Abstract
Objective
This study assesses swallowing function following chemoradiotherapy and neck dissection in head and neck cancer patients and investigates clinical, treatment and neck dissection factors associated with dysphagia.
Study Design
Case series with chart review
Setting
Tertiary Care Center
Subjects and Methods
88 patients undergoing neck dissection after chemoradiotherapy for advanced head and neck cancer were reviewed. Dysphagia outcome measures included weight loss, diet, gastrostomy tube-dependency and video swallow findings of aspiration or stenosis. Additionally we created a Diet/GT Scale, score 1–5. Univariate and multivariate analysis of clinical, treatment or neck dissection factors potentially associated with dysphagia outcome measures was undertaken.
Results
Peak mean weight loss was 17% at 6 months after chemoradiotherapy. At 12 months a soft/regular diet was taken by 78/88 (89%) and only 1/88 (1%) of patients were nil per os. Gastrostomy tube-dependence at 6, 12, 24 months was 53%, 25%, and 10%. Diet/GT score was 5 (gastrostomy tube removed and soft/regular diet) for 47% at 6 months, 74% at 12 months and 89% at 24 months. Multivariate analyses revealed that higher tumor stage was associated with a lower Diet/GT score at 12 months (p=0.02) and gastrostomy-dependence at 12 (p=0.01) and 24 months (p=0.04).
Conclusion
Despite the addition of neck dissection to chemoradiotherapy, nearly all patients took a soft or regular diet, reached a Diet/GT score of 5 and only 1% remained nil per os. A higher tumor stage is associated with a lower Diet/GT score and gastrostomy tube-dependency beyond 12 months.
Introduction
Treatment for advanced head and neck cancer (HNC) often involves aggressive chemoradiotherapy (CRT). Multiple studies have demonstrated that intensive CRT regimens are effective in the management of HNC with improved survival and organ preservation rates.1,2 Late toxicities and quality of life have surfaced as important issues for survivors.3 Dysphagia occurs in 37% – 82% of patients after CRT and has been evaluated in several studies.4–10 Dysphagia after CRT limits oral diet and often necessitates a gastrostomy feeding tube (GT) for adequate nutrition. Long term GT dependence after CRT adversely influences quality of life in HNC patients.11
The additional impact of neck dissection (ND) after CRT on swallowing function is less well understood. Reports by Machtay et al12 and Lango et al13 have suggested increased late toxicity and GT dependence in patients undergoing ND after CRT.
The goals of this retrospective study are to characterize the swallowing ability in HNC patients undergoing ND after CRT at our institution and to evaluate the impact of clinical, treatment and ND factors on dysphagia measures in these patients.
Methods
All eligible patients who underwent ND after treatment with CRT for an advanced stage squamous cell carcinoma of the oropharynx, hypopharynx, larynx or unknown primary at the Brigham and Women’s Hospital or Dana Farber Cancer Institute between January 1998 and June 2008 were reviewed from electronic and paper chart medical records. Patients with cancers of the oral cavity, sinonasal cavity, nasopharynx or salivary glands were excluded as were patients with recurrent disease or history of other malignancies. All patients included had a primary site complete response to CRT at the time of ND and a follow up of at least 24 months after CRT without a recurrence during this time. The protocol was approved by the Dana-Farber Cancer Institute institutional review board.
Neck Dissection
ND was advised for patients who did not achieve a complete response in the neck. Additionally, during a portion of this time period, some patients with initial extensive N2b or N3 neck disease underwent ND regardless of response to treatment.
The following ND variables were tabulated: ND timing from completion of CRT, type of ND, and ND complications. ND were either radical neck dissection (RND), modified radical neck dissection (MRND) or selective neck dissection (SND), depending on neck stage, response to CRT and surgeon preference. ND complications were categorized as “at least one wound complication” and “at least one complication”. The category “at least one wound complication” captures all minor wound infections such as fluid collections, wound dehiscence, flap necrosis and also major wound complications, defined as those requiring hospital readmission, a return to the operating room or any chyle leak. The second category, “at least one complication,” summarizes all complications including wound, airway and systemic complications. Patients undergoing tracheotomy for perioperative airway stabilization were also recorded.
Dysphagia Outcome Measures
Dysphagia assessments included: weight loss, diet, GT status, and Diet/GT Score. These measures were assessed at 6, 12, and 24 months. Weight loss was compared to pretreatment weight. Diet was reviewed and described with the following five categories: nil per os (NPO), liquid, pureed, soft and regular diet. In this retrospective study pretreatment diet was not consistently recorded and therefore not included in the analyses. All patients were advised to undergo placement of a GT before the start of concurrent chemoradiotherapy. Length of GT dependency was calculated from completion of CRT. A Diet/GT scale, score 1–5, was newly constructed, combining GT status and diet and is described in Table 1.
