Abstract
We previously identified claudin-2 as a functional mediator of breast cancer liver metastasis. We now confirm that claudin-2 levels are elevated in liver metastases, but not in skin metastases, compared to levels in their matched primary tumors in patients with breast cancer. Moreover, claudin-2 is specifically expressed in liver-metastatic breast cancer cells compared to populations derived from bone or lung metastases. The increased liver tropism exhibited by claudin-2-expressing breast cancer cells requires claudin-2-mediated interactions between breast cancer cells and primary hepatocytes. Furthermore, the reduction of the claudin-2 expression level, either in cancer cells or in primary hepatocytes, diminishes these heterotypic cell-cell interactions. Finally, we demonstrate that the first claudin-2 extracellular loop is essential for mediating tumor cell-hepatocyte interactions and the ability of breast cancer cells to form liver metastases in vivo. Thus, during breast cancer liver metastasis, claudin-2 shifts from acting within tight-junctional complexes to functioning as an adhesion molecule between breast cancer cells and hepatocytes.
INTRODUCTION
Metastatic tumor cells must overcome numerous barriers in order to successfully disseminate to distant organs. The metastatic cascade consists of multiple steps, including local tumor cell migration, invasion and intravasation into the lymphatic system or bloodstream, survival in the circulation, adhesion/extravasation at the metastatic site, evasion of immunosurveillance, and eventual growth in a foreign microenvironment (29, 30). Of these numerous steps, interactions between tumor cells and resident cells within the metastatic microenvironment represent the most important determinants contributing to the ability of cancer cells to metastasize to specific organs (14, 24).
The liver represents a common site of metastasis for solid cancers and represents the third most common site for breast cancer metastasis (12). Unique structural features of the liver, including the open fenestrae that characterize the sinusoidal endothelium coupled with the lack of an organized subendothelial basement membrane, have a great impact on tumor interactions within the hepatic microenvironment. Early in vivo electron microscopy studies revealed that breast cancer cells, upon seeding the liver, can extend cellular projections through the fenestrated endothelium into the space of Disse, making direct contact with hepatocytes (36). Subsequent in vitro studies revealed that breast cancer cells form electron-dense structures at points of contact between hepatocytes, which are reminiscent of tight-junctional complexes (37). The importance of cancer cell-hepatocyte interactions has been reinforced by the observation that colorectal cancer cells also interact directly with hepatocytes during liver metastasis (31, 32). However, the mechanisms underlying these heterotypic cell-cell interactions are largely unexplored.
Claudins are key components within tight junctions, and they participate in homo- and heteromeric interactions between adjacent cells. They contain four transmembrane domains, which create two extracellular loops that direct homotypic claudin interactions. Claudin-2 is the most divergent member of the family and is unique, given that its expression is restricted to leaky epithelia (40, 48). Roles for claudin-2 in promoting cancer growth have recently been reported. Indeed, the claudin-2 expression level has been shown to increase with colorectal cancer progression (7, 21), and high claudin-2 levels have been observed in fibrolamellar hepatocellular carcinomas and gastric cancers (15, 33). In breast cancer cells, claudin-2 expression is downregulated in invasive breast carcinomas associated with lymph node metastasis (20, 43, 44). However, our recently reported data showed that claudin-2 is readily detected in breast cancer liver metastases and promotes a liver-metastatic phenotype in breast cancer cells (45). In the current study, we demonstrate a functional requirement for claudin-2 in promoting breast cancer metastasis to the liver through a mechanism that involves enhanced adhesion to resident hepatocytes via claudin-2–claudin-2 interactions.
MATERIALS AND METHODS
Cell culture and transfections.
The 4T1 and MDA-MB-231 cell lines were obtained from the American Type Culture Collection (ATCC) and cultured as previously described (45). Claudin-4 and the chimeric constructs were kindly provided by J. M. Anderson and were described previously (5). These constructs were subcloned into the pMSCV-blasticidin vector. Pooled stable 4T1 populations were generated by infecting cells using a murine stem cell virus (MSCV) retroviral expression system according to the manufacturer's protocol (Clontech). Pooled stable populations were maintained under antibiotic selection with 1 μg/ml puromycin and 4 μg/ml blasticidin.
The generation of 4T1-derived liver-weak cell populations that overexpress claudin-2 was described previously (45). HEK-293 and Mv1Lu cells were kindly provided by J. Massagué (Memorial Sloan-Kettering Cancer Center), and HaCaT cells were kindly provided by J. J. Lebrun (McGill University) and were described previously (23, 26).
Claudin-2 immunohistochemistry.
Immunohistochemical staining for claudin-2 was performed as previously described (45). Briefly, paraffin sections were subjected to antigen retrieval in 10 mM citrate buffer (pH 6.0) for 10 min at subboiling temperatures. Slides were incubated overnight at 4°C with a polyclonal rabbit anti-claudin-2 antibody (1:25 dilution) (catalog number 516100; Invitrogen). Following incubation with the primary antibody, a secondary biotin-conjugated antibody was applied for 30 min. After washing with distilled water, slides were developed with diaminobenzidine (Dako) as the chromogen. All slides were counterstained by using Harris hematoxylin. The scoring of claudin-2 staining (percent positivity and intensity) was performed by two independent reviewers. Claudin-2 staining in the primary tumors and metastases were calibrated against claudin-2 staining (scored as +3) observed in normal tissues within the breast (mammary duct), skin (sweat gland), or liver (bile duct) parenchyma that was adjacent to the lesions (data not shown).
Human clinical samples.
Two matched breast tumor and liver metastases as well as five matched breast tumor and skin metastasis samples were obtained from the Princess Margaret Hospital (Toronto, Canada). Access to samples was provided after institutional review board (IRB) approval. Three additional matched breast tumor and liver metastasis samples were obtained from patients with metastatic breast cancer who were enrolled in a study at the Segal Cancer Centre according a protocol approved by the Jewish General Hospital (Montreal, Canada) research ethics committee. After informed consent had been obtained, the patients underwent ultrasound-guided liver biopsy for diagnostic reasons. Paraffin-embedded tissues were sectioned and subjected to claudin-2 immunohistochemistry as described above.
Immunoblotting.
Membranes were processed as previously described (45) and subjected to immunoblot analysis using the following antibodies: claudin-2 (1:5,000) (catalog number 325600; Invitrogen), claudin-4 (1:5,000) (catalog number 329400; Invitrogen), and α-tubulin (1:10,000) (catalog number T9626; Sigma) antibodies. The blots were incubated with the appropriate horseradish peroxidase (HRP)-conjugated anti-IgG secondary antibodies (Jackson ImmunoResearch Laboratories) and visualized with a chemiluminescent HRP substrate (Immobilon).
Primary hepatocyte isolation and culture.
Hepatocytes were isolated from mice according to a modified version of a two-step collagenase method described previously (27, 28). Briefly, cells were plated at a density of 1.5 × 105 cells/cm2 on fibronectin-coated dishes (BD Biosciences) in Dulbecco's modified Eagle's medium (DMEM)–F-12 modified medium (Wisent) supplemented with sodium selenite (5 ng/liter), insulin (5 mg/liter), transferrin (5 mg/liter) (Invitrogen), streptomycin (100 μg/ml), and penicillin (100 U/ml) (Wisent). Hepatocytes were allowed to adhere for 4 h, after which the culture medium was replaced by the same medium supplemented with dexamethasone (10−7 M) and epidermal growth factor (EGF) (20 ng/ml). Hepatocytes were cultured for 24 to 48 h, until they had formed a monolayer.
