Abstract
The use of bone grafting is a well-established way to enhance initial implant fixation in situations with reduced bone stock. Ceramic bone substitutes are inferior alternatives to autogenous or allogeneic bone graft. Improvement of bone graft substitutes is needed. We investigated whether biomechanical implant fixation and osseointegration of experimental implant grafted with β-TCP granules (Conduit) could be improved by soaking the β-TCP granules in bisphosphonate (zoledronate).
In 10 dogs, a pair of titanium coated implants surrounded by a 2.5 mm gap was inserted into the proximal part of each tibia. The gap was grafted with β-TCP granules either soaked with zoledronate or saline. At 12 weeks, the implants were evaluated with biomechanical push-out test and histomorphometrical analysis.
We found that bisphosphonate increased one of the three biomechanical parameters, but found no difference in the amount of new bone or β-TCP granules between the two treatment groups.
This study indicates that local treatment of β-TCP granules with zoledronate not only has the potential to increase implant fixation but also calls for further experimental research in order to optimize the dose of zoledronate.
Keywords: Implant fixation, bisphosphonate, biomechanical fixation, histomorphometry, bone graft substitute.
INTRODUCTION
Long-term survival of hip arthroplasties is dependent on early and secure fixation to bone [1, 2]. In situations with reduced bone stock at the implantation site, the use of bone graft is a well established way of optimizing the early implant fixation [3, 4]. Autograft is considered as the gold standard [5]. However, the use of autograft is associated with donor site morbidity and limited availability. An alternative to autograft is allograft. However, the use of allograft is associated with the risk of disease transfer, inconsistent quality, and immunological host response that might inhibit osseointegration. Alternatives for auto- and allograft are bone graft substitutes [6]. Studies have shown that these graft substitutes are inferior to allograft when comparing osseointegration and implant fixation [7, 8]. It is of interest to optimize the osseointegrated properties of bone graft substitutes.
A potential way to increase osseointegration of bone graft substitutes could be with the use of bisphosphonates. Studies have investigated the use of bisphosphonates as adjuvant to bone graft substitutes [9-11]. The general findings were a decreased osteoclastic activity and an enhanced new bone formation. However, little is still known about the properties of bisphosphonates to enhance osseointegration and implant fixation of implants surrounded with bone graft substitutes.
We tested the hypothesis that grafting beta-tricalcium phosphate granules around an experimental implant, after they had been soaked in bisphosphonate and subsequently rinsed, would increase biomechanical implant fixation, enhance new bone formation and preserve beta-tricalcium phosphate granules.
MATERIALS AND METHODS
Study Design
We used 10 skeletally mature female hound dogs with a median weight of 21 kg (range, 19-25 kg). This study was approved by our institution’s Animal Care and Use Committee. NIH guidelines for the care and use of laboratory animals have been observed.
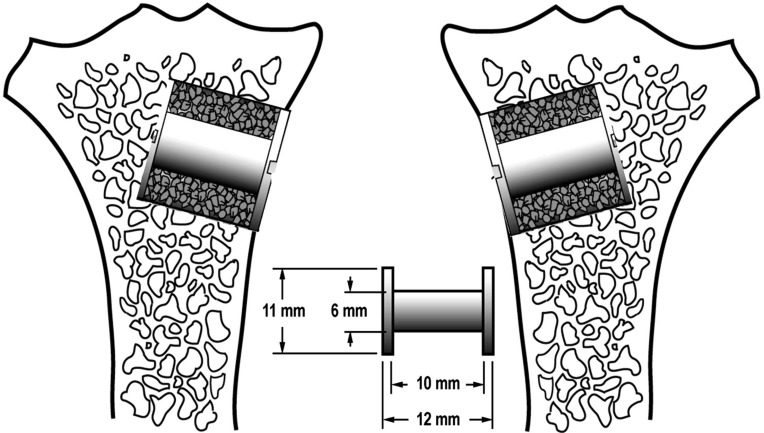
One porous-coated implant was inserted into the proximal part of each tibia. Each implant was surrounded by a 2.5 mm gap filled with beta-tricalcium phosphate (β-TCP) granules soaked in either a zoledronate solution or saline (Fig. 1). The two treatment groups were systematically alternated between left and right tibia with random start. The observation period was 12 weeks.
Fig. (1).
