Abstract
We review the mechanical origin of auditory-nerve excitation, focusing on comparisons of the magnitudes and phases of basilar-membrane (BM) vibrations and auditory-nerve fiber responses to tones at a basal site of the chinchilla cochlea with characteristic frequency ≈ 9 kHz located 3.5 mm from the oval window. At this location, characteristic frequency thresholds of fibers with high spontaneous activity correspond to magnitudes of BM displacement or velocity in the order of 1 nm or 50 μm/s. Over a wide range of stimulus frequencies, neural thresholds are not determined solely by BM displacement but rather by a function of both displacement and velocity. Near-threshold, auditory-nerve responses to low-frequency tones are synchronous with peak BM velocity toward scala tympani but at 80–90 dB sound pressure level (in decibels relative to 20 microPascals) and at 100–110 dB sound pressure level responses undergo two large phase shifts approaching 180°. These drastic phase changes have no counterparts in BM vibrations. Thus, although at threshold levels the encoding of BM vibrations into spike trains appears to involve only relatively minor signal transformations, the polarity of auditory-nerve responses does not conform with traditional views of how BM vibrations are transmitted to the inner hair cells. The response polarity at threshold levels, as well as the intensity-dependent phase changes, apparently reflect micromechanical interactions between the organ of Corti, the tectorial membrane and the subtectorial fluid, and/or electrical and synaptic processes at the inner hair cells.
In the mammalian cochlea, the bulk of auditory information is transmitted to the brain via the inner hair cells, which provide the sole synaptic inputs to 90–95% of the afferent fibers of the auditory nerve (1). Auditory-nerve excitation is triggered by the depolarization of inner hair cells upon deflection of their “hair” bundles toward the taller “hairs” or stereocilia (2, 3). Presumably, the forces that deflect the stereocilia are derived from the vibrations of the basilar membrane (BM) but it is not known how these vibrations are transmitted to the inner hair cells (4). Although it is clear that the BM and auditory-nerve fibers are similarly tuned at frequencies close to the characteristic frequency (CF) (5, 6), there is no consensus on whether neural thresholds correspond to a constant magnitude of BM displacement, velocity, or some function of these variables. Neither is it known with certainty what phases of BM vibrations trigger auditory-nerve excitation.
Our ignorance on the relationship between the magnitudes and phases of cochlear vibrations and auditory-nerve responses stems in part from the dearth of measurements of BM vibration in relatively healthy cochleae, which until recently were available from a single site in the cochlea of each species. Even when adequate mechanical data are available, comparisons with the responses of auditory-nerve fibers have been perfunctory, typically involving a frequency-threshold tuning curve from a single auditory-nerve fiber in one subject and BM data for another individual of the same species. Given the variability of both neural (e.g., ref. 7) and mechanical responses (e.g., refs. 5 and 6) as well as the difficulty of fully controlling the experimental conditions, such comparisons are bound to be imprecise and inconclusive.
Here we review the cochlear mechanical bases of auditory-nerve excitation as revealed by comparisons of the magnitudes and phases of BM and auditory-nerve-fiber responses to tones recorded from a basal site of the chinchilla cochlea with CF ≈ 9 kHz located about 3.5 mm from the oval window. To date, this site is the only location for which systematic and extensive sets of BM and auditory-nerve-fiber responses are available in any species. Using results from the literature as well as previously unpublished data we address three issues, namely frequency tuning at threshold levels, the timing of auditory-nerve excitation at threshold, and the intensity dependence of auditory-nerve response phases. These issues are investigated by using two complementary approaches. One approach consists of comparing averages of BM and auditory-nerve fiber data (magnitudes or phases) obtained from separate sets of normal subjects. The second approach consists of carrying out the comparisons by using BM and neural data collected consecutively in the same individual cochleae, under identical conditions.
Methods
The data of Figs. 1, 3A, 5, and 6 have not been previously published. The new BM and auditory-nerve data were obtained by using methods described, respectively, in refs. 8 and 9. For the purpose of relating BM and neural response phases, the latter have been corrected for an estimated 1-ms (frequency independent) delay introduced by synaptic processes and neural conduction time (for justification see refs. 9 and 10). Therefore, the corrected neural phases of Figs. 4–6 indicate, to a good first approximation, the phases of depolarization of inner hair cells.
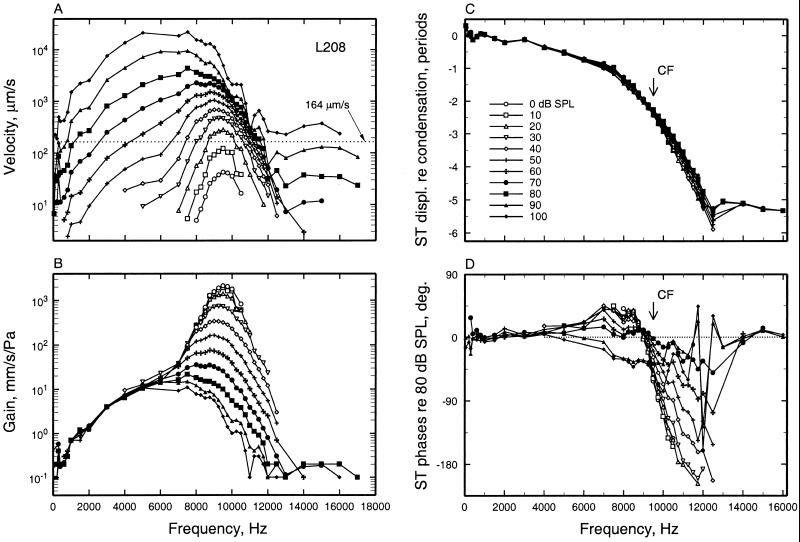
Figure 1.