Table 1.
Diet/GT Score
| Diet/GT Score | Diet and GT1-status |
|---|---|
| 1 | GT Present and NPO2 |
| 2 | GT Present and Liquid/Pureed Diet |
| 3 | GT Present and Soft/Regular Diet |
| 4 | No GT and Liquid/Pureed Diet |
| 5 | No GT and Soft/Regular Diet |
Abbreviations:
=gastrostomy tube,
=nil per os
Video swallow studies were performed in patients with significant swallowing difficulties after treatment. The presence of aspiration was graded according to the Penetration-Aspiration scale (PAS).14 We considered a PAS of ≥4 to represent significant aspiration. Strictures were also noted and patients were considered to have significant stenosis if dilation was required.
Statistics
Univariate and multivariate analyses were carried out using logistic regression (GT status and stenosis outcomes) and ordinal logistic regression (Diet/GT Score outcome) to assess whether clinical, treatment and neck dissection variables were associated with the dysphagia outcome measures. Clinical variables used for analysis included age, gender, tumor site, tumor stage (T-stage) and neck stage. Treatment variables considered were type of chemotherapy (induction versus concurrent CRT) and type of radiation therapy. ND variables included type of ND (RND versus MRND/SND), time of ND (<12weeks versus ≥12 weeks after CRT), ND complications and need for tracheotomy. The Wald Chi-Square test for logistic regression was used to investigate the statistical significance of associations. Variables that could not be analyzed using logistic regression because of small cell numbers were analyzed with the Fisher’s exact test. Potential predictors identified in univariate analysis were further entered in multiple regression analyses using a p value of <0.25 for entry. The final model was chosen by Akaike Information Criterion (AIC). The multivariate analysis also included age and type of chemotherapy. A two-sided p value <0.05 was considered to be significant.
All statistical analyses were perfomed using SAS for Windows software, Version 9.2 (SAS Institute).
Results
Clinical variables
One hundred and seventeen patients were identified. 19 were excluded due to recurrence and 10 were excluded because of insufficient follow up (<24 months) resulting in 88 eligible patients for this analysis. The mean follow up from end of CRT for all patients was 70 months with a range from 24 to 136 months. Clinical characteristics are listed in Table 2. All patients had advanced stage disease with 87/88 stage IV and 1/88 stage III. The majority of patients, 82%, had oropharyngeal primaries.
Table 2.
Clinical Characteristics
| # | % | |
|---|---|---|
| Gender | ||
| Male | 75 | 85.2 |
| Female | 13 | 14.8 |
| Age | ||
| Median, Range | 53.5, 39–78 | |
| Tumor Site | ||
| Oropharynx | 72 | 81.8 |
| Hypopharynx | 4 | 4.6 |
| Larynx | 2 | 2.3 |
| Unknown Primary | 10 | 11.4 |
| Tumor Stage | ||
| Tx | 10 | 11.4 |
| T1 | 28 | 31.8 |
| T2 | 23 | 26.1 |
| T3 | 17 | 19.3 |
| T4 | 10 | 11.4 |
| Nodal Stage | ||
| N1 | 1 | 1.2 |
| N2a | 23 | 26.1 |
| N2b | 34 | 38.6 |
| N2c | 17 | 19.3 |
| N3 | 13 | 14.8 |
| Overall Stage | ||
| I | 0 | 0 |
| II | 0 | 0 |
| III | 1 | 1 |
| IV | 87 | 99 |
Treatment Variables
Treatment characteristics are listed in Table 3. Sixty-one patients received platinum-based induction chemotherapy followed by concurrent CRT with the majority receiving weekly carboplatin. Twenty-seven patients received primary concurrent CRT with weekly carboplatin and paclitaxel or bolus cisplatin. External beam radiation was delivered using a 3-field or conformal technique until approximately 2004, after which point intensity modulated radiotherapy (IMRT) was most commonly used. The total radiation dose to the primary and grossly involved nodes was 70–72 Gray at 1.8–2 Gray per day. Information about radiation technique and total dose was not available for 12 of 88 (13.6%) patients.
Table 3.