Assay of adhesion to epithelial cells or hepatocytes.
To assess the abilities of 4T1-derived or MDA-MB-231 cells to adhere to kidney-, lung-, and skin-derived epithelial cell lines or hepatocytes, 1 × 105 carboxy-fluorescein diacetate, succinimidyl ester (CFDA SE; Invitrogen)-labeled cells were seeded onto a monolayer of primary hepatocytes. Cells were then incubated at 37°C for 1 h. The plates were washed twice in phosphate-buffered saline (PBS) and fixed in 10% formalin (Fisher Scientific) for 20 min. After 2 washes in PBS, attached cells were visualized by using epifluorescence. For each experiment, data are expressed as the number of fluorescent cells per field. Data represent the averages of data from at least 3 independent experiments (5 fields/well and 3 wells/experiment).
Cell/extracellular matrix attachment assays.
Assays to quantify breast cancer cell adhesion to type IV collagen and fibronectin were performed as previously described (45).
Hepatocyte adhesion assay using blocking antibodies.
For attachment assays using integrin complex-blocking antibodies, 5 × 105 trypsinized cells suspended in PBS were incubated on ice for 45 min using increasing concentrations of α2β1 (catalog number MAB1998; Millipore)-, α5β1 (catalog number MAB1969; Millipore)-, or β1 (catalog number MAB2253; Millipore)-blocking antibodies or an isotype control antibody (catalog number 12-371; BD Biosciences) prior to the performance of the hepatocyte adhesion assays described above.
Immunoprecipitation/immunoblotting.
Four million cells from the indicated cell populations were labeled with 1.5 mg sulfo-N-hydroxysuccinimide (NHS) long-chain (LC) biotin (catalog number 21335; Thermo Scientific, Pittsburgh, PA) for 30 min on ice. Unincorporated biotin was removed, and the cells were subsequently lysed. Claudin-2 or claudin-4 chimeras were immunoprecipitated with the appropriate antibodies (catalog numbers 516100 and 329400; Invitrogen) and immunoblotted with streptavidin-HRP to detect biotinylated proteins.
siRNA transfections.
For claudin-2 knockdowns, primary cultured hepatocytes were transfected with two small interfering RNAs (siRNAs) targeting mouse claudin-2 or a scrambled dicer substrate duplex siRNA by using INTERFERin reagent (Polyplus transfection). claudin-2 siRNA sequences were as follows: rCrUrC rArUrA rCrArG rCrCrU rGrArC rUrGrG rGrUrA rU for C2#1 and rUrGrC rGrArU rArUrC rUrArC rArGrU rArCrC rCrUrU rU for C2#2. Cells were serially transfected a total of three times: once in the morning immediately following cell plating, once in the evening of the same day, and once in the morning of the following day. A portion of the siRNA-transfected cells was maintained and lysed following the completion of the experiment to monitor the efficacy and duration of the transient claudin-2 knockdowns.
Experimental metastasis assays.
Experimental metastasis assays (splenic injections) and liver metastatic burden were assessed as previously described (45). The mice were housed in facilities managed by the McGill University Animal Resources Centre, and all animal experiments were conducted under a McGill University-approved animal use protocol in accordance with guidelines established by the Canadian Council on Animal Care.
Statistical analysis.
Statistical significance values (P values) for short-term splenic injection assays, fibronectin or type IV collagen adhesion assays, hepatocyte adhesion assays, or liver metastasis formation following splenic injection were obtained by performing a two-sample unequal-variance Student t test.
RESULTS
Claudin-2 levels are elevated in liver metastases from breast cancer patients.
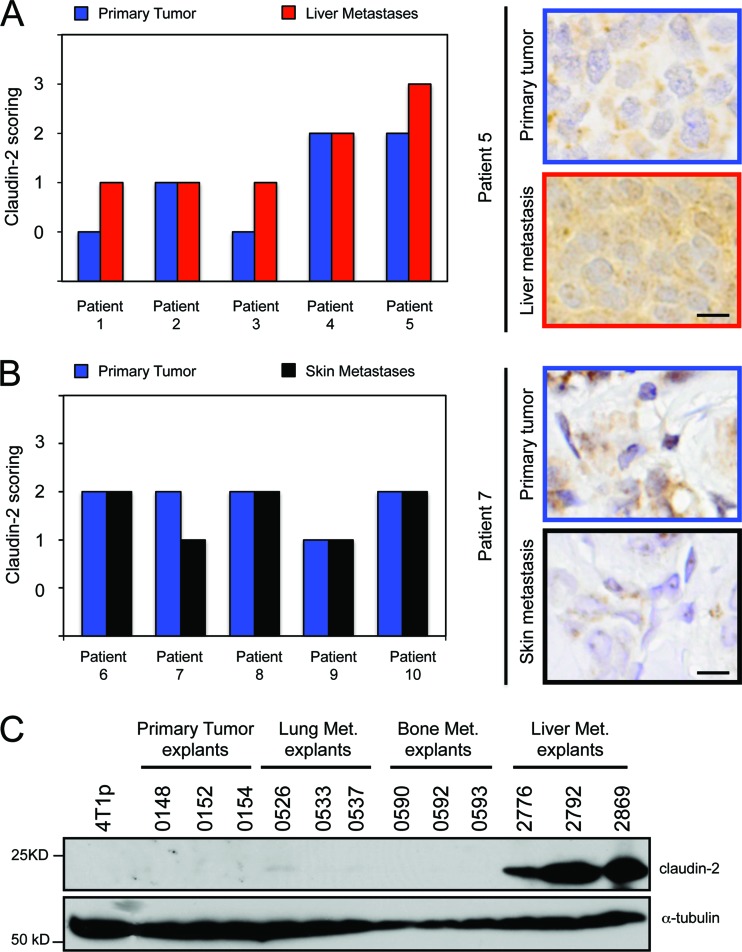
We previously demonstrated that claudin-2 expression is readily detected in breast cancer liver metastases (45). To extend these analyses, we assessed claudin-2 expression in five primary breast tumors and their matched liver metastases. As previously reported, claudin-2 staining exhibited membrane and cytoplasmic staining in carcinoma cells (20, 45; data not shown). Interestingly, the intensity and abundance of staining were enriched in 60% (3/5) of the liver metastases relative to the primary tumor (Fig. 1A). Conversely, claudin-2 levels were comparable between the five skin metastases compared to their matched primary breast tumors (Fig. 1B). In order to validate these clinical data, we employed a series of 4T1-derived breast cancer cell lines, which were selected in vivo for their ability to specifically metastasize to the lung, bone, or liver. We demonstrated that claudin-2 expression is observed only for liver-metastatic breast cancer cells (45) and is undetectable in bone or lung metastatic populations (38, 39) (Fig. 1C). These data indicate that claudin-2 expression in breast cancer metastases is clinically relevant and is selectively associated with liver metastasis.
Fig 1.
