Abstract
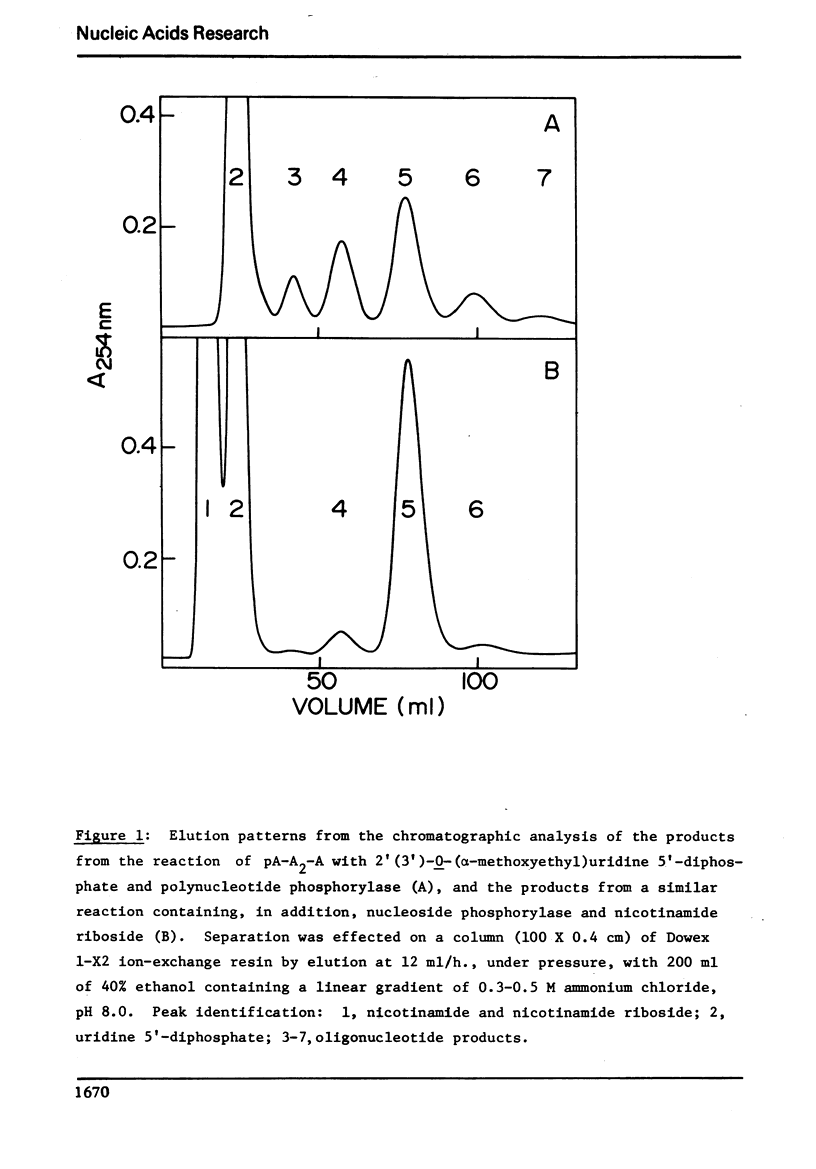
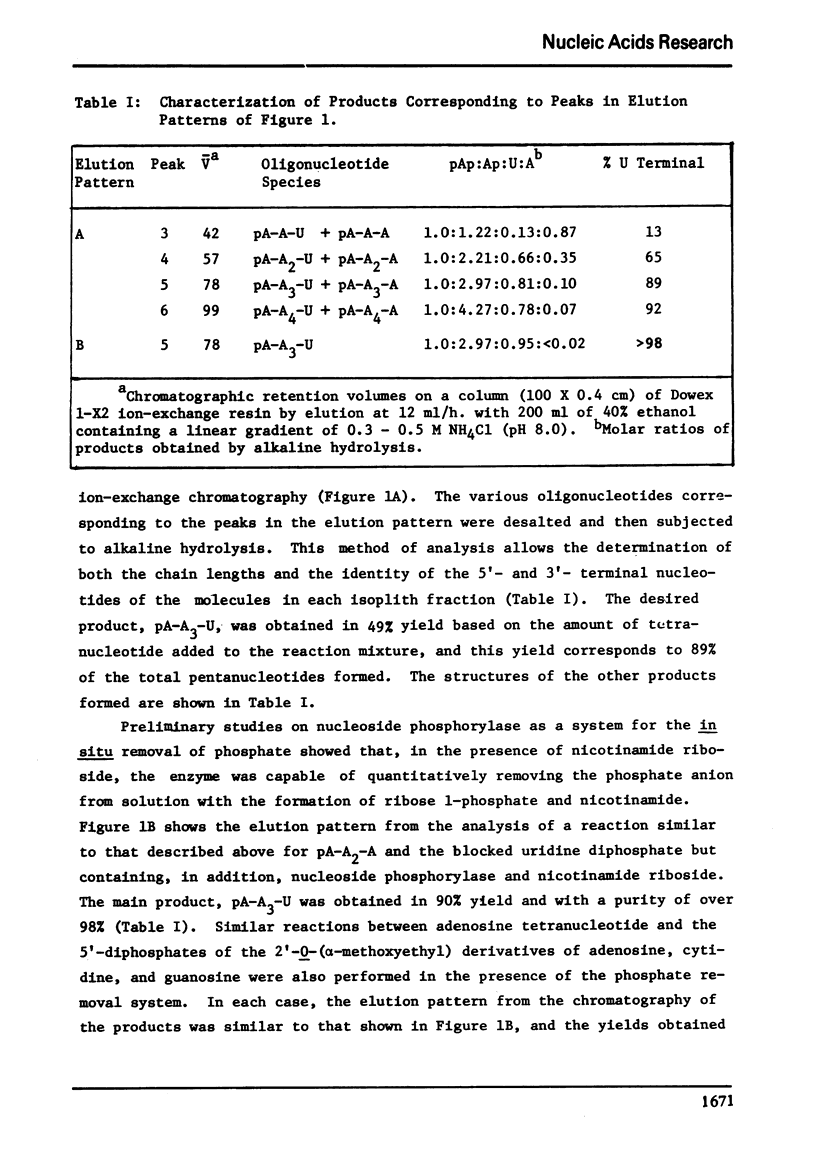
The reaction of the tetranucleotide, pA-A2-A, with 2′(3′)-0-(α-methoxyethyl)uridine 5′-diphosphate, Mg2+ ions, and M. luteus polynucleotide phosphorylase followed by mild acid treatment to remove the blocking groups results in a 49% yield of the desired single addition product, pA-A3-U, together with smaller amounts of pA-A-U, pA-A-A, pA-A2-U, pA-A2-A, pA-A3-A, pA-A4-U, and pA-A4-A. The side products are thought to arise from the phosphorolysis of the acceptor molecule by the inorganic phosphate formed in the reaction mixture and from subsequent additions to the various oligonucleotide species by the resulting adenosine 5′-diphosphate. A system developed for the removal of inorganic phosphate as it is formed in the synthesis involves the addition to the reaction mixture of calf spleen nucleoside phosphorylase and nicotinamide riboside and, under these conditions, pA-A3-U can be prepared in 90% yield with essentially no side products. Under similar conditions, pA-A3-A, pA-A3-G, and pA-A3-C may be prepared from pA-A2-A and the appropriate blocked nucleoside diphosphate in yields of 85-94%. The incubation of pA-A2-A alone with polynucleotide phosphorylase exhibits the phenomenon of “transnucleotidation” in that the molecule is partially converted to oligonucleotides of smaller and larger chain lengths. In the presence of the phosphate removal system, however, the tetranucleotide is not attacked by the enzyme, and thus, “transnucleotidation” appears to be simply a combination of phosphorolytic and addition reactions catalyzed by trace amounts of inorganic phosphate contaminating the enzyme and/or the substrate.