Treatment Characteristics
| # | % | |
|---|---|---|
| Treatment | ||
| Induction Chemotherapy then CRT1 | 61 | 69.3 |
| Concurrent CRT | 27 | 30.7 |
| Radiation Technique | ||
| 3Field or Conformal Daily | 14 | 15.9 |
| 3 Field or Conformal BID2 | 10 | 11.4 |
| 3 Field Concomitant Boost | 36 | 40.9 |
| IMRT3 Daily | 16 | 18.2 |
| NA4 | 12 | 13.6 |
Abbreviations:
=chemoradiotherapy,
=twice daily,
=intensity modulated radiation therapy,
=not available
Neck Dissection Variables
ND characteristics are listed in Table 4. NDs were performed at a median of 10 weeks, range 4–19.7 weeks after CRT. Eighty four patients underwent unilateral ND and 4 patients had simultaneous bilateral NDs. Twenty eight of 88 (32%) patients experienced “at least one complication” after ND and 17/88 (19%) patients had “at least one wound complication.” Tracheotomy was required in 10/88 (11%) patients; 8/10 patients required a tracheotomy at the time of ND and 2/10 in the postoperative period.
Table 4.
Neck Dissection Characteristics
| # | % | |
|---|---|---|
| Type of ND1 | ||
| RND2 | 22 | 24 |
| MRND3* | 66 | 72 |
| Selective ND | 4 | 4 |
| ND complications | ||
| At least one complication | 28 | 32 |
| At least one wound complication | 17 | 19.3 |
| Airway complication | ||
| Tracheotomy | 11 | 12.5 |
Four patients underwent simultaneous bilateral NDs.
Abbreviations:
=neck dissection,
=radical neck dissection,
=modified radical neck dissection,
Dysphagia Outcome Measures
Weight Loss
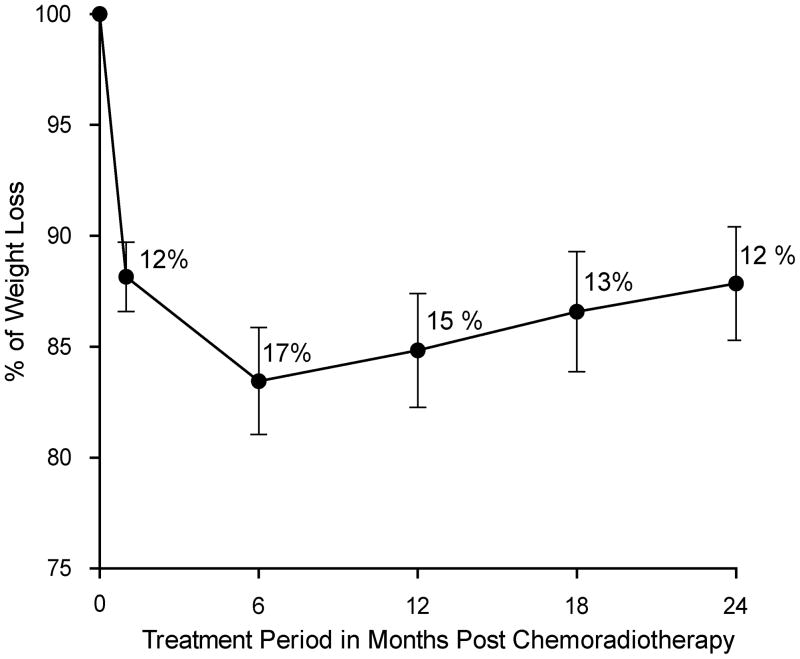
Weight changes were reviewed over the follow up period of 24 months. A peak mean weight loss of 17% was observed at 6 months after CRT. The course of mean weight loss from start of treatment until 24 months after CRT is shown in Figure 1. Among patients with available weight data, the weight loss was ≥10% of the pretreatment weight in 77% (46/60) at 6 months, in 73% (44/60) at 12 months and in 58% (35/60) at 24 months.
Figure 1.
Mean weight loss from start of treatment until 24 months post chemoradiotherapy in all available patients (n=60).
Diet
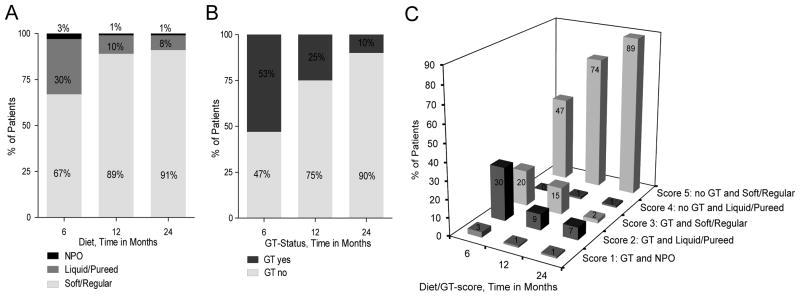
The following number of patients were able to take a soft or regular diet at 6, 12 and 24 months: 59/88 (67%), 78/88 (89%), 80/88 (91%). At both 12 and 24 months only 1/88, (1%) of patients was NPO and the remaining 87/88 (99%) were able to take an oral diet (Figure 2a).