Claudin-2 expression is enriched in human breast cancer liver metastases compared with matched primary breast tumors. (A and B) Paraffin-embedded sections from five matched primary breast tumors and liver metastases (A) and five matched primary breast tumors and skin metastases (B) were subjected to immunohistochemical staining with an anti-claudin-2 antibody. The scoring of claudin-2 staining was performed by two independent reviewers. (A) Claudin-2 staining was enriched in three of five liver metastases compared to the matched primary breast tumors. The scale bar (bottom) represents 10 μm. (B) No enrichment in claudin-2 staining was observed for the skin metastases compared to the matched primary breast tumors. The scale bar (bottom) represents 10 μm. (C) Immunoblot analysis of claudin-2 demonstrates expression specifically in 4T1-derived explant populations isolated from liver metastases compared to those derived from lung or bone metastases. As a loading control, total cell lysates were blotted for α-tubulin.
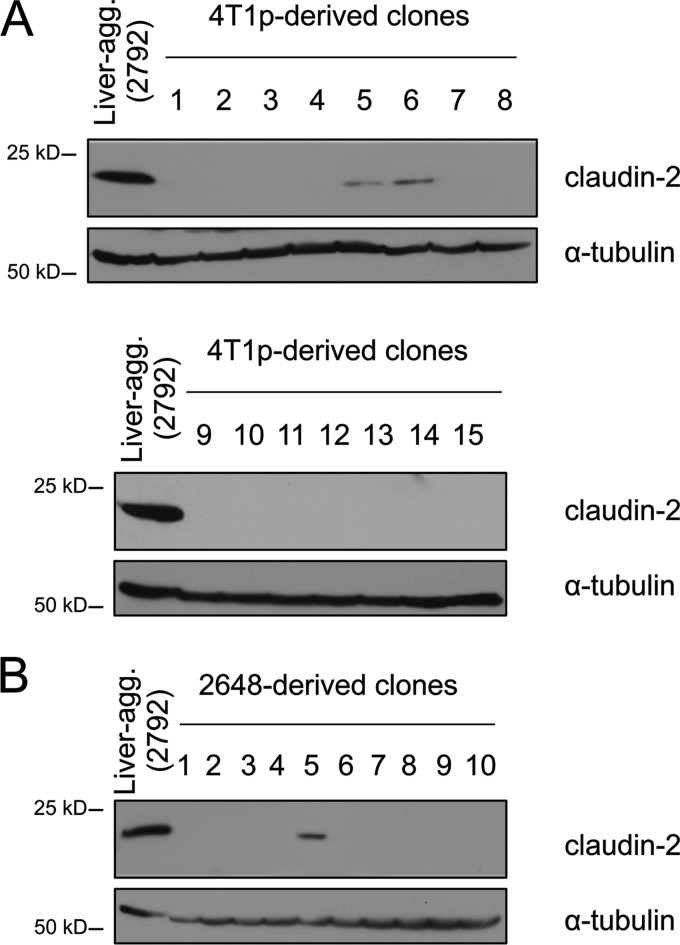
Claudin-2-expressing 4T1-derived populations preexist prior to selection of a liver-metastatic phenotype.
We recently described the establishment of 4T1-derived subpopulations that have acquired a liver-aggressive phenotype and further demonstrated a functional role for claudin-2 in promoting breast cancer liver metastasis (45). To assess whether this in vivo selection approach enriched for claudin-2-expressing cells that preexisted in the parental 4T1 population, we established individual clones from the 4T1 parental or the 2648 liver-weak cell population through in vitro dilution cloning. We observed that while the vast majority of clones derived from these cell populations were claudin-2 negative, 2/15 of the parental 4T1-derived clones (13%) and 1/10 of the 2648-derived clones (10%) were claudin-2 positive (Fig. 2). Thus, a subpopulation of claudin-2-expressing cells preexists in the 4T1 parental population and is enriched during the in vivo selection process. This selection for claudin-2-expressing cells is likely to account for the enhanced liver-metastatic phenotype associated with the in vivo-selected breast cancer cells, given the functional role that claudin-2 plays in this process (45).
Fig 2.
In vitro dilution cloning reveals that claudin-2-positive cells represent a small percentage of liver-weak derived cell populations. Shown are immunoblot analyses of claudin-2 expression in the parental 4T1-derived clones (A) and 2648-derived clones (B). As a loading control, total cell lysates were blotted for α-tubulin.
Claudin-2 promotes survival of early breast cancers in the liver.
Claudin-2 expression in breast cancer cells is required for the formation of liver metastases (45). Here, we explored the molecular mechanisms by which claudin-2 promotes this phenotype. Given that elevated claudin-2 levels do not enhance primary mammary tumor growth (45) and are further enriched in liver metastases (Fig. 1A), we reasoned that claudin-2 does not regulate general features of the tumorigenic phenotype but selectively enhances breast cancer cell adhesion and/or survival within the liver parenchyma. Interestingly, we discovered that the extent of tumor cell proliferation (Ki67), apoptosis (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling [TUNEL]), and endothelial cell recruitment (CD31) is not altered in end-stage hepatic metastases derived from breast cancer cells expressing high or low levels of claudin-2 (data not shown). Thus, claudin-2 is likely to function at an early stage following the seeding of the liver to promote breast cancer colonization and metastasis formation.
These observations prompted us to examine whether liver-aggressive breast cancer cells displayed enhanced adhesion to the sinusoidal endothelium, which represents the earliest step once breast cancer cells reach the liver. This was achieved via intravital imaging at different time points after tumor cell injection (see Fig. S1 in the supplemental material). No differences in the numbers of tumor cells that adhered to the sinusoidal endothelium were observed at 30, 45, and 60 min postinjection. Thus, we conclude that the significant differences in liver burden observed with our liver-aggressive breast cancer cells are not due to enhanced tumor cell-endothelial cell adhesion.
We next asked if claudin-2 expression in the liver-aggressive populations plays a role in the survival of early cancer cells following seeding in the liver. The splenic injection of CFDA SE-labeled claudin-2-expressing or -deficient cells allowed us to quantify the number of fluorescent breast cancer cells surviving in the liver at early and later time points (3 h, 27 h, and 51 h) (see Fig. S2 in the supplemental material). Significantly fewer claudin-2-deficient breast cancer cells remained in the liver than the vector controls, at both 27 h (23% reduction) and 51 h (68% reduction) postinjection. Taken together, these observations suggest that claudin-2 confers an early survival advantage to breast cancer cells following the seeding of the liver.
Claudin-2 promotes breast cancer cell adhesion to hepatocytes.
Another mechanism by which claudin-2 may facilitate the formation of liver metastases is by enhancing breast cancer cell adhesion to resident hepatocytes. Indeed, the endothelial lining of the liver sinusoids possesses numerous open fenestrae that provide immediate access of tumor cells to hepatocytes. Furthermore, previous electron microscopy-based studies have shown that breast cancer cells can form “tight-junction-like” structures with hepatocytes (36, 37).
