim et al. 2001). Rice farmers in California converted entirely from dry seeding to water seeding in the early part of the 20th century mainly to manage Echinochloa crus-galli (Hill et al. 2001). Water depth alone exerts a dominant effect on the structure of weed communities and the fate of weeds recruited into the growing crop (Janiya et al. 1999). Flooding depth and duration also have a differential effect on the survival and growth of weed species. In Africa, Kent and Johnson (2001) reported that flooding to 2–8 cm increased the density of Sphenoclea zeylanica and decreased that of Echinochloa colona and E. crus-pavonis compared with saturated soil conditions. Increased flood duration from either 2 or 4 days within every 7 days to continuous flooding had no effect on S. zeylanica, but decreased the numbers of E. colona and E. crus-pavonis. Gealy (1998) reported that deep flooding may reduce Ipomoea spp. infestation. Different weed species seem to respond differently to water management and either selection of tolerant ecotypes for some weeds associated with rice or shifts in weed species composition means that traditional soil puddling and flood management may no longer be effective for their control. Understanding the mechanisms associated with adaptation to such aquatic systems in rice and associated weeds could facilitate the development of management strategies that favour rice establishment but simultaneously suppress weeds in flooded soils.
Substantial progress has been made in understanding the mechanisms of tolerance of anaerobiosis in different plant species during the last few decades (Kennedy et al. 1992; Vartapetian and Jackson 1997). This review attempts to summarize the major adaptive features of rice for low-oxygen stress during seed germination and early growth. It further highlights some of the mechanisms probably associated with tolerance of a few wetland weeds and weeds that recently became associated with lowland paddy conditions. The review further discusses the prospects of using knowledge gained in plant adaptation to anaerobic conditions for application to optimize crop establishment in rice.
Rice adaptation to anaerobic systems
Flooding stress in lowland rice ecosystems
Despite being a semi-aquatic species, rice is sensitive to various types of flooding stress and this sensitivity varies with the genotype, stage of development, duration and depth of flooding, and floodwater conditions (Jackson and Ram 2003; Sarkar et al. 2006; Das et al. 2009; Mackill et al. 2012). Even shallow flooding of the soil caused by heavy rainfall soon after seeding can reduce germination and result in poor crop establishment, since rice is sensitive to flooding during germination (Yamauchi et al. 1993; Ismail et al. 2009). During vegetative growth of rice, the most common and damaging type of flooding is short-term complete inundation (up to 2 weeks), also referred to as flash floods. This type of flooding currently affects more than 20 million ha of rice-growing areas in Asia (Mackill et al. 1996; Bailey-Serres et al. 2010), and can result in severe damage and plant mortality if it is sustained for longer than a week. The extent of damage caused by complete submergence during the vegetative stage is largely modulated by environmental conditions, with higher temperatures, greater water turbidity and lower solar radiation worsening the severity of the stress (Das et al. 2009).
Rice genotypes tolerant of complete submergence at the vegetative stage, such as the Indian landrace FR13A, were identified that can survive submergence for over 2 weeks, and a single gene responsible for tolerance (SUB1A) was cloned and its role in conferring tolerance established (Xu et al. 2006; Bailey-Serres et al. 2010). Furthermore, a marker-assisted backcrossing system was developed and used to transfer SUB1 into several popular rice varieties, and some of them have already been released for commercial use in several countries in Asia. These varieties showed a yield advantage of 1 to >3 t ha−1 over the original varieties following submergence for a few days to 18 days (Neeraja et al. 2007; Septiningsih et al. 2009; Singh et al. 2009; Mackill et al. 2012).
Longer-term partial or stagnant flooding is also common in low-lying areas, where water accumulates through most of the growing season and at various depths (Mackill et al. 1996; Singh et al. 2011). A water depth of 30–50 cm is common in flood-affected rainfed areas, and is referred to as medium-deep or stagnant floods (SF). These water depths depress yields by hindering tillering and plant growth, and can result in lodging and poor grain quality. Under more extreme conditions, water depths can reach several metres, and this is referred to as deepwater or floating rice (Catling 1992). Farmers in the latter ecosystems grow specific varieties that undergo rapid internode elongation to maintain the top of the plants above the water surface. Recently, two genes, ‘SNORKEL1’ and ‘SNORKEL2’, that are responsible for this internode elongation under deepwater conditions were cloned (Hattori et al. 2009).
Regardless of the challenges posed by submergence, rice has evolved features that allow it to flourish in a wide range of environments affected by flooding. However, most of the traits associated with tolerance of different types of flooding stress identified so far are expressed better in traditional landraces being grown by farmers. These landraces have low yield potential with poor combining ability, making progress to transfer these characteristics into high-yielding genotypes through conventional breeding relatively slow. However, faster progress is now being witnessed after recent developments in molecular and genomics tools and approaches (Xu et al. 2006; Bailey-Serres et al. 2010).
Genetic variation in flooding tolerance during germination and early growth in rice
Breeding for better germination, greater seedling vigour and higher tolerance of waterlogging in rice has been attempted before (Yamauchi et al. 1993; Redoña and Mackill 1996; Biswas and Yamauchi 1997), but with limited success because genotypes with sufficient tolerance were not available (Ling et al. 2004).
In a quest for submergence tolerance at the seedling stage, Yamauchi et al. (1993) screened 258 accessions from the International Rice Research Institute (IRRI) gene bank and 404 from the International Network for the Genetic Evaluation of Rice (INGER), using seeds pre-germinated for 2 days and then sown at 25-mm soil depth and submerged with 20–50 mm of water. Using this system, 12 genotypes were identified as tolerant, with emergence in the range of 54–78 %, compared with 7–19 % for the sensitive genotypes. Furthermore, these authors observed that tolerant genotypes produce longer coleoptiles under hypoxia, in a manner independent of O2 and ethylene concentrations; however, their mesocotyls and shoots elongate faster in response to ethylene than those of sensitive genotypes (Yamauchi et al. 1994; Yamauchi and Winn 1996; Biswas and Yamauchi 1997).
Ling et al. (2004) evaluated 359 accessions from different sources, including indica and japonica accessions, using a water depth of 20 cm at 30 °C. They used shoot elongation (coleoptiles) as the criterion for selecting tolerant accessions and identified reasonable variation in coleoptile length after 5 days under these conditions, although all genotypes failed to produce visible roots under such conditions. They further selected two contrasting genotypes, one with slow shoot elongation (DV85; 0.3 cm) and the other with faster shoot elongation (Kinmaze; 3.1 cm), and crossed them to develop a set of 81 recombinant inbred lines. These lines were subsequently genotyped to construct a genetic map, and phenotyped using the same criteria, leading to the detection of five quantitative trait loci (QTL), one each on chromosomes 1, 2 and 7, and two on chromosome 5. The method used for screening in these studies can detect variation in the ability of the coleoptiles to elongate under flooded conditions, but not necessarily variation in ability to produce shoots and roots and survive the shallow water depths commonly experienced in flooded fields. Tolerance of these genotypes and the effectiveness of these QTLs will therefore warrant further validation.
Recently, a small number of rice genotypes with greater tolerance of flooding during germination and early seedling growth were identified following large-scale screening of >8000 genebank accessions and breeding lines at IRRI (Ismail et al. 2009; Angaji et al. 2010). These accessions were phenotyped by sowing dry seeds in soil and then flooding with 80–100 mm of water; this is more stringent than the screening methods of Yamauchi et al. (1993) using pre-germinated seeds and shallower water depth. Furthermore, tolerant genotypes were selected based on ability to generate roots and shoots, including new leaves, and to survive flooding by emerging from the floodwater within 3 weeks after sowing. In contrast, Ling et al. (2004) assessed only variation in coleoptile growth. One of these lines, ‘Khao Hlan On’, was crossed with a widely grown lowland variety, ‘IR64’, and a BC2F2 population was developed and used for genetic analysis. Five QTLs were identified, one each on chromosomes 1, 3 and 7, and two on chromosome 9, explaining from 18 to 34 % of the phenotypic variation. Cultivar ‘Khao Hlan On’ contributed the tolerant alleles at all QTL loci. Two of these QTLs, on chromosomes 7 and 9, are considered major QTLs and are currently being fine-mapped and cloned to facilitate their use in breeding. Additional mapping populations were developed using other tolerant lines to identify additional QTLs and to facilitate combining multiple genes for higher tolerance. Together, these efforts reveal a vast genetic variation in flooding tolerance during germination and early seedling growth in lowland rice, which can be further explored through breeding.
Traits associated with tolerance of anaerobic conditions during germination and early seedling growth in rice
Seeds of major crops such as maize, wheat, barley, oats and sorghum are more sensitive to anaerobic conditions during germination, and they fail to germinate and lose viability when seeded in flooded or waterlogged soils, resulting in poor crop establishment (Perata et al. 1997; Setter et al. 1997; Vartapetian and Jackson 1997). Of the major cereals, rice is the only crop capable of germination under water (Taylor 1942; Yamauchi et al. 1993; Ella and Setter 1999; Angaji et al. 2010), but further growth is limited to the coleoptiles (Biswas and Yamauchi 1997). Plant tissues in germinating seeds of rice can suffer from hypoxia or even anoxia in submerged paddy fields (Setter et al. 1988; Drew 1990; Greenway and Setter 1996), which could severely limit crop establishment in such soils because the lack of oxygen could hinder the functions of the enzymes involved in mobilizing stored carbohydrates and generating energy by oxidative pathways. Several growth, anatomical and physiological traits are identified that are associated with germination and survival of flooded soils.
Coleoptile extension and its regulation
Rice coleoptiles can elongate faster at low O2 concentrations than in air, but germinating seeds normally fail to form leaves and roots (Alpi and Beevers 1983; Ishizawa and Esashi 1984). However, variation in the rate of coleoptile elongation under low oxygen (0–5 %) was observed among 10 rice genotypes with maximum elongation observed at 0 % oxygen (Turner et al. 1981). Rapid elongation of coleoptiles could facilitate contact with air in waterlogged or flooded soils and subsequent aeration of the growing embryo. Traits associated with coleoptile elongation of pre-germinated seeds under anoxia have been investigated before, and this growth was found to be independent of ethylene synthesis (Pearce and Jackson 1991; Pearce et al. 1992), but dependent on the rate of ethanol synthesis (Setter et al. 1994), suggesting the importance of anaerobic metabolism during germination in flooded soils.