Figure 2.
Figure 2a. Diet assessment at 6, 12, 24 months after chemoradiotherapy
Figure 2b. GT dependence at 6, 12, 24 months after chemoradiotherapy.
Figure 2c. Diet/GT Score at 6, 12, 24 months.
GT Status
A GT was placed before the start of concurrent CRT in 86/88 patients. GT status at different time points is listed in Figure 2b. The median time to GT removal after completion of CRT was 7 months. GT dependence at 6, 12 and 24 months was 47/88 (53%), 22/88 (25%), and 9/88 (10%). Four of the 9 patients with GTs at 24 months subsequently had their GTs removed, between 27 and 41 months after CRT. Only 5/88 (6%) patients retained their GTs throughout the entire study period, with a follow up among these 5 patients ranging from 50 – 129 months.
Diet/GT Score
The Diet/GT Scores are shown in Figure 2c. At 6, 12, and 24 months a score of 5 (no GT and soft/regular diet) was achieved in 41/88 (47%), 65/88 (74%) and 78/88 (89%) of patients. During the same time points a score of 1 (GT present and NPO) was identified in 3/88 (3%), 1/88 (1%), and 1/88 (1%).
Video Swallow Aspiration and Stenosis Results
Significant swallowing difficulties prompted video swallow studies in 58/88 (66%) patients, at a median of 11.1 weeks post ND.
On the video swallow study, 43/58 (74%) patients were found to have a PAS ≥ 4. Severe aspiration with insufficient or absent cough reflex, PAS score 7 or 8, was observed in 34/58 (58%) patients. Despite high aspiration scores, only 9/43 (21%) were made NPO at the time of video swallow, and 6/9 began an oral diet within 3 months after the videoswallow. 5/43 (12%) developed issues with pneumonia that were effectively medically managed. There were no deaths related to aspiration pneumonia. Ultimately 39/43 (91%) had their GTs removed and tolerated an oral diet.
Pharyngoesophagael stenosis requiring dilation was identified in 26/88 (30%) patients on video swallow or endoscopy during ND. In 10/26 patients the stenosis was diagnosed before or at neck dissection and in 16/26 patients the stenosis was first described after ND. Video swallow studies pre ND and esophagoscopies at the time of ND were not always performed therefore some of the 16 patients might have also had their stenosis present prior to their ND. All 26 patients underwent dilation and 24/26 (92%), successfully had their GTs removed and 21/26 (81%) returned to a soft or regular diet.
Clinical, Treatment and Neck Dissection Factors Influence on Dysphagia Outcome Measures
Univariate and multivariate analyses were undertaken to determine if clinical, treatment or neck dissection variables were associated with GT status, Diet/GT Score or stenosis at different time points. The type of radiation therapy could not be considered for statistical analysis because of the small number of patients undergoing the different radiation techniques and because of missing radiation information for 12/88 patients. Diet/GT Score groups with low patient numbers were combined for optimal statistical analysis. Specifically, patients with scores 1 and 2 were collapsed into one group, because they both represent GT-dependent patients with very restricted oral intake. Score 3 had achieved adequate patient numbers and was used as a stand alone group. Score groups 4 and 5, representing GT independent patients taking nutrition completely per os were collapsed into one group, thus leaving 3 Diet/GT groups used for statistical analysis.
GT Status
The univariate results for GT status are shown in Table 5. T-stage was significantly associated with long term GT dependence at 12 (p=0.003) and 24 (p=0.03) months. T-stage remained significant when adjusting for neck stage, time of neck dissection and type of chemotherapy (p=0.01 at 12 months and p=0.04 at 24 months). Among ND variables, only timing of ND was significant in the univariate analyses (p=0.04), but failed to reach significance in the multivariate setting. Other ND variables, including type of ND and ND complications showed no significant association with GT dependence. Tracheotomy was borderline significantly associated with GT dependence at 24 months (p=0.06) using the Fisher’s exact test but could not be tested in a logistic model due to the small size of this group.
Table 5.