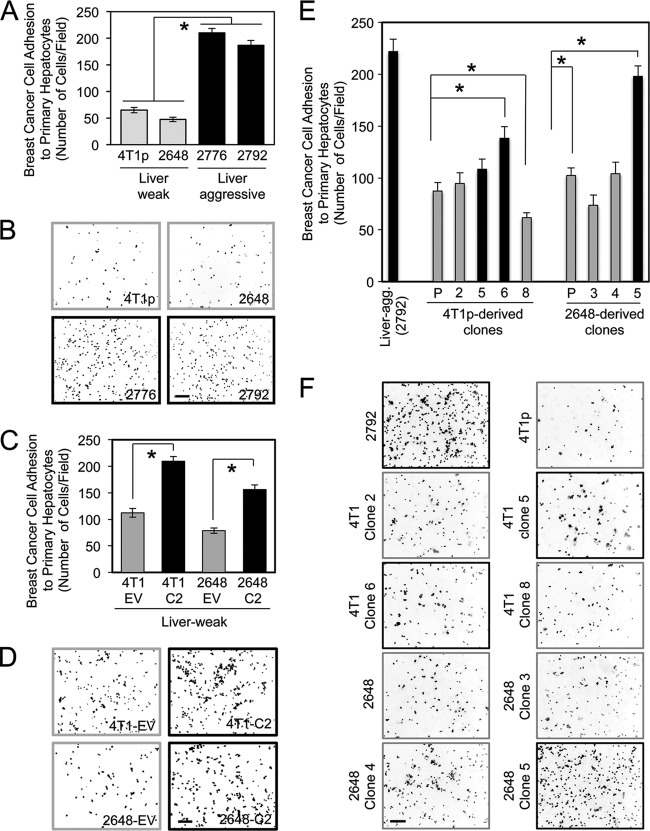
Using a cell attachment assay, we demonstrate that liver-aggressive cells display a 2.8- to 4.5-fold-higher propensity to adhere to primary hepatocyte monolayers than their weakly liver-aggressive counterparts (Fig. 3A and B). To further examine a potential role of claudin-2 in hepatocyte adhesion, we overexpressed claudin-2 in parental 4T1 cells (4T1p) or in a liver-weak cell population (2648) (45). Elevated claudin-2 expression levels resulted in a 1.8- to 2-fold increase in adhesion to hepatocytes compared to empty-vector control cells (Fig. 3C and D). In agreement with these observations, the level of endogenous claudin-2 expression observed for the 4T1-derived clones or the 2648-derived clones (Fig. 2) correlated directly with their ability to interact with hepatocytes (Fig. 3E and F).
Fig 3.
Liver-metastatic 4T1 explant populations display an enhanced ability to adhere to primary hepatocytes. (A) Liver-weak (4T1p and 2648) and liver-aggressive (2776 and 2792) breast cancer cells were analyzed for their abilities to adhere to primary hepatocyte monolayers. Statistically significant increases in hepatocyte adhesion were observed for all liver-aggressive explants (∗, P < 0.001, when liver-weak cells lines were compared with liver-aggressive cells). (B) Representative images of each cell population following adhesion to a monolayer of primary hepatocytes. The scale bar (bottom right) represents 200 μm and applies to all panels. (C) Liver-weak cell populations engineered to overexpress claudin-2 (C2) and their corresponding empty vector controls (EV) were analyzed for their abilities to adhere to primary hepatocyte monolayers. Statistically significant increases in hepatocyte adhesion were observed with populations overexpressing claudin-2 (∗, P < 0.0001). (D) Representative images of each cell population following adhesion to a monolayer of primary hepatocytes. (E) Clonal cell lines derived from liver-weak cell populations were assessed for their abilities to adhere to primary hepatocytes. A correlation between the ability of breast cancer cells to interact with hepatocytes and increasing claudin-2 levels was observed (∗, P < 0.02). (F) Representative images of each cell population following adhesion to a monolayer of primary hepatocytes. The scale bar (bottom left) represents 200 μm.
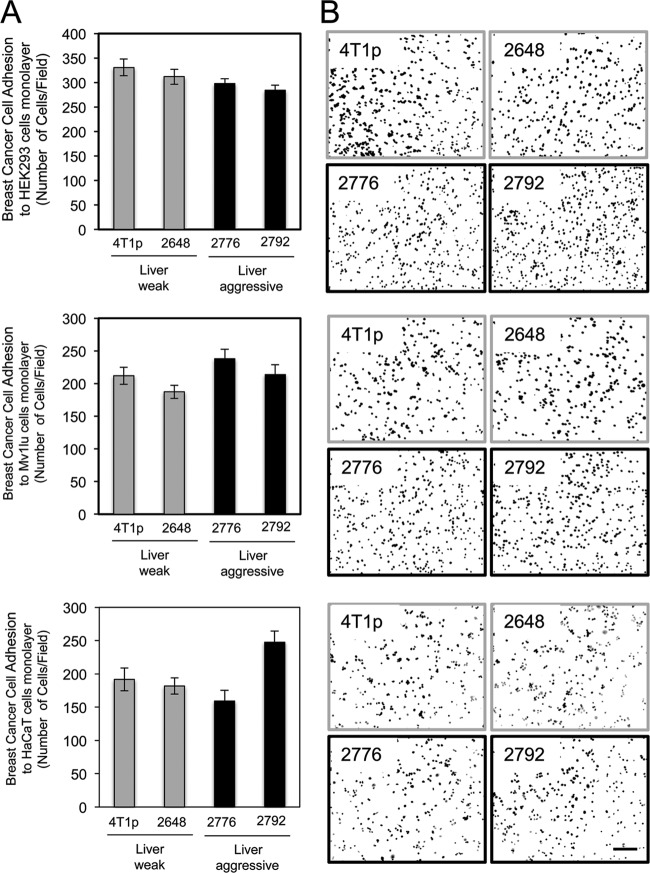
To assess whether the liver-aggressive 4T1 subpopulations possessed generally enhanced characteristics of adhesion to a variety of epithelial cells, we characterized their abilities to adhere to HEK-293 kidney epithelial cells, Mv1Lu lung epithelial cells, or HaCaT skin keratinocytes. No significant changes in adhesion to these cell lines were observed between weakly and highly liver-aggressive breast cancer cells (Fig. 4). In contrast to primary hepatocytes, claudin-2 is not expressed in kidney, lung, or skin epithelial cells (data not shown).
Fig 4.
Liver-aggressive breast cancer cells do not preferentially adhere to kidney-, lung-, or keratinocyte-derived cell lines. (A) Liver-weak (4T1p and 2648) and liver-aggressive (2776 and 2792) breast cancer cells were analyzed for their abilities to adhere to a monolayer of HEK-293 (kidney), Mv1lu (lung), or HaCaT (skin) cells. (B) Representative images of each breast cancer cell population following adhesion to a monolayer of HEK-293, Mv1lu, or HaCaT cells are shown. The scale bar (bottom right) represents 200 μm and applies to all panels.
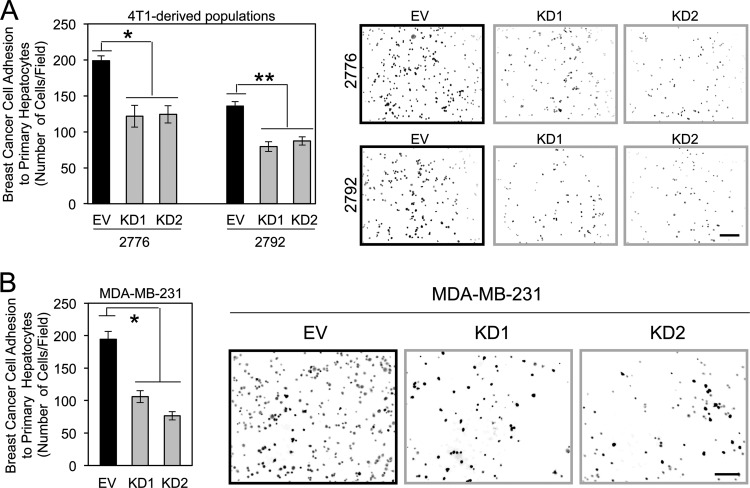
Next, we generated 4T1-derived liver-metastatic and MDA-MB-231 breast cancer cells with diminished claudin-2 expression levels using short hairpin RNA (shRNA) approaches (45). The reduction of claudin-2 expression levels in 4T1-derived liver-aggressive cells resulted in a 1.6- to 1.7-fold decrease in adhesion to hepatocytes compared to control cells (Fig. 5A). A similar result was obtained by using MDA-MB-231 human breast cancer cells as an independent model system that endogenously expresses claudin-2. Attachment assays revealed that diminished claudin-2 expression levels resulted in an average 1.8- to 2.5-fold reduction in the ability of MDA-MB-231 cells to adhere to hepatocytes (Fig. 5B).
