Abstract
Recent genetic association studies have identified the A-allele of rs1006737 within CACNA1C as a risk factor for schizophrenia as well as mood disorders. Some evidence suggests that this polymorphism plays a role in cognitive function both in schizophrenia patients and healthy individuals; however, the precise nature of this association remains unclear. Here we investigated the possible association of this polymorphism with a wide range of neurocognitive functions in schizophrenia patients and in healthy subjects. Schizophrenia patients exhibited significantly poorer performance on all the cognitive domains as compared to healthy controls. In patients, A-allele carriers demonstrated significantly worse logical memory performance than the G-allele homozygotes. In controls, no significant association was observed between the genotype and any of the cognitive domains examined. These results add to the literature suggesting that rs1006737 may be associated with schizophrenia through its detrimental effect on endophenotypic traits.
CACNA1C on chromosome 12p13 encodes an α-1 subunit of the voltage-dependent L-type gated calcium channel CAv1.2, which mediates the influx of calcium ions into the cell upon membrane polarisation and plays an important role in dendritic development, neuronal survival, synaptic plasticity and memory/learning1. Genome-wide association studies have detected an intronic single nucleotide polymorphism (SNP) rs1006737 of CACNA1C as a risk factor for bipolar disorder2,3. Subsequent studies have demonstrated that this SNP is also associated with unipolar major depressive disorder4,5,6 and schizophrenia5,7,8.
It is now well established that schizophrenia is a highly heritable disorder. One of the cardinal features of this disorder is wide-ranging cognitive impairments including profound memory deficits9,10. It has been postulated that susceptibility genes for a neuropsychiatric disorder could mediate liability for the disorder at least partly by influencing endophenotypes such as neurophysiological, neuroanatomical and neurocognitive features11. A heritability study on a collection of endophenotypes for schizophrenia suggests that cognitive function is one of such endophenotypic markers worthy of consideration in attempting to identify the genetic basis of schizophrenia12. Supporting this, many (but not all) studies have reported an association between SNPs in schizophrenia susceptibility genes, such as catechol-O-methyltransferase (COMT), dysbindin 1 (DTNBP1) and zinc finger protein 804A (ZNF804A), and cognitive performance both in schizophrenia patients13,14,15 and healthy people16,17,18,19. Several recent studies have also shown that the psychiatric risk A-allele at rs1006737 within CACNA1C is associated with impaired working memory in schizophrenia patients20 as well as with decreased attention21, working memory20 and verbal fluency22 in healthy adults. However, these studies examined relatively limited cognitive domains, and no studies to date have employed a comprehensive cognitive test battery to investigate the association of CACNA1C with neurocognition in schizophrenia patients or healthy people.
The present study aimed to investigate an association of the CACNA1C rs1006737 polymorphism with an array of neurocognitive functioning in schizophrenia patients and healthy control subjects. Our secondary purpose was to examine whether this SNP would be associated with schizophrenia as no case-control studies for this association have been performed in a Japanese population.
Results
Association of CACNA1C rs1006737 with the development of schizophrenia
In the case-control genetic association sample, age (t = 3.0, P = 0.002) and gender distribution (χ2 = 67.2, P < 0.001) significantly differed between the two diagnostic groups. Genotype frequencies did not deviate from Hardy-Weinberg equilibrium in schizophrenia patients or healthy controls (Table 1). No significant differences were seen in genotype or allele frequencies between the patients and controls (Table 1); however, we would like to note that the odds ratio of the minor allele (A-allele) for schizophrenia was 1.15 (95% confidence interval: 0.86–1.54). Table 2 presents a comparison of our data and those of the four previous studies5,7,8,20 which conducted a genetic association analysis between schizophrenia and the CACNA1C polymorphism rs1006737.