Univariate Analysis of Clinical, Treatment and Neck Factors Association with GT Status
| Clinical, Treatment, Neck Dissection Characteristics | GT1 Dependence at 12 Months | GT Dependence at 24 Months | ||
|---|---|---|---|---|
| p-value | OR2 (95% CI) | p-value | OR (95% CI) | |
| Primary Tumor Site (Oropharyx vs. Other) | 0.52 | 0.7 (0.2 – 2.2) | 0.57 | 1.9 (0.2 – 16.2) |
| T Stage (T1–T4) | 0.003 | 2.3 (1.3 – 3.9) | 0.03 | 2.2 (1.1 – 4.5) |
| N Stage (N1, N2a, N2b, N2c, N3) | 0.08 | 1.5 (1.0 – 2.5) | 0.69 | 1.1 (0.6 – 2.2) |
| Chemotherapy Technique (Induction vs. CRT3) | 0.35 | 1,7 (0.6 – 5.2) | 0.86 | 0.9 (0.2 – 3.8) |
| Type of ND4 (RND5 vs. MRND6) | 0.77 | 0.8 (0.3 – 2.7) | 0.3 | 0.3 (0.04 – 2.7) |
| Complications after ND: | ||||
| At Least One Complication | 0.60 | 1.3 (0.5 – 3.6) | 0.4 | 1.8 (0.5 – 7.4) |
| At Least One Wound Complication | 0.27 | 2.3 (0.5 – 10.4) | 0.27 | 2.3 (0.5 – 10.4) |
| Time of ND (ND before or after 12 weeks after CRT) | 0.20 | 1.9 (0.7 – 5.2) | 0.04 | 4.6 (1.1 – 19.9) |
| Weight Loss from Start of CRT (≥10% vs. <10%) | 0.34 | 0.6 (0.2 – 1.8) | 0.48 | 0.6 (0.1 – 2.5) |
| Tracheotomy | 0.11 | 0.06 | (1.1, 25.2) | |
Abbreviations:
GT= gastrostomy tube,
OR= odds ratio,
CRT= chemoradiotherapy,
ND= neck dissection,
RND=radical neck dissection,
MRND= modified radical neck dissection
Diet/GT Score
A higher T-stage (p=0.01) was associated with a lower Diet/GT Score in the univariate analyses at 12 months, Table 6. T-stage continued to be significantly associated with a lower Diet/GT Score at 12 months when adjusted for neck stage, time of neck dissection and type of chemotherapy (p=0.02). Tracheotomy was significantly associated with Diet/GT Score at 12 months using the Fisher’s exact test (0.05).
Table 6.
Univariate Analysis of Clinical, Treatment and Neck Factors Association with Diet/GT Score
| Clinical, Treatment and Neck Dissection Characteristics | Dysphagia at 6 Months | Dysphagia at 12 Months | ||
|---|---|---|---|---|
| p-value | OR1 (95% CI) | p-value | OR (95% CI) | |
| Tumor Site (Oropharyx vs. Other) | 0.3 | 1.8 (0.6 – 5.0) | 0.54 | 0.7 (0.2 – 2.2) |
| T Stage (T1–T4) | 0.17 | 1.3 (0.9 – 2.0) | 0.01 | 2.0 (1.2 – 3.4) |
| N Stage (N1, N2a, N2b, N2c, N3) | 0.92 | 1.0 (0.7 – 1.5) | 0.08 | 1.5 (0.9 – 2.4) |
| Chemotherapy Technique (Induction vs. CRT2) | 0.29 | 1.6 (0.7 – 3.8) | 0.42 | 1.6 (0.5 – 4.8) |
| Type of ND3 (RND4 vs. MRND5) | 0.42 | 0.7 (0.3 – 1.7) | 0.76 | 0.8 (0.3 – 2.6) |
| Complications after ND: | ||||
| At Least One Complication | 0.22 | 1.7 (0.7 – 3.9) | 0.53 | 1.4 (0.5 – 3.7) |
| At Least One Wound Complication | 0.31 | 1.7 (0.6 – 4.5) | 0.53 | 1.4 (0.5 – 4.6) |
| Tracheotomy | 0.46 | 1.7 (0.5 – 5.9) | 0.05 | 4.0 (1.1 – 14.5) |
| Time of ND (ND before or after 12 weeks after CRT) | 0.77 | 1.1 (0.5 – 2.6) | 0.21 | 1.9 (0.7 – 5.0) |
| Weight loss from start of CRT (≥10% vs. <10%) | 0.45 | 1.5 (0.5 – 4.6) | 0.31 | 0.6 (0.2 – 1.7) |
Abbreviations:
OR= odds ratio,
CRT= chemoradiotherapy,
ND= neck dissection,
RND= radical neck dissection,
MRND= modified radical neck dissection
Pharyngoesophagel Stenosis
Analyses of multiple clinical and treatment factors was performed for association with pharyngoesophageal stenosis identified before and after ND. In the univariate analyses only induction chemotherapy was statistical significance, p=0.05. Among the 26 patients that developed stenosis, 22 underwent induction chemotherapy and 4 received primary CRT. Induction chemotherapy proved to be associated significantly with stenosis (p=0.04) in the multivariate model, that also included T-stage as well as type of chemotherapy.