Fig 5.
Claudin-2 functions to promote breast cancer adhesion to hepatocytes. (A) The indicated breast cancer cells were plated onto primary hepatocyte monolayers, and adhesion was quantified after 1 h. Liver-aggressive cells infected with two independent Cldn2 shRNA expression vectors (knockdown [KD1 and KD2]) or harboring the empty vector (EV) were analyzed. Diminished claudin-2 levels in liver-aggressive cells (KD1 and KD2) resulted in statistically significant decreases in hepatocyte adhesion compared to control cells (empty vector) (∗, P = 0.016; ∗∗, P = 0.033). Representative images of each cell population following adhesion to a monolayer of primary hepatocytes are shown. The scale bar (bottom right) represents 200 μm. (B) Human MDA-MB-231 breast cancer cells were infected with two independent human Cldn2 shRNA expression vectors (KD1 and KD2) or an empty vector and analyzed for their abilities to adhere to primary hepatocyte monolayers. Diminished claudin-2 levels in MDA-MB-231 cells (KD1 and KD2) resulted in statistically significant decreases in hepatocyte adhesion compared to control cells (empty vector) (∗, P = 0.015). Shown are representative images of each cell population following adhesion to a monolayer of primary hepatocytes. The scale bar (right) represents 200 μm.
Claudin-2-mediated adhesion of breast cancer cells to hepatocytes is independent of integrin function.
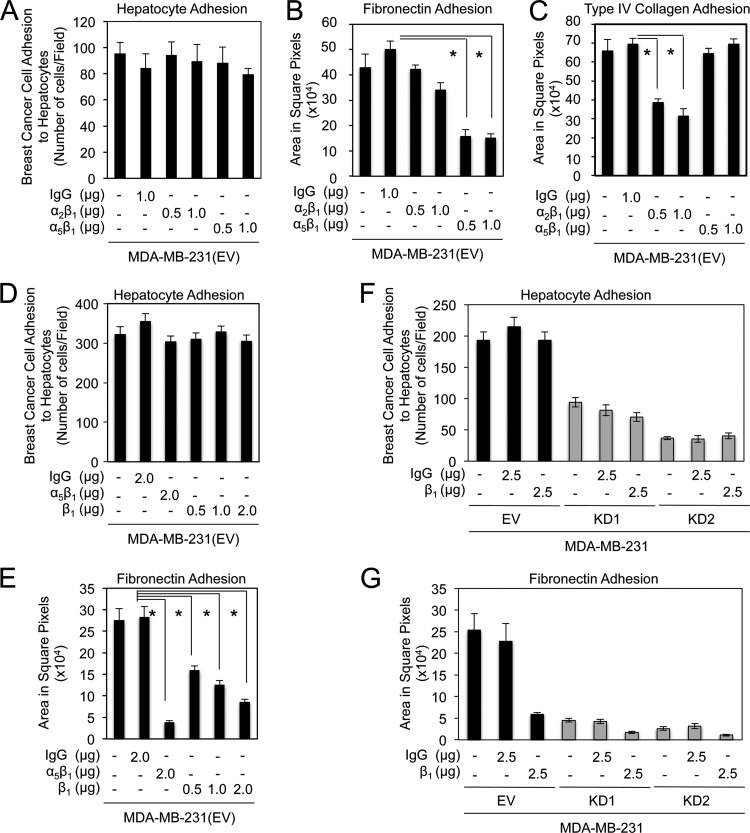
Previous studies have suggested that integrins may play an important role in cancer cell interactions with hepatocytes (17–19, 31). Our previous studies suggested that claudin-2 can influence breast cancer cell adhesion to extracellular matrices, such as type IV collagen and fibronectin, in an integrin-dependent fashion (45). To investigate the potential role of integrins in promoting breast cancer cell interactions with hepatocytes in our MDA-MB-231 breast cancer model, we employed neutralizing antibodies to various integrin receptors or integrin subunits. Blocking antibodies against integrin-α2β1 (type IV collagen) and integrin-α5β1 (fibronectin) did not affect breast cancer cell attachment to primary hepatocytes (Fig. 6A) but were effective in reducing breast cancer cell adhesion to type IV collagen and fibronectin, respectively (Fig. 6B and C). To further assess the potential role played by other β1-containing integrin complexes, a similar experiment was conducted by using an independent blocking antibody against integrin-β1. In agreement with the results obtained by using the α2β1- or α5β1-blocking antibodies, no effect on breast cancer cell attachment to primary hepatocytes was observed with β1-neutralizing antibodies (Fig. 6D). In contrast, this antibody effectively impaired breast cancer cell adhesion to fibronectin (Fig. 6E). To assess whether a potential cooperative mechanism, involving both claudin-2 and integrin-containing complexes, might account for breast cancer cell adhesion to hepatocytes, we next conducted an experiment in which we combined β1-neutralizing antibodies with MDA-MB-231 breast cancer cells possessing diminished claudin-2 expression levels. While no synergistic effect on breast cancer cell attachment to primary hepatocytes was observed when the β1-neutralizing antibodies were combined with either MDA-MB-231 population harboring the claudin-2 knockdowns (Fig. 6F), this antibody effectively impaired breast cancer cell adhesion to fibronectin (Fig. 6G). Taken together, these results suggest that claudin-2 facilitates tumor cell adhesion to hepatocytes independently of its reported role in promoting the membrane localization of α2β1- or α5β1-integrin complexes (45).
Fig 6.
β1-containing integrins do not participate in claudin-2-mediated breast cancer cell adhesion to hepatocytes. (A to C) The numbers of MDA-MB-231 breast cancer cells that adhered to a monolayer of primary hepatocytes (A), fibronectin (B), or type IV collagen (C) following incubation with a control isotype or increasing concentrations of blocking antibodies against α2β1- and α5β1-integrin complexes were quantified. Blocking antibodies against α2β1- and α5β1-integrin complexes effectively prevented adhesion to collagen type IV and fibronectin, respectively. These antibodies failed to impair MDA-MB-231 breast cancer cell adhesion to primary hepatocytes. (D and E) The number of MDA-MB-231 breast cancer cells that adhered to a monolayer of primary hepatocytes (D) or fibronectin (E) following incubation with a control isotype or increasing concentrations of blocking antibodies against integrin-β1 were quantified. While blocking antibodies against the integrin-β1 subunit or the α5β1-integrin complex prevented adhesion to fibronectin, they had no effect on breast cancer adhesion to primary hepatocytes (∗, P < 0.001). (F and G) The numbers of MDA-MB-231 breast cancer cells that possessed normal or diminished claudin-2 expression levels, which adhered to a monolayer of primary hepatocytes (F) or fibronectin (G) following incubation with a control isotype or blocking antibodies against β1-integrin, were quantified. Blocking antibodies against β1-integrin-containing complexes effectively prevented breast cancer cell adhesion to fibronectin, but failed to impair MDA-MB-231 breast cancer cell adhesion to primary hepatocytes. For panel F, the P value was < 0.0001 for KD1 or KD2 versus the empty vector with no treatment (∗), KD1 or KD2 versus the empty vector with 2.5 μg IgG isotype control antibody (∗∗), and KD1 or KD2 versus the empty vector with 2.5 μg β1-neutralizing antibody (∗∗∗). For panel G, the P value was <0.0001 (∗).