Tolerance of early flooding, where rapid coleoptile growth is considered important, therefore contrasts with tolerance of flooding at the later vegetative stages of growth, when submergence injury is exacerbated by rapid growth and use of carbohydrate reserves (Jackson and Ram 2003; Baily-Serres et al. 2010). However, our recent work (D. J. Mackill and A. M. Ismail, unpublished) showed that it is possible to combine both tolerance of anaerobic conditions during germination and during early growth with that during the vegetative stage conferred by the SUB1 gene, involving inactivation of shoot elongation. This raises the question of the specific time point and conditions during growth and development when either of these opposing mechanisms could dominate and how signalling is mediated to trigger the expression of one mechanism or the other. One possible argument is that tolerance during germination and early growth is regulated by low oxygen during the early stages. At these early stages, the germinating and growing embryo is dependent on stored carbohydrates in the seed endosperm and when absence of aerial or underwater photosynthesis prevents the generation of oxygen under water, and while aerenchyma tissue is still not well developed to provide aeration of the growing tissue. However, once seedlings become autotrophic, oxygen is made available either through underwater photosynthesis or direct contact with air. By then, the mechanisms associated with tolerance of flooding during germination will no longer dominate. Other mechanisms such as rapid underwater elongation, as in deepwater rice varieties, or the dormancy strategy mediated by the SUB1 gene in submergence-tolerant varieties, become functional when the seedlings are flooded. Substantial efforts are still needed to resolve this enigma, and reveal the signalling mechanisms that regulate the shifts in metabolic pathways and functioning of the genes involved in each type of flooding tolerance.
Mobilization of stored carbohydrates
Generally, seeds with carbohydrate reserves, such as cereals, are known to be more tolerant of hypoxia during germination than seeds with fatty acid reserves (Al-Ani et al. 1985; Raymond et al. 1985). Moreover, several studies investigated the processes of starch breakdown under aerobic conditions (Beck and Zeigler 1989; Fincher 1989), yet the importance of activities and levels of expression of starch-degrading enzymes under low-oxygen stress has not been sufficiently established. Amylases are believed to play a major role in starch breakdown in cereal seeds (Murata et al. 1968), and rice seeds retain their ability to break down starch into readily fermentable carbohydrates when germinating under hypoxic or even anoxic conditions (Atwell and Greenway 1987). Rice seed has the complete set of enzymes needed for degradation of starch and its use for growth and maintenance of the growing embryo; however, the activities of these enzymes are affected by oxygen availability. The importance of α-amylases in starch degradation when O2 is limiting was reported in previous studies (Guglielminetti et al. 1995; Perata et al. 1997; Hwang et al. 1999), which indicate that both expression and translation can occur under anoxia (Perata et al. 1992, 1993). Studies also indicated that the induction of amylases is controlled by either plant hormones or glucose starvation (Umemura et al. 1998; Loreti et al. 2003).
These studies demonstrated the unique ability of rice to break down starch reserves during germination in anaerobic soils compared with other cereals. Furthermore, genetic variation in the ability to break down starch into usable soluble sugars was also reported within rice. Genotypes that are more tolerant of flooding during germination seem to have better capabilities for breaking starch into simple sugars, as demonstrated by faster depletion of starch in their germinating seeds compared with sensitive genotypes (Ismail et al. 2009). Tolerant genotypes also showed greater total amylase activity in germinating seeds, with a progressive increase in activity from sowing to reach several-fold at 3 days after seeding in flooded soil. This higher amylase activity was also associated with maintenance of higher soluble sugar concentrations in germinating seeds, greater starch depletion, better shoot and root growth, and higher seedling survival (Ismail et al. 2009). Tolerant varieties also seem to store a higher proportion of carbohydrates in their seeds as soluble sugars, and maintain higher concentrations with time during germination and early growth (Fig. 1).
Fig. 1.
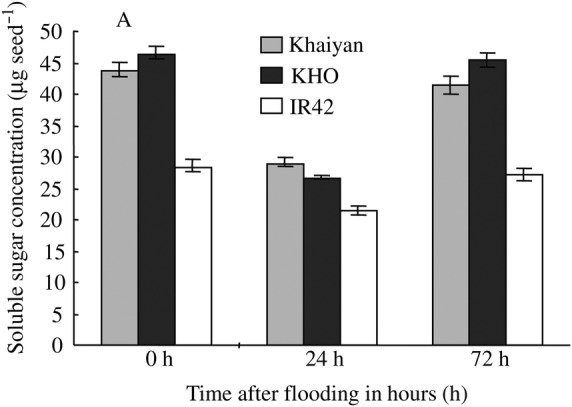
Soluble sugar concentrations in seeds of flooding-tolerant Khaiyan and Khao Hlan On (KHO) and intolerant IR42 after 0 (dry), 24 and 72 h of hypoxia. Seeds were incubated in 0.1 % sterile agar solution maintained under hypoxia (0.03 mol O2 m−3) by bubbling with N2 gas. Analysis was performed as described in Ismail et al. (2009). Data are means of two experiments, each with two biological replicates, and vertical bars are ±SE.
Expression analyses using primers specific for various members of the amylase gene family indicated that only RAmy3D is differentially expressed in tolerant and sensitive genotypes under hypoxia, with stronger expression in a tolerant genotype (‘Khaiyan’) than in a sensitive genotype (‘IR42’) from 24 to 72 h under hypoxia (Ismail et al. 2009). RAmy3D is important for oligosaccharide degradation (Terashima et al. 1997) and is regulated by sugar availability (Loreti et al. 2003). Hwang et al. (1999) found that anoxia diminishes the expression of Amy1 and Amy2 gene subfamilies, but up-regulates many of the Amy3 subfamily members in rice embryos. RAmy1A has a gibberellic acid (GA)-responsive element in its promoter that is absent in the promoter of RAmy3D (Lu et al. 1998) and this is probably why RAmy1A is affected by residual GAs present in epithelium layers (Kaneko et al. 2002). Terashima et al. (1997) also showed that the role of RAmy1A is mainly in the hydrolysis of soluble starch, whereas the barrel structure of RAmy3D helps degrade complex oligosaccharides. The intolerant lines showing RAmy1A expression may still have sufficient residual GA for induction during anoxia, although GA action is likely to be arrested in anaerobic tissues.
Considering that α-amylase 3 constitutes ∼60 % of the total amylase mRNA in rice cells starved of glucose (Lu et al. 1998), RAmy3D may play a significant role in tolerant cultivars during hypoxia and, together with other active amylases, may help promote rapid starch hydrolysis to stimulate alcohol fermentation and sustain the energy required during germination. The variations in α-amylase activity under air, hypoxia and anoxia probably suggest that the ability of germinating rice seeds to sense sugar availability is a complex control mechanism that is regulated differently in tolerant and intolerant rice cultivars. Further studies are needed to unravel these regulatory mechanisms. Understanding the allelic variation in the structural and regulatory mechanisms of this gene family could help in exploiting its use for breeding to enhance rice seed germination in flooded soils.
Anaerobic metabolism
Under anoxia, carbohydrate catabolism shifts from aerobic to anaerobic pathways (alcoholic fermentation) for generating ATP for growth and maintenance processes. However, ATP production is 18-fold less efficient in this process than is aerobic respiration (Greenway and Setter 1996). Nevertheless, an increased rate of anaerobic respiration still remains one of the most important mechanisms to alleviate the adverse effects of reduced ATP supply during oxygen deficiency, and several reports demonstrated its importance in plants (Avadhani et al. 1978; Jackson et al. 1982; Waters et al. 1991; Setter and Ella 1994; Gibbs et al. 2000). We observed genetic variation in the extent of anaerobic respiration in rice cultivars contrasting in tolerance of anaerobic conditions during germination and early seedling growth (Ismail et al. 2009). Activities of both pyruvate decarboxylase (PDC) and alcohol dehydrogenase (ADH) increased significantly within 12 h of imbibition in both tolerant and sensitive genotypes, but remained progressively higher in the tolerant genotypes with time. Pdc1 and Pdc2 and Adh1 and Adh2 showed greater expression under hypoxia but with no apparent differences between tolerant and sensitive lines. Altogether, the data suggest that anaerobic respiration is probably one of the important attributes contributing to the superior performance of tolerant rice genotypes, and that the genetic variation in the extent of induction of this pathway is probably regulated post-transcriptionally. Further studies should confirm whether this variation in activity of PDC and ADH is associated with the variation in tolerance observed between rice cultivars.
Role of plant hormones
The plant hormone ethylene promotes rice seedling growth under flooded conditions through effects mediated by GA (Fukao et al. 2006), and this flooding-induced elongation is one of the escape mechanisms that help submerged plants regain contact with air. This elongation is beneficial in early growth as elongation of the coleoptiles (Ku et al. 1970) and mesocotyls (Suge 1971), but a disadvantage for seedlings if the duration of flooding is short because of the tendency of elongating plants to fall over after the water recedes (Ella et al. 2003; Das et al. 2009). Excessive shoot elongation with failure to reach the water surface can also lead to exhaustion of carbohydrate reserves, leading to plant death (Jackson and Ram 2003; Sarkar et al. 2006). The elongation-promoting effect of ethylene in rice is not only on the coleoptile and mesocotyl of young seedlings and internodes of older plants, but also on other plant parts such as leaves (Jackson et al. 1987) and roots (Konings and Jackson 1979). Several studies show the importance of ethylene interactions with other plant hormones such as abscisic acid (ABA), auxins and gibberellins under low-oxygen stress; however, the effects of these plant hormones are hard to predict and sometimes contentious, probably because of various environmental factors and floodwater characteristics that could alter these responses. In the absence of oxygen, ethylene, auxins and GA are typically inactive in rice (Raskin and Kende 1984; Pegoraro et al. 1988); however, auxins can stimulate coleoptile elongation under aerobic conditions (Pegoraro et al. 1988). Carbon dioxide that accumulates in plant tissue in the absence of photosynthetic carbon fixation can also promote elongation (Suge and Kusanagi 1975; Raskin and Kende 1984).