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett G. N., Mackey J. K., Wiebers J. L., Gilham P. T. 2'-O-(alpha-methoxyethyl)nucleoside 5'-diphosphates as "single-addition" substrates in the synthesis of specific oligoribonucleotides with polynucleotide phosphorylase. Biochemistry. 1973 Sep 25;12(20):3956–3962. doi: 10.1021/bi00744a027. [DOI] [PubMed] [Google Scholar]
- Chou J. Y., Singer M. F. The effect of chain length on the phosphorolysis of oligonucleotides by polynucleotide phosphorylase. J Biol Chem. 1970 Mar 10;245(5):1005–1011. [PubMed] [Google Scholar]
- GAREN A., LEVINTHAL C. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 1960 Mar 11;38:470–483. doi: 10.1016/0006-3002(60)91282-8. [DOI] [PubMed] [Google Scholar]
- Ho N. W., Gilham P. T. The analysis of polydeoxyribonucleotides by digestion with phosphatase and phosphodiesterases. Biochim Biophys Acta. 1973 Apr 21;308(7):53–58. doi: 10.1016/0005-2787(73)90121-4. [DOI] [PubMed] [Google Scholar]
- Mackey J. K., Gilham P. T. New approach to the synthesis of polyribonucleotides of defined sequence. Nature. 1971 Oct 22;233(5321):551–553. doi: 10.1038/233551a0. [DOI] [PubMed] [Google Scholar]
- McCutchan T. F., Gilham P. T. Studies on polynucleotides containing hybrid sequences. Synthesis of oligonucleotides containing thymidine, adenosine, and a single deoxyribonucleotidyl-(3'-5')-ribonucleotide linkage. Biochemistry. 1973 Nov 20;12(24):4840–4846. doi: 10.1021/bi00748a005. [DOI] [PubMed] [Google Scholar]
- ROWEN J. W., KORNBERG A. The phosphorolysis of nicotinamide riboside. J Biol Chem. 1951 Dec;193(2):497–507. [PubMed] [Google Scholar]