The magnitudes and phases of BM responses to tones at the 3.5-mm site of the chinchilla cochlea. (A) Velocity magnitude as a function of stimulus frequency (abscissa) and level (parameter). The dotted line indicates the velocity corresponding to the CF threshold of the auditory-nerve fiber in Fig. 3B. (B) As in A, but normalized to stimulus pressure. (C) Response phases of peak displacement toward scala tympani, relative to peak condensation at the external ear canal, as a function of stimulus frequency and level. (D) As in C, but normalized to the phases of responses to 80-dB stimuli. Data from cochlea L208, recorded by using a laser vibrometer (59).
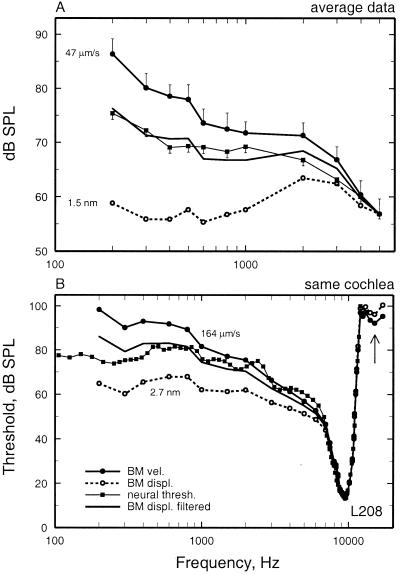
Figure 3.
Frequency tuning of BM vibrations and auditory-nerve fibers at the 3.5-mm site of the chinchilla cochlea. (A) An average frequency-threshold curve for auditory-nerve fibers is compared with the average tuning of BM responses in several cochleae, expressed as three curves indicating the stimulus levels at which BM vibration attains a displacement of 1.5 nm, a velocity of 47 μm/s, and a constant displacement high-pass filtered at a rate of 3.83 dB/octave. The three curves are equated to neural threshold at 5 kHz. BM data were measured in 18 cochleae by using laser velocimetry. The threshold averages of auditory-nerve fibers were based on data for 183 fibers with CF = 8–12 kHz recorded in 77 chinchillas. The data consisted of frequency-threshold tuning curves, measured with an automated adaptive algorithm (7, 60), and 0.5-s samples of responses to low-frequency tones (≤1 kHz) (9). Vertical bars indicate the SEM. (B) Frequency tuning of responses to tones of a BM site and an auditory-nerve fiber with similar characteristic frequency recorded in the same cochlea. The neural frequency-threshold tuning curve is compared with BM tuning curves indicating constant displacement (2.7 nm), constant velocity (164 μm/s), and displacement high-pass filtered at a rate of 3.81 dB/octave. The auditory-nerve fiber had spontaneous activity of 11.2 spikes/s. (B) Redrawn from figure 1B of Narayan et al. (8).
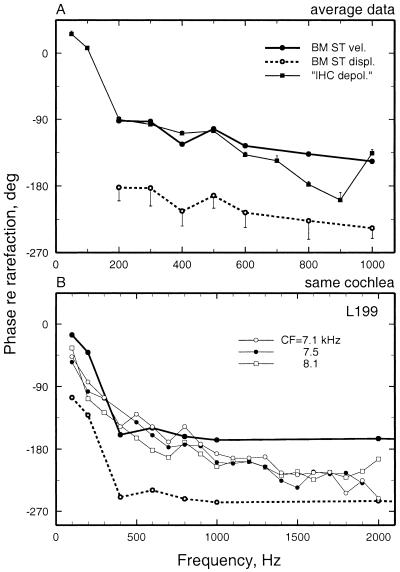
Figure 5.
The phases of BM and auditory-nerve-fiber responses to low-frequency tones. (A) Average response phases of auditory-nerve fibers and BM vibrations at the base of the chinchilla cochlea. Neural data are averages from 13–52 fibers (depending on frequency) with CF 8–12 kHz. BM responses were measured in 8–18 cochleae (depending on frequency). Vertical bars indicate SEM. (B) The phases of responses to tones of a BM site and auditory-nerve fibers with similar CF recorded in the same cochlea. The auditory-nerve phases have been corrected for 1 ms (the sum of neural and synaptic delays) so that they indicate the presumed phases of peak depolarization of the inner hair cells (9). The same BM data are represented in two curves, depicting the phases of peak velocity toward scala tympani and of peak displacement toward scala tympani. Spontaneous rates were 105, 62, and 73 spikes/s, respectively. Thresholds at CF were 11, 4, and 1 dB SPL, respectively. Stimuli for the auditory-nerve fibers were 10 repetitions of 75-dB, 100-ms tone pips presented every 300 ms. The data points for BM phases represent averages for responses to 50–100 dB SPL, depending on frequency. Averaging is justified because, as shown in Fig. 6B, BM response phases did not vary significantly as a function of stimulus intensity.
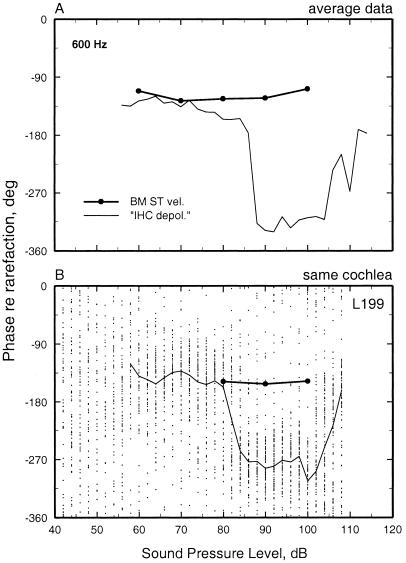
Figure 6.
