Abstract
Objectives
It is unknown if a relationship exists between multiple sclerosis and chronic cerebrospinal venous insufficiency and if this venous pathology is a causal factor for multiple sclerosis or is a product of a neurological disease. Even so, one should expect that if multiple sclerosis were the cause for venous lesions, then patients with an extended history of the disease would present with a more severe venous pathology.
Design
Retrospective analysis of catheter venography of the azygous and internal jugular veins, and duration of clinical history of the disease in multiple sclerosis patients.
Setting
Mono-profile specialist hospital.
Participants
353 multiple sclerosis patients, with duration of the disease: 0.5-41 years (median: 10 years).
Main outcome measures
We performed statistical analysis of the correlations between the duration of multiple sclerosis and the degree and number of venous lesions revealed using catheter venography.
Results
We observed weak, statistically insignificant correlations between the severity of chronic cerebrospinal venous insufficiency and the duration of multiple sclerosis. For the cumulated scores of venous lesions, Spearman and Kendall's tau correlation coefficients were 0.03 and 0.02, respectively; for maximal scores of venous lesions, coefficients were 0.06 and 0.05, while for the number of diseased veins they were 0.007 and 0.006, respectively. Consequently, this analysis did not yield any data supporting the idea that MS is the cause of venous lesions.
Conclusion
The results of our survey indicated that venous malformations are most likely congenital, and multiple sclerosis had no significant impact on the development of venous pathology.
Introduction
The aim of this study was to evaluate a potential role for multiple sclerosis (MS) in the development of venous malformations. It has recently been revealed that MS, a chronic disease of the central nervous system, is often associated with chronic cerebrospinal venous insufficiency. Chronic cerebrospinal venous insufficiency is a unique venous pathology, which is comprised of stenoses and occlusions in extracranial veins draining the brain and spinal cord.1–9 The discovery of chronic cerebrospinal venous insufficiency has shed new light on a potential pathogenesis of MS.10–12 Presently, however, it remains unknown if a relationship exists between the two pathologies and, importantly, if chronic cerebrospinal venous insufficiency is a causal factor for MS or a product of MS. Even so, one could expect that if MS is the cause of chronic cerebrospinal venous insufficiency, then patients with an extended history of the disease would present with a more severe venous pathology.
Patients and methods
This survey was part of a clinical trial on endovascular treatments for chronic cerebrospinal venous insufficiency. The entire study was designed to assess safety and efficacy of endovascular procedures performed to alleviate venous outflow blockages in the main veins draining the central nervous system. The study was approved by the Bioethical Committee of the Regional Silesian Board of Physicians in Katowice, Poland (approval number 7/2010). The study was registered at ClinicalTrials.gov, identifier NCT01264848.
All patients provided written consent to undergo the procedures and tests. A total of 353 MS patients were evaluated who, previously, using Doppler sonography and magnetic resonance venography, had been diagnosed with chronic cerebrospinal venous insufficiency. The patients’ ages ranged from 15 to 70 years, with a median age of 43 years. The patients had suffered from MS for 0.5–41 years, with a median duration of the disease of 10 years. Catheter venography was performed under mild sedation and local anaesthesia. The details of the venographic protocol can be found in our previous paper.4 The following anatomical structures were evaluated: the internal jugular veins, the brachiocephalic veins and the azygous vein. The following venographic flow patterns were categorized into four grades and were regarded as abnormal:
Grade 1: venous outflow slowed down, no reflux detected;
Grade 2: venous outflow slowed down, mild reflux and/or pre-stenotic dilation of the vein;
Grade 3: venous outflow slowed down, with reflux and outflow through collaterals;
Grade 4: no outflow through the vein, huge outflow through collaterals.
To evaluate correlations between the venous outflow pathology and the duration of the disease, the degree of venous abnormalities was measured in three ways:
An accumulated score of the venous lesions involved the sum of the grades of venous blockages (e.g. if the right internal jugular vein was grade 2 and the azygous vein was grade 4, then the accumulated score was 6; all outflow blockages in the left brachiocephalic veins were arbitrary scored as 2 because in this big vein the categorized grading system was difficult to use to establish a value);
A maximal score of the venous lesions was determined; a grade was given to the most affected vein;
The total number of the veins with confirmed outflow pathology was determined.