Synergistic and counteracting interactions of various plant hormones are apparent in several growth systems of plants (Davies 1995). For example, ethylene promotes internode elongation in deepwater rice, causing rapid elongation when plants are submerged (Suge 1974; Metraux and Kende 1983), and this effect is mediated through an ethylene-induced increase in the responsiveness of the internode tissue to GA. Ethylene seems to mediate this effect through damping endogenous ABA, the potent antagonist of GA, and therefore the extent of growth becomes dependent on the relative concentrations of endogenous GA and ABA (Hoffmann-Benning and Kende 1992). Moreover, ethylene was reported to enhance sucrose transport from the scutellum to the growing coleoptile in germinating rice seeds, in which sucrose is cleaved into glucose and fructose (Ishizawa and Esashi 1988). The promoting effect of ethylene on coleoptile growth seems to be mediated at later stages of seedling germination. Earlier, Pearce et al. (1992) reported that coleoptile elongation in media devoid of oxygen or enriched with CO2 is independent of ethylene. In our recent work, we also observed a delay in endogenous ethylene synthesis by germinating rice seeds, but ethylene increased significantly only 3 days following imbibition under flooded conditions, with a substantially greater increase in concentration with time afterward in the tolerant genotype (Ismail et al. 2009). These data suggest that the role of ethylene is probably more important during coleoptile and shoot elongation than during germination under hypoxia.
Additional traits probably involved in the survival of anaerobic soils
Rapid cell expansion is necessary for fast growth under flooding and low-oxygen stress in germinating seeds. Expansins are a group of non-enzymatic proteins known to regulate cell wall expansion. These proteins are encoded by a gene superfamily with at least two major groups: α-expansins and β-expansins. Their role in cell extensibility has been reported in many crops, including rice (Huang et al. 2000). Transcript expression patterns of Os-EXP1, Os-EXP2, Os-EXP3 and Os-EXP4 genes were often highly correlated with elongation (Cho and Kende 1997; Huang et al. 2000). Transcript profiling of anoxia-grown rice seedlings revealed that EXPA7 and EXPB12 are likely to be involved in rice coleoptile elongation under anoxia (Lasanthi-Kudahettige et al. 2007).
Another group of proteins known to be involved in regulating cell wall expansion are the peroxidases. These enzymes are responsible for the assembly of lignins and proteins in the cell wall (Whitmore 1978) and for the binding of ferulic acid to the cell wall by the formation of diferuloyl cross-links to matrix polysaccharides (Fry 1979), subsequently reducing wall extensibility and restricting cell elongation. Higher peroxidase activity is closely associated with reduced elongation in plants such as mung bean (Goldberg et al. 1987) and peanut (Zheng and van Huystee 1992). In their study on the effect of ethylene and ethylene inhibitors, Lee and Lin (1996) reported that ethylene enhanced coleoptile elongation but decreased peroxidase activity, especially the wall-bound form, and the introduction of an ethylene action inhibitor blocked both ethylene-mediated coleoptile elongation and the decline in peroxidase activity. The authors further speculated that the activity of peroxidases, especially the cell-wall-bound form, is inversely related to the rate of coleoptile elongation in rice. Seeds of tolerant rice genotypes germinating under anoxic conditions showed substantially lower peroxidase activity than the intolerant genotypes (Ismail et al. 2009). Furthermore, the activity of peroxidases in germinating embryos correlated negatively with coleoptile growth and seedling survival. Together, peroxidases and expansins could play a role in regulating cell-wall growth and elongation during germination in flooded soils.
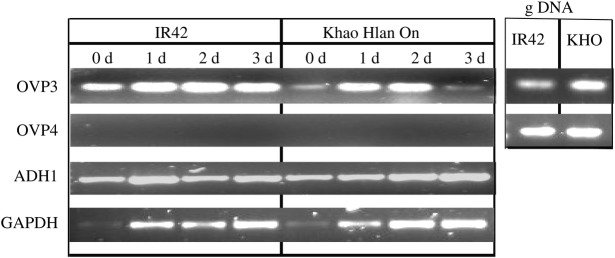
Specific genes that are involved in energy metabolism or sensing may also be important for germination under low-oxygen stress. Using rice genotypes contrasting in tolerance of anoxia during germination, Liu et al. (2010) studied the expression of the H+-vacuolar pyrophosphatase (OVP) genes, six of which were identified in the rice genome. Out of these six genes, only OVP3 was found to be responsive to anoxia during germination. This was attributed in part to the promoter region of this gene, which contains more anoxia-inducible motifs than the putative promoters of the other five OVP genes. Comparing the expression of OVP3 in a tolerant variety (Kho Hlan On) and a sensitive variety (IR42), we observed transient expression during the first 2 days following sowing in hypoxic conditions in the tolerant line, but constitutive expression in the sensitive line (Fig. 2). The functional role of this gene in anoxia tolerance is yet to be known, but is speculated to be associated with energizing proton transport into the vacuoles, thus mitigating membrane depolarization (Liu et al. 2010).
Fig. 2.
Expression of vacuolar H+-pyrophosphatase (OVP3) under hypoxia in Khao Hlan On and IR42 at 0 (dry), 24, 48 and 72 h of hypoxia. Seeds were incubated in 0.1 % sterile agar solution maintained under hypoxia (0.03 mol O2 m−3) by bubbling with N2 gas. RNA extraction and reverse transcriptase-polymerase chain reaction analysis was performed as described in Ismail et al. (2009). The following primers were used: OVP3F, 5′-AATTTGAGGACGGACGGAGAT-3′; OVP3R, 5′-GGCTCAGGCAGACAGAAACT-3′; OVP4F, 5′CTGGGACAATGCCAAGAAAT-3′; OVP4R, 5′-ATGATTGTTTACTCCGTGCG-3′ (Liu et al. 2010). Adh1 expression was included as a check for weak housekeeping gene GAPDH at 0 (dry) time-point and genomic DNA (gDNA) for OVP3 and OVP4 was used to check for effectiveness of the primers and gene presence. The Adh1 primers used were: Adh1F, 5′- CCAGTTCAGCAGGTACTTGC -3′; Adh1R, 5′- CAGGATACACAGAAGAACCG -3′ (Fukao et al. 2006).
These studies suggest that numerous mechanisms are potentially involved in rice adaptation to anaerobic conditions during germination and early growth. Exploiting these traits in germplasm enhancement will be possible if sufficient genetic variation is available in the cultivated rice gene pool or wild relatives. More efforts are also needed to establish which of these mechanisms and the genes involved could be used effectively to develop rice varieties tolerant of anaerobic soils.
Seed and field conditions that favour the expression of tolerance traits
The viability of seeds and their ability to germinate in flooded soils can be considerably affected by the specific conditions in which the seeds were handled and stored prior to sowing, and by how the seedbed was prepared. Knowledge of the practices that prolong seed longevity prior to sowing, as well as optimum seedbed conditions at sowing, can enhance the performance of tolerant cultivars. Our recent studies pinpoint some strategies and seedbed management options that can improve seedling establishment in flooded soils (Ella et al. 2010, 2011). Furthermore, these studies show that even a slight deterioration in seed quality and viability caused by poor pre-sowing conditions can have serious consequences when seeds are sown in flooded soils, though these effects are not detectable under control conditions. Furthermore, the studies highlight the benefits of combining good management practices with genetic tolerance for better crop establishment, as tolerant genotypes seem to respond better to these manipulations than sensitive ones.
Older seeds and seeds stored at warmer temperatures had lower germination, and seedling establishment in flooded soils was poorer (Ella et al. 2010). Seedling survival and growth were highest at floodwater temperatures of 24–26 °C, but significantly less at lower (18–20 °C) or higher (30–32 °C) temperatures, although this range in optimum temperature for germination in flooded soils seems surprisingly narrow. Survival is also greater at shallower water depth and in water with less algal growth. In another set of trials (Ella et al. 2011), the influence of pre-soaking of seeds for 24 h prior to sowing or of priming (soaking for 24 h, followed by drying) was tested. Both treatments accelerated and improved germination and seedling survival in flooded soils, especially of the tolerant genotypes. Priming reduced lipid peroxidation and increased the activity of superoxide dismutase (SOD) and catalase (CAT). It also enhanced the activity of total amylases, hastening the breakdown of starch. Survival after flooding correlated positively with amylase activity but negatively with the extent of lipid peroxidation prior to sowing. Under all conditions, a reduction in survival was strongly associated with higher lipid peroxidation and with reduced activity of some enzymes associated with scavenging of reactive oxygen species (ROS), such as SOD and CAT, as well as with reduced activity of amylase enzymes that are involved in carbohydrate mobilization in germinating seeds.
Generation of ROS is known to occur during the dehydration of various plant tissues, such as during seed maturation and seed storage (Smirnoff 1993), and the peroxidative change in polyunsaturated fatty acids is likely to be one of the major causes of deterioration of stored seeds (Wilson and McDonald 1986; Hendry 1993; Sung 1996). Oxidative stress could also inhibit growth and development by arresting cell division in the growing embryo (Reichheld et al. 1999). Numerous studies reported the presence of several antioxidative and hydrolytic enzymes in dry seeds, and the activity of certain enzymes rises considerably after the start of imbibition, such as peroxidases in tomato (Morohashi 2002), which are known to play an important role in protecting seeds from oxidative damage besides other functions (Moosavi et al. 2009), and polyphenol oxidase in wheat (Demeke et al. 2001).
Seed priming has long been established as an effective method for ensuring rapid and uniform seed germination in cereals and vegetables sown in aerated soils (e.g. Lee et al. 1998; Farooq et al. 2006). In most of these studies, seed priming was reported to increase seed vigour and extend seed longevity, and was associated with improvement in activity of several enzymes associated with the germination process, besides enhancing synthesis of nucleic acids and triggering faster cell division upon hydration. Repair of DNA and cellular membranes likely to be damaged during dehydration of seeds was also thought to take place during seed priming (Dell'Aquila and Taranto 1986; Fu et al. 1988). These preparatory and repair processes are probably more important when seeds are to be sown under less favourable conditions, as is the case with flooded soils. Besides, both priming and pre-soaking seem to accelerate germination and seedling emergence from flooded soils by 1–2 days. Seed priming will be more effective for areas likely to be affected by uncontrolled flooding, as in rainfed areas, where primed seeds can be dried and stored until sufficient rainwater accumulates in the field. However, pre-soaking seeds before sowing will be effective in areas where water resources are secure and controlled at the time of sowing.
