Abstract
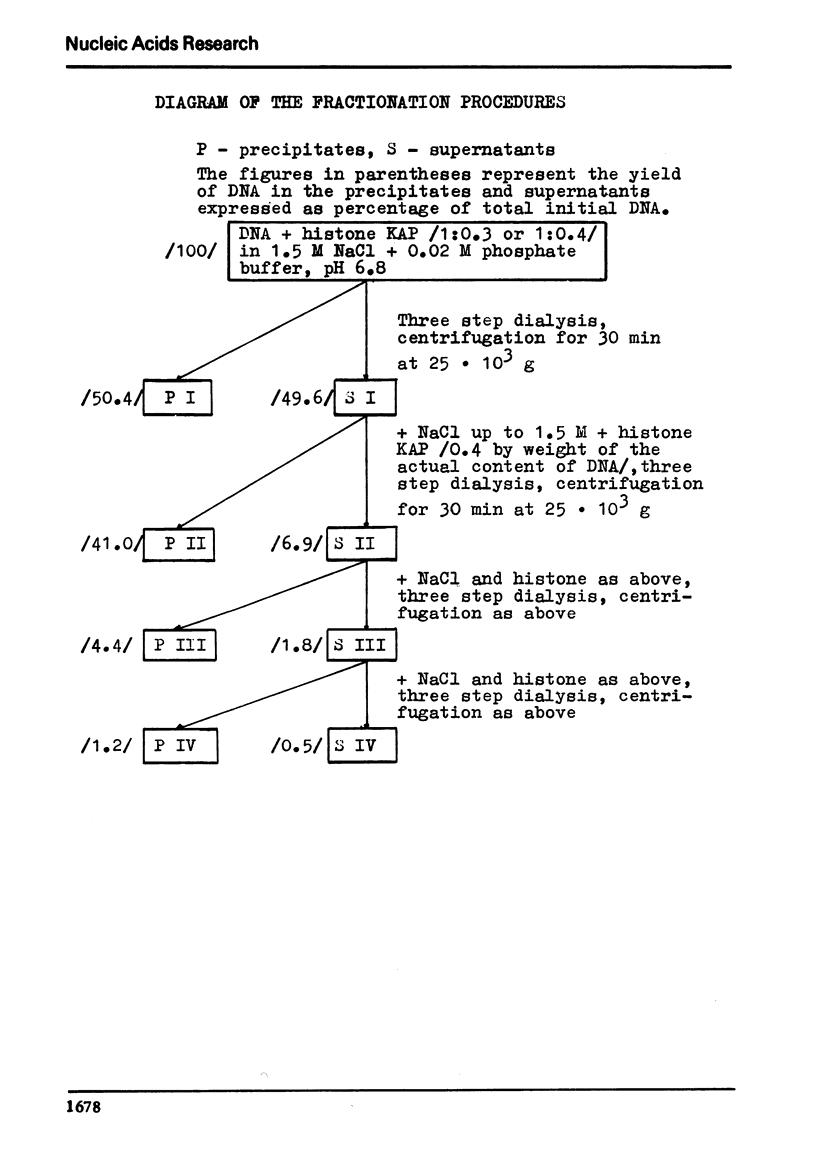
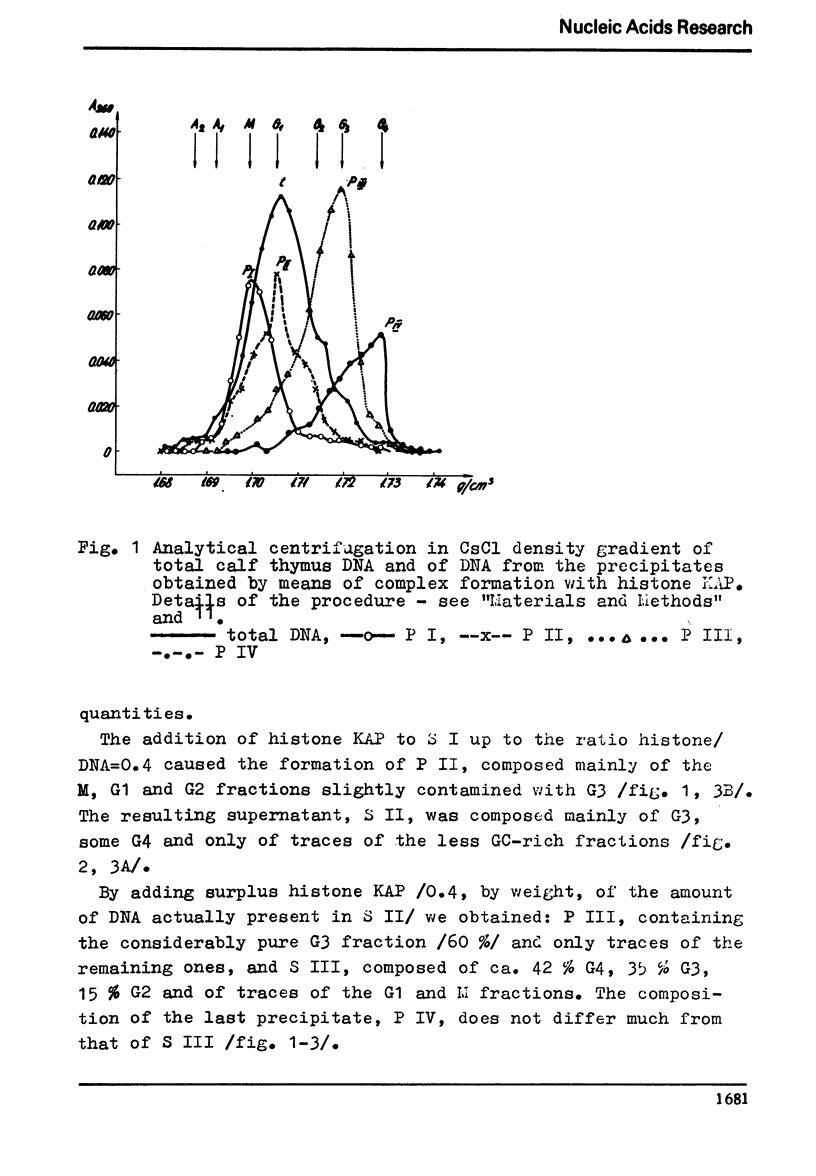
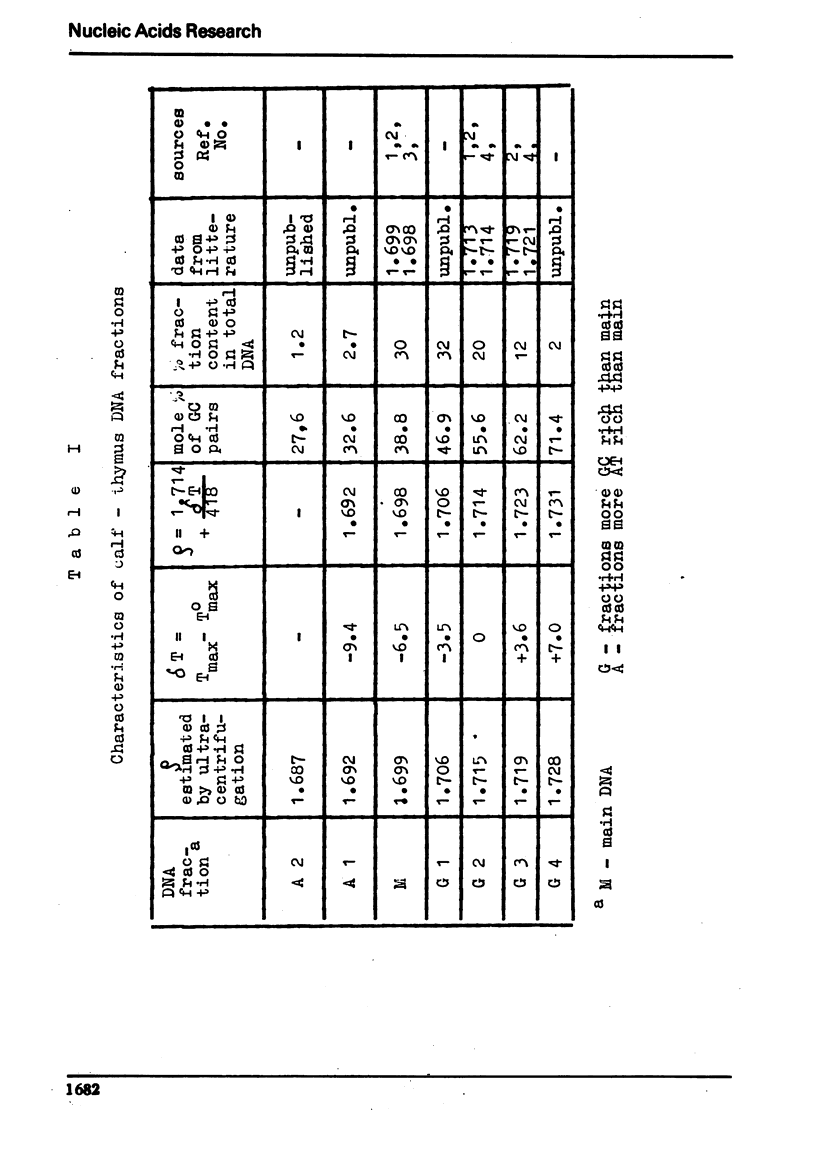
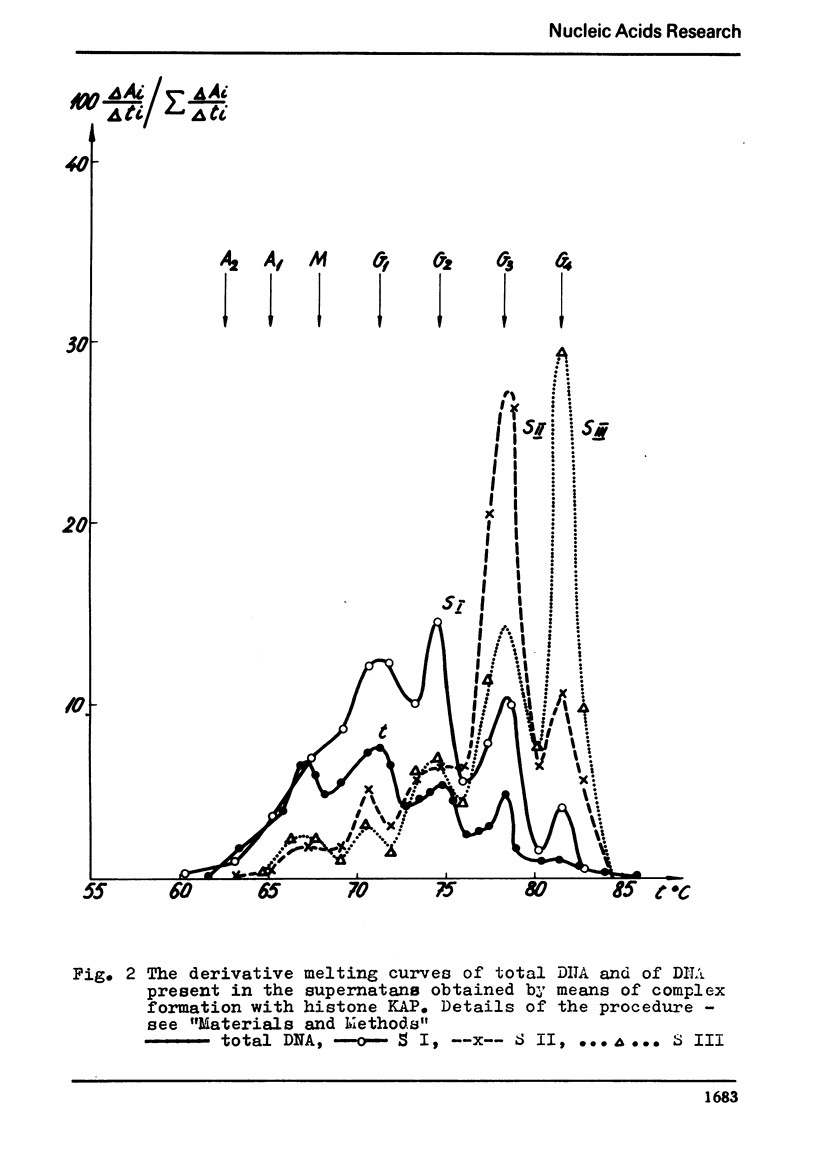
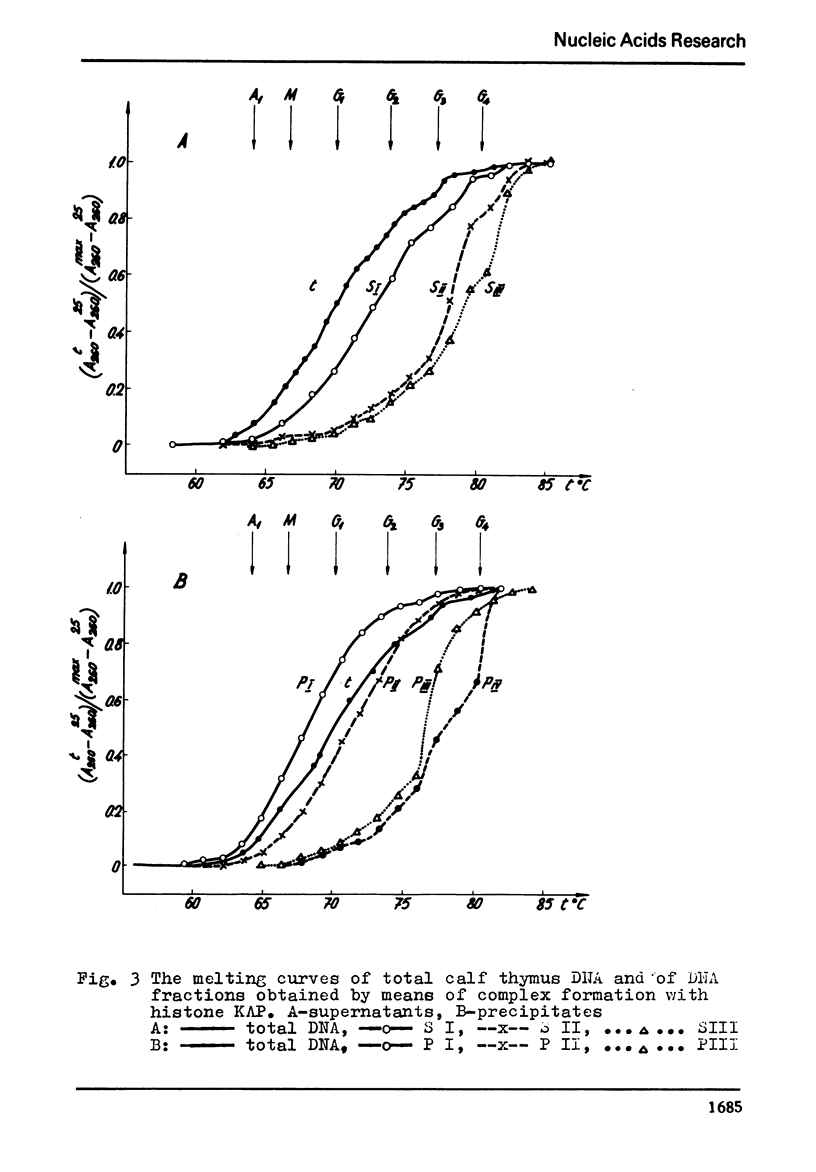
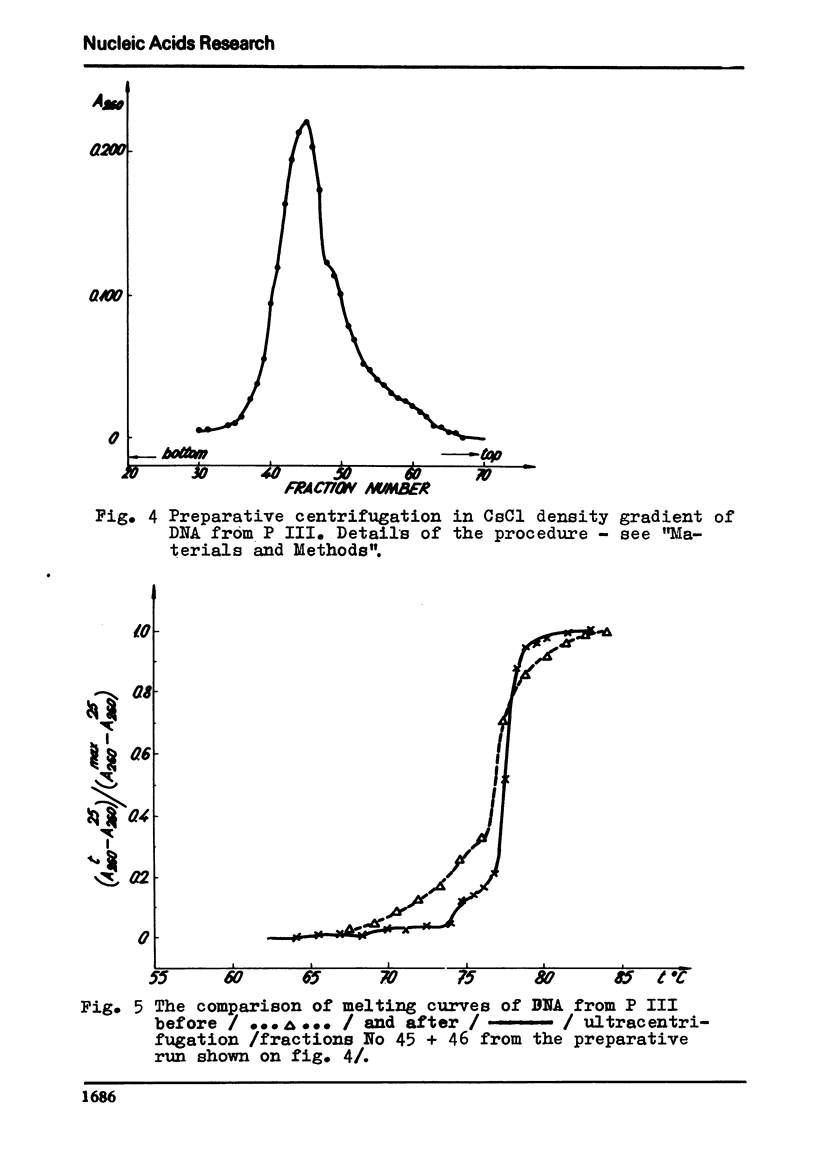
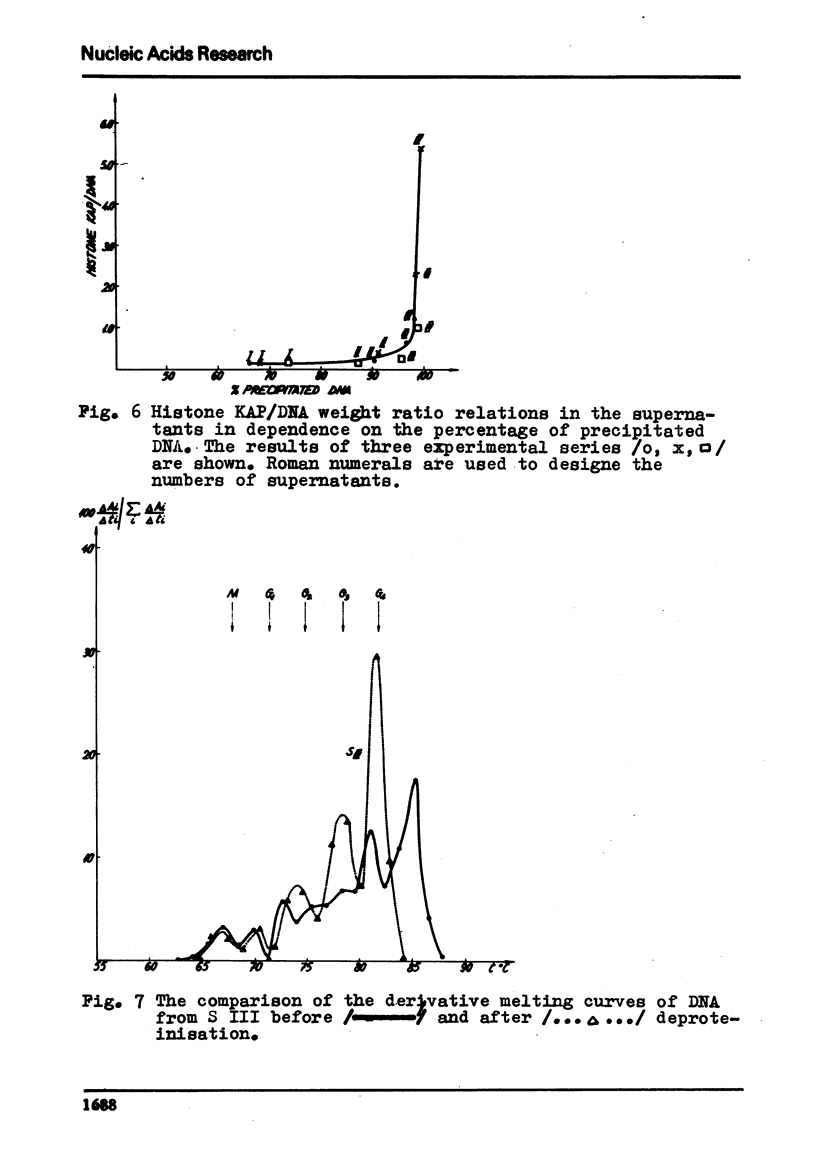
The process of fractionation of total calf thymus DNA using a step precipitation of DNA by means of increasing concentrations of the homologous histone KAP was investigated. In addition to the known fractions three so far undescribed ones/in thymus/,characterized by buoyant densities in CsCl equal 1.692, 1.706 and 1.728 g/ccm, were identified. Considerable amounts of