All clinical data (age, duration of the disease) were obtained from the history of the patients. The scoring of venous lesions using the above scale was performed by one of the authors who was not performing the venography, and this assessment was done exclusively basing on the movies recorded during the venography procedures.
Statistical analysis involved the evaluation of correlations between the duration of MS history and the degree and number of venous lesions. To assess the strength of these correlations, Spearman and Kendall's tau correlation coefficients were calculated. A correlation coefficient value below 0.4 was considered to represent a weak correlation. Also, statistical significances of the coefficients were calculated. The significance of the P values was set at P < 0.05.
Results
We obtained weak correlations between the severity of chronic cerebrospinal venous insufficiency measured by the number and degree of venous lesions and the duration of MS. For the cumulated scores of venous lesions, Spearman and Kendall's tau correlation coefficients were 0.03 and 0.02, respectively; for maximal scores of venous lesions, the coefficients were 0.06 and 0.05, while the coefficients for the number of diseased veins were 0.007 and 0.006, respectively. Further statistical analysis did not reveal any significance (P > 0.05) of these correlations. Consequently, the analysis did not yield any data supporting the idea that MS is the cause of chronic cerebrospinal venous insufficiency. The details of the data are presented in Figures 1–3.
Figure 1.
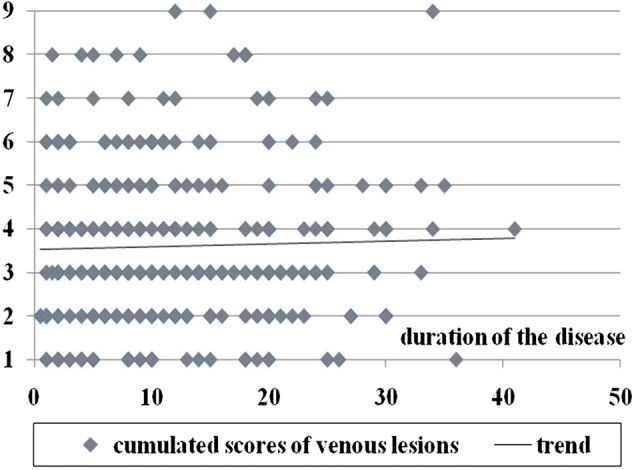
Duration of MS compared with cumulated scores of venous lesions; P values for correlation coefficients are >0.05 (Spearman's rank coefficient test = 0.63, Spearman's permutation test = 0.63, Kendall tau rank coefficient test = 0.59, Kendall tau permutation test = 0.63)
Figure 3.
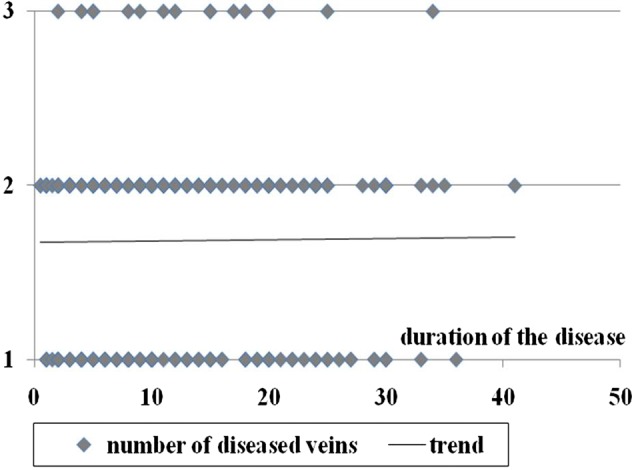
Duration of MS compared to number of diseased veins; P values for correlation coefficients are >0.05 (Spearman's rank coefficient test = 0.90, Spearman's permutation test = 0.91, Kendall tau rank coefficient test = 0.87, Kendall tau permutation test = 0.91)
Figure 2.