The phases of BM and auditory-nerve-fiber responses to low-frequency tones as a function of stimulus level. (A) Average phases of responses to 600-Hz tones recorded at the BM of eight cochleae and from 27 auditory-nerve fibers with CF 8–12 kHz. (B) The responses to 600-Hz tones of a BM site and an auditory-nerve fiber (CF = 7. 1 kHz; spontaneous rate = 105 spikes/s) with similar CF recorded in the same cochlea. The phases of peak BM velocity toward scala tympani are indicated by ● connected by a thick line. The neural responses are depicted as a scatter diagram of phase vs. level, with each dot representing one spike. Stimuli were five repetitions of 100-ms tones, presented every 300 ms at random levels, with 2-dB steps. The mean phases of the neural responses are indicated by a thin line.
Figure 4.
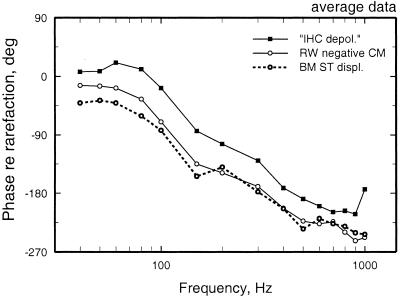
Average response phases of auditory-nerve fibers, cochlear microphonics (recorded at the round window), and BM displacement at the base of the chinchilla cochlea. Redrawn from the data of figure 5 of Ruggero et al. (16).
Frequency Tuning of BM and Auditory-Nerve Responses to Tones
Fig. 1 summarizes the responses to tones of a BM site located 3.5 mm away from the oval window in a nearly normal chinchilla cochlea. Vibration magnitudes (Fig. 1 A and B) and phases (Fig. 1 C and D) are plotted as a function of frequency (abscissa) and stimulus level (ordinate). The magnitudes grow at compressive rates at frequencies near CF (9.5 kHz) but linearly at other frequencies, with the result that sensitivity at CF decreases and frequency tuning becomes broader as a function of increasing stimulus level (Fig. 1 A and B). Response phases increasingly lag as stimulus frequency increases, with a relatively shallow slope at low frequencies and steep slopes near CF (Fig. 1C), a frequency region within which phases shift systematically with stimulus level (Fig. 1D). At frequencies well apart from CF, response phases do not vary with stimulus levels.
To compare frequency tuning at various stages of auditory-signal transduction it is convenient and customary to construct equal-response curves (e.g., Fig. 2) expressing the sound pressure level (SPL) required to produce a constant response magnitude. In the case of auditory-nerve fibers, frequency tuning usually is specified in terms of threshold. Such frequency-threshold curves may be compared with equal-velocity or equal-displacement curves computed from BM responses (e.g., Fig. 2) by determining the SPLs required to elicit a given magnitude of velocity (e.g., dotted line in Fig. 1A) or displacement. The first explicit comparison between neural thresholds and essentially normal BM vibrations was performed for a site of the guinea pig cochlea with CF ≈18 kHz (5). The thresholds for a single auditory-nerve fiber recorded in one cochlea were thought to correspond to a fixed magnitude of BM velocity, about 40 μm/s (5). However, on the basis of the same data it was later argued that neural thresholds correspond to a fixed BM displacement (11). A comparison for responses from the hook region of the cat cochlea led to a similar conclusion, with neural threshold corresponding to BM vibrations amounting to about 1 nm (12). In the chinchilla, average tuning curves for auditory-nerve fibers were compared with BM data recorded at sites with CFs of 9 and 10 kHz in two individual cochleae (13). Near CF there was an excellent match between neural and mechanical tuning, with neural thresholds corresponding to 39 and 73 μm/s (or, equivalently, 0.62 and 1.3 nm). However, because the comparisons were carried out over a limited range of frequencies (>2 kHz), no firm conclusion was reached regarding the precise relationship between mechanical and neural tuning.
Figure 2.
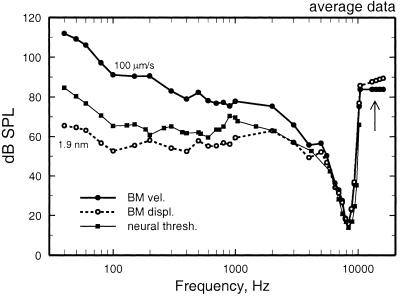
Frequency tuning of BM vibrations and auditory-nerve fibers at the 3.5-mm site of the chinchilla cochlea. An average frequency-threshold curve computed from responses of many auditory-nerve fibers is compared with the average tuning of BM responses in several cochleae, expressed as the stimulus levels at which BM vibration attains a displacement of 1.9 nm or a velocity of 100 μm/s. BM data were measured by using the Mössbauer technique. Redrawn from the data of figure 22 of Ruggero et al. (17).
Mechanical tuning also has been compared with tuning curves for inner hair cells. Sellick et al. (14) compared BM isovelocity and isodisplacement curves from guinea pig cochleae with one inner hair cell tuning curve (based on a constant DC receptor potential). Perusal of those data does not suggest any obvious conclusion on whether isovelocity or isodisplacement provides a better match to the receptor potentials. However, another study in guinea pig (15) found that tip-to-tail ratios for the receptor potential of one inner hair cell were substantially higher than those for displacement magnitude of BM vibrations.
Comparison of Averaged Mechanical and Neural Responses at the 3.5-mm Site of the Chinchilla Cochlea.