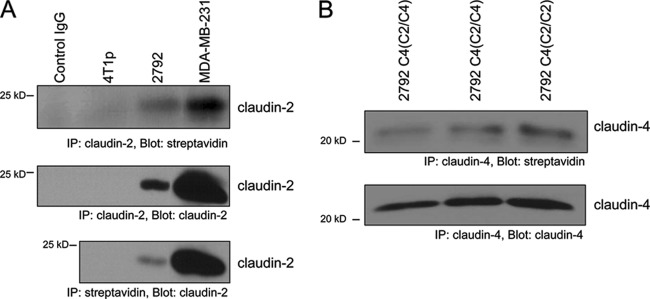
The notion that claudin-2 can promote breast cancer cell adhesion to hepatocytes would suggest that it remains on the plasma membrane, even though it is no longer properly localized to tight junctions. To investigate this possibility, we immunoprecipitated claudin-2 or claudin-2/claudin-4 chimeras following the biotinylation of cell surface proteins in both MDA-MB-231 and 4T1-derived liver-aggressive breast cancer cells. Our results indicate that biotinylated claudin-2, as well as biotinylated chimeric proteins, could be detected in both breast cancer cell models, confirming that a proportion of claudin-2 in the cells is localized to the plasma membrane (Fig. 7).
Fig 7.
Claudin-2 is localized at the cell membrane in liver-aggressive breast cancer cells. Following the biotinylation of cell surface molecules, whole-cell lysates were immunoprecipitated with an anti-claudin-2, anti-claudin-4, or antistreptavidin antibody. Immunoblot analyses were performed by using antibodies against claudin-2 (A), claudin-4 (B), or streptavidin (A and B), as indicated.
The first extracellular loop of claudin-2 is sufficient to promote breast cancer cell adhesion to hepatocytes.
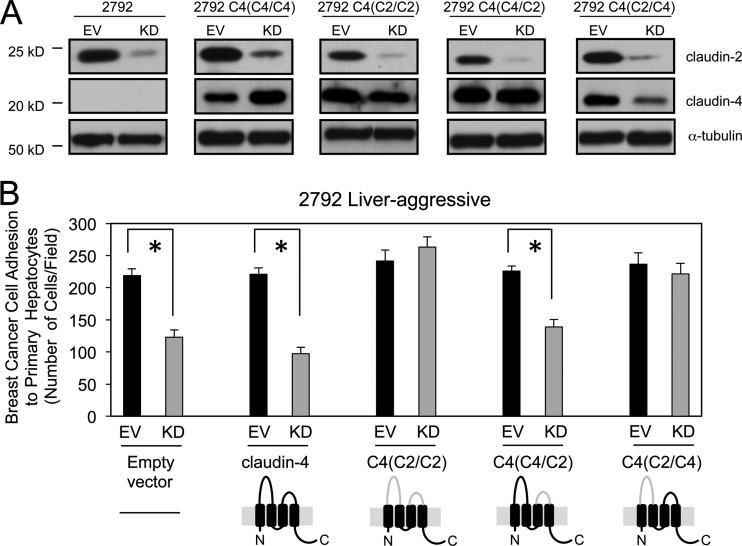
When functioning as tight-junctional proteins, claudin-claudin interactions between adjacent cells are mediated via their extracellular loops (5). We therefore asked whether these regions of claudin-2 also promote the hepatocyte adhesion phenotype exhibited by liver-aggressive breast cancer cells. To achieve this, we employed chimeric constructs that substitute both extracellular loops of claudin-4 with those of claudin-2 [C4(C2/C2)], only the first extracellular loop of claudin-2 [C4(C2/C4)], or only the second extracellular loop of claudin-2 [C4(C4/C2)] (5). In two independent liver-aggressive breast cancer populations, immunoblot analysis confirmed that endogenous claudin-2 levels were effectively reduced by using shRNA approaches and that claudin-4 and the claudin-4/2 chimeras were similarly expressed (Fig. 8A, and see Fig. S3A in the supplemental material). Attachment assays confirmed that reduced claudin-2 expression levels result in the decreased ability of liver-aggressive breast cancer cells to interact with primary hepatocytes (Fig. 8B, and see Fig. S3B in the supplemental material). The expression of wild-type claudin-4 fails to rescue the ability of claudin-2-deficient breast cancer cell lines to adhere to hepatocytes (Fig. 8B, and see Fig. S3B in the supplemental material). This observation underscores the specificity for claudin-2 in promoting breast cancer-hepatocyte interactions. In contrast, the reconstitution of the claudin-2 extracellular loops within the claudin-4/2 chimera rescued breast cancer cell adhesion to primary hepatocytes (Fig. 8B, and see Fig. S3B in the supplemental material). Finally, the rescue of endogenous claudin-2 knockdown by the expression of the C4(C2/C4) chimera was sufficient to rescue the hepatocyte adhesion phenotype, while the C4(C4/C2) chimera failed to do so (Fig. 8B, and see Fig. S3B in the supplemental material). Our results demonstrate that cell-cell interactions between liver-metastatic breast cancer cells and hepatocytes are mediated primarily through the first extracellular loop of claudin-2.
Fig 8.
The first extracellular loop of claudin-2 is sufficient to promote breast cancer cell adhesion to hepatocytes. (A) Immunoblot analysis of claudin-2, claudin-4, or the chimeric proteins demonstrates expression levels in cells infected with one Cldn2 shRNA expression vector (KD) or in cells harboring the empty vector (EV). As a loading control, total cell lysates were blotted for α-tubulin. The gray lines in the claudin schematics indicate the extracellular loops derived from claudin-2 within a claudin-4 backbone (solid line). (B) The number of breast cancer cells that adhered to a monolayer of primary hepatocytes was quantified. Diminished adhesion was observed for cells with reduced claudin-2 levels in which an empty vector, claudin-4, or the C4(C4/C2) chimera was expressed (∗, P < 0.05). However, a complete rescue of the adhesive phenotype was observed when the C4(/C2/C2) or C4(C2/C4) chimera was expressed.
Claudin-2 expression is required for primary hepatocytes to promote breast cancer cell adhesion.