Adaptation of weeds to flooding during establishment
Weeds have a major economic importance for rice production in South and Southeast Asia, and their control constitutes up to 30 % of the total production cost. More than 1800 plant species were reported as weeds of rice in Asia (Moody 1989), and those of the Cyperaceae and Poaceae are predominant (Rao et al. 2007). Relatively few of these are considered pan-tropical, with those tolerant of flooding regimes commonly found in lowland rice (Table 1). Of the grass weeds affecting rice, E. crus-galli is one of the most widespread, and studies have shown a range of mechanisms that allow some subspecies or ecotypes to germinate and establish in flooded conditions. This species is likely to have attracted the particular attention of researchers due to its wide distribution, lack of effective control measures and the considerable crop losses that its infestations can cause. Other species probably have adaptive mechanisms, but these have not been adequately studied yet.
Table 1.
Examples of some common weeds associated with paddy rice and possible traits associated with tolerance of anaerobic conditions.
| Species | Tolerance of oxygen deficiency | Seed/tuber germination under hypoxia | Adaptive traits | References |
|---|---|---|---|---|
| Cyperus difformis L. | Tolerant | No | Rapid early growth, underwater photosynthesis | Sanders (1994) |
| Cyperus rotundus L.Purple nutsedge ‘Lowland ecotype’ | Tolerant | No | High amylase activity; increased PDC and ADH after 24 h flooding; bigger tubers, CHO reserves | Peña-Fronteras et al. (2009) |
| ‘Upland ecotype’ | Intolerant | No | High amylase activity; increased PDC and ADH after 48 h flooding | |
| Diplachne fusca (L.) Beauv. | Tolerant | Yes | Rapid elongation of fourth and fifth leaves | McIntyre et al. (1989) |
| Echinochloa colona L. Barnyardgrass/jungle grass | Intolerant | No | Poor coleoptile growth; lower PDC and ADH; poor coleoptile growth | L.P. Estioko et al. (unpublished) |
| Echinochloa crus-galli (L.) Beauv. | ||||
| Barnyardgrass | Tolerant | Yes | Increased PDC and ADH; fast coleoptile growth | L.P. Estioko et al. (unpublished) |
| var. oryzicola | Tolerant | Yes | NADP Malic increased; PEP C decreased | |
| Cockspur grass var. ‘formosensis’ | Tolerant | Yes | Increased PDC and ALDH; increased aldolase | Rumpho and Kennedy (1981) |
| var. ‘practicola’ | Intolerant | No | Same ADH, enolase, SUS | Fukao et al. (2003) |
| Leptochloa chinensis L. | Intolerant | No | Tolerates only shallow flooding (<20 mm) germination/seedling stage | Chauhan and Johnson (2008) |
| Zizania sp. Wild rice | Tolerant | Yes | Increased ADH and LDH; lysigenous spaces in roots | Muench et al. (1993); Stover(1928) |
Wetland areas tend to show greater commonality across geographical areas than dryland systems (Sculthorpe 1967). Further, in wetlands, it is usual for moist, saturated and flooded conditions to exist, providing a range of micro-environments for flood-tolerant species to germinate and establish (Etherington 1983). These conditions are not unlike those found in many lowland rice fields and this may help to explain the widespread distribution of many lowland rice weeds, as well as the range of ecotypes that exist. As a likely result, several of the widespread weeds of rice are among those described as being the world's worst weeds: E. crus-galli, E. colona, Cyperus difformis, C. iria, F. miliacea, Ischaemum rugosum and S. zeylanica (Holm et al. 1977).
Flooding as a control measure for weeds in paddy fields
Despite many rice weeds being well adapted to lowland conditions, flooding has a major suppressive effect on the emergence of most rice weeds and has long been used as an effective measure for weed control, as in transplanted and water-seeded rice. The need to allow the rice to establish aerobically in direct-seeded systems makes early flooding ineffective, and gives opportunities for weeds to establish. Some species, such as F. miliacea and E. colona, show little or no innate or induced dormancy and germinate rapidly on the surface of saturated soils (Kim and Moody 1989), but can be suppressed by shallow water. Likewise, germination of the invasive grass weed Leptochloa chinensis is strongly suppressed by standing water (>15 mm), and establishment was found to be prohibited by water depths of >50 mm (Mortimer et al. 2005; Chauhan and Johnson 2008). Others, such as C. difformis, exhibit polymorphism in germination responses to flooding, which is possibly a ‘bet-hedging’ survival tactic against unpredictable flooding events.
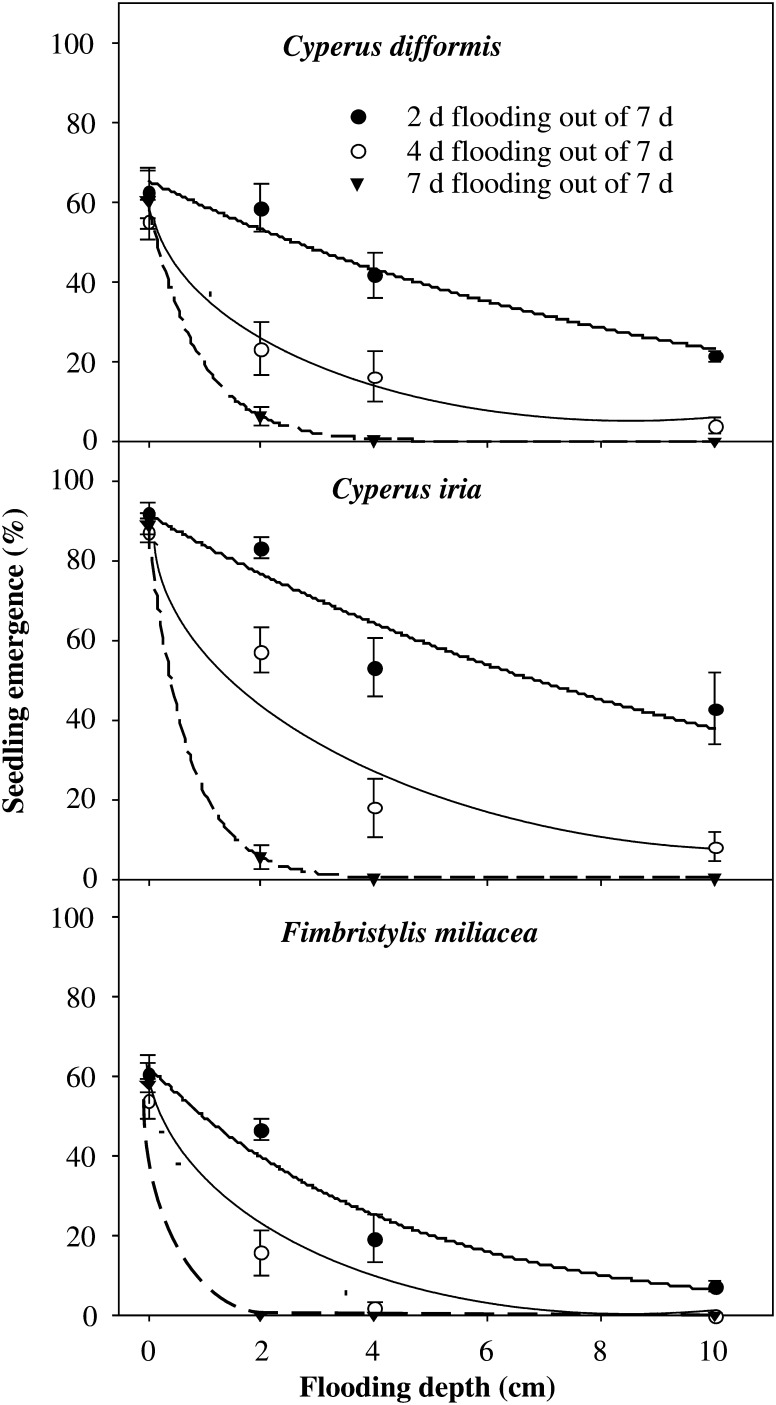
In a study involving three common sedge weeds of rice, C. difformis, C. iria and F. miliacea, Chauhan and Johnson (2009) observed that continuous flooding of 2 cm depth was sufficient to reduce the emergence of C. difformis to <10 % of that of seeds sown in saturated soils, and 4 cm of flooding was sufficient to completely inhibit the emergence and growth of C. difformis (Fig. 3). Intermittent shallow flooding did not reduce seedling emergence, but, with the water depth increased to 10 cm, emergence decreased by 97 %. Flooding affected seedling emergence of F. miliacea more than that of C. difformis and C. iria, and a flooding depth of 4 cm for 4 days out of each 7 days is more effective than just waterlogging (0 cm standing water). However, seedlings did not emerge when continuous flooding was maintained at 2 cm depth or more. These data demonstrate the considerable variation in the responses of various weed species to flooding during establishment; however, little is known about the basis for these variable responses. Further, distinctions between functional groups of weed species based on germination response may be blurred by ecotypic differentiation (Mortimer et al. 2005).
Fig. 3.
Effects of flooding depth and duration on seedling emergence (%) of C. difformis, C. iria and F. miliacea (adapted from Chauhan and Johnson 2009).
Adaptation to anaerobic conditions in rice weeds
The lack of information on the mechanisms of tolerance of aquatic environments for major weeds of rice, such as C. difformis, was highlighted before (Sanders 1994). Despite its very small seed size, C. difformis is able to grow much faster than rice during the early growth stages; the author further speculated that submerged and aerial leaves may have evolved differing biochemical processes to support photosynthesis. Echinochloa crus-galli var. oryzicola is able to germinate in an oxygen-free environment, and it produced a 2- to 3-cm shoot after 7 days growth in the light or dark under N2 (Rumpho and Kennedy 1981).
Contrasting responses to low-oxygen stress were observed when comparing Echinochloa oryzoides (barnyard grass) and rice (Pearce and Jackson 1991; Pearce et al. 1992). When grown in media short of oxygen or enriched with CO2, extension of the shoot of E. oryzoides was slowed due to reduced growth of the mesocotyl, while that of the rice shoot was promoted through enhanced growth of the coleoptile. Interestingly, these contrasting responses were not caused by altered ethylene synthesis, since ethylene concentration was not affected by high CO2 and was even depressed by oxygen deficiency in both species. Moreover, neither exogenous ethylene nor an ethylene action inhibitor exerted any effects on shoot extension in anoxic seedlings of both species, but the endogenous concentrations of the ethylene precursor l-aminocyclopropane-l-carboxylic acid increased, resulting in fast ethylene formation upon aeration. This led the authors to speculate that ethylene may have no regulatory role in the absence of oxygen, and that this post-anoxic ethylene production may have contributed to the faster extension by rice coleoptiles and slower extension by mesocotyls of E. oryzoides under low-oxygen stress. Our recent studies also showed that the increase in endogenous ethylene in rice seeds germinating under hypoxia took place a few days after imbibition, and that the delay was more obvious in sensitive genotypes with slower coleoptile elongation (Ismail et al. 2009), suggesting that ethylene synthesis and its effect are probably occurring after the coleoptiles gain contact with air or with upper floodwater layers where the oxygen concentration is relatively high. Whether this process is regulated by regaining contact with air or light remains to be established.