The problems inherent in comparing data from different individual subjects are ameliorated by making comparison using averages of grouped data. To date, such comparisons have been carried out only for the 3.5-mm site of the chinchilla cochlea (Fig. 2). An average frequency-threshold curve, computed from responses of many auditory-nerve fibers recorded in one group of chinchillas, was compared with tuning curves computed for velocity and displacement of (nearly normal) BM responses recorded with the Mössbauer technique in another group of chinchillas. The comparison indicated that, at CF (≈8.5 kHz), neural threshold corresponded to a displacement of about 2 nm or, equivalently, 100 μm/s. However, when the comparison was carried out over a range of stimulus frequencies encompassing more than two decades (over which the magnitudes of velocity and displacement diverge widely, at a rate of 6 dB per octave or 20 dB per decade), the frequency tuning of auditory-nerve fibers did not match a constant BM displacement or velocity, but rather corresponded to intermediate values (6, 16, 17).
An updated comparison of averaged neural and BM tuning for the same site of the chinchilla cochlea now can be carried out by using new BM measurements obtained with a laser vibrometer, as well as a larger database of frequency-threshold curves for auditory-nerve fibers. We want to ascertain that the conclusion drawn from Fig. 2 was unsullied by inclusion of BM responses that might have been abnormally insensitive at near-CF frequencies because of cochlear damage. Therefore, although it is desirable to compare mechanical and neural responses over the widest possible range of stimulus frequencies, we limit the present comparison to frequencies lower than 5 kHz, at which BM responses are linear and resistant to cochlear insults (5, 6, 13). The lower limits of the frequency range of measurements are dictated by the insensitivity of both neural and mechanical responses to low-frequency stimuli and, in the case of laser velocimetry recordings, by the presence of an artifact (because of motion of the perilymph meniscus overlying the BM measurements site) whose effects increase with decreasing frequency (12, 13, 18).
Average neural thresholds and BM vibration magnitudes as a function of frequency are compared in Fig. 3A. Average rate thresholds were computed for auditory-nerve fibers with spontaneous activity >18 spikes/s and CFs in the range of 8 to 12 kHz. Fibers with high spontaneous activity were selected because, in contrast with fibers with medium or low spontaneous activity, their thresholds are uniform in any individual ear at any given CF region (7). BM vibration magnitudes are presented in Fig. 3A as curves of constant velocity or constant displacement, which were arbitrarily equated at 5 kHz. At this frequency, neural threshold corresponds to 1.5 nm or 47 μm/s. Fig. 3A makes it clear that, in the range of 200 to 5,000 Hz, neural thresholds do not correspond to a constant BM displacement. Rather, neural thresholds closely approximate displacement magnitudes that have undergone high-pass filtering at a rate of +3.83 dB/octave.
Comparison of Mechanical and Neural Responses Recorded in the Same Chinchilla Cochleae.
In two exceptional cases, we succeeded in recording from auditory-nerve fibers and BM sites with corresponding CFs in the same chinchilla cochleae (8). The BM data of Fig. 1 were obtained from one of these cochleae, in which four auditory-nerve fibers were encountered with CFs (7.8–9.5 kHz) comparable to the CF of the BM recording site (9.5 kHz). Comparison of responses is especially straightforward in the case of the 9.5-kHz fiber (Fig. 3B), which presumably innervated an inner hair cell immediately adjacent to the BM recording site. At the fiber's CF threshold (13 dB SPL), BM vibrations had a peak velocity of 164 μm/s (dotted line in Fig. 1A) or, equivalently, a peak displacement of 2.7 nm. These values were used to specify mechanical isodisplacement and isovelocity tuning curves. At frequencies between CF and 1 kHz, there was a good match between neural thresholds and a constant BM velocity. However, taking into account the entire frequency range of measurements, neural thresholds were better fit by mechanical displacements subjected to high-pass filtering at a rate of 3.81 dB/octave. The tuning curves for the three other fibers with similar CF were well fit by BM displacement high-pass filtered at rates of 3.9–4.1 dB/octave. A similar comparison was carried out in another cochlea, in which the BM recording site had a CF of 9 kHz and four fibers were found with comparable CFs. The tuning curves of these fibers also were well matched by high-pass filtered BM displacement (at rates of 2.7–6 dB/octave).
Thus, at near-threshold stimulus levels, the frequency tuning of auditory-nerve fibers in both cochleae closely resembled that of BM displacement modified by high-pass filtering. However, neural tuning curves lacked the high-frequency plateaus (arrows in Figs. 2 and 3B) usually demonstrable in BM responses (6, 12, 17, 19, 20), suggesting that such vibrations are not transmitted to the stereocilia of inner hair cells (21).
Timing of Auditory-Nerve Threshold Responses to Low-Frequency Tones in Relation to BM Motion
The traditional view of auditory-nerve excitation was that spikes are generated when the BM is displaced toward scala vestibuli (22). This view was based on the functional polarity of the hair cell stereociliar bundles (which indicates that depolarization should occur when stereocilia are deflected away from the modiolus) and the likelihood that shear must be generated between the reticular lamina and the tectorial membrane sites contacted by the tips of the stereocilia (23, 24). An updated version of this view, partly based on the likelihood that inner hair cell stereocilia are not firmly connected to the tectorial membrane (25, 26), is that inner hair cells are depolarized (and auditory-nerve fibers excited) when the BM is moving at maximum speed from scala tympani toward scala vestibuli (27, 28). At the apex of the cochlea, both intracellular recordings from guinea pig inner hair cells (29) as well as responses of chinchilla auditory-nerve fibers (9, 10, 30) support the notion that depolarization of the inner hair cells occurs when the cochlear partition is in motion toward scala vestibuli.