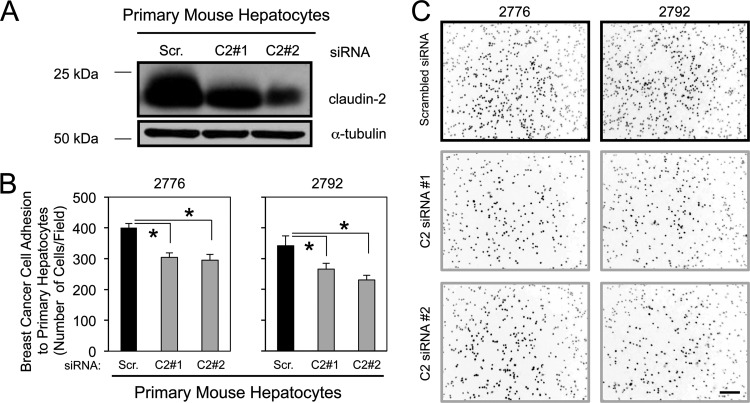
Claudin-2 forms trans-homotypic interactions with claudin-2 molecules expressed in adjacent cells (10). Of interest, claudin-2 is known to be highly expressed by hepatocytes (22). Thus, we next determined if the ability of breast cancer cells to adhere to hepatocytes was mediated through a trans-homotypic interaction between claudin-2 molecules expressed in both cell types. We have established conditions to transiently reduce claudin-2 levels in primary cultured hepatocytes. Using two independent siRNAs targeting claudin-2 (C2#1 and C2#2), we achieved a 25 to 50% reduction in claudin-2 levels over 4 independent experiments (Fig. 9A). Reduced claudin-2 expression levels in primary hepatocytes attenuated the adhesion of two independent liver-aggressive breast cancer cell populations (Fig. 9B and C). Taken together, our results demonstrate that breast cancer cells expressing claudin-2 interact, in part, with resident hepatocytes through the formation of trans-homotypic claudin-2–claudin-2 interactions between these cell types.
Fig 9.
Claudin-2 knockdown in primary hepatocytes decreases the adhesion of cancer cells to the hepatocyte monolayer. (A) Immunoblot analysis of claudin-2 expression in primary cultured hepatocytes transfected with either a scrambled siRNA (Scr.) or two independent Cldn2 siRNAs (C2#1 or C2#2). As a loading control, total cell lysates were blotted for α-tubulin. (B) Liver-aggressive cell populations were analyzed for their abilities to adhere to primary hepatocyte monolayers with diminished claudin-2 expression levels. Decreases in hepatocyte adhesion were observed for hepatocyte cultures with diminished expression levels of claudin-2 (∗, P < 0.0001). (C) Representative images of each cell population following adhesion to a monolayer of primary hepatocytes. The scale bar (bottom right) represents 200 μm.
The first extracellular loop of claudin-2 is sufficient for its pro-liver-metastatic functions.
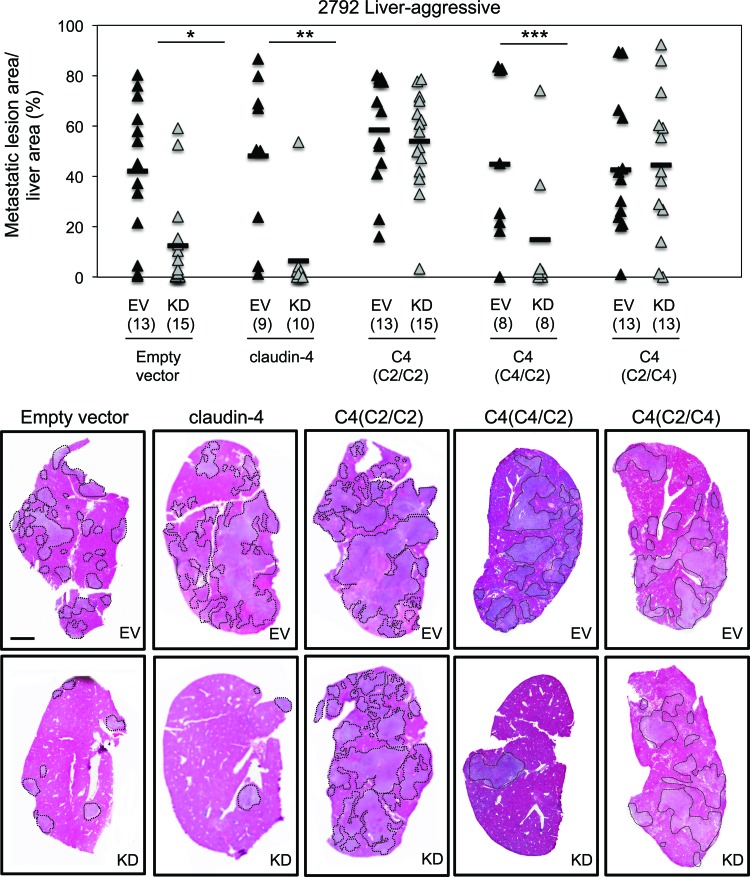
To examine whether the claudin-2 extracellular loops are also important to enhance the liver-metastatic phenotype of breast cancer cells, we performed splenic injections using 4T1-derived breast cancer cells in which the endogenous claudin-2 level was reduced and claudin-4 or the individual claudin-4–claudin-2 chimeras were expressed [(C4(C2/C2), C4(C2/C4), and C4(C4/C2)]. As previously reported (45), reduced claudin-2 expression levels in liver-aggressive breast cancer cells significantly decreased the liver-metastatic burden compared to controls (Fig. 10, and see Fig. S4 in the supplemental material). In agreement with the results generated by the hepatocyte adhesion assay, claudin-4 expression failed to rescue breast cancer liver metastasis in a claudin-2-deficient background. In contrast, the introduction of a the claudin-4 chimera possessing both claudin-2 extracellular loops [C4(C2/C2)] fully rescued the liver-metastatic phenotype (Fig. 10, and see Fig. S4 in the supplemental material). Finally, only the C4(C2/C4) chimera, and not the C4(C4/C2) chimera, rescued the liver-metastatic phenotype (Fig. 10, and see Fig. S4 in the supplemental material). Together, our results demonstrate that claudin-2 promotes breast cancer liver metastasis in part by promoting cell-cell interactions between breast cancer cells and hepatocytes. These interactions are driven by trans-homotypic complexes between claudin-2 expressed by the cancer cell and claudin-2 expressed by the hepatocytes, which require the first extracellular loop.
Fig 10.
The first extracellular loop of claudin-2 is required to promote the breast cancer liver-metastatic phenotype. Quantification of the tumor burden (tumor area/tissue area) within the cardiac liver lobe following the splenic injection of the indicated cell populations is shown. The number of mice analyzed in each cohort is indicated in parentheses. Decreases in liver-metastatic burden were observed when the empty vector-, claudin-4-, or C4(C4/C2)-expressing cells, in the context of diminished claudin-2 levels, were injected into the spleen (∗, P < 0.002; ∗∗, P < 0.001; ∗∗∗, P < 0.04). However, the liver-metastatic phenotype was rescued when the C4(C2/C2) or the C4(C2/C4) chimera was expressed. Hematoxylin- and eosin-stained images of the cardiac liver lobe are shown for mice injected with each of the cell populations. Dotted lines circumscribe breast cancer metastatic lesions within the liver. The scale bar represents 2 mm.