Fukao et al. (2003) compared anaerobic metabolism during germination in anoxia-tolerant and -intolerant E. crus-galli subspecies, and assessed the activity of a set of associated enzymes. The activity of all enzymes studied was elevated during anoxia in both tolerant and sensitive subspecies. However, only aldehyde dehydrogenase (ALDH) activity was more strongly induced under anoxia in the anoxia-tolerant subspecies. Our recent studies comparing accessions contrasting in tolerance of low-oxygen stress during germination and early growth within rice and weed species showed similar trends, with ALDH strongly induced only in tolerant lines in both rice and weeds (L. P. Estioko, A. M. Baltazar, F. E. Merca, A. M. Ismail and D. E. Johnson, unpublished). The role of ALDH in detoxifying acetaldehyde that accumulates upon re-aeration of submerged rice plants was established before (Tsuji et al. 2003a), and two mitochondrial genes, Aldh2a and Aldh2b, were cloned, but only Aldh2a, mapped to the long arm of chromosome 2, was found to be induced by submergence (Nakazono et al. 2000; Tsuji et al. 2003b). Aldehyde dehydrogenase may therefore play a role in detoxifying acetaldehyde formed through alcoholic fermentation during anaerobic germination and/or in recycling of carbon for other metabolic processes.
In recent years, some weeds such as C. rotundus sedge have become problem weeds in lowland rice grown in rotation with vegetables in the Philippines and other Asian countries. These weeds are common problems with upland crops but, in paddy fields, they had been effectively controlled by prolonged flooding. The shift in the ability of these weeds to grow vigorously in flooded soils suggests their adaptation to wetland conditions and that ordinary flooding of rice fields is no longer effective against them. Elucidating the mechanisms that permit these upland weeds to tolerate flooded soil conditions could help develop better management and control strategies to curtail this shift and also provide the means for better weed management. We compared growth habit, carbohydrate reserves and metabolism, and activities of some of the enzymes involved in anaerobic respiration in an upland and a lowland ecotype of these weeds (Peña-Fronteras et al. 2009). The lowland ecotype has much larger tubers than the upland ecotype, providing more energy for use during anaerobic catabolism upon flooding. Furthermore, the tolerant ecotype maintains greater amylase activity and soluble sugars in its tubers than does the upland sedge in flooded conditions. The study further suggests that the adaptive shift in lowland sedges could be attributed to numerous traits, including large carbohydrate reserves and the ability to mobilize them to grow new sprouts, coupled with the modulation of anaerobic respiration during germination, possibly to control the use of carbohydrate reserves and sustain substrate supply to avoid starvation and seedling death during prolonged flooding. One interesting finding in this study is the inability of the tubers of both upland and lowland sedges to sprout under flooded conditions, suggesting that sprouting probably happens during land preparation for transplanted rice, a practice essentially intended for the control of these weeds.
In a subsequent study to further elucidate the physiological differences between the lowland and upland ecotypes of these sedges, Fuentes et al. (2010) observed that the lowland ecotype develops even larger tubers with increasing floodwater depth, probably as an adaptive feature for flooded-soil conditions. The lowland ecotype also develops thicker stems with larger aerenchyma air spaces than the upland ecotype, which is useful for aerating submerged plant parts. Voesenek et al. (2006) summarized various adaptive responses in wetland plants to avoid the adverse effects of submergence, including underwater photosynthesis, aerenchyma formation and enhanced shoot elongation. Underwater photosynthesis can generate more oxygen, and aerenchyma tissues facilitate its diffusion to oxygen-deprived plant parts such as roots in flooded soils. The lowland ecotypes of C. rotundus also retain their capability to mobilize and use starch reserves under hypoxia, and have lower lactate dehydrogenase activity under flooded conditions than the upland types, a feature necessary to avoid accumulation of lactic acid and the consequent cellular acidosis, which is associated with cell death under low-oxygen stress (Roberts et al. 1984). These adaptive features in the lowland ecotype are constitutively expressed; however, some of them, such as tuber growth and aerenchyma formation, are enhanced with increased severity of stress (Fuentes et al. 2010). By demonstrating the shift in adaptation in these weeds to flooded soil conditions, these studies suggest the need for alternative methods to control these weeds, other than the traditional flooding used in paddy fields.
Besides the traits described above, other plant species adapted to wetland conditions seem to possess similar or additional mechanisms that allow them to survive in these excess-water environments. Young plants of Taxodium distichum, for instance, exhibit an avoidance mechanism in reduced soil conditions by increasing aerenchyma formation and rhizosphere oxygenation that enable them to tolerate flooding in later stages (Kludze et al. 1994). Rumex species are one of the well-studied aquatic plants, and traits associated with their adaptation to such conditions include the development of large numbers of adventitious roots and petiole elongation to maintain contact with air (Blom et al. 1994). These adaptive features are influenced by differential responses to plant hormones such as ethylene and auxin, and reduced oxygen upon submergence might serve as a signalling factor. Likewise, Visser et al. (2000) reported increased porosity in adventitious roots of two monocotyledonous species (Carex sp. and Juncus sp.) and two dicotyledonous species (Ranunculus sp. and Rumex sp.) after growth in oxygen-deficient conditions. These studies also reported substantial radial oxygen loss in the basal root zone of the dicot species, which would decrease longitudinal diffusion of oxygen to the root apex and limit root growth. Huber et al. (2009) reported variation in flooding-tolerance-related traits among genotypes of Trifolium sp. and observed that both constitutive and flooding-induced variations affect genotype performance during soil flooding. Increases in root porosity and petiole length were related to better performance during shallow and prolonged soil flooding. Apparently, plant species in wetland habitats seem to have developed various strategies to survive such conditions. Some of these traits are common across a wide range of species, including rice and associated weeds.
Tolerance of anaerobic conditions is of benefit in rice but a nuisance in weeds
The renewed interest in the potential for selecting rice germplasm tolerant of flooding during germination and early seedling growth is relevant for most direct-seeded systems (Yamauchi et al. 1993), particularly to control problematic grasses and sedges in wet- and water-seeding systems (Hill et al. 2001). Direct seeding will help overcome the problems of labour shortages and associated increase in wages, reduce the need for excessive use of water for land preparation prior to transplanting, and shorten the cropping cycle, which will further help in enhancing system productivity and resource use. Using early flooding to combat problematic rice weeds will also facilitate a reduction in herbicide use and the associated health and environmental hazards. The progress made in identifying sources of tolerance in rice and in advancing knowledge on the genetics and physiology of tolerance seems encouraging, and breeding lines with enhanced ability to germinate and establish in flooded soils are within reach. Future efforts should focus on important traits in rice, such as accelerated germination, faster shoot extension, ability to produce functional roots and leaves under flooded conditions, and probably ability of seedlings to develop aerenchyma tissue to aerate submerged plant parts at earlier stages. Knowledge of the genetics, physiology and regulatory processes associated with these traits, and possibly others, will facilitate the development of better rice varieties and relevant effective seed and seedbed management practices for a reliable direct-seeding system.
Because some weed species are adapted to flooded paddy fields and wetland conditions, this presents a challenge to the current and future efficacy of weed management. Although herbicides are likely to play an important role, the evolution of herbicide resistance, declining rates of commercial release of new herbicide molecules, and concerns about environmental health and weed species shifts will militate against sole reliance on herbicides. Farmers will then be required to move towards greater integration of herbicides with cultural control measures. Among these, water management and flooding are likely to remain the most important means of cultural weed management. Enabling effective weed suppression raises the question, ‘How do we manage flooding to maximize its differential effects to enable good rice establishment while effectively suppressing weed growth?’. In turn, a second question is, ‘What else do we need to know about weed ecology to maximize the use of flooding as a tool for effective weed management in direct-seeded systems?’. To answer these inquiries one requires knowledge of the factors stimulating weed seed germination under lowland conditions—for instance, some species, including grasses and sedges (e.g. Leptochloa sp. and Fimbristylis sp.), germinate as the field is drained while others, such as Diplachne sp., have higher germination with shallow flooding. Understanding the impact of early events and conditions on seedling emergence, growth rates and mortality will help guide the development of more effective weed management approaches. Clearly, the availability of rice cultivars with greater tolerance of flooding, particularly at germination and early growth stages, will provide opportunities for more robust weed management systems.
Conclusions and forward look
Improving rice tolerance of flooding during germination and seedling growth will facilitate the use of direct seeding for crop establishment in lowland rice ecosystems. Direct seeding brings numerous benefits to farmers, including lower labour costs, reduced water use, early harvest and less reliance on herbicides. Rice demonstrated exploitable variation in tolerance of flooding during germination and at early stages of plant growth, with some progress made in understanding the basis of tolerance. However, more efforts are needed to identify the genes involved for use in breeding. Weeds associated with lowland rice have similar adaptive traits to those of rice. Some weeds, such as the lowland ecotypes of C. rotundus, have developed additional adaptive mechanisms such as larger carbohydrate reserves and thicker stems with larger aerenchyma air spaces. Better understanding is still required to maximize the role of controlled flooding as a tool for the control of these lowland weeds. Research issues that are relevant include establishing a better understanding of tolerance mechanisms, the relationship between water management and weed recruitment, and developing rice germplasm and practices to improve rice-seedling emergence under flooding. Furthermore, the development of sufficient understanding of weed responses to flooding at the field level would assist in targeting particular weed species to allow for precise management practices such as time and depth of flooding and drainage, which could be effective against particular weeds without hindering rice establishment.
Sources of funding
This work was partly supported by the German Federal Ministry for Economic Cooperation and Development (BMZ); the Bill & Melinda Gates Foundation; and the Irrigated Rice Research Consortium of the International Rice Research Institute.
Contributions by the authors
A.M.I. and D.E.J. wrote the manuscript; E.S.E., G.V.V. and A.M.B. contributed some of the information and to editing.
Conflict of interest statement
None declared.