A diagram illustrating the implants inserted into the proximal part of the tibia. Implant placement was systematically rotated with random start.
Implants
The 20 implants used consisted of a titanium alloy core (Ti-6Al-4V) onto which a 0.75 mm porous bead-coated coating was obtained by sintering spherical beads (commercially pure Ti). The porous-bead coating had an average pore size of 250-300 μm and porosity range of 40-50%. The implants had a nominal diameter of 6.0 mm and length of 10.0 mm. The porous-bead coating was applied by Depuy Orthopaedics, Inc. (Warsaw, IN, USA).
Conduit
Conduit (Conduit, Depuy Orthopaedics, Inc., Warsaw, IN, USA) is a commercially available bone graft substitute for the repair of bony defects. It is made of pure β-TCP and complies with ASTM F1088-87. Conduit is delivered from the manufacturer in the form of granules of approximately 1.5-3.0mm in diameter with interconnecting pores occupying approximately 70% of the volume.
Surgery
Before each surgery two portion of β-TCP granules weighing 0.71g (range, 0.68-0.74g) were soaked in either 1 mL bisphosphonate solution (0.05 mg zoledronate pr. mL saline) or 1 mL saline for 3 min, and then squeezed for excess fluid before being rinsed in 2 ml saline for 3 min and again squeezed for excess fluid. The bisphosphonate solution was made by diluting Zometa® (Novartis, Basel, Switzerland) obtained through our hospital pharmacy with saline. One vial of Zometa® contains 4.0 mg zoledronate, 220 mg mannitol, and 24 mg sodiumcitrate in 5 mL distillated water.
All surgery was done using sterile conditions and with the dogs under general anesthesia. The proximal anteromedial surface of the tibia was exposed using a medial incision. Then a 2.5 mm K wire was inserted perpendicular into the surface of the proximal part of tibia. The K wire was inserted 20 mm distal of the tibia plateau. Over the K-wire, a cannulated drill with a diameter of 11.0 mm was used to drill a 12.0 mm deep hole. After removing bone debris and irrigating the bone cavity, a 6.0 mm implant with a footplate was inserted.
Specimen Preparation
Two specimens containing the implant and surrounding bone were cut from each tibia perpendicular to the long axis of the implant using a water-cooled band saw (Exact Apparatebau, Nordenstedt, Germany). The first and most superficial specimen with a thickness of 3.5 mm was stored at -20°C and used for later biomechanical testing. The second specimen with a thickness of 6.5 mm was fixed in 70% ethanol and used for later histomorphometrical analysis.
Biomechanical Testing
Implants were tested till their failure by axial push-out test on an MTS Bionics Test Machine (MTS, Eden Prairie, MN, USA). The specimens were placed on a metal support jig with a 7.4-mm diameter central opening. Centering the implant over the opening assured a 0.7-mm distance between the implant and support jig as recommended [12]. Implants were pushed from the peripheral side towards the inside of the bone. A preload of 3 N defined the start of the test. We used a displacement rate of 5 mm/minute, and continuous load vs displacement data were recorded. Maximum shear strength (MPa) was determined from the maximum force applied until failure of the bone-implant interface. Maximum shear stiffness (MPa/mm) was obtained from the slope of the linear section of the load vs displacement curve. Total energy absorption (J/m2) was calculated until the area under the load displacement curve failed. All push-out parameters were normalized by the cylindrical surface area of the transverse implant section tested.
Histomorphometry
Specimens were dehydrated gradually in ethanol (70–100%) containing basic fuchsin, and embedded in methylmethacrylate. Four vertical uniform random sections were cut with a hard tissue microtome (KDG-95, MeProTech, Heerhugowaard, The Netherlands) around the central part of each implant as described by Overgaard et al. [13]. Before making the sections, the implant was randomly rotated around its long axis. The sections were cut parallel to this axis. The 50-μm thick sections were cut with a distance of 400 μm, and counterstained with 2% light-green (BDH Laboratory Supplies, Poole, England). With this protocol, bone was stained green and non-mineralized tissue red.
Blinded histomorphometrical analysis was done using a stereological software program (CAST grid, Olympus Denmark A/S, Ballerup, Denmark). Tissue-to-surface contact was defined as new bone, fibrous tissue, or β-TCP granules in contact with the implant surface and estimated using sine-weighted lines. Volume fractions of new bone, β-TCP granules, or fibrous tissue were estimated by point counting in the gap around the implant.