A rather different situation holds for basal sites of the cochlea, where recordings from inner hair cells and auditory-nerve fibers are in conflict. As at the apex, depolarization responses of basal inner hair cells to low-frequency stimuli are in phase with peak BM velocity toward scala vestibuli (31–33). In contrast, responses of auditory-nerve fibers to low-frequency, near-threshold stimulation appear to be synchronous with BM displacement or motion toward scala tympani. Traditional views of inner hair cell stimulation were first challenged by studies of the responses of gerbil and guinea pig auditory-nerve fibers to very low-frequency stimuli (<100 Hz), designed to produce displacement steps or trapezoids at the BM (34–36). These studies, which deduced BM displacement from cochlear microphonics recorded at the round window, concurred that the timing of auditory-nerve fiber excitation differs significantly between basal and apical cochlear locations and suggested that fibers innervating the cochlear base are stimulated when the BM is displaced or in motion toward scala tympani. Later investigations, although often disagreeing in many details and also relying on cochlear microphonics to deduce BM motion, generally supported these conclusions, which were found to also apply to tonal stimuli with much higher frequencies (30, 37, 38).
Comparison of Averaged Phases for Mechanical and Neural Responses Recorded at the Base of the Cochlea.
The first recordings of BM vibration in the chinchilla cochlea, obtained with the Mössbauer technique, yielded response phases for low-frequency stimuli that were consistent with the phases of cochlear microphonics recorded at the round window (Fig. 4) (30, 39). Fig. 4 compares the phases of BM vibrations and auditory-nerve fiber excitation in response to low frequency (40–1,000 Hz) tones presented at near-threshold levels (16). [Comparisons are restricted to responses to low-frequency stimuli because phase locking in the auditory nerve diminishes rapidly above 1 kHz (40, 41) and also because instrumental and neural jitter greatly disturb the determination of phase at higher frequencies.] According to Fig. 4, neural excitation is triggered at an instant intermediate between the times of peak BM displacement and velocity toward scala tympani (i.e., leading BM displacement toward scala tympani; Fig. 4) (6, 10, 17). This response polarity was surprising because it conflicted with the phases of inner hair cell depolarization responses to low-frequency tones at the base of the guinea pig cochlea (31, 32). The paradoxical difference between the polarity of responses of inner hair cells and auditory-nerve fibers is not caused by species differences, because it also holds for inner hair cell and auditory-nerve data recorded at the base of guinea pig cochleae (38).
Fig. 5A shows average phases for the BM responses (of 8–18 cochleae, depending on frequency, newly recorded by using laser velocimetry) whose magnitudes are shown in Fig. 3A, together with average phases of near-threshold responses of auditory-nerve fibers with CFs of 8–12 kHz taken from a database of Ruggero et al. (9). Throughout the 200- to 1,000-Hz range of stimulus frequencies, peak inner hair cell depolarization (deduced from the timing of neural excitation) was nearly synchronous with peak BM velocity toward scala tympani, confirming the response polarity of Fig. 4 but indicating a somewhat larger phase lead relative to BM displacement.
Comparison of BM and Auditory-Nerve Fiber Responses Recorded at the Base of the Same Cochleae.
Fig. 5B compares the phases of neural and mechanical responses to tones in one of two cochleae from which responses were recorded from both the BM and auditory nerve fibers (8). Among the latter, three had CFs (7.1–8.1 kHz) similar to the CF (9 kHz) of the BM recording site. At stimulus frequencies <1 kHz, the neural response phases matched closely those of peak BM velocity toward scala tympani, consistent with the comparison of averaged data of Fig. 5A. Results in the other cochlea in which both neural and BM data were recorded (represented in Figs. 1 and 3B) were similar to those of Fig. 5B.
Intensity Dependence of Response Phases of Auditory-Nerve Fibers
Many years ago, Nelson Kiang and colleagues described striking intensity-dependent irregularities in the responses of cat auditory-nerve fibers (40, 42). These consisted of abrupt phase shifts, a bimodal distribution of excitation within each period of the response to a low frequency tone (peak splitting), and a notch in rate-intensity functions. Similar phenomena have been subsequently studied in cat (43–46), guinea pig (38), and chinchilla (9, 30). In chinchilla the basic phenomenon, a 90–180° phase shift, occurs almost universally, at 85–90 dB SPL, in responses of auditory-nerve fibers to low-frequency tones. An example of the phase shift for responses to 600-Hz tones is shown in Fig. 6B. At the exact intensity of the phase shift, period histograms also may exhibit peak splitting and, less often, response rates may dip sharply. Interpretation of these phenomena usually has been based on postulating the existence of two modes of excitation, which grow at different rates, have different phases, and can cause mutual cancellation (44, 45). In the case of high-CF auditory-nerve fibers, the possibility that similar phase shifts might take place in BM vibrations has been considered but usually rejected because of the linear nature of basal BM responses to stimuli with frequency well below CF (see Fig. 1). Nevertheless, until now we did not consider the matter closed because the existence of such phase shifts in BM responses was seldom addressed explicitly and was not well tested. In particular, BM recordings using the Mössbauer technique rarely permitted obtaining reliable responses spanning the intensity range over which neural phase shifts occur, because of its relative insensitivity combined with the insensitivity of BM responses to low-frequency tones at basal (high-CF) sites. We recently have revisited this subject by using a more sensitive technology (laser velocimetry).
Comparison of Averaged Phases for Mechanical and Neural Responses Recorded at the Base of the Cochlea.