Acknowledgements
The assistance of James Egdane, Lamberto Licardo and Melencio Apostol is gratefully acknowledged. We also thank Bill Hardy for critically reading the manuscript.
References
- Al-Ani A, Bruzau F, Raymond P, Saint-Ges V, Leblanc JM, Pradet A. Germination, respiration, and adenylate energy charge of seeds at various oxygen partial pressures. Plant Physiology. 1985;79:885–890. doi: 10.1104/pp.79.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpi A, Beevers H. Effect of O2 concentration on rice seedlings. Plant Physiology. 1983;71:30–34. doi: 10.1104/pp.71.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angaji SA, Septiningsih EM, Mackill DJ, Ismail AM. Identification of QTLs associated with tolerance of anaerobic conditions during germination in rice (Oryza sativa L.) Euphytica. 2010;172:159–168. [Google Scholar]
- Atwell BJ, Greenway H. Carbohydrate metabolism of rice seedlings grown in oxygen-deficient solution. Journal of Experimental Botany. 1987;38:466–478. [Google Scholar]
- Avadhani PN, Greenway H, Lefroy R, Prior L. Alcoholic fermentation and malate metabolism in rice germinating at low oxygen concentrations. Australian Journal of Plant Physiology. 1978;5:15–25. [Google Scholar]
- Bailey-Serres J, Fukao T, Ronald P, Ismail AM, Heuer S, Mackill D. Submergence tolerant rice: SUB1's journey from landrace to modern cultivar. Rice. 2010;3:138–147. [Google Scholar]
- Balasubramanian V, Hill JE. Direct seeding of rice in Asia: emerging issues and strategic research needs for the 21st century. In: Pandey S, Mortimer M, Wade L, Tuong TP, Lopez K, Hardy B, editors. Direct seeding: research strategies and opportunities. Los Baños, Philippines: International Rice Research Institute; 2002. pp. 15–39. [Google Scholar]
- Beck E, Zeigler P. Biosynthesis and degradation of starch in higher plants. Annual Review of Plant Physiology and Plant Molecular Biology. 1989;40:95–117. [Google Scholar]
- Biswas JK, Yamauchi M. Mechanism of seedling establishment of direct-seeded rice (Oryza sativa L.) under lowland conditions. Botanical Bulletin of the Academia Sinica. 1997;38:29–32. [Google Scholar]
- Blom CWPM, Voesenek LACJ, Banga M, Engelaar WMHG, Rijnders JHGM, Van de Steeg HM, Visser EJW. Physiological ecology of riverside species: adaptive responses of plants to submergence. Annals of Botany. 1994;74:253–263. [Google Scholar]
- Catling D. Rice in deep water. Manila, Philippines: International Rice Research Institute; 1992. [Google Scholar]
- Chauhan BS, Johnson DE. Germination ecology of Chinese sprangletop (Leptochloa chinensis) in the Philippines. Weed Science. 2008;56:820–825. [Google Scholar]
- Chauhan BS, Johnson DE. Ecological studies on Cyperus difformis, Cyperus iria and Fimbristylis miliacea: three troublesome annual sedge weeds of rice. Annals of Applied Biology. 2009;155:103–112. [Google Scholar]
- Chin DV, Mortimer M. Weed management in direct-seeded rice in South Vietnam. In: Pandey S, Mortimer M, Wade L, Tuong TP, Lopez K, Hardy B, editors. Direct seeding: research strategies and opportunities. Los Baños, Philippines: International Rice Research Institute; 2002. pp. 349–356. [Google Scholar]
- Cho HT, Kende H. Expression of expansin genes is correlated with growth in deepwater rice. The Plant Cell. 1997;9:1661–1671. doi: 10.1105/tpc.9.9.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das KK, Panda D, Sarkar RK, Reddy JN, Ismail AM. Submergence tolerance in relation to variable floodwater conditions in rice. Environmental and Experimental Botany. 2009;66:425–434. [Google Scholar]
- Davies PJ. Plant hormones: physiology, biochemistry and molecular biology. Dordrecht: Kluwer Academic Publishers; 1995. pp. 1–35. [Google Scholar]
- Dell'Aquila A, Taranto G. Cell division and DNA synthesis during osmopriming treatments and following germination in aged wheat embryos. Seed Science and Technology. 1986;14:333–341. [Google Scholar]
- Demeke T, Chang H-G, Morris CF. Effect of germination, seed abrasion and seed size on polyphenol oxidase assay activity in wheat. Plant Breeding. 2001;120:369–373. [Google Scholar]
- Drew MC. Sensing soil oxygen. Plant, Cell and Environment. 1990;13:681–693. [Google Scholar]
- Du LV, Tuong TP. Enhancing performance of dry-seeded rice: effects of seed priming, seeding rate, and time of seeding. In: Pandey S, Mortimer M, Wade L, Tuong TP, Lopez K, Hardy B, editors. Direct seeding: research strategies and opportunities. Los Baños, Philippines: International Rice Research Institute; 2002. pp. 241–256. [Google Scholar]
- Ella ES, Setter TL. Importance of carbohydrates in rice seedling establishment under anoxia. Acta Horticulturae. 1999;504:209–216. [Google Scholar]
- Ella ES, Kawano N, Yamauchi Y, Tanaka K, Ismail AM. Blocking ethylene perception during submergence reduced chlorophyll degradation and improved seedling survival in rice. Functional Plant Biology. 2003;30:813–819. doi: 10.1071/FP03049. [DOI] [PubMed] [Google Scholar]
- Ella ES, Dionisio-Sese ML, Ismail AM. Proper management improves seedling survival and growth during early flooding in contrasting rice (Oryza sativa L.) genotypes. Crop Science. 2010;50:1997–2008. [Google Scholar]
- Ella ES, Dionisio-Sese ML, Ismail AM. Seed pretreatment in rice reduces damage, enhances carbohydrate mobilization and improves emergence and seedling establishment under flooded conditions. AoB PLANTS. 2011 doi: 10.1093/aobpla/plr007. 2011: plr007; 10.1093/aobpla/plr007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherington JR. Wetland ecology. London: Edward Arnold; 1983. The Institute of Biology: Studies in Biology 154. [Google Scholar]
- Farooq M, Basra SMA, Tabassum R, Afzal I. Enhancing the performance of direct-seeded fine rice by seed priming. Plant Production Science. 2006;9:446–456. [Google Scholar]
- Fincher GB. Molecular and cellular biology associated with endosperm mobilization in germinating cereal grains. Annual Review of Plant Physiology and Plant Molecular Biology. 1989;40:305–346. [Google Scholar]
- Fry SC. Phenolic components of the primary cell wall and their possible role in the hormonal regulation of growth. Planta. 1979;146:343–351. doi: 10.1007/BF00387807. [DOI] [PubMed] [Google Scholar]
- Fu JR, Lu SH, Chen RZ, Zhang BZ, Liu ZS, Cai DY. Osmoconditioning of peanut (Arachis hypogaea L.) seed with PEG to improve vigor and some biochemical activities. Seed Science and Technology. 1988;16:197–212. [Google Scholar]
- Fuentes RG, Baltazar AM, Merca FE, Ismail AM, Johnson DE. Morphological and physiological responses of lowland purple nutsedge (Cyperus rotundus L.) to flooding. AoB PLANTS. 2010;2010 doi: 10.1093/aobpla/plq010. 10.1093/aobpla/plq010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukao T, Kennedy RA, Yamasue Y, Rumpho ME. Genetic and biochemical analysis of anaerobically-induced enzymes during seed germination of Echinochloa crus-galli varieties tolerant and intolerant of anoxia. Journal of Experimental Botany. 2003;54:1421–1429. doi: 10.1093/jxb/erg140. [DOI] [PubMed] [Google Scholar]
- Fukao T, Xu K, Ronald PC, Bailey-Serres J. A variable cluster of ethylene response factor-like genes regulates metabolic and developmental acclimation responses to submergence in rice. The Plant Cell. 2006;18:2021–2034. doi: 10.1105/tpc.106.043000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gealy DR. Differential response of palmleaf morningglory (Ipomoea wrightii) and pitted morningglory (Ipomoea lacunosa) to flooding. Weed Science. 1998;46:217–224. [Google Scholar]
- Gibbs J, Morell S, Valdez A, Setter TL, Greenway H. Regulation of alcoholic fermentation in coleoptiles of two rice cultivars differing in tolerance for anoxia. Journal of Experimental Botany. 2000;51:785–796. [PubMed] [Google Scholar]
- Goldberg R, Liberman M, Mathieu C, Peirron M, Catesson AM. Development of epidermal cell wall peroxidase along the mung bean hypocotyls: possible involvement in the cell wall stiffening process. Journal of Experimental Botany. 1987;38:1378–1390. [Google Scholar]
- Greenway H, Setter TL. Is there anaerobic metabolism in submerged rice plants? A view point. In: Singh VP, Singh RK, Singh BB, Zeigler RS, editors. Physiology of stress tolerance in rice. Manila, Philippines: Narendra Deva University of Agriculture and Technology and International Rice Research Institute; 1996. pp. 11–30. [Google Scholar]
- Guglielminetti L, Yamaguchi J, Perata P, Alpi A. Amylolytic activities in cereal seeds under aerobic and anaerobic conditions. Plant Physiology. 1995;109:1069–1076. doi: 10.1104/pp.109.3.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori Y, Nagai K, Furukawa S, Song X-J, Kawano R, Sakakibara H, Wu J, Matsumoto T, Yoshimura A, Kitano H, Matsuoka M, Mori H, Ashikari M. The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature. 2009;460:1026–1031. doi: 10.1038/nature08258. [DOI] [PubMed] [Google Scholar]
- Hendry GAF. Oxygen free radical processes and seed longevity. Seed Science Research. 1993;3:141–153. [Google Scholar]
- Hill JE, Mortimer AM, Namuco OS, Janiya JD. Water and weed management in direct-seeded rice: are we headed in the right direction? In: Peng S, Hardy B, editors. Rice research for food security and poverty alleviation. Los Baños, Philippines: International Rice Research Institute; 2001. pp. 491–510. [Google Scholar]