Table 1. Genotype and allele distributions in schizophrenia patients and healthy controls for the CACNA1C polymorphism rs1006737.
| Genotype counts (frequency) | Allele counts (frequency) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | AA | AG | GG | χ2 P-value1 (df = 1) | A | G | OR (95%CI) | χ2 P-value (df = 1) | HWE P-value (df = 1) | |
| Schizophrenia | 552 | 2 (0.00) | 70 (0.13) | 480 (0.87) | 0.36 | 74 (0.07) | 1030 (0.93) | 1.15 (0.86–1.54) | 0.35 | 0.74 |
| Controls | 1132 | 3 (0.00) | 127 (0.11) | 1002 (0.89) | 133 (0.06) | 2131 (0.94) | 0.62 | |||
Abbreviations: CI, confidence interval; HWE, Hardy-Weinberg Disequilibrium; OR, odds ratio; df, degree of freedom.
1P-value was calculated after combining AA and AG genotypes as a group.
Table 2. Summary of genetic association studies of schizophrenia for the CACNA1C polymorphism rs1006737.
| Genotype counts (AA/AG/GG) | Allele frequency (A-allele) | |||||||
|---|---|---|---|---|---|---|---|---|
| n (SZ/CON) | SZ | CON | χ2 P-value | SZ | CON | χ2 P-value | OR (95%CI) | |
| The present study | 552/1132 | 2/70/480 | 3/127/1002 | 0.36 5 | 0.067 | 0.059 | 0.35 | 1.15 (0.86–1.54) |
| Green et al. (2010)1 | 479/11361 | 66/208/205 | 1252/4949/5160 | 0.034 | 0.355 | 0.328 | 0.083 | 1.15 (0.99–1.32) |
| Nyegaard et al. (2010)2 | 976/1489 | 130/444/402 | 158/675/656 | 0.015 | 0.361 | 0.333 | 0.044 | 1.13 (1.003–1.275) |
| Bigos et al. (2010)3 | 282/440 | NA | NA | 0.03 | NA | NA | NA | NA |
| Zhang et al. (2012)4 | 318/401 | NA | NA | > 0.05 | NA | NA | NA | NA |
Abbreviations: SZ, schizophrenia patients; CON, control individuals; CI, confidence interval; OR, odds ratio; NA: not available.
1P-value for allele frequency was calculated based on their data.
2P-value for allele frequency, odds ratio and its 95% confidence interval were calculated based on their data.
3Odds ratio (95% confidence interval) for A-allele homozygotes was reported to be 1.77 (1.07–2.91).
4Detailed data are not presented in this report.
5P-value was calculated after combining AA and AG genotypes as a group.
Association of CACNA1C rs1006737 with cognition
Demographic characteristics and clinical variables for patients and controls included in the cognitive endophenotype analysis are presented in Table 3. No significant differences between the genotypes were seen in any of the variables examined for either patients or controls. Therefore we controlled only for age and gender in the following ANCOVA analyses.
Table 3. Demographic and clinical variables of schizophrenia patients and healthy controls included in the cognitive experiment, stratified by the CACNA1C rs1006737 genotype.
| Schizophrenia patients (n = 202) | Healthy controls (n = 706) | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | AG+AA (n = 31) | GG (n = 171) | Statistics | P-value | AG+AA (n = 87) | GG (n = 619) | Statistics | P-value |