Discusssion
The goals of this study were to characterize dysphagia in patients undergoing ND after CRT and to identify factors contributing to swallowing difficulty. Dysphagia was common during the first 6 months after treatment. There was substantial improvement in swallowing ability between 6 and 12 months and there was slight further improvement between 12 and 24 months after treatment. Ultimately, at 24 months nearly all patients regained their ability to take an oral diet and had their GTs removed. T-stage was identified as a significant factor contributing to dysphagia in patients undergoing ND after CRT.
The need and benefit gained from post CRT ND has been called into question,15–18 leading to further scrutiny regarding the risks and complications from ND after CRT.19,20 In contrast to the well investigated impact of CRT on swallowing,4,8,9 less is known about the additional contribution of neck dissection to post CRT dysphagia. Graner et al6 suggested that further fibrosis after ND may increase pharyngeal dysfunction and consequently potentiate post CRT dysphagia. An analysis by the Radiation Therapy Onocology Group12 revealed a significantly higher rate of severe late toxicities, described as feeding tube dependence > 2 years and pharyngeal or laryngeal related toxicity, among patients undergoing ND after CRT as compared to patients treated with CRT and no ND. These findings were corroborated by Lango et al,13 who found an increased risk for GT dependence beyond 18 months in patients undergoing post CRT ND as compared to those not undergoing ND. Graner et al6 also found worse dysphagia Performance Status Scale scores in patients treated with ND post intra-arterial CRT as compared to those without ND. These reports raise concern that ND may further increase swallowing difficulty in patients receiving CRT for advanced head and neck cancer.
Comparison of reports regarding swallowing function after CRT are often confounded by utilization of different dysphagia measures at varying time points. We utilized weight loss, diet, GT-status, video swallow results and the newly created Diet/GT Score at 6, 12 and 24 months following CRT.
Our diet assessment revealed that 89% and 91% patients were able to take a soft or regular diet at 12 and 24 months. We were unable to identify any other ND post CRT diet results for direct comparison. Furthermore, even among post CRT swallowing reports, very few investigators have described the type of diet tolerated at 12 and 24 months after CRT. Lazarus et al21 reported that 94% of patients were able to eat a soft diet at 12 months post treatment. Rademaker et al22 found 86% of patients tolerated a soft diet and 56% ate all consistencies after CRT. These post CRT soft diet assessments compare favorably to our ND post CRT results. This limited comparison suggests that ND did not further adversely impact diet at 12 months. The Diet/GT Score findings highlight this result with 74% and 89% of patients achieving score 5 at 12 and 24 months. Results from the Diet/GT Score further demonstrate that the greatest rate of swallowing improvement was between 6 and 12 months but there was still some improvement between 12 and 24 months following CRT.
We identified GT dependency rates of 25% and 10% at 12 and 24 months. Literature review discovered only one other report that decribes GT dependency separately for patients undergoing ND following CRT.13 Lango et al13 similarly found a GT dependence at 12 months of 29% for patients receiving ND and 24% for patients without a ND. Other reports combine CRT and post CRT ND patients and describe GT dependency rates of 19%–25% at 12 months4,5,10 and 10%–16%% at 24 months.4,5,23 Higher rates of GT dependency at 24 months of 22% and 30% have also been reported by Staar et al24 and Ang et al.25 Our GT dependency rates at 12 and 24 months were similar to other post ND reports and no worse than other reports that combined patients undergoing CRT with and without ND.
An important potential contributor to post CRT dysphagia is pharyngoesophageal stenosis which occurred in 26/88 (30%) of our patients. Nearly all of our patients underwent successful dilation, regained oral intake and had their GTs removed. This stenosis rate is comparable to current literature findings reporting the incidences from 9% to 37% in studies that included patients with and without post CRT ND.4,26–28
Overall our ND following CRT swallowing results compared favorably to reports in the literature for ND as well as non ND post CRT patients. Type of oral diet4,21,22, rates of GT dependence4,5,10,23 and incidence of pharyngoesophageal stenosis4,26–28 were similar between our patient population and prior reports. The addition of ND did not appear to adversely influence swallow results.