- Hoffmann-Benning S, Kende H. On the role of abscisic acid and gibberellin in the regulation of growth in rice. Plant Physiology. 1992;99:1156–1161. doi: 10.1104/pp.99.3.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm LG, Plucknett DL, Pancho JV, Herberger JR. The World's worst weeds: distribution and biology. Honolulu, HI: East West Center Press; 1977. p. 609 pp. [Google Scholar]
- Huang J, Takano T, Akita S. Expression of α-expansin genes in young seedlings of rice. Planta. 2000;211:467–473. doi: 10.1007/s004250000311. [DOI] [PubMed] [Google Scholar]
- Huber H, Jacobs E, Visser EW. Variation in flooding-induced morphological traits in natural populations of white clover (Trifolium repens) and their effects on plant performance during soil flooding. Annals of Botany. 2009;103:377–386. doi: 10.1093/aob/mcn149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang YS, Thomas BR, Rodriguez RL. Differential expression of α-amylase genes during seedling development under anoxia. Plant Molecular Biology. 1999;40:911–920. doi: 10.1023/a:1006241811136. [DOI] [PubMed] [Google Scholar]
- International Rice Research Institute. Rice almanac. 2nd edn. Manila, Philippines: International Rice Research Institute; 1997. 181 pp. [Google Scholar]
- Ishizawa K, Esashi Y. Gaseous factors involved in the enhanced elongation of rice coleoptiles under water. Plant, Cell and Environment. 1984;7:239–245. [Google Scholar]
- Ishizawa K, Esashi Y. Action mechanism of ethylene in the control of sugar translocation in relation to rice coleoptile growth. I. Sucrose metabolism. Plant, Cell and Environment. 1988;29:131–141. [Google Scholar]
- Ismail AM, Ella ES, Vergara GV, Mackill DJ. Mechanisms associated with tolerance for flooding during germination and early seedling growth in rice (Oryza sativa) Annals of Botany. 2009;103:197–209. doi: 10.1093/aob/mcn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M, Ram PC. Physiological and molecular basis of susceptibility and tolerance of rice plants to complete submergence. Annals of Botany. 2003;91:227–241. doi: 10.1093/aob/mcf242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MB, Herman B, Goodenough A. An examination of the importance of ethanol in causing injury to flooded plants. Plant, Cell and Environment. 1982;5:163–172. [Google Scholar]
- Jackson MB, Waters I, Setter T, Greenway H. Injury to rice plants caused by complete submergence: a contribution by ethylene (ethene) Journal of Experimental Botany. 1987;38:1826–1838. [Google Scholar]
- Janiya JD, Dizon MA, Mortimer M, Piggin C, Hill J. The impact of cropping practices on rice-weed communities. Proceedings of the 17th Asian Pacific Weed Sciences Society Conference; Bangkok, Thailand: 1999. pp. 146–151. [Google Scholar]
- Kaneko M, Itoh H, Ueguchi-Tanaka M, Ashikari M, Matsuoka M. The α-amylase induction in endosperm during rice seed germination is caused by gibberellin synthesized in epithelium. Plant Physiology. 2002;128:1264–1270. doi: 10.1104/pp.010785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy RA, Rumpho ME, Fox TC. Anaerobic metabolism in plants. Plant Physiology. 1992;100:1–6. doi: 10.1104/pp.100.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent RJ, Johnson DE. Influence of flood depth and duration on biology and on growth of lowland rice weeds, Côte d'Ivoire. Crop Protection. 2001;20:691–694. [Google Scholar]
- Khush GS. Manila, Philippines: International Rice Research Institute; 1984. Terminology of rice growing environments. 5–10. [Google Scholar]
- Kim JK, Kang YS, Lee MH, Kim SS, Park ST. Wet-seeded rice cultivation technology in Korea. In: Peng S, Hardy B, editors. Rice research for food security and poverty alleviation. Los Baños, Philippines: International Rice Research Institute; 2001. pp. 545–560. [Google Scholar]
- Kim SC, Moody K. Germination of two rice cultivars and several weed species. Korean Journal of Weed Science. 1989;9:116–122. [Google Scholar]
- Kludze HK, Pezeshki SR, Delaune RD. Evaluation of root oxygenation and growth in bald-cypress in response to short-term soil hypoxia. Canadian Journal of Forest Research. 1994;24:804–809. [Google Scholar]
- Konings H, Jackson MB. A relationship between rates of ethylene production by roots and the promoting or inhibiting effects of exogenous ethylene and water on root elongation. Zeitschrift für Pflanzenphysiologie. 1979;92:385–397. [Google Scholar]
- Ku HS, Suge H, Rappaport L, Pratt HK. Stimulation of rice coleoptile growth by ethylene. Planta. 1970;90:333–339. doi: 10.1007/BF00386385. [DOI] [PubMed] [Google Scholar]
- Lasanthi-Kudahettige R, Magneschi L, Loret E, Gonzali S, Licausi F, Novi G, Beretta O, Vitulli F, Alpi A, Perata P. Transcript profiling of the anoxic rice coleoptile. Plant Physiology. 2007;144:218–231. doi: 10.1104/pp.106.093997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Kim JH, Hong SB, Yun SH. Effect of humidification and hardening treatment on seed germination of rice. Korean Journal of Crop Science. 1998;43:157–160. [Google Scholar]
- Lee TM, Lin YH. Peroxidase activity in relation to ethylene-induced rice (Oryza sativa L.) coleoptile elongation. Botanical Bulletin of Academia Sinica. 1996;37:239–245. [Google Scholar]
- Ling J, Ming-Yu H, Chun-Ming W, Jian-Min W. Quantitative trait loci and epistatic analysis of seed anoxia germinability in rice (Oryza sativa) Rice Science. 2004;11:238–244. [Google Scholar]
- Liu Q, Shang Q, Burton RA, Shirley NJ, Atwell BJ. Expression of vacuolar H+-pyrophosphatase (OVP3) is under control of an anoxia-inducible promoter in rice. Plant Molecular Biology. 2010;72:47–60. doi: 10.1007/s11103-009-9549-z. [DOI] [PubMed] [Google Scholar]
- Loreti E, Yamaguchi J, Alpi A, Perata P. Sugar modulation of α-amylase genes under anoxia. Annals of Botany. 2003;91:143–148. doi: 10.1093/aob/mcf117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CA, Lim EK, Yu SM. Sugar response sequence in the promoter of a rice α-amylase gene serves as a transcriptional enhancer. Journal of Biological Chemistry. 1998;273:10120–10131. doi: 10.1074/jbc.273.17.10120. [DOI] [PubMed] [Google Scholar]
- Mackill DJ, Coffman WR, Garrity DP. Rainfed lowland rice improvement. Los Baños, Philippines: International Rice Research Institute; 1996. 242 pp. [Google Scholar]
- Mackill DJ, Ismail AM, Singh US, Labios RV, Paris TR. Development and rapid adoption of submergence-tolerant (Sub1) rice varieties. Advances in Agronomy. 2012;115:303–356. [Google Scholar]
- McIntyre S, Mitchell DS, Ladiges PY. Seedling mortality and submergence in Diplachne fusca: a semi-aquatic weed of rice fields. Journal of Applied Ecology. 1989;26:537–549. [Google Scholar]
- Metraux JP, Kende H. The role of ethylene in the growth response of submerged deepwater rice. Plant Physiology. 1983;72:441–446. doi: 10.1104/pp.72.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody E. Weeds reported in rice in South and Southeast Asia. Philippines: International Rice Research Institute; 1989. 424 pp. [Google Scholar]
- Moosavi A, Tavakkol Afshari R, Sharif-Zadeh F, Aynehband A. Effect of seed priming on germination characteristics, polyphenoloxidase and peroxidase activities of four amaranth cultivars. International Journal of Food, Agriculture and Environment. 2009;7:353–358. [Google Scholar]
- Morohashi Y. Peroxidase activity develops in the micropylar endosperm of tomato seeds prior to radical protrusion. Journal of Experimental Botany. 2002;53:1643–1650. doi: 10.1093/jxb/erf012. [DOI] [PubMed] [Google Scholar]
- Mortimer AM, Namuco OS, Johnson DE. Seedling recruitment in direct-seeded rice: weed biology and water management. In: Toriyama K, Heong KL, Hardy B, editors. Rice is life: scientific perspectives for the 21st century. Los Baños, Philippines, Tsukuba, Japan: International Rice Research Institute, Japan International Research Center for Agricultural Sciences; 2005. pp. 202–205. CD. [Google Scholar]
- Muench DG, Archibold OW, Good AG. Hypoxic metabolism in wild rice (Zizania palustris): enzyme induction and metabolite production. Physiologia Plantarum. 1993;89:165–171. [Google Scholar]
- Murata T, Akazawa T, Fukuchi S. Enzymic mechanism of starch breakdown in germinating rice seeds. I. An analytical study. Plant Physiology. 1968;43:1899–1905. doi: 10.1104/pp.43.12.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazono M, Tsuji H, Li Y, Saisho D, Arimura S, Tsutsumi N, Hirai A. Expression of a gene encoding mitochondrial aldehyde dehydrogenase in rice increases under submerged conditions. Plant Physiology. 2000;124:587–598. doi: 10.1104/pp.124.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeraja C, Maghirang-Rodriguez R, Pamplona A, Heuer S, Collard B, Septiningsih E, Vergara G, Sanchez D, Xu K, Ismail AM, Mackill D. A marker-assisted backcross approach for developing submergence-tolerant rice cultivars. Theoretical and Applied Genetics. 2007;115:767–776. doi: 10.1007/s00122-007-0607-0. [DOI] [PubMed] [Google Scholar]
- Pandey S, Mortimer M, Wade L, Tuong TP, Lopez K, Hardy B, editors. Direct seeding: research strategies and opportunities. Proceedings of the International Workshop on Direct Seeding in Asian Rice Systems: Strategic Research Issues and Opportunities, 25–28 January 2000, Bangkok, Thailand; Los Baños, Philippines: International Rice Research Institute; 2002. [Google Scholar]
- Pearce DM, Jackson MB. Comparison of growth responses of barnyard grass (Echinochloa oryzoides) and rice (Oryza sativa) to submergence, ethylene, carbon dioxide and oxygen shortage. Annals of Botany. 1991;68:201–209. [Google Scholar]