| Age, years: mean ± SD | 40.9 ± 13.1 | 42.0 ± 13.2 | t = 0.40 | 0.69 | 46.7 ± 16.0 | 44.1 ± 15.4 | t = 1.47 | 0.14 |
| Gender, female: n (%) | 12 (38.7) | 64 (37.4) | χ2 = 0.02 | 0.89 | 65 (74.7) | 455 (73.5) | χ2 = 0.06 | 0.81 |
| Education, years: mean ± SD | 13.1 ± 2.2 | 13.0 ± 2.7 | t = 0.12 | 0.90 | 15.0 ± 2.9 | 15.1 ± 2.7 | t = 0.35 | 0.72 |
| Family history of any psychiatric disorder: yes, n (%) | 14 (45.2) | 72 (42.1) | χ2 = 0.10 | 0.75 | 12 (13.8) | 84 (13.6) | χ2 = 0.003 | 0.95 |
| Age at onset, years: mean ± SD | 23.2 ± 9.0 | 24.0 ± 8.4 | t = 0.51 | 0.61 | NA | NA | NA | NA |
| Duration of illness, years: mean ± SD | 18.8 ± 12.0 | 17.7 ± 11.9 | t = 0.45 | 0.65 | NA | NA | NA | NA |
| Outpatients/inpatients: n (%) of outpatients | 23 (74.2) | 133 (77.8) | χ2 = 0.19 | 0.66 | NA | NA | NA | NA |
| Number of hospitalisations: mean ± SD | 3.0 ± 3.3 | 2.3 ± 2.4 | t = 1.40 | 0.16 | NA | NA | NA | NA |
| Lifetime ECT: yes, n (%) | 2 (6.5) | 22 (12.9) | Fisher's exact | 0.54 | NA | NA | NA | NA |
| Medication dosage1, mg/day: mean ± SD | 913.4 ± 685.7 | 711.2 ± 658.8 | t = 1.51 | 0.13 | NA | NA | NA | NA |
| PANSS positive score2: mean ± SD | 12.5 ± 7.0 | 13.4 ± 5.4 | t = 0.65 | 0.51 | NA | NA | NA | NA |
| PANSS negative score2: mean ± SD | 17.7 ± 7.9 | 18.3 ± 7.7 | t = 0.31 | 0.76 | NA | NA | NA | NA |
| PANSS general psychopathology score2: mean ± SD | 27.2 ± 7.2 | 29.2 ± 8.5 | t = 1.04 | 0.30 | NA | NA | NA | NA |
Abbreviations: ECT, electroconvulsive therapy; PANSS, Positive and Negative Syndrome Scale; NA, not applicable.
1Chlorpromazine equivalents.
2Data were available for 130 patients with schizophrenia.
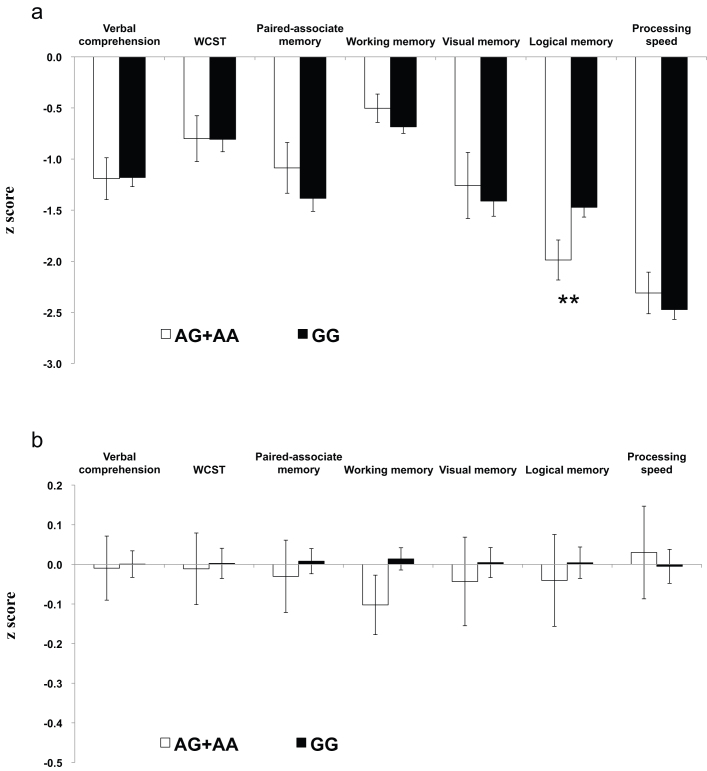
Results of the association between genotype and cognition are shown in Fig. 1a for schizophrenia patients and Fig. 1b for healthy controls. The initial two-way ANCOVA controlling for age and gender showed that the main effect of diagnosis was significant for all the seven cognitive factors (all P-values < 0.001), confirming the association of schizophrenia with pervasive neurocognitive deficits. The most pronounced impairment was found for processing speed (more than 2 SD below the mean of the controls), followed by logical memory. The main effect of genotype was marginally significant only for logical memory [F(1,846) = 6.64, P = 0.010]. Genotype-by-diagnosis interaction was significant only for logical memory [F(1,846) = 8.68, P = 0.003]. The one-way ANCOVA in schizophrenia patients, controlling for age and gender, revealed that the main effect of genotype was significant for logical memory [F(1,169) = 8.39, P = 0.006], but not for the other six factors (all P-values > 0.1) (Fig. 1a). In the one-way ANCOVA in control subjects the main effect of genotype was not significant for any of the seven factors (all P-values > 0.1) (Fig. 1b).