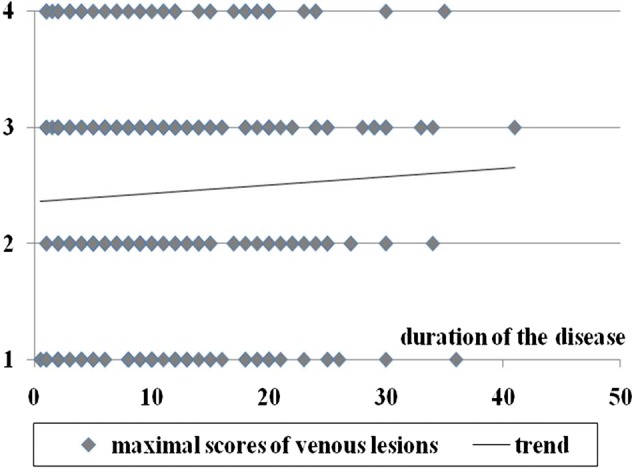
Duration of MS compared with maximal scores of venous lesions; P values for correlation coefficients are >0.05 (Spearman's rank coefficient = 0.27, Spearman's permutation test = 0.28, Kendall tau rank coefficient test = 0.19, Kendall tau permutation test = 0.27)
Discussion
In this study using catheter venography for the assessment of chronic cerebrospinal venous insufficiency, there were no correlations observed between the duration of MS and the severity of venous pathology. Thus, the results were in contrast to the idea of a neurological pathology being the primary cause for the development of the vascular lesions.
The use of catheter venography, which at the moment has been regarded as gold standard for the assessment of vascular pathologies, can be seen as the strong point of our study. However, it has been weakened by our scale for the grading of chronic cerebrospinal venous insufficiency,4 which is not yet widely accepted. Also, there were additional limitations to our study. It should be noted that our study has only been an indirect evaluation of a hypothetical clinical course of MS based on an analysis of a big cohort. Also, we were unable to rule out a potential selection bias (i.e. our patient cohort did not represent the authentic random sample of MS patients). Moreover, the sensitivity of diagnostics and the grading of chronic cerebrospinal venous insufficiency could potentially contain errors, especially in the cases of the azygous vein lesions. However, a relatively large number of evaluated patients have potentially contributed to the minimization of some of the above-presented biases.
Vascular specialists, as stated in the Consensus Document of the International Union of Phlebology on the diagnosis and treatment of venous malformations, have interpreted chronic cerebrospinal venous insufficiency as vascular malformations of congenital origin.13 On the contrary, the neurological community has been rather sceptical about the interpretation from the vascular specialists. There have been published reports that deny the existence of chronic cerebrospinal venous insufficiency,14,15 but these studies appear to be improperly designed.16
Alternative studies were published that tried to resolve the dilemma between the primary vs. secondary nature of chronic cerebrospinal venous insufficiency by studying patients with clinically isolated syndrome (CIS).
In a study by Yamout et al.,17 the authors had found that the patients with CIS, a neurological syndrome that does not meet the criteria of MS, but can precede MS, have low prevalence of chronic cerebrospinal venous insufficiency (9%), while 92% of clinically overt MS patients present with venous abnormalities. Therefore, the data from Yamout et al. support the secondary nature of chronic cerebrospinal venous insufficiency and assumes that chronic cerebrospinal venous insufficiency may develop due to the action of proinflammatory agents released by a diseased brain or brain atrophy. However, Yamout et al. have assessed a number of patients: 11 patients have CIS and 13 have late-stage MS.17 In addition, the venographic criteria of chronic cerebrospinal venous insufficiency chosen by Yamout et al. appear to be debatable . Similarly, Baracchini et al. assessed 50 patients with CIS by Doppler sonography and found chronic cerebrospinal venous insufficiency flow patterns in 16% of the patients, while less severe sonographic abnormalities are even more common (52% of the patients).18 Also the authors explained that the venous pathology is secondary to MS. Although the studies contained weak arguments, the results cannot be ignored; however, the results could be interpreted differently. It has been known that only 30–70% of the CIS cases progress to MS.19 Thus, it is possible that only patients with clinically silent chronic cerebrospinal venous insufficiency could progress into MS. In addition, it is known that many healthy individuals can demonstrate disturbances of venous outflow through the internal jugular veins,20 leading to the unlikely possibility that cerebral atrophy is the cause of chronic cerebrospinal venous insufficiency.