Fig. 6A displays the average phases of responses to 600-Hz tones of 27 chinchilla auditory-nerve fibers with CFs of 8–12 kHz, plotted as a function of stimulus intensity. Response phases are constant over a wide range of intensities, from threshold to 86 dB SPL, but undergo a reversal as the intensity is stepped from 86 to 88 dB SPL. Phases stay constant in the range of 88 to 105 dB but undergo another reversal at 105–110 dB. Similar shifts were found for stimulus frequencies 100–1,000 Hz (9). For comparison, Fig. 6A also shows average phases for responses of a basal BM site with CF of 9–10 kHz to 600-Hz tones presented at 60, 70, 80, 90, and 100 dB SPL. Although absolute phases varied substantially from cochlea to cochlea (not shown), in any single cochlea, for a given stimulus frequency, the variation of BM phase with intensity was insignificant (average standard deviation: 7°). The slight variations with intensity of the average phases of Fig. 6A reflects almost entirely a combination of the variation of response phase across cochleae and differences in the sample size across stimulus levels. Comparison for other stimulus frequencies in the range of 100 to 1,000 Hz yielded similar results in that the neural responses underwent phase reversals but BM responses did not.
The Phases of BM and Auditory-Nerve Fiber Responses Recorded at the Base of the Same Cochleae.
A stringent test for the existence of BM counterparts of the intensity phase shifts seen in neural responses was carried out by recording BM and auditory-nerve-fiber responses to the same stimuli in the same cochleae (Fig. 6B). Fig. 6B displays the responses to 600-Hz tones of a fiber (CF: 7.1 kHz) recorded in one of these cochleae, as a function of stimulus intensity. Each dot represents an action potential. At intensities between threshold and 80 dB, most discharges were synchronous with peak BM velocity toward scala tympani. At 80–84 dB, the timing of discharge shifted abruptly, by about 140°. A second abrupt shift took place at about 100 dB, with phases returning to their near-threshold values. In contrast with these phase shifts, the phases of BM vibration did not change significantly as a function of stimulus intensity. Similar findings were obtained for stimulus frequencies 200–800 Hz in other fibers in the same and in another cochlea (8).
Summary Discussion
Systematic comparisons of the responses of auditory-nerve fibers and BM vibrations have been carried out only for the 3.5-mm site of the chinchilla cochlea. Thus, it remains to be seen whether the present findings apply to other sites of the cochlea of the chinchilla as well as other species.
Frequency Tuning.
The similar frequency tuning exhibited by BM vibrations and auditory-nerve fiber thresholds at the 3.5-mm site of the chinchilla cochlea refutes lingering arguments in defense of the existence in mammalian cochleae of a “second filter” (e.g., refs. 47 and 48), analogous to the process that sharpens the frequency tuning of hair cells in the basilar papilla of turtles (49, 50). However, the comparisons of BM and neural response magnitudes and phases suggest that frequency-threshold neural tuning curves are not simply determined by BM displacement, but rather by a function of both BM displacement and velocity. A role of BM velocity in neural excitation is reasonable if the stereocilia of inner hair cells are not attached to the tectorial membrane (25, 26), because in that case deflection of the stereocilia would be accomplished by their motion relative to the surrounding fluid (27, 28). Such a velocity dependence of receptor potentials has been demonstrated in basal inner hair cells, with an upper-frequency limit of velocity sensitivity perhaps as high as 1,600 Hz (see figure 7 of ref. 33). Nevertheless, it is prudent to think of the terms displacement and velocity more as shorthand expressions rather than as literal indicators of how inner hair cell stereocilia are stimulated, because it is likely that more central stages of cochlear signal processing (i.e., transduction currents, receptor potentials, calcium currents, transmitter release, and spike generation) act as (or mimic the effects of) frequency filters.
Polarity of Auditory-Nerve Fiber Excitation.
The polarity of auditory-nerve fiber excitation relative to BM vibrations at the base of the cochlea remains the most puzzling of our findings. First, the polarity of auditory-nerve fiber excitation at threshold levels seems to be incompatible with recordings of inner hair cell receptor potentials. This discrepancy may reflect dissimilar stimulation conditions (e.g., stimulus intensity) or may indicate that the recordings from inner hair cells are flawed (e.g., because the microelectrode interferes with cochlear micromechanics; ref. 51). Second, the polarity of excitation at threshold is opposite that predicted by the standard assumption that the stereocilia of hair cells (including inner hair cells) are deflected toward the taller stereocilia (the depolarizing direction) by rotation of the reticular lamina toward scala vestibuli (23, 24). Several schemes have been put forth to explain the response phases of basal auditory-nerve fibers, including their dependence on stimulus level, on the basis of micromechanics, electrical filtering of receptor potentials by the basolateral-membrane of inner hair cells, and a possible influence of extracellular microphonics (9, 32, 38, 52, 53). However, it seems fair to state that none have succeeded in accounting for the full complexity of the findings [see reviews by Ruggero et al. (9) and Cheatham and Dallos (53)].
Intensity Dependence of Auditory-Nerve Fiber Response Phases.
The drastically different behaviors of BM and neural response phases as a function of stimulus level rule out the possibility that the intensity-dependent phase shifts of auditory-nerve fibers reflect multiple modes in BM vibrations (54–57) but suggest that multiple vibration modes exist in the micromechanics of the organ of Corti/tectorial membrane complex (9, 44, 45, 58).
Acknowledgments
We were supported by Grant DC-00419 from the National Institute on Deafness and Other Communication Disorders.