preparations seriously enriched in individual satellite fractions were obtained. The ability of GC-rich satellite DNAs to form more soluble complexes with histone KAP is suggested as reason for the observed fractionation.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansevin A. T., Brown B. W. Specificity in the association of histones with deoxyribonucleic acid. Evidence from derivative thermal denaturation profiles. Biochemistry. 1971 Mar 30;10(7):1133–1142. doi: 10.1021/bi00783a006. [DOI] [PubMed] [Google Scholar]
- Brunk C. F., Leick V. Rapid equilibrium isopycnic CsC1 gradients. Biochim Biophys Acta. 1969 Mar 18;179(1):136–144. doi: 10.1016/0005-2787(69)90129-4. [DOI] [PubMed] [Google Scholar]
- Corneo G., Ginelli E., Polli E. Renaturation properties and localization in heterochromatin of human satellite DNA's. Biochim Biophys Acta. 1971 Nov 19;247(4):528–534. doi: 10.1016/0005-2787(71)90689-7. [DOI] [PubMed] [Google Scholar]
- Ilyin Y. V., Varshavsky A. Y., Mickelsaar U. N., Georgiev G. P. Studies on deoxyribonucleoprotein structure. Redistribution of proteins in mixtures of deoxyribonucleoproteins, DNA and RNA. Eur J Biochem. 1971 Sep 24;22(2):235–245. doi: 10.1111/j.1432-1033.1971.tb01537.x. [DOI] [PubMed] [Google Scholar]