Statistical Analysis
We used Intercooled Stata 9.0 (Stata Inc., College Station, TX, USA) for statistical analysis. Biomechanical data were normally distributed and analyzed with Student’s paired t-test. Histomorphometrical data were not normally distributed and analyzed with Wilcoxon signed rank test. Two tailed p<0.05 were considered significant.
RESULTS
All dogs were fully weight-bearing within 3 days of surgery. No dogs were excluded during the observation period. No clinical signs of infection were present at the implantation site at time of euthanization.
We found a 2.5-fold increase in maximum shear stiffness of the implants surrounded with TCP granules soaked in zoledronate (Table 1). No significant differences were found in shear strength or energy absorption.
Table 1.
Biomechical Implant Fixation
| Maximum Shear Stiffness (MPa/mm) | Maximum Shear Strength (MPa) | Total Energy Absorption (kJ/m2) | |
|---|---|---|---|
| Control | 5.32 (1.53;9.11) | 1.00 (0.44;1.56) | 0.21 (0.13;0.29) |
| Zoledronate | 17.09 (3.95;30.23) | 2.45 (0.70;2.22) | 0.38 (0.12;0.65) |
| Zoledronate/Control | 2.57 (1.08;6.13) | 1.98 (0.80;4.93) | 1.26 (0.46;33.45) |
| p-value | 0.036 | 0.123 | 0.61 |
Data are presented as mean for each treatment group (Control or Zoledronate) or median for the paired ratios (Zoledronate/Control). 95%CI in parentheses.
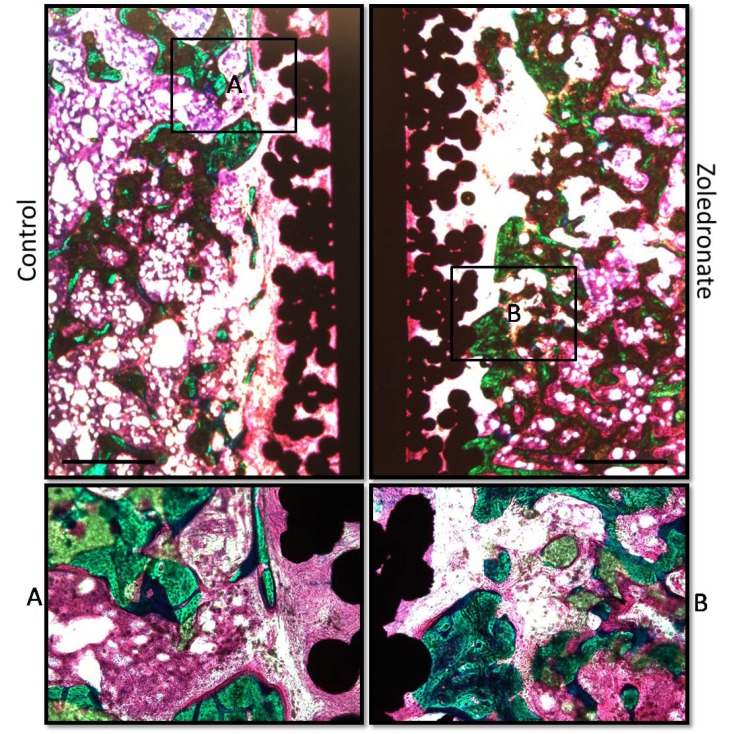
We found no significant differences in any of the histomorphometrical parameters between the treatment groups (Tables 2 and 3). The surface on the implants from both treatments groups were primary covered by fibrous tissue. The gaps around all implants were filled with homogenous distributed β-TCP granules covered with new bone. Fibrous tissue in the gap was primary observed in the proximity of the implant surface (Fig. 2).
Table 2.
Tissue Surface Fractions
| New Bone (%) | TCP (%) | Fibrous Tissue (%) | |
|---|---|---|---|
| Control | 0.88 (0.60;4.83) | 0.00 (0.00;0.00) | 94.14 (87.38;97.41) |
| Zoledronate | 0.72 (0.00;5.18) | 0.00 (0.00;0.00) | 95.36 (89.07;100.00) |
| p-value | 0.68 | na | 0.64 |
Data are presented as median for each treatment group with interquartile range in Parenthesis.