Figure 1. Comparisons of performance on the seven neurocognitive domains between A-allele carriers (white bars) and G-allele homozygotes (black bars) in patients with schizophrenia (a) and control subjects (b).
**P = 0.006 (according to the ANCOVA controlling for age and gender). Error bars represent standard errors of the mean.
Discussion
We can summarize our findings as follows. For the genetic association analysis, we did not find significant differences in genotype or allele frequencies of the CACNA1C rs1006737 polymorphism between the patients and controls, with the odds ratio of the risk A-allele for schizophrenia being 1.15 (95% confidence interval: 0.86–1.54). In the neurocognitive endophenotype analysis we found a significant association between the risk allele and lower logical memory performance in schizophrenia patients. No significant association between the genotype and cognitive function was found in healthy controls.
The present study is the first to examine the effect of the psychiatric risk gene CACNA1C on a wide range of neurocognitive domains in schizophrenia patients or healthy controls. In the endophenotype analysis we confirmed the pervasive neurocognitive impairments in schizophrenia. Our finding of the especially profound deficits in processing speed and logical (verbal) memory corresponds well to the literature9,10,23. The fact that verbal memory is one of the most severely impaired cognitive domains in schizophrenia might be related to the present finding of a significant effect of rs1006737 on this particular domain. The present finding also accords with the rodent data associating CAv1.2 with memory function24,25. In healthy controls, we did not find a significant effect of this genotype on any of the cognitive domains. While we can only speculate on the reason for these discrepant results between schizophrenia patients and healthy controls, this type of differential association was also reported for other schizophrenia candidate genes, such as COMT15 and ZNF804A14. Previous studies investigating the effect of rs1006737 on neurocognitive function have observed somewhat different results from the present one. Krug et al. found rs1006737 to be associated with decreased semantic verbal fluency in healthy men22, although our neuropsychological test battery did not include this domain. Zhang et al. showed an association between the A-allele and impaired working memory in both schizophrenia patients and healthy controls (but not in patients with bipolar disorder)20, whereas we did not find the significant association between rs006737 and working memory either in schizophrenia patients or healthy individuals. It should be noted, however, that their test battery to assess working memory consisted of the n-back and dot pattern expectancy (DPX) tasks, which were substantially different from our subtests pertaining to the working memory factor, ie, digit span and spatial memory span. It may be that working memory performance as measured by the former tasks is more sensitive to the effect of the CACNA1C genotype.
With respect to the association between schizophrenia and the rs1006737 polymorphism of CACNA1C, we failed to find the significant association reported in previous studies5,7,8. Nonetheless, the odds ratio observed here was comparable to those reported in the two previous genetic association studies on CACNA1C rs1006737 for schizophrenia5,8 (Table 2). It is therefore conceivable that our relatively small sample size for the genetic association study may have caused the non-significant result, as is also indicated by the power calculation below. Thus the present result should not be interpreted as contradictory to the literature linking CACNA1C rs1006737 to various psychiatric disorders including schizophrenia. Indeed, the current consensus in genetic association studies for common diseases including schizophrenia and mood disorders is that a very large sample size is required to discover common risk loci, and the identified polymorphisms in candidate genes for any particular disease impart only a small risk to the overall heritability of the disease. It should also be noted that Zhang et al. did not find a significant association between rs1006737 and schizophrenia in the Han Chinese population20. Given that the frequency of the risk A-allele is markedly lower in Asian than in Caucasian populations (Table 2), it is possible that the association between rs1006737 and schizophrenia could be influenced by the ethnic difference.