- Johns E. W. Studies on histones. 7. Preparative methods for histone fractions from calf thymus. Biochem J. 1964 Jul;92(1):55–59. doi: 10.1042/bj0920055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurnit D. M., Shafit B. R., Maio J. J. Multiple satellite deoxyribonucleic acids in the calf and their relation to the sex chromosomes. J Mol Biol. 1973 Dec 15;81(3):273–284. doi: 10.1016/0022-2836(73)90141-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Li H. J., Brand B., Rotter A. Thermal denaturation of calf thymus DNA: existence of a GC-richer fraction. Nucleic Acids Res. 1974 Feb;1(2):257–265. doi: 10.1093/nar/1.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panusz H., Bartkowiak J., Krzywiec A., Joustra M., Plucienniczak A. The fractionation of calf thymus DNA on histone KAP-Sepharose 4B columns. Nucleic Acids Res. 1974 Sep;1(9):1143–1151. doi: 10.1093/nar/1.9.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plucienniczak A., Bartkowiak J., Krzywiec A., Panusz H. Fractionation of calf thymus DNA based on its interaction with homologeous f-1 histone. Melting curves of the obtained fractions. Biochem Biophys Res Commun. 1974 Feb 4;56(3):799–806. doi: 10.1016/0006-291x(74)90676-7. [DOI] [PubMed] [Google Scholar]
- Polli E., Ginelli E., Bianchi P., Corneo G. Renaturation of calf thymus satellite DNA. J Mol Biol. 1966 May;17(1):305–308. doi: 10.1016/s0022-2836(66)80114-6. [DOI] [PubMed] [Google Scholar]