Table 3.
Tissue Volume Fractions
| New Bone (%) | TCP (%) | Fibrous Tissue (%) | |
|---|---|---|---|
| Control | 14.19 (13.00;17.25) | 10.29 (9.53;11.43) | 16.44 (8.21;18.54) |
| Zoledronate | 16.84 (12.34;19.23) | 12.11 (8.39;14.23) | 14.70 (5.55;22.13) |
| p-value | 0.58 | 0.80 | 0.88 |
Data are presented as median for each treatment group with interquartile range in parenthesis.
Fig. (2).
Pictures display representative histological samples from the same animal. The surface on the implants from both treatments groups were primary covered by fibrous tissue. The gaps around all implants were filled with homogenous distributed β--TCP granules covered with new bone. Fibrous tissue in the gap was primary observed in the proximity of the implant surface. Bar = 1.0 mm.
DISCUSSION
The purpose of this study was to investigate the effect of soaking β-TCP granules in zoledronate before grafting them around an experimental titanium-coated implant. We found that zoledronate increased the maximum shear stiffness, but without increasing or decreasing the amount of newly formed bone or β-TCP granules.
Our experimental model was designed to study the early fixation and osseointegration of an implant surrounded by artificial bone graft and placed in cancellous. The canine was chosen as the test animal since its bone closely resembles human bone [14]. The paired design of the study allowed us to eliminate the biological difference between animals. The model is limited by the lack of clinical relevant joint fluid access and direct load bearing. Furthermore, the design is limited by the reduced ability of the β-TCP granules to withstand the dynamic loading conditions of a clinical implant. Furthermore, only ten animals were included in this study. Given the relative statistical power, any statistically non-significant difference should be interpreted with caution.
We used a pure β-TCP as a representative of a ceramic bone graft substitute. The aim of this experimental study was to show a principal effect of adding a bisphosphonate to a ceramic bone graft substitute on new bone formation and implant fixation. The β-TCP granules used in this study may not be able to withstand the compressive forces of a clinical loaded implant [6]. Zoledronate was chosen as a bisphosphonate representative since it was the most potent commercially available bisphosphonate at the time of surgery.
Bisphosphonate is known to inhibit osteoclastic induced resorption [15]. Furthermore, it has previouslu been shown in vitro that resorption of dentin slices can be decreased when calcium phosphate with chemically associated zoledronate is present [9]. An expected outcome of this study would be decreased resorption of β-TCP granules. Furthermore, it has previously been shown in vivo that resorption of allograft rinsed in bisphosphonate is decreased while the formation of new bone within the allograft is increased [16-21]. An expected outcome of this study would be an increased bone formation leading to an increased implant fixation. We found an increase in one of three biomechanical parameters, but no difference was found in the amount of β-TCP granules or new bone.
The increase in one of the three biomechanical parameters without an increase in new bone formation is in contrast to previous studies where a correlation was found between these variables [17, 19]. The previous studies emphasis that bone density is important for implant fixation. The present study indicates that others factors besides bone density influence implant fixation. One explanation for the increase in biomechanical implant fixation without corresponding increase in new bone formation could be a result of an optimized composition of the mineral or matrix phase of the newly formed bone due to the local bisphosphonate treatment [22]. Another explanation for the increase in biomechanical implant fixation without the increase in new bone formation could be a decrease in bone turnover, leading to a prolonged period of bone formation [23]. This prolonged period of bone formation might enhance the quality of the newly formed bone due to an optimized mineralization.
We expected to find an increased formation of new bone, but did not find any difference in the amount of new bone between the two treatment groups. This is in contrast to other studies where local treatment with a bisphosphonate increases new bone formation [10, 21]. One explanation for this discrepancy could be the used concentration of zoledronate in this study. The dose of zoledronate used in this study was based upon the study by Tanzer et al. [24]. We have previously demonstrated a dose-response relationship between the concentration of zoledronate and implant fixation, when soaking morselized allograft in zoledronate [25]. It could be that another concentration of zoledronate would increase new bone formation. However, it is important to notice that the used concentration of zoledronate in this study did not decrease new bone formation as shown in other studies [26, 27]. Another explanation for the lack of increased formation of new bone could be a too short observation period.