A few limitations to the present study should be acknowledged. First, the limited power of the present sample for the case-control association study is likely to have caused a type II error. Power calculation was performed based on the present sample of 552 patients and 1132 controls with the minor allele frequency of 0.059 in controls, revealing that our study had 90% power to detect a significant difference at the α = 5% level if the allelic odds ratio was 1.59 or more. Given that two studies have found the odds ratio to be around 1.155,8, the present sample size is considered insufficient to detect a significant difference, although our data may contribute to future meta-analytic studies. The current sample size for the cognitive endophenotype analysis may also have been under-powered to detect a small, if any, effect of genotype on cognition, particularly in the patient group; post-hoc power calculations revealed that, for the sample of 175 schizophrenia patients (ie, 25 A-allele carriers v. 150 G-allele homozygotes), this study had 90% power to detect a mean difference of 0.7 (with a standard deviation of 1.0) at the α = 5% level, and for the sample of 620 controls (ie, 75 A-allele carriers v. 545 G-allele homozygotes), this study had 90% power to detect a mean difference of 0.4 (with a standard deviation of 1.0) at the α = 5% level. Still, our sample size was comparable to or larger than those of previous studies20,21,22 that have investigated the effect of CACNA1C rs1006737 on neurocognitive/neuroimaging correlates. Further studies that examine the effect of this SNP on neurocognition in a larger sample are needed to draw definitive conclusions. Second, although we employed a neuropsychological test battery that covered wide-ranging neurocognitive domains, it still omitted some domains implicated in the cognitive pathology of schizophrenia such as verbal fluency and inductive reasoning.
In summary, the present finding suggests that CACNA1C may be involved in the pathophysiology of schizophrenia, at least in part by influencing verbal memory function. Since growing evidence demonstrates that CACNA1C is a shared susceptibility gene that crosses currently available diagnostic categories, future studies are warranted to test the effect of CACNA1C on endophenotypic traits in various psychiatric disorders, particularly in psychotic and mood disorders.
Methods
Subjects
Subjects of the genetic association part of our study consisted of 552 patients with schizophrenia (304 men and 248 women; mean age ± standard deviation (SD): 43.7 ± 13.9 years) and 1132 healthy controls (387 men and 745 women; mean age ± SD: 46.0 ± 16.2 years). All subjects were biologically unrelated Japanese. Patients were recruited from the outpatient clinic and inpatient ward of the National Center of Neurology and Psychiatry Hospital, Japan, which is located at the western part of Tokyo, or through advertisements in free local magazines and our website announcement. Most of the patients recruited via advertisements or website announcement were regularly attending to a nearby hospital or clinic located in the same geographical area, ie, the western part of Tokyo. Consensus diagnosis by at least two psychiatrists was made for each patient according to the Diagnostic and Statistical Manual of Mental Disorders, 4th edition criteria26, on the basis of unstructured interviews and information from medical records.
Healthy controls were recruited from the same geographical area via the free local magazines and our website announcement. They were interviewed using the Japanese version of the Mini-International Neuropsychiatric Interview27,28 by a research psychiatrist, and only those who demonstrated no current Axis I psychiatric disorders were enrolled in the study. In addition, those individuals who demonstrated one or more of the following conditions in a non-structured interview performed by an experienced psychiatrist were excluded: past or current regular contact to psychiatric services, having a history of regular use of psychotropics or substance abuse/dependence, presenting other obvious self-reported signs of past primary psychotic and mood disorders, and having a prior medical history of central nervous system disease or severe head injury.
Of the subjects genotyped, neuropsychological data were available for 202 patients and 706 controls. For these patients included in the cognitive assessment, daily doses of antipsychotics, including depot antipsychotics, were converted to chlorpromazine equivalents using guidelines29,30. Schizophrenic symptoms were assessed by a research psychiatrist in 130 (64%) of the 202 patients, using the Positive and Negative Syndrome Scale (PANSS)31; this yields positive, negative, and general psychopathology subscale scores.