We also assessed whether specific clinical, treatment or neck dissection factors were associated with swallow outcome measures. T-stage was associated with a worse Diet/GT Score and increased GT dependence. Others have also identified an association between T-stage and adverse swallowing outcomes.12,29,30 Machtay et al12 showed that T-stage, T1/2 versus T3/4, was a significant variable for severe late toxicities. Nguyen et al29 found patients with large tumors, T3/4 versus T1/2, had an increased risk for aspiration. Additional trends identified among our patients included a borderline association between tracheotomy and Diet/GT score as well as induction chemotherapy and stenosis.
Limitations of our study include its retrospective nature and the lack of a direct comparison to a group of patients that did not undergo ND. Video swallow studies were not available for all patients before and after ND and this limits assessment of the incidence of stenosis and aspiration. Additionally, due to small sample size, our assessment of primary site, radiation technique and tracheotomy impact on swallow outcome was limited.
Conclusion
We conclude that despite the addition of ND to CRT nearly all of our patients regained their swallowing ability and were able to take an oral diet and have their GT’s removed by 12 – 24 months after CRT. The addition of ND did not appear to substantially diminish swallow ability as compared to reports in the literature. Higher T-stage was associated with a worse swallow outcome.
Footnotes
Poster Presentation, American Academy of Otolaryngology - Head and Neck Surgery Annual Meeting, Boston, Massachusetts, U.S.A., September 26–29, 2010.
References
- 1.Posner MR, Hershock DM, Blajman CR, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357:1705–15. doi: 10.1056/NEJMoa070956. [DOI] [PubMed] [Google Scholar]
- 2.Pignon JP, Bourhis J, Domenge C, et al. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-Analysis of Chemotherapy on Head and Neck Cancer. Lancet. 2000;355:949–55. [PubMed] [Google Scholar]
- 3.Nguyen NP, Frank C, Moltz CC, et al. Impact of dysphagia on quality of life after treatment of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2005;61:772–8. doi: 10.1016/j.ijrobp.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 4.Goguen LA, Posner MR, Norris CM, et al. Dysphagia after sequential chemoradiation therapy for advanced head and neck cancer. Otolaryngol Head Neck Surg. 2006;134:916–22. doi: 10.1016/j.otohns.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Caudell JJ, Schaner PE, Meredith RF, et al. Factors associated with long-term dysphagia after definitive radiotherapy for locally advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2009;73:410–5. doi: 10.1016/j.ijrobp.2008.04.048. [DOI] [PubMed] [Google Scholar]
- 6.Graner DE, Foote RL, Kasperbauer JL, et al. Swallow function in patients before and after intra-arterial chemoradiation. Laryngoscope. 2003;113:573–9. doi: 10.1097/00005537-200303000-00033. [DOI] [PubMed] [Google Scholar]
- 7.Rosenthal DI, Lewin JS, Eisbruch A. Prevention and treatment of dysphagia and aspiration after chemoradiation for head and neck cancer. J Clin Oncol. 2006;24:2636–43. doi: 10.1200/JCO.2006.06.0079. [DOI] [PubMed] [Google Scholar]
- 8.Eisbruch A, Lyden T, Bradford CR, et al. Objective assessment of swallowing dysfunction and aspiration after radiation concurrent with chemotherapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2002;53:23–8. doi: 10.1016/s0360-3016(02)02712-8. [DOI] [PubMed] [Google Scholar]
- 9.Salama JK, Stenson KM, List MA, et al. Characteristics associated with swallowing changes after concurrent chemotherapy and radiotherapy in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 2008;134:1060–5. doi: 10.1001/archotol.134.10.1060. [DOI] [PubMed] [Google Scholar]
- 10.Caglar HB, Tishler RB, Othus M, et al. Dose to larynx predicts for swallowing complications after intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72:1110–8. doi: 10.1016/j.ijrobp.2008.02.048. [DOI] [PubMed] [Google Scholar]
- 11.Ronis DL, Duffy SA, Fowler KE, et al. Changes in quality of life over 1 year in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 2008;134:241–8. doi: 10.1001/archoto.2007.43. [DOI] [PubMed] [Google Scholar]