In conclusion, this study demonstrated that local treatment of β-TCP granules with zoledronate has the potential to increase biomechanical implant fixation. However, the results also emphasize the need for further preclinical studies in order to optimize the dose of zoledronate. The study demonstrated the importance of combining biomechanical testing and histomorphometrical analyses when investigating experimental implant fixation.
ACKNOWLEDGEMENTS
We wish to thank Jane Pauli and Anette Milton, Orthopaedic Research Laboratory, Aarhus University Hospital, for their technical expertise.
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflicts of interest.
REFERENCES
- 1. Kärrholm J, Borssén B, Löwenhielm G, Snorrason F. Does early micromotion of femoral stem prostheses matter? 4-7-year stereoradiographic follow-up of 84 cemented prostheses. J Bone Joint Surg Br. 1994;76(6 ):912–7. [PubMed] [Google Scholar]
- 2. Ryd L, Albrektsson BE, Carlsson L, et al. Roentgen stereophotogrammetric analysis as a predictor of mechanical loosening of knee prostheses. J Bone Joint Surg Br. 1995;77(3 ):377–83. [PubMed] [Google Scholar]
- 3. Gie GA, Linder L, Ling RS, Simon JP, Slooff TJ, Timperley AJ. Impacted cancellous allografts and cement for revision total hip arthroplasty. J Bone Joint Surg Br. 1993;75(1 ):14–21. doi: 10.1302/0301-620X.75B1.8421012. [DOI] [PubMed] [Google Scholar]
- 4. Slooff TJ, Huiskes R, van Horn J, Lemmens AJ. Bone grafting in total hip replacement for acetabular protrusion. Acta Orthop Scand. 1984;55(6 ):593–6. doi: 10.3109/17453678408992402. [DOI] [PubMed] [Google Scholar]
- 5. Toms AD, Barker RL, Jones RS, Kuiper JH. Impaction bone-grafting in revision joint replacement surgery. J Bone Joint Surg Am. 2004;86-A(9 ):2050–60. doi: 10.2106/00004623-200409000-00028. [DOI] [PubMed] [Google Scholar]
- 6. Hak DJ. The use of osteoconductive bone graft substitutes in orthopaedic trauma. J Am Acad Orthop Surg. 2007;15(9 ):525–36. doi: 10.5435/00124635-200709000-00003. [DOI] [PubMed] [Google Scholar]
- 7. Jensen TB, Overgaard S, Lind M, Rahbek O, Bünger C, Søballe K. Osteogenic protein-1 increases the fixation of implants grafted with morcellised bone allograft and ProOsteon bone substitute: an experimental study in dogs. J Bone Joint Surg Br. 2007;89(1 ):121–6. doi: 10.1302/0301-620X.89B1.17077. [DOI] [PubMed] [Google Scholar]
- 8. Baas J, Elmengaard B, Bechtold J, Chen X, Søballe K. Ceramic bone graft substitute with equine bone protein extract is comparable to allograft in terms of implant fixation: a study in dogs. Acta Orthop . 2008;79(6 ):841–50. doi: 10.1080/17453670810016948. [DOI] [PubMed] [Google Scholar]
- 9. Josse S, Faucheux C, Soueidan A, et al. Novel biomaterials for bisphosphonate delivery. Biomaterials. 2005;26(14 ):2073–80. doi: 10.1016/j.biomaterials.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 10. Välimäki VV, Moritz N, Yrjans JJ, Vuorio E, Aro HT. Effect of zoledronic acid on incorporation of a bioceramic bone graft substitute. Bone. 2006;38(3 ):432–43. doi: 10.1016/j.bone.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 11. Tanaka T, Saito M, Chazono M, et al. Effects of alendronate on bone formation and osteoclastic resorption after implantation of beta-tricalcium phosphate. J Biomed Mater Res A. 2010;93(2 ):469–74. doi: 10.1002/jbm.a.32560. [DOI] [PubMed] [Google Scholar]
- 12. Dhert WJ, Verheyen CC, Braak LH, et al. A finite element analysis of the push-out test: influence of test conditions. J Biomed Mater Res . 1992;26(1 ):119–30. doi: 10.1002/jbm.820260111. [DOI] [PubMed] [Google Scholar]
- 13. Overgaard S, Søballe K, Jørgen H, Gundersen G. Efficiency of systematic sampling in histomorphometric bone research illustrated by hydroxyapatite-coated implants: optimizing the stereological vertical-section design. J Orthop Res. 2000;18(2 ):313–21. doi: 10.1002/jor.1100180221. [DOI] [PubMed] [Google Scholar]