In our study, we attempted to resolve the problem of primary vs. secondary nature of chronic cerebrospinal venous insufficiency by an alternate method. The endovascular treatments for chronic cerebrospinal venous insufficiency were preceded by the detailed assessment of the treated veins primarily by means of catheter venography. This preprocedural evaluation, in addition to obvious clinical information, provides additional data that can be used to better understand the phenomenon of venous malformations in MS. Our analysis focused on the correlations between the duration of MS and the characteristics of venous lesions. Statistical analysis of the lesion parameters could potentially answer the following question: can inflammation or other processes associated with MS influence the severity of chronic cerebrospinal venous insufficiency (i.e. is chronic cerebrospinal venous insufficiency a product of MS). If this result was observed, then one can expect more severe and numerous lesions in the patients with a longer history of the disease; however, no correlation was observed between the duration of the history of MS and the degree of chronic cerebrospinal venous insufficiency. Consequently, the results of our survey indicated that the venous malformations were most likely congenital and that MS did not appear to have a significant impact on the degree and progression of the venous pathology.
However, the possibility that a neurological process could play a role in the progression of chronic cerebrospinal venous insufficiency cannot be completely excluded, even if our analysis did not confirm such a scenario (e.g. due to the limitation of our diagnostic methods). It should be expected that a vasoactive substance (e.g. endothelin-121–24 or another vasoconstrictor) rather than a proinflammatory substance could be a causal factor. It could be possible that a vasoactive agent would act on a previously susceptible segment of blood vessel (i.e. on a preclinical malformed venous valve; the most common location of chronic cerebrospinal venous insufficiency pathology). For example, it has been known that in aortic valve stenosis, endothelin-1, a potent vasoconstrictor, plays a role in the progression of the disease.25,26 Alternatively, one could hypothesize that inflammatory and neurodegenerative processes within nervous parenchyma and the impairment of venous outflow should act together to result in clinically overt MS. This hypothetical scenario would explain the phenomenon why many healthy individuals that present with chronic cerebrospinal venous insufficiency have no obvious clinical consequences. Undoubtedly, these uncertainties should be revealed by ongoing studies.
DECLARATIONS
Competing interests
None declared
Funding
None
Ethical approval
The study has been approved by the Bioethical Committee of the Regional Silesian Board of Physicians in Katowice, Poland (approval number 7/2010). The study has been registered at ClinicalTrials.gov, identifier NCT01264848
Guarantor
MS
Contributorship
All authors contributed equally
Acknowledgements
None
Reviewer
Ali Yagan
References
- 1.Zamboni P, Galeotti R, Menegatti E, et al. Chronic cerebrospinal venous insufficiency in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 2009;80:392–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zamboni P, Menegatti E, Galeotti R, et al. The value of cerebral Doppler venous haemodynamics in the assessment of multiple sclerosis. J Neurol Sci 2009;282:21–7 [DOI] [PubMed] [Google Scholar]
- 3.Simka M, Kostecki J, Zaniewski M, Majewski E, Hartel M Extracranial Doppler sonographic criteria of chronic cerebrospinal venous insufficiency in the patients with multiple sclerosis. Int Angiol 2010;29:109–14 [PubMed] [Google Scholar]
- 4.Ludyga T, Kazibudzki M, Simka M, et al. Endovascular treatment for chronic cerebrospinal venous insufficiency: is the procedure safe? Phlebology 2010;25:286–95 [DOI] [PubMed] [Google Scholar]
- 5.Simka M, Kostecki J, Zaniewski M, Majewski E, Szewczyk-Urgacz D Preliminary report on pathologic flow patterns in the internal jugular and vertebral veins of patients with multiple sclerosis. Przegl Flebol 2009;17:61–4 [Google Scholar]