The present experiments on our participants were conducted in accordance with the Declaration of Helsinki. After the nature of the study procedures had been fully explained, written informed consent was obtained from all participants. The study was approved by the ethics committee of the National Center of Neurology and Psychiatry, Japan.
Genotyping
Genomic DNA was prepared from venous blood according to standard procedures. Rs1006737 was genotyped using the TaqMan 5′-exonuclease allelic discrimination assay (assay ID: C___2584015_10). The thermal cycling conditions for polymerase chain reaction were as follows: 1 cycle at 95°C for 10 min followed by 50 cycles of 92°C for 15 s and 60°C for 1 min. The allele-specific fluorescence was measured with ABI PRISM 7900 Sequence Detection Systems (Applied Biosystems, Foster City, CA). Ambiguous genotype data were not included in the analysis.
Neurocognitive test battery
A neurocognitive test battery, comprising full Japanese versions of the Wechsler Memory Scale-Revised (WMS-R)32,33 and the Wechsler Adult Intelligence Scale-Revised (WAIS-R)34,35 and the Wisconsin Card Sorting Test (WCST)36,37, was administered. This battery was identical to that employed in our previous study38. It took approximately 4 hours to complete the battery, and this battery was administered over two or three days. The result of the digit span subtest in the WAIS-R is not reported here as its more comprehensive version (ie, spatial memory span in addition to digit span) is included in the WMS-R. Table 1 lists the subtests included in these three tests. A portion of the subjects did not complete the whole neuropsychological test battery due to several reasons such as fatigue or unexpected change of his/her schedule; however, those who completed at least one of the three neuropsychological tests were included in the present analyses. Consequently, WMS-R, WAIS-R and WCST were completed by 173 (85.6%), 178 (88.1%) and 177 (87.6%) of the total 202 schizophrenia patients and by 679 (96.2%), 618 (87.5%) and 664 (94.1%) of the total 706 control subjects, respectively (detailed in Supplementary Table S1 online).
Considering that there were as many as 27 subtests in the three neuropsychological tests, main analyses were conducted using the seven cognitive factors that we had identified in the previous report38 to avoid the problem of multiple testing as well as to make the interpretation of results easier. The seven factors were: verbal comprehension, WCST, paired-associate memory, working memory, visual memory, logical memory, and processing speed. These seven factors corresponded closely to the factors identified in previous studies that applied a factor analytic technique to neurocognitive functioning in schizophrenia39. Results of all subtest scores on the three neuropsychological tests and the WAIS-R IQs, stratified by diagnostic groups and CACNA1C rs1006737 genotype, are provided in Supplementary Table S1 online. Subtests that loaded onto each factor are indicated in the footnote of this table.
Analysis
Averages are reported as means ± SD. For demographic and clinical characteristics, categorical variables were compared using the χ2 test or Fisher's exact test where appropriate. The t-test was used to examine differences between groups. Deviations of genotype distributions from Hardy-Weinberg equilibrium (HWE) were assessed using the χ2 test for goodness of fit. Genotype and allele distributions were compared between patients and controls by using the χ2 test for independence. Due to the small number of the AA genotype (2 in the schizophrenia group and 3 in the healthy control group), we combined the AA genotype with the AG genotype in all of the analysis, as in previous studies20,40.
The association between the SNP rs1006737 and cognition was examined by the following steps. First, mean scores of the seven cognitive factors were normalised to the z-score using the control group data. The two-way analysis of covariance (ANCOVA), with genotype (minor allele ‘A’ carriers v. major allele ‘G’ homozygotes) and diagnosis (schizophrenia v. controls) as fixed factors and age and gender as covariates, was then performed to compare the seven cognitive factor scores. Lastly, we conducted one-way ANCOVA with potential confounders, in addition to age and gender, as covariates, separately for schizophrenia and control groups, to further explore the simple effect of genotype.
Statistical significance was set at two-tailed P < 0.05. In the cognitive endophenotype analysis we used a Bonferroni-corrected P-value of 0.007 (0.05/7) for statistical significance, given that there were seven cognitive factors that were simultaneously examined. Analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 18.0 (SPSS Japan, Tokyo).