- 14. Aerssens J, Boonen S, Lowet G, Dequeker J. Interspecies differences in bone composition, density, and quality: potential implications for in vivo bone research. Endocrinology. 1998;139(2 ):663–70. doi: 10.1210/endo.139.2.5751. [DOI] [PubMed] [Google Scholar]
- 15. Fleisch H. Development of bisphosphonates. Breast Cancer Res. 2002;4(1 ):30–4. doi: 10.1186/bcr414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Agholme F, Aspenberg P. Experimental results of combining bisphosphonates with allograft in a rat model. J Bone Joint Surg Br . 2009;91(5 ):670–5. doi: 10.1302/0301-620X.91B5.21867. [DOI] [PubMed] [Google Scholar]
- 17. Jakobsen T, Baas J, Kold S, Bechtold JE, Elmengaard B, Søballe K. Local bisphosphonate treatment increases fixation of hydroxyapatitecoated implants inserted with bone compaction. J Orthop Res. 2009; 27(2 ):189–94. doi: 10.1002/jor.20745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jakobsen T, Kold S, Bechtold JE, Elmengaard B, Søballe K. Local alendronate increases fixation of implants inserted with bone compaction: 12-week canine study. J Orthop Res. 2007;25(4 ):432–41. doi: 10.1002/jor.20276. [DOI] [PubMed] [Google Scholar]
- 19. Jakobsen T, Kold S, Bechtold JE, Elmengaard B, Søballe K. Effect of topical alendronate treatment on fixation of implants inserted with bone compaction. Clin Orthop Relat Res. 2006;444:229–34. doi: 10.1097/01.blo.0000191273.34786.40. [DOI] [PubMed] [Google Scholar]
- 20. Astrand J, Aspenberg P. Systemic alendronate prevents resorption of necrotic bone during revascularization. A bone chamber study in rats. BMC Musculoskelet Disord. 2002;3:19–0. doi: 10.1186/1471-2474-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Webb CL, Nguyen NM, Schoen FJ, Levy RJ. Bone allografts pretreated with a bisphosphonate are not resorbed. Acta Orthop Scand . 2002;73(1 ):20–3. doi: 10.1080/000164702317281350. [DOI] [PubMed] [Google Scholar]
- 22. Zioupos P, Currey JD, Hamer AJ. The role of collagen in the declining mechanical properties of aging human cortical bone. J Biomed Mater Res. 1999;45(2 ):108–16. doi: 10.1002/(sici)1097-4636(199905)45:2<108::aid-jbm5>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 23. Astrand J, Harding AK, Aspenberg P, Tägil M. Systemic zoledronate treatment both prevents resorption of allograft bone and increases the retention of new formed bone during revascularization and remodelling. A bone chamber study in rats. BMC Musculoskelet Disord. 2006;7:63. doi: 10.1186/1471-2474-7-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tanzer M, Karabasz D, Krygier JJ, Cohen R, Bobyn JD. The Otto Aufranc Award: bone augmentation around and within porous implants by local bisphosphonate elution. Clin Orthop Relat Res. 2005;441:30–9. doi: 10.1097/01.blo.0000194728.62996.2d. [DOI] [PubMed] [Google Scholar]
- 25. Jakobsen T, Baas J, Bechtold JE, Elmengaard B, Søballe K. The effect of soaking allograft in bisphosphonate: a pilot dose-response study. Clin Orthop Relat Res. 2010;468(3 ):867–74. doi: 10.1007/s11999-009-1099-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Baas J, Elmengaard B, Jensen TB, Jakobsen T, Andersen NT, Soballe K. The effect of pretreating morselized allograft bone with rhBMP-2 and/or pamidronate on the fixation of porous Ti and HA-coated implants. Biomaterials. 2008;29(19 ):2915–22. doi: 10.1016/j.biomaterials.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 27. Jakobsen T, Baas J, Bechtold JE, Elmengaard B, Søballe K. Soaking morselized allograft in bisphosphonate can impair implant fixation. Clin Orthop Relat Res. 2007;463:195–201. doi: 10.1097/BLO.0b013e31813c6696. [DOI] [PubMed] [Google Scholar]