- 6.Al-Omari MH, Rousan LA Jugular vein morphology and hemodynamics in patients with multiple sclerosis. Int Angiol 2010;29:115–20 [PubMed] [Google Scholar]
- 7.Zamboni P, Consorti G, Galeotti R, et al. Venous collateral circulation of the extracranial cerebrospinal outflow routes. Curr Neurovasc Res 2009;6:204–12 [DOI] [PubMed] [Google Scholar]
- 8.Bartolomei I, Salvi F, Galeotti R, et al. Hemodynamic pattern of chronic cerebrospinal venous insufficiency in multiple sclerosis. Correlation with symptoms at onset and clinical course. Int Angiol 2010;29:183–8 [PubMed] [Google Scholar]
- 9.Zamboni P, Galeotti R, Menegatti E, et al. Endovascular treatment of chronic cerebrospinal venous insufficiency, A prospective open-label study. J Vasc Surg 2009;50:1348–58 [DOI] [PubMed] [Google Scholar]
- 10.Haacke EM Chronic cerebral spinal venous insufficiency in multiple sclerosis. Expert Rev Neurother 2011;11:5–9 [DOI] [PubMed] [Google Scholar]
- 11.Simka M Blood brain barrier compromise with endothelial inflammation may lead to autoimmune loss of myelin during multiple sclerosis. Curr Neurovasc Res 2009;6:132–9 [DOI] [PubMed] [Google Scholar]
- 12.Simka M, Zaniewski M Reinterpreting the magnetic resonance signs of hemodynamic impairment in the brains of multiple sclerosis patients from the perspective of a recent discovery of outflow block in the extracranial veins. J Neurosc Res 2010;88:1841–5 [DOI] [PubMed] [Google Scholar]
- 13.Lee BB, Bergan J, Gloviczki P, et al. Diagnosis and treatment of venous malformations Consensus Document of the International Union of Phlebology (IUP)- 2009. Int Angiol 2009;28:434–51 [PubMed] [Google Scholar]
- 14.Sundström P, Wåhlin A, Ambarki K, Birgander R, Eklund A, Malm J Venous and cerebrospinal fluid flow in multiple sclerosis: A case-control study. Ann Neurol 2010;68:255–9 [DOI] [PubMed] [Google Scholar]
- 15.Doepp F, Friedemann P, Valdueza JM, Schmierer K, Schreiber SJ No cerebrocervical venous congestion in patients with multiple sclerosis. Ann Neurol 2010;68:173–83 [DOI] [PubMed] [Google Scholar]
- 16.Zamboni P Regarding “no cerebrospinal venous congestion in patients with multiple scleroisis. Intraluminal jugular septation”. Ann Neurol 2010;68:969 [DOI] [PubMed] [Google Scholar]
- 17.Yamout B, Herlopian A, Issa Z, et al. Extracranial venous stenosis is an unlikely cause of multiple sclerosis. Mult Scler 2010;16:1341–8 [DOI] [PubMed] [Google Scholar]
- 18.Baracchini C, Perini P, Calabrese M, Causin F, Rinaldi F, Gallo P No evidence of chronic cerebrospinal venous insufficiency at multiple sclerosis onset. Ann Neurol 2011;69:90–9 [DOI] [PubMed] [Google Scholar]
- 19.Miller D, Barkhof F, Montalban X, Thompson A, Filippi M Clinically isolated syndromes suggestive of multiple sclerosis, part I: natural history, pathogenesis, diagnosis and progression. Lancet Neurol 2005;4:281–8 [DOI] [PubMed] [Google Scholar]
- 20.Stoquart-ElSankari S, Lehmann P, Villette A, et al. A phase-contrast MRI study of physiologic cerebral venous flow. J Cerebr Blood F Met 2009;29:1208–15 [DOI] [PubMed] [Google Scholar]
- 21.Pache M, Kaiser HJ, Akhalbedashvili N, et al. Extraocular blood flow and endothelin-1 plasma levels in patients with multiple sclerosis. Eur Neurol 2003;49:164–8 [DOI] [PubMed] [Google Scholar]
- 22.Haufschild T, Shaw SG, Kesselring J, Flammer J Increased endothelin-1 plasma levels in patients with multiple sclerosis. J Neuroophthalmol 2001;21:37–8 [DOI] [PubMed] [Google Scholar]
- 23.Speciale L, Sarasella M, Ruzzante S, et al. Endothelin and nitric oxide levels in cerebrospinal fluid of patients with multiple sclerosis. J Neurovirol 2000;6:S62–6 [PubMed] [Google Scholar]
- 24.Dashwood MR, Loesch A Endothelin-1 as a neuropeptide: neurotransmitter or neurovascular effects? J Cell Commun Signal 2010;4:51–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peltonen T, Taskinen P, Näpänkangas J, et al. Increase in tissue endothelin-1 and ETA receptor levels in human aortic valve stenosis. Eur Heart J 2009;30:242–9 [DOI] [PubMed] [Google Scholar]
- 26.El-Hamamsy I, Balachandran K, Yacoub MH, et al. Endothelium-dependent regulation of the mechanical properties of aortic valve cusps. J Am Coll Cardiol 2009;53:1448–55 [DOI] [PubMed] [Google Scholar]