- 12.Machtay M, Moughan J, Trotti A, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol. 2008;26:3582–9. doi: 10.1200/JCO.2007.14.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lango MN, Egleston B, Ende K, et al. Impact of neck dissection on long-term feeding tube dependence in patients with head and neck cancer treated with primary radiation or chemoradiation. Head Neck. 32:341–7. doi: 10.1002/hed.21188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenbek JC, Robbins JA, Roecker EB, et al. A penetration-aspiration scale. Dysphagia. 1996;11:93–8. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- 15.Goguen LA, Posner MR, Tishler RB, et al. Examining the need for neck dissection in the era of chemoradiation therapy for advanced head and neck cancer. Arch Otolaryngol Head Neck Surg. 2006;132:526–31. doi: 10.1001/archotol.132.5.526. [DOI] [PubMed] [Google Scholar]
- 16.Brizel DM, Prosnitz RG, Hunter S, et al. Necessity for adjuvant neck dissection in setting of concurrent chemoradiation for advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2004;58:1418–23. doi: 10.1016/j.ijrobp.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Nouraei SA, Upile T, Al-Yaghchi C, et al. Role of planned postchemoradiotherapy selective neck dissection in the multimodality management of head and neck cancer. Laryngoscope. 2008;118:797–803. doi: 10.1097/MLG.0b013e318165e33e. [DOI] [PubMed] [Google Scholar]
- 18.Goguen LACC, Sher DJ, Israel DA, Blinder RA, Norris CM, Tishler RB, Haddad RI, Annino JD. Utilizing Computed Tomography as Road Map for Designing Selective and Superselective Neck Dissection after Chemoradiotherapy. Otolaryngol Head Neck Surg. 2010 doi: 10.1016/j.otohns.2010.04.020. in press. [DOI] [PubMed] [Google Scholar]
- 19.Davidson BJ, Newkirk KA, Harter KW, et al. Complications from planned, posttreatment neck dissections. Arch Otolaryngol Head Neck Surg. 1999;125:401–5. doi: 10.1001/archotol.125.4.401. [DOI] [PubMed] [Google Scholar]
- 20.Goguen LACC, Li Y, Zhao SD, Annino JD. Neck Dissection after Chemoradiatherapy – Timing and Complications. Arch Otolaryngol Head Neck Surg. 2010 doi: 10.1001/archoto.2010.188. in press. [DOI] [PubMed] [Google Scholar]
- 21.Lazarus C, Logemann JA, Pauloski BR, et al. Effects of radiotherapy with or without chemotherapy on tongue strength and swallowing in patients with oral cancer. Head Neck. 2007;29:632–7. doi: 10.1002/hed.20577. [DOI] [PubMed] [Google Scholar]
- 22.Rademaker AW, Vonesh EF, Logemann JA, et al. Eating ability in head and neck cancer patients after treatment with chemoradiation: a 12-month follow-up study accounting for dropout. Head Neck. 2003;25:1034–41. doi: 10.1002/hed.10317. [DOI] [PubMed] [Google Scholar]
- 23.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091–8. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 24.Staar S, Rudat V, Stuetzer H, et al. Intensified hyperfractionated accelerated radiotherapy limits the additional benefit of simultaneous chemotherapy--results of a multicentric randomized German trial in advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;50:1161–71. doi: 10.1016/s0360-3016(01)01544-9. [DOI] [PubMed] [Google Scholar]
- 25.Ang KK, Harris J, Garden AS, et al. Concomitant boost radiation plus concurrent cisplatin for advanced head and neck carcinomas: radiation therapy oncology group phase II trial 99–14. J Clin Oncol. 2005;23:3008–15. doi: 10.1200/JCO.2005.12.060. [DOI] [PubMed] [Google Scholar]
- 26.Citrin D, Mansueti J, Likhacheva A, et al. Long-term outcomes and toxicity of concurrent paclitaxel and radiotherapy for locally advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2009;74:1040–6. doi: 10.1016/j.ijrobp.2008.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee WT, Akst LM, Adelstein DJ, et al. Risk factors for hypopharyngeal/upper esophageal stricture formation after concurrent chemoradiation. Head Neck. 2006;28:808–12. doi: 10.1002/hed.20427. [DOI] [PubMed] [Google Scholar]
- 28.Lawson JD, Otto K, Grist W, et al. Frequency of esophageal stenosis after simultaneous modulated accelerated radiation therapy and chemotherapy for head and neck cancer. Am J Otolaryngol. 2008;29:13–9. doi: 10.1016/j.amjoto.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen NP, Frank C, Moltz CC, et al. Analysis of factors influencing aspiration risk following chemoradiation for oropharyngeal cancer. Br J Radiol. 2009;82:675–80. doi: 10.1259/bjr/72852974. [DOI] [PubMed] [Google Scholar]
- 30.Caudell JJ, Schaner PE, Desmond RA, et al. Dosimetric factors associated with long-term dysphagia after definitive radiotherapy for squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 76:403–9. doi: 10.1016/j.ijrobp.2009.02.017. [DOI] [PubMed] [Google Scholar]