Abstract
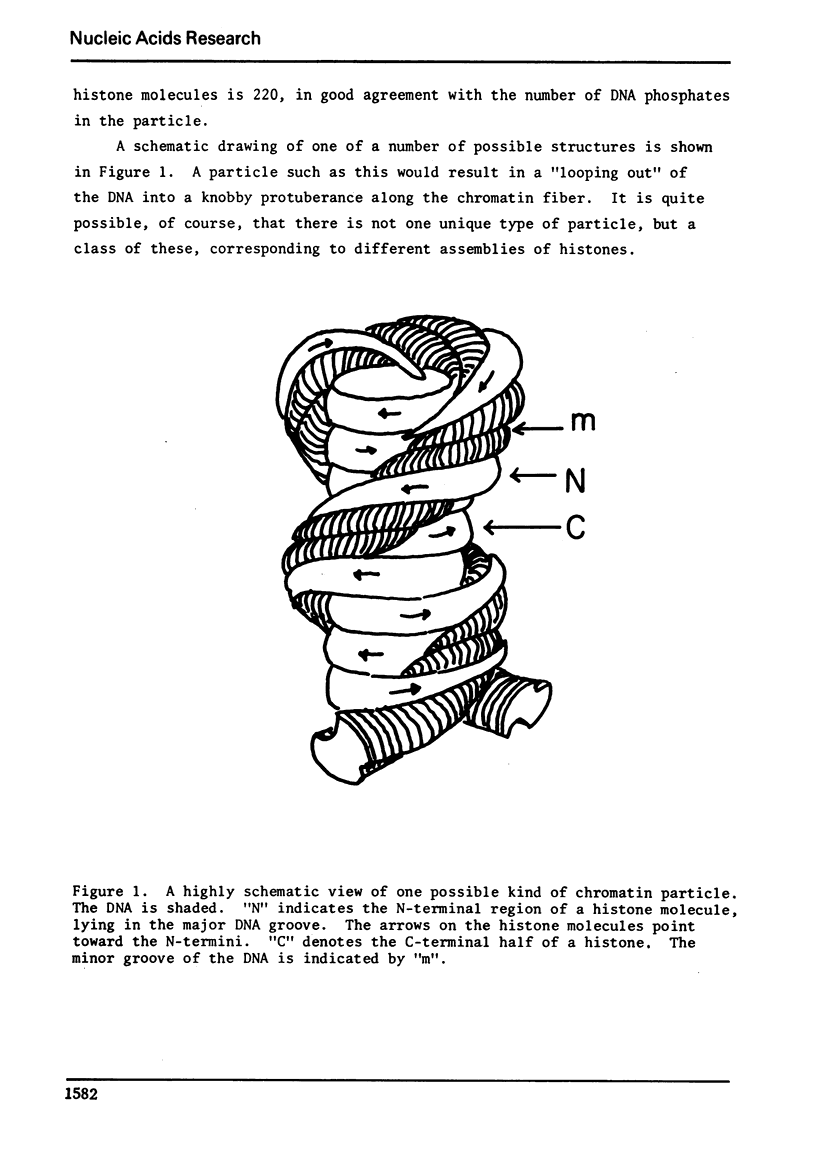
A model is proposed for the structure of nuclease-resistant chromatin particles. The model is novel in that it proposes that the DNA in such a particle is wound about a protein core, made up of the hydrophobic regions of histone molecules.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler A. J., Ross D. G., Chen K., Stafford P. A., Woiszwillo M. J., Fasman G. D. Interaction of deoxyribonucleic acid with histone f2b and its half-molecules. Circular dichroism studies. Biochemistry. 1974 Jan 29;13(3):616–623. doi: 10.1021/bi00700a033. [DOI] [PubMed] [Google Scholar]
- Bartley J. A., Chalkley R. The viscosity of nucleohistone in urea. Biochim Biophys Acta. 1968 Jun 26;160(2):224–228. doi: 10.1016/0005-2795(68)90090-1. [DOI] [PubMed] [Google Scholar]
- Boublík M., Bradbury E. M., Crane-Robinson C. An investigation of the conformational changes in histones F1 and F2a1 by proton magnetic resonance spectroscopy. Eur J Biochem. 1970 Jul;14(3):486–497. doi: 10.1111/j.1432-1033.1970.tb00315.x. [DOI] [PubMed] [Google Scholar]
- Bradbury E. M., Molgaard H. V., Stephens R. M., Bolund L. A., Johns E. W. X-ray studies of nucleoproteins depleted of lysine-rich histone. Eur J Biochem. 1972 Dec 18;31(3):474–482. doi: 10.1111/j.1432-1033.1972.tb02555.x. [DOI] [PubMed] [Google Scholar]
- Bradbury E. M., Rattle H. W. Simple computer-aided approach for the analyses of the nuclear-magnetic-resonance spectra of histones. Fractions F1, Fsa1, F2B, cleaved halves of F2B and F2B-DNA. Eur J Biochem. 1972 May 23;27(2):270–281. doi: 10.1111/j.1432-1033.1972.tb01836.x. [DOI] [PubMed] [Google Scholar]
- Bram S., Ris H. On the structure of nucleohistone. J Mol Biol. 1971 Feb 14;55(3):325–336. doi: 10.1016/0022-2836(71)90321-4. [DOI] [PubMed] [Google Scholar]
- Crick F. General model for the chromosomes of higher organisms. Nature. 1971 Nov 5;234(5323):25–27. doi: 10.1038/234025a0. [DOI] [PubMed] [Google Scholar]
- D'Anna J. A., Jr, Isenberg I. A complex of histones IIb2 and IV. Biochemistry. 1973 Mar 13;12(6):1035–1043. doi: 10.1021/bi00730a003. [DOI] [PubMed] [Google Scholar]
- D'Anna J. A., Jr, Isenberg I. Interactions of histone LAK (f2a2) with histones KAS (f2b) and GRK (f2a1). Biochemistry. 1974 May 7;13(10):2098–2104. doi: 10.1021/bi00707a016. [DOI] [PubMed] [Google Scholar]
- Hayashi H., Iwai K. Calf thymus alanine-rich, leucine-rich histone: sequences of the tryptic peptides and characteristic distributions of the basic and other residues in the molecule. J Biochem. 1971 Sep;70(3):543–547. doi: 10.1093/oxfordjournals.jbchem.a129670. [DOI] [PubMed] [Google Scholar]
- Johns E. W. Histones, chromatin structure and RNA synthesis. Nat New Biol. 1972 May 17;237(72):87–88. doi: 10.1038/newbio237087a0. [DOI] [PubMed] [Google Scholar]
- Johnson R. S., Chan A., Hanlon S. Mixed conformations of deoxyribonucleic acid in intact chromatin isolated by various preparative methods. Biochemistry. 1972 Nov 7;11(23):4347–4358. doi: 10.1021/bi00773a023. [DOI] [PubMed] [Google Scholar]