Author Contributions
H.H. designed the study, managed the literature searches, collected the data, undertook the statistical analyses, and wrote the draft of the manuscript. N.Y. performed the genotyping. J.M., Yum.K. and Yuk.K. administered the neuropsychological tests. T.F., T.T., D.S., M.O., K.H., M.T. and K.A. contributed to the data collection and gave critical comments on the manuscript. H.K. supervised the entire project and gave critical comments on the manuscript. All authors contributed to and have approved the final manuscript.
Supplementary Material
Supplementary Table S1
Acknowledgments
We would like to thank all participants who took part in the study. This study was supported by Health and Labour Sciences Research Grants, Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS), Intramural Research Grant for Neurological and Psychiatric Disorders of NCNP, (H.K.), and Grant-in-Aid for Young Scientists from the JSPS (H.H.).
References
- Bhat S. et al. CACNA1C (Ca(v)1.2) in the pathophysiology of psychiatric disease. Prog Neurobiol in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira M. A. et al. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet 40, 1056–1058 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar P. et al. Whole-genome association study of bipolar disorder. Mol Psychiatry 13, 558–569 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casamassima F. et al. Phenotypic effects of a bipolar liability gene among individuals with major depressive disorder. Am J Med Genet B Neuropsychiatr Genet 153B, 303–309 (2010). [DOI] [PubMed] [Google Scholar]
- Green E. K. et al. The bipolar disorder risk allele at CACNA1C also confers risk of recurrent major depression and of schizophrenia. Mol Psychiatry 15, 1016–1022 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J. et al. Genome-wide association study of recurrent early-onset major depressive disorder. Mol Psychiatry 16, 193–201 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigos K. L. et al. Genetic variation in CACNA1C affects brain circuitries related to mental illness. Arch Gen Psychiatry 67, 939–945 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyegaard M. et al. CACNA1C (rs1006737) is associated with schizophrenia. Mol Psychiatry 15, 119–121 (2010). [DOI] [PubMed] [Google Scholar]
- Saykin A. J. et al. Neuropsychological function in schizophrenia. Selective impairment in memory and learning. Arch Gen Psychiatry 48, 618–624 (1991). [DOI] [PubMed] [Google Scholar]
- Heinrichs R. W. & Zakzanis K. K. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology 12, 426–445 (1998). [DOI] [PubMed] [Google Scholar]
- Gottesman II & Gould T. D. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry 160, 636–645 (2003). [DOI] [PubMed] [Google Scholar]
- Greenwood T. A. et al. Initial heritability analyses of endophenotypic measures for schizophrenia: the consortium on the genetics of schizophrenia. Arch Gen Psychiatry 64, 1242–1250 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward N. D., Jayathilake K. & Meltzer H. Y. COMT val108/158met genotype, cognitive function, and cognitive improvement with clozapine in schizophrenia. Schizophr Res 90, 86–96 (2007). [DOI] [PubMed] [Google Scholar]
- Hashimoto R. et al. The impact of a genome-wide supported psychosis variant in the ZNF804A gene on memory function in schizophrenia. Am J Med Genet B Neuropsychiatr Genet 153B, 1459–1464 (2010). [DOI] [PubMed] [Google Scholar]
- Wirgenes K. V. et al. Catechol O-methyltransferase variants and cognitive performance in schizophrenia and bipolar disorder versus controls. Schizophr Res 122, 31–37 (2010). [DOI] [PubMed] [Google Scholar]
- Barnett J. H., Jones P. B., Robbins T. W. & Müller U. Effects of the catechol-O-methyltransferase Val158Met polymorphism on executive function: a meta-analysis of the Wisconsin Card Sort Test in schizophrenia and healthy controls. Mol Psychiatry 12, 502–509 (2007). [DOI] [PubMed] [Google Scholar]