- Pearce DM, Hall KC, Jackson MB. The effects of oxygen, carbon dioxide and ethylene on ethylene biosynthesis in relation to shoot extension in seedlings of rice (Oryza sativa) and barnyard grass (Echinochloa oryzoides) Annals of Botany. 1992;69:441–447. [Google Scholar]
- Pegoraro R, Mapelli S, Torti G, Bertani A. Indole-3-acetic acid and rice coleoptile elongation under anoxia. Journal of Plant Growth Regulation. 1988;7:85–94. [Google Scholar]
- Peña-Fronteras JT, Villalobos MC, Baltazar AM, Merca FE, Ismail AM, Johnson DE. Adaptation to flooding in upland and lowland ecotypes of Cyperus rotundus, a troublesome sedge weed of rice: tuber morphology and carbohydrate metabolism. Annals of Botany. 2009;103:295–302. doi: 10.1093/aob/mcn085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perata P, Pozueta-Romero J, Akazawa T, Yamaguchi J. Effects of anoxia on starch breakdown in rice and wheat seeds. Planta. 1992;188:611–618. doi: 10.1007/BF00197056. [DOI] [PubMed] [Google Scholar]
- Perata P, Geshi N, Yamaguchi J, Akazawa T. Effect of anoxia on the induction of α-amylase in cereal seeds. Planta. 1993;191:402–408. [Google Scholar]
- Perata P, Guglielminetti L, Alpi A. Mobilization of endosperm reserves in cereal seeds under anoxia. Annals of Botany. 1997;79(Supplement A):49–56. [Google Scholar]
- Rao AN, Johnson DE, Sivaprasad B, Ladha JK, Mortimer AM. Weed management in direct-seeded rice. Advances in Agronomy. 2007;93:153–255. [Google Scholar]
- Raskin I, Kende H. The role of gibberellin in the growth response of submerged deep water rice. Plant Physiology. 1984;76:947–950. doi: 10.1104/pp.76.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond P, Al-Ani A, Pradet A. ATP production by respiration and fermentation, and energy charge during aerobiosis and anaerobiosis in twelve fatty and starchy germinating seeds. Plant Physiology. 1985;79:9879–9884. doi: 10.1104/pp.79.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redoña ED, Mackill DJ. Genetic variation for seedling-vigor traits in rice. Crop Science. 1996;36:285–290. [Google Scholar]
- Reichheld J-P, Vernoux T, Lardon F, Van Montagu M, Inze D. Specific checkpoints regulate plant cell cycle progression in response to oxidative stress. The Plant Journal. 1999;17:647–656. [Google Scholar]
- Roberts JKM, Callis J, Jardetzky O, Walbot V, Freeling M. Cytoplasmic acidosis as a determinant of flooding intolerance in plants. Proceedings of the National Academy of Sciences of the USA. 1984;81:6029–6033. doi: 10.1073/pnas.81.19.6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumpho ME, Kennedy RA. Anaerobic metabolism in germinating seeds of Echinochloa crus-galli (barnyardgrass): metabolite and enzyme studies. Plant Physiology. 1981;68:165–168. doi: 10.1104/pp.68.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders BA. The life cycle and ecology of Cyperus difformis (rice weed) in temperate Australia: a review. Australian Journal of Experimental Agriculture. 1994;34:1031–1038. [Google Scholar]
- Sarkar RK, Reddy JN, Sharma SG, Ismail AM. Physiological basis of submergence tolerance in rice and implications for crop improvement. Current Science. 2006;91:899–906. [Google Scholar]
- Sculthorpe CD. The biology of aquatic vascular plants. London: Edward Arnold; 1967. [Google Scholar]
- Septiningsih EM, Pamplona AM, Sanchez DL, Neeraja CN, Vergara GV, Heuer S, Ismail AM, Mackill DJ. Development of submergence-tolerant rice cultivars: the Sub1 locus and beyond. Annals of Botany. 2009;103:151–160. doi: 10.1093/aob/mcn206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setter TL, Ella ES. Relationship between coleoptile elongation and alcoholic fermentation in rice exposed to anoxia. I. Importance of treatment conditions and different tissues. Annals of Botany. 1994;74:265–271. [Google Scholar]
- Setter TL, Kupkanchanakul T, Kupkanchanakul K, Bhekasut P, Wiengweera A, Greenway H. Environmental factors in deepwater rice areas in Thailand: O2, CO2, and ethylene. Proceeding of Symposium of the 1987 International Deepwater Rice Workshop; Los Baños, Philippines: International Rice Research Institute; 1988. pp. 55–66. [Google Scholar]
- Setter TL, Ella ES, Valdez AP. Relationship between coleoptile elongation and alcoholic fermentation in rice exposed to anoxia. II. Cultivar differences. Annals of Botany. 1994;74:273–279. [Google Scholar]
- Setter TL, Ellis M, Laureles EV, Ella ES, Senadhira D, Mishra SB, Sarkarung S, Datta S. Physiology and genetics of submergence tolerance in rice. Annals of Botany. 1997;79(Supplement A):67–77. [Google Scholar]
- Singh S, Mackill DJ, Ismail AM. Responses of SUB1 rice introgression lines to submergence in the field: yield and grain quality. Field Crops Research. 2009;113:12–23. [Google Scholar]
- Singh S, Mackill DJ, Ismail AM. Tolerance of longer-term partial stagnant flooding is independent of the SUB1 locus in rice. Field Crops Research. 2011;121:311–323. [Google Scholar]
- Smirnoff N. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytologist. 1993;125:27–58. doi: 10.1111/j.1469-8137.1993.tb03863.x. [DOI] [PubMed] [Google Scholar]
- Stover EL. The roots of wild rice Zizania aquatica L. Ohio Journal of Science. 1928;28:43–59. as cited by Sculthorpe, 1967. [Google Scholar]
- Suge H. Stimulation of oat and rice mesocotyl growth by ethylene. Plant and Cell Physiology. 1971;12:831–837. [Google Scholar]
- Suge H. Synergistic action of ethylene with gibberellins in the growth of rice seedlings. Proceedings of Crop Science Society of Japan. 1974;43:83–87. [Google Scholar]
- Suge H, Kusanagi T. Ethylene and carbon dioxide: Regulation of growth in two perennial aquatic plants, arrowhead and pondweed. Plant and Cell Physiology. 1975;16:65–72. [Google Scholar]
- Sung JM. Lipid peroxidation and peroxide-scavenging in soybean seeds during aging. Physiologia Plantarum. 1996;97:85–89. [Google Scholar]
- Taylor DL. Influence of oxygen tension on respiration, fermentation and growth of wheat and rice. American Journal of Botany. 1942;29:721–738. [Google Scholar]
- Terashima M, Hosono M, Katoh S. Functional roles of protein domains on rice α-amylase activity. Applied Microbiology and Biotechnology. 1997;47:364–367. doi: 10.1007/s002530050941. [DOI] [PubMed] [Google Scholar]
- Tsuji H, Meguro N, Suzuki Y, Tsutsumi N, Hirai A, Nakazono M. Induction of mitochondrial aldehyde dehydrogenase by submergence facilitates oxidation of acetabldehyde during re-aeration in rice. FEBS Letters. 2003a;546:369–373. doi: 10.1016/s0014-5793(03)00631-8. [DOI] [PubMed] [Google Scholar]
- Tsuji H, Tsutsumi N, Sasaki T, Hirai A, Nakazono M. Organ-specific expression and chromosomal locations of two mitochondrial aldehyde dehydrogenase genes from rice (Oryza sativa L.) ALDH2a and ALDH2b. Gene. 2003b;305:195–204. doi: 10.1016/s0378-1119(03)00383-4. [DOI] [PubMed] [Google Scholar]
- Tuong TP, Pablico PP, Yamauchi M, Confesor R, Moody K. Increasing water productivity and weed suppression of wet-seeded rice: effect of water management and rice genotypes. Experimental Agriculture. 2000;36:71–89. [Google Scholar]
- Turner FT, Chen CC, McCauley GN. Morphological development of rice seedlings in water at controlled oxygen levels. Agronomy Journal. 1981;73:566–570. [Google Scholar]
- Umemura T, Perata P, Futsuhara Y, Yamaguchi J. Sugar sensing and α-amylase repression in rice embryos. Planta. 1998;204:420–428. doi: 10.1007/s004250050275. [DOI] [PubMed] [Google Scholar]
- Vartapetian BB, Jackson MB. Plant adaptations to anaerobic stress. Annals of Botany. 1997;79(Supplement A):3–20. [Google Scholar]
- Visser EJW, Colmer TD, Blom CWPM, Voesenek LACJ. Changes in growth, porosity, and radial oxygen loss from adventitious roots of selected mono- and dicotyledonous wetland species with contrasting types of aerenchyma. Plant, Cell and Environment. 2000;23:1237–1245. [Google Scholar]
- Voesenek LACJ, Colmer TD, Pierik R, Millenaar FF, Peeters AJM. How plants cope with complete submergence. New Phytologist. 2006;170:213–226. doi: 10.1111/j.1469-8137.2006.01692.x. [DOI] [PubMed] [Google Scholar]
- Waters I, Morrell S, Greenway H, Colmer TD. Effects of anoxia on wheat seedlings. II. Influence of O2 supply prior to anoxia on tolerance to anoxia, alcoholic fermentation, and sugar levels. Journal of Experimental Botany. 1991;42:1437–1447. [Google Scholar]
- Whitmore FW. Lignin-protein complex catalyzed by peroxidase. Plant Science Letters. 1978;13:241–245. [Google Scholar]
- Wilson DO, McDonald MB. The lipid peroxidation model of seed ageing. Seed Science and Technology. 1986;14:269–300. [Google Scholar]
- Xu K, Xu X, Fukao T, Canlas P, Maghirang-Rodriquez R, Huer S, Ismail AM, Bailey-Serres J, Ronald PC, Mackill DJ. Sub1A is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature. 2006;442:705–708. doi: 10.1038/nature04920. [DOI] [PubMed] [Google Scholar]
- Yamauchi M, Winn T. Rice seed vigor and seedling establishment in anaerobic soil. Crop Science. 1996;36:680–686. [Google Scholar]
- Yamauchi M, Aguilar AM, Vaughan DA, Seshu DV. Rice (Oryza sativa L.) germplasm suitable for direct sowing under flooded soil surface. Euphytica. 1993;67:177–184. [Google Scholar]
- Yamauchi M, Herradura PS, Aguilar AM. Genotype difference in rice post-germination growth under hypoxia. Plant Science. 1994;100:105–113. [Google Scholar]
- Zheng X, van Huystee RB. Peroxidase-regulated elongation of segments from peanut hypocotyls. Plant Science. 1992;81:47–56. [Google Scholar]