Abbreviations
- BM
basilar membrane
- CF
characteristic frequency
- SPL
sound pressure level
Footnotes
This paper was presented at the National Academy of Sciences Colloquium “Auditory Neuroscience: Development, Transduction and Integration,” held May 19–21, 2000, at the Arnold and Mabel Beckman Center in Irvine, CA.
References
- 1.Spoendlin H. In: Physiology of the Ear. Jahn A F, Santos-Sacchi J, editors. New York: Raven; 1988. pp. 201–219. [Google Scholar]
- 2.Hudspeth A J, Corey D P. Proc Natl Acad Sci USA. 1977;74:2407–2411. doi: 10.1073/pnas.74.6.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russell I J, Richardson G P, Cody A R. Nature (London) 1986;321:517–519. doi: 10.1038/321517a0. [DOI] [PubMed] [Google Scholar]
- 4.Ruggero M A. In: Active Hearing. Flock A, Ottoson D, Ulfendahl M, editors. Oxford: Pergamon; 1995. pp. 321–336. [Google Scholar]
- 5.Sellick P M, Patuzzi R, Johnstone B M. J Acoust Soc Am. 1982;72:131–141. doi: 10.1121/1.387996. [DOI] [PubMed] [Google Scholar]
- 6.Robles L, Ruggero M A, Rich N C. J Acoust Soc Am. 1986;80:1364–1374. doi: 10.1121/1.394389. [DOI] [PubMed] [Google Scholar]
- 7.Liberman M C. J Acoust Soc Am. 1978;63:442–455. doi: 10.1121/1.381736. [DOI] [PubMed] [Google Scholar]
- 8.Narayan S S, Temchin A N, Recio A, Ruggero M A. Science. 1998;282:1882–1884. doi: 10.1126/science.282.5395.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruggero M A, Rich N C, Shivapuja B G, Temchin A N. Audit Neurosci. 1996;2:159–185. [Google Scholar]
- 10.Ruggero M A, Rich N C. J Neurophysiol. 1987;58:379–403. doi: 10.1152/jn.1987.58.2.379. [DOI] [PubMed] [Google Scholar]
- 11.Neely S T, Kim D O. Hear Res. 1983;9:123–130. doi: 10.1016/0378-5955(83)90022-9. [DOI] [PubMed] [Google Scholar]
- 12.Cooper N P, Rhode W S. Hear Res. 1992;63:163–190. doi: 10.1016/0378-5955(92)90083-y. [DOI] [PubMed] [Google Scholar]
- 13.Ruggero M A, Rich N C, Recio A, Narayan S S, Robles L. J Acoust Soc Am. 1997;101:2151–2163. doi: 10.1121/1.418265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sellick P M, Patuzzi R, Johnstone B M. Hear Res. 1983;10:93–100. doi: 10.1016/0378-5955(83)90019-9. [DOI] [PubMed] [Google Scholar]
- 15.Russell I J, Kössl M, Murugasu E. In: Advance in Hearing Research. Manley G A, Klump G M, Köppl C, Fastl H, Oeckinghaus H, editors. Singapore: World Scientific; 1995. pp. 136–144. [Google Scholar]
- 16.Ruggero M A, Robles L, Rich N C. J Acoust Soc Am. 1986;80:1375–1383. doi: 10.1121/1.394390. [DOI] [PubMed] [Google Scholar]
- 17.Ruggero M A, Rich N C, Robles L, Shivapuja B G. J Acoust Soc Am. 1990;87:1612–1629. doi: 10.1121/1.399409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Recio A, Rich N C, Narayan S S, Ruggero M A. J Acoust Soc Am. 1998;103:1972–1989. doi: 10.1121/1.421377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rhode W S. J Acoust Soc Am. 1971;49:1218–1231. doi: 10.1121/1.1912485. [DOI] [PubMed] [Google Scholar]
- 20.Nuttall A L, Dolan D F. J Acoust Soc Am. 1996;99:1556–1565. doi: 10.1121/1.414732. [DOI] [PubMed] [Google Scholar]
- 21.Wilson J P. In: Auditory Physiology and Perception. Cazals Y, Demany L, Horner K, editors. Oxford: Pergamon; 1992. pp. 71–84. [Google Scholar]
- 22.Goldstein M H., Jr . In: Medical Physiology. Mouncastle V B, editor. St. Louis: Mosby; 1968. pp. 1465–1498. [Google Scholar]
- 23.ter Kuile E. Pflügers Arch Ges Physiol Menschen Tiere. 1900;79:146–157. [Google Scholar]
- 24.Davis H. Cold Spring Harb Symp Quant Biol. 1965;30:181–189. doi: 10.1101/sqb.1965.030.01.020. [DOI] [PubMed] [Google Scholar]
- 25.Lim D. J Acoust Soc Am. 1980;67:1686–1695. doi: 10.1121/1.384295. [DOI] [PubMed] [Google Scholar]
- 26.Lim D. Hear Res. 1986;22:117–146. doi: 10.1016/0378-5955(86)90089-4. [DOI] [PubMed] [Google Scholar]
- 27.Dallos P, Billone M C, Durrant J D, Wang C-Y, Raynor S. Science. 1972;177:356–358. doi: 10.1126/science.177.4046.356. [DOI] [PubMed] [Google Scholar]
- 28.Billone M, Raynor S. J Acoust Soc Am. 1973;54:1143–1156. doi: 10.1121/1.1914361. [DOI] [PubMed] [Google Scholar]