- Hashimoto R. et al. Association between the dysbindin gene (DTNBP1) and cognitive functions in Japanese subjects. Psychiatry Clin Neurosci 63, 550–556 (2009). [DOI] [PubMed] [Google Scholar]
- Hashimoto R. et al. A genetic variation in the dysbindin gene (DTNBP1) is associated with memory performance in healthy controls. World J Biol Psychiatry 11, 431–438 (2010). [DOI] [PubMed] [Google Scholar]
- Balog Z., Kiss I. & Kéri S. ZNF804A may be associated with executive control of attention. Genes Brain Behav 10, 223–227 (2010). [DOI] [PubMed] [Google Scholar]
- Zhang Q. J. et al. The effects of CACNA1C gene polymorphism on spatial working memory in both healthy controls and patients with schizophrenia or bipolar disorder. Neuropsychopharmacology 37, 677–684 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimm M. et al. Effects of a CACNA1C genotype on attention networks in healthy individuals. Psychol Med 41, 1551–1561 (2011). [DOI] [PubMed] [Google Scholar]
- Krug A. et al. Effect of CACNA1C rs1006737 on neural correlates of verbal fluency in healthy individuals. Neuroimage 49, 1831–1836 (2010). [DOI] [PubMed] [Google Scholar]
- Dickinson D., Ramsey M. E. & Gold J. M. Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch Gen Psychiatry 64, 532–542 (2007). [DOI] [PubMed] [Google Scholar]
- Moosmang S. et al. Role of hippocampal Cav1.2 Ca2+ channels in NMDA receptor-independent synaptic plasticity and spatial memory. J Neurosci 25, 9883–9892 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. A. et al. Conditional forebrain deletion of the L-type calcium channel Ca V 1.2 disrupts remote spatial memories in mice. Learn Mem 15, 1–5 (2008). [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. DSM-IV: Diagnostic and Statistical Manual of Mental Disorders, 4th edn. (American Psychiatric Association, 1994). [Google Scholar]
- Sheehan D. V. et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59 (suppl.20), 22–57 (1998). [PubMed] [Google Scholar]
- Otsubo T. et al. Reliability and validity of Japanese version of the Mini-International Neuropsychiatric Interview. Psychiatry Clin Neurosci 59, 517–526 (2005). [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Practice Guidelines for the Treatment of Patients with Schizophrenia (American Psychiatric Press, 1997). [Google Scholar]
- Inagaki A., Inada T., Fujii Y. & Yagi G. Equivalent Dose of Psychotropics (Seiwa Shoten, 1999). (in Japanese). [Google Scholar]
- Kay S. R., Fiszbein A. & Opler L. A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13, 261–276 (1987). [DOI] [PubMed] [Google Scholar]
- Sugishita M. Japanese Wechsler Memory Scale- (Nihonbunkakagakusha, 2001). (in Japanese). [Google Scholar]
- Wechsler D. Wechsler Memory Scale Manual, Revised (Psychological Corporation, 1987). [Google Scholar]
- Shinagawa F., Kobayashi S., Fujita K. & Maekawa H. Japanese Wechsler Adult Intelligence Scale-Revised (Nihonbunkakagakusha, 1990). (in Japanese). [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale, Revised (Psychological Corporation, 1981). [Google Scholar]
- Heaton R. K. The Wisconsin Card Sorting Test (Manual) (Psychological Assessment Resources, 1981). [Google Scholar]
- Kashima H. et al. in Cerebral Dynamics, Laterality and Psychopathology (eds R. Takahashi, P. Flor-Henry, J. Gruzelier & S. Niwa) 337–345 (Elsevier, 1987). [Google Scholar]
- Hori H. et al. Schizotypy and genetic loading for schizophrenia impact upon neuropsychological status in bipolar II and unipolar major depressive disorders. J Affect Disord in press. [DOI] [PubMed] [Google Scholar]
- Nuechterlein K. H. et al. Identification of separable cognitive factors in schizophrenia. Schizophr Res 72, 29–39 (2004). [DOI] [PubMed] [Google Scholar]
- Wang F., McIntosh A. M., He Y., Gelernter J. & Blumberg H. P. The association of genetic variation in CACNA1C with structure and function of a frontotemporal system. Bipolar Disord 13, 696–700 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1