- Kelley R. I. Isolation of a histone IIb1-IIb2 complex. Biochem Biophys Res Commun. 1973 Oct 15;54(4):1588–1594. doi: 10.1016/0006-291x(73)91168-6. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D. Chromatin structure: a repeating unit of histones and DNA. Science. 1974 May 24;184(4139):868–871. doi: 10.1126/science.184.4139.868. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D., Thomas J. O. Chromatin structure; oligomers of the histones. Science. 1974 May 24;184(4139):865–868. doi: 10.1126/science.184.4139.865. [DOI] [PubMed] [Google Scholar]
- Li H. J., Bonner J. Interaction of histone half-molecules with deoxyribonucleic acid. Biochemistry. 1971 Apr 13;10(8):1461–1470. doi: 10.1021/bi00784a030. [DOI] [PubMed] [Google Scholar]
- Littau V. C., Burdick C. J., Allfrey V. G., Mirsky S. A. The role of histones in the maintenance of chromatin structure. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1204–1212. doi: 10.1073/pnas.54.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malchy B., Kaplan H. Reactive properties of the amino groups of histones in calf thymus chromatin. J Mol Biol. 1974 Feb 5;82(4):537–545. doi: 10.1016/0022-2836(74)90247-2. [DOI] [PubMed] [Google Scholar]
- Olins A. L., Olins D. E. Spheroid chromatin units (v bodies). Science. 1974 Jan 25;183(4122):330–332. doi: 10.1126/science.183.4122.330. [DOI] [PubMed] [Google Scholar]
- Pardon J. F., Wilkins M. H., Richards B. M. Super-helical model for nucleohistone. Nature. 1967 Jul 29;215(5100):508–509. doi: 10.1038/215508a0. [DOI] [PubMed] [Google Scholar]
- Richards B. M., Pardon J. F. The molecular structure of nucleohistone (DNH). Exp Cell Res. 1970 Sep;62(1):184–196. doi: 10.1016/0014-4827(79)90519-6. [DOI] [PubMed] [Google Scholar]
- Rill R., Van Holde K. E. Properties of nuclease-resistant fragments of calf thymus chromatin. J Biol Chem. 1973 Feb 10;248(3):1080–1083. [PubMed] [Google Scholar]
- Roark D. E., Geoghegan T. E., Keller G. H. A two-subunit histone complex from calf thymus. Biochem Biophys Res Commun. 1974 Jul 24;59(2):542–547. doi: 10.1016/s0006-291x(74)80014-8. [DOI] [PubMed] [Google Scholar]
- Sahasrabuddhe C. G., Van Holde K. E. The effect of trypsin on nuclease-resistant chromatin fragments. J Biol Chem. 1974 Jan 10;249(1):152–156. [PubMed] [Google Scholar]
- Simpson R. T. Interaction of a repotter molecule with chromatin. Evidence suggesting that the proteins of chromatin do not occupy the minor groove of deoxyribonucleic acid. Biochemistry. 1970 Nov 24;9(24):4814–4819. doi: 10.1021/bi00826a028. [DOI] [PubMed] [Google Scholar]
- Simpson R. T. Modification of chromatin by trypsin. The role of proteins in maintainance of deoxyribonucleic acid conformation. Biochemistry. 1972 May 23;11(11):2003–2008. doi: 10.1021/bi00761a002. [DOI] [PubMed] [Google Scholar]
- Skandrani E., Mizon J., Sautière P., Biserte G. Etude de la fraction F2b des histones de thymus de veau. Biochimie. 1972;54(10):1267–1272. doi: 10.1016/s0300-9084(72)80067-1. [DOI] [PubMed] [Google Scholar]
- Slayter H. S., Shih T. Y., Adler A. J., Fasman G. D. Electron microscopy and circular dichroism studies on chromatin. Biochemistry. 1972 Aug 1;11(16):3044–3054. doi: 10.1021/bi00766a016. [DOI] [PubMed] [Google Scholar]