- 29.Santos-Sacchi J, Dallos P. Hear Res. 1983;9:317–326. doi: 10.1016/0378-5955(83)90034-5. [DOI] [PubMed] [Google Scholar]
- 30.Ruggero M A, Rich N C. J Acoust Soc Am. 1983;73:2096–2108. doi: 10.1121/1.389577. [DOI] [PubMed] [Google Scholar]
- 31.Nuttall A L, Brown M C, Masta R I, Lawrence M. Brain Res. 1981;211:171–174. doi: 10.1016/0006-8993(81)90078-0. [DOI] [PubMed] [Google Scholar]
- 32.Russell I J, Sellick P M. J Physiol (London) 1983;338:179–206. doi: 10.1113/jphysiol.1983.sp014668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patuzzi R, Yates G. Hear Res. 1987;30:83–98. doi: 10.1016/0378-5955(87)90186-9. [DOI] [PubMed] [Google Scholar]
- 34.Konishi T, Nielsen D W. Jpn J Physiol. 1978;28:291–307. doi: 10.2170/jjphysiol.28.291. [DOI] [PubMed] [Google Scholar]
- 35.Zwislocki J J, Sokolich W G. Science. 1973;182:64–66. doi: 10.1126/science.182.4107.64. [DOI] [PubMed] [Google Scholar]
- 36.Sokolich W G, Hamernik R P, Zwislocki J J, Schmiedt R A. J Acoust Soc Am. 1976;59:963–974. doi: 10.1121/1.380955. [DOI] [PubMed] [Google Scholar]
- 37.Oshima W, Strelioff D. Hear Res. 1983;12:167–184. doi: 10.1016/0378-5955(83)90104-1. [DOI] [PubMed] [Google Scholar]
- 38.Sellick P M, Patuzzi R, Johnstone B M. Hear Res. 1982;7:199–222. doi: 10.1016/0378-5955(82)90014-4. [DOI] [PubMed] [Google Scholar]
- 39.Ruggero M A, Robles L, Rich N C. J Acoust Soc Am. 1986;79:1491–1498. doi: 10.1121/1.393763. [DOI] [PubMed] [Google Scholar]
- 40.Johnson D H. J Acoust Soc Am. 1980;68:1115–1122. doi: 10.1121/1.384982. [DOI] [PubMed] [Google Scholar]
- 41.Weiss T, Rose C. Hear Res. 1988;33:167–174. doi: 10.1016/0378-5955(88)90029-9. [DOI] [PubMed] [Google Scholar]
- 42.Kiang N Y S, Moxon E C. Ann Otol Rhinol Laryngol. 1972;81:714–730. doi: 10.1177/000348947208100513. [DOI] [PubMed] [Google Scholar]
- 43.Gifford M L, Guinan J J., Jr J Acoust Soc Am. 1983;74:115–123. doi: 10.1121/1.389728. [DOI] [PubMed] [Google Scholar]
- 44.Kiang N Y S. In: Handbook of Physiology—The Nervous System: Sensory Processes. Darian-Smith I, editor. Bethesda, MD: Am. Physiol. Soc.; 1984. pp. 639–674. [Google Scholar]
- 45.Kiang N Y S. Hear Res. 1990;49:1–16. doi: 10.1016/0378-5955(90)90091-3. [DOI] [PubMed] [Google Scholar]
- 46.Liberman M C, Kiang N Y S. Hear Res. 1984;16:75–90. doi: 10.1016/0378-5955(84)90026-1. [DOI] [PubMed] [Google Scholar]
- 47.Allen J B, Neely S T. Physics Today. 1992;45:40–47. [Google Scholar]
- 48.Allen J B, Fahey P F. J Acoust Soc Am. 1993;94:809–816. doi: 10.1121/1.408182. [DOI] [PubMed] [Google Scholar]
- 49.Crawford A C, Fettiplace R. J Physiol (London) 1981;312:377–412. doi: 10.1113/jphysiol.1981.sp013634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Art J J, Fettiplace R. J Physiol (London) 1987;385:207–242. doi: 10.1113/jphysiol.1987.sp016492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zwislocki J J. Acta Oto-Laryngol. 1985;100:201–209. doi: 10.3109/00016488509104782. [DOI] [PubMed] [Google Scholar]
- 52.Cheatham M A, Dallos P. J Acoust Soc Am. 1998;104:356–369. doi: 10.1121/1.423245. [DOI] [PubMed] [Google Scholar]
- 53.Cheatham M A, Dallos P. J Acoust Soc Am. 1999;105:799–810. doi: 10.1121/1.426269. [DOI] [PubMed] [Google Scholar]
- 54.Kolston P J. Proc Natl Acad Sci USA. 1999;96:3676–3681. doi: 10.1073/pnas.96.7.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hubbard A. Science. 1993;259:68–71. doi: 10.1126/science.8418496. [DOI] [PubMed] [Google Scholar]
- 56.Mountain D C, Cody A R. Hear Res. 1999;132:1–14. doi: 10.1016/s0378-5955(99)00013-1. [DOI] [PubMed] [Google Scholar]
- 57.Nilsen K E, Russell I J. Nat Neurosci. 1999;2:642–648. doi: 10.1038/10197. [DOI] [PubMed] [Google Scholar]
- 58.Lin T, Guinan J J., Jr J Acoust Soc Am. 2000;107:2615–2630. doi: 10.1121/1.428648. [DOI] [PubMed] [Google Scholar]
- 59.Ruggero M A, Rich N C. Hear Res. 1991;51:215–230. doi: 10.1016/0378-5955(91)90038-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wiederhold M L, Kiang N Y. J Acoust Soc Am. 1970;48:950–965. doi: 10.1121/1.1912234. [DOI] [PubMed] [Google Scholar]