- Prosser J., Moar M., Bobrow M., Jones K. W. Satellite sequences in chimpanzee (Pan troglodytes). Biochim Biophys Acta. 1973 Aug 24;319(2):122–134. doi: 10.1016/0005-2787(73)90003-8. [DOI] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- Skinner D. M., Beattie W. G. Cs2S04 gradients containing both Hg2+ and Ag+ effect the complete separation of satellite deoxyribonucleic acids having identical densities in neutral CsCl gradients. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3108–3110. doi: 10.1073/pnas.70.11.3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touvet-Poliakow M. C., Daune M. P., Champagne M. H. Interactions entre l'acide désoxyribonucléique et les histones. Eur J Biochem. 1970 Nov;16(3):414–423. doi: 10.1111/j.1432-1033.1970.tb01096.x. [DOI] [PubMed] [Google Scholar]
- VINOGRAD J., HEARST J. E. Equilibrium sedimentation of macromolecules and viruses in a density gradient. Fortschr Chem Org Naturst. 1962;20:373–422. [PubMed] [Google Scholar]
- Yasmineh W. G., Yunis J. J. Satellite DNA in calf heterochromatin. Exp Cell Res. 1971 Jan;64(1):41–48. doi: 10.1016/0014-4827(71)90190-x. [DOI] [PubMed] [Google Scholar]
- ZBARSKII I. B., SAMARINA O. P. [Fractionation and incorporation of glycine-1-C-14 into nucleoproteins]. Biokhimiia. 1962 May-Jun;27:557–564. [PubMed] [Google Scholar]