DISCUSSION
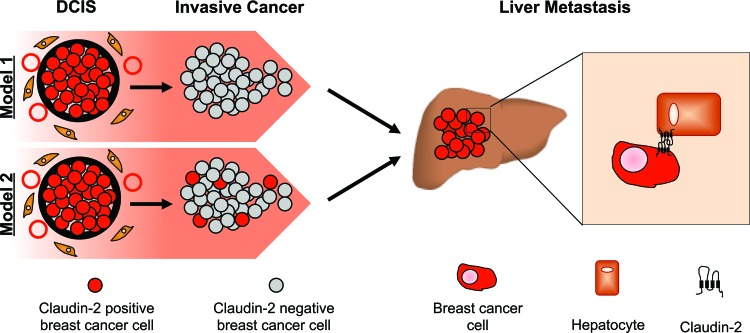
This study provides mechanistic insights into the observation that claudin-2 enhances breast cancer liver metastasis. Claudin-2 expression has been demonstrated for 52% of breast carcinomas (43); however, its expression was recently shown to be downregulated in invasive ductal carcinomas of the breast that are associated with lymph node metastasis and high clinical stages (20). Claudin-2 was also shown to be more highly expressed in invasive lobular carcinomas than in matched axillary lymph node metastases (44). We have recently reported that, while weakly expressed in primary human breast cancer cells, claudin-2 expression was readily detected in liver metastases (45). Our current immunohistochemistry staining of liver metastases and matched primary breast tumors further supports our findings that claudin-2 expression is enhanced in the majority of liver metastases. It is conceivable that claudin-2 expression may be lost during certain stages of the metastatic process, such as early dissemination from the primary tumor, but is regained at later stages, which allows the establishment of breast cancer cells in the liver (Fig. 11, model 1). However, we demonstrate that approximately 10% of clones derived from liver-weak cell populations (parental 4T1 and 2648 cells) expressed claudin-2, suggesting that claudin-2-expressing cells might be selected due to the ability of claudin-2 to promote interactions between breast cancer cells and hepatocytes (Fig. 11, model 2). Interestingly, the ability of claudin-2-positive clones to interact with hepatocytes correlates with the level of claudin-2 expression. However, these claudin-2-positive clones express lower claudin-2 levels than those observed for the in vivo-selected liver-aggressive cell populations (Fig. 2). Thus, we cannot preclude the possibility that claudin-2 may also be further upregulated in liver-aggressive cells in response to signals coming from the liver microenvironment.
Fig 11.
Schematic depicting the potential roles for claudin-2 in breast cancer liver metastasis. Low levels of claudin-2 expression, and its loss in invasive breast cancers, may be required for local invasion and escape from the primary tumor (model 1). Alternatively, a small percentage of claudin-2-positive cells may persist in invasive cancer cells in the primary tumor (model 2). Elevated expression levels of claudin-2, or selection for preexisting claudin-2-positive breast cancer cells, within liver metastases may serve to enhance the survival of breast cancer cells by promoting interactions between the tumor cell and resident hepatocytes.
Interestingly, high claudin-2 levels were recently reported for a subset of hepatocellular carcinomas (33). Moreover, claudin-2 has been observed for gastric carcinomas and colorectal cancer, which are both highly metastatic to the liver (1, 15, 21). It is conceivable that claudin-2 expression is selected for in primary liver cancer and in solid cancer cells that display a propensity to metastasize to the liver. In this respect, claudin-2 is known to be expressed at high levels in hepatocytes (47). Recent data have demonstrated that colorectal cancer cells lodge in the liver due to mechanical restriction and that endothelial cells retract to permit the direct interaction of cancer cells with hepatocytes (31, 32). These observations are consistent with our own intravital imaging data, which demonstrate that liver-metastatic breast cancer cells do not preferentially adhere to the sinusoidal endothelium compared to weakly metastatic breast cancer cells. Electron microscopy studies of metastatic breast cancer cells within the liver revealed that they form intimate interactions with hepatocytes (36). Moreover, breast cancer cells that were allowed to adhere to primary hepatocyte monolayers in vitro formed “tight-junction-like” complexes with hepatocytes (37). The intravital imaging experiments were performed over very short time periods, extending only to an hour after tumor cell injection. In contrast, the imaging of fluorescently tagged breast cancer cells surviving in the liver was conducted over a period of 2 days after the tumor cell injection. Thus, it is likely that the liver-weak and liver-aggressive cells show the same ability to adhere to sinusoidal endothelial cells, and these very early adhesive events are independent of tumor cell/extracellular matrix and tumor cell/hepatocyte adhesion that are critical for breast cancer cell survival following the seeding of the liver. At later time points, between 24 and 48 h postinjection, breast cancer cells harboring a claudin-2 knockdown are at a disadvantage relative to cells expressing claudin-2 and may die by apoptosis due to their inability to effectively bind to the extracellular matrix or interact with hepatocytes. These observations argue that cancer cell-hepatocyte interactions are critical for the ability of breast cancer cells to seed and colonize the liver.
Epithelial-to-mesenchymal transitions (EMTs) in cancer cells contribute to increased invasion and cancer cell dissemination (46). Once cancer cells have seeded the metastatic site, a mesenchymal-to-epithelial transition (MET) occurs, which is key to the productive colonization and growth of the subsequent metastases (35). Indeed, at the sites of metastases, disseminated cells often recapitulate the pathology of their corresponding primary tumors (13). Recently, it was demonstrated that prostate and breast cancer cells undergo an MET, as measured by E-cadherin upregulation, upon interactions with hepatocytes (2, 3, 51). Thus, one may expect that the formation of claudin-2-mediated cell-cell interactions between cancer cells and hepatocytes may induce an MET that is needed to successfully establish liver metastases.
While cancer cell-hepatocyte interactions are clearly beneficial to the establishment of liver metastases, the precise mechanisms that govern such heterotypic cell-cell interactions remain poorly defined. A recent study suggested that the adhesion of CC531s colon cancer cells to hepatocytes correlates with increased expression levels of the integrin subunits αv, α6, and β1. However, the requirement for these integrin subunits for directly facilitating interactions between colon cancer cells and hepatocytes was not established (31). Such results are in keeping with previous studies suggesting that breast cancer cell adhesion to hepatocytes involves integrin-mediated adhesion to hepatocytes (19). Indeed, the TA3/Ha and TA3/St breast cancer cell lines were originally derived from the same tumor and form liver metastases following intraportal injection. While TA3/Ha cells employ α6β4-integrins to promote adhesion to hepatocytes in vitro (18), the adhesion of TA3/St cells requires integrin-α5β1, which binds to fibronectin on the hepatocyte cell surface (19).
These data are interesting in light of our recent results showing that claudin-2 expression facilitates cell-matrix interactions by increasing the cell surface expression levels of α2β1- and α5β1-integrin complexes in breast cancer cells (45). However, our current data suggest that claudin-2 promotes breast cancer cell adhesion to hepatocytes independently of β1-containing integrin complexes. Our data clearly demonstrate that blocking antibodies to specific integrin receptors, or the β1 subunit, fail to impair breast cancer cell adhesion to hepatocytes, even though they effectively block breast cancer cell adhesion to type IV collagen or fibronectin. Our results support a mechanism by which claudin-2 participates in homotypic interactions involving claudin-2 expressed on breast cancer cells and claudin-2 expressed on hepatocytes (Fig. 11). Thus, breast cancer cells seeding the liver survive the early steps of colonization through claudin-2-dependent interactions with extracellular matrix components, via integrin engagement, and through breast cancer cell-hepatocyte interactions that rely on claudin-2 homotypic interactions.
Several studies have demonstrated homo- and/or heterotypic interactions between members of the claudin family (6, 34, 40, 42). Claudin-2 was reported previously to interact with claudin-3 but not claudin-1 (11), and, in agreement with our results, claudin-2 was shown to form homotypic complexes (10). However, little is known about the molecular mechanisms that govern these interactions. For instance, the second extracellular loop was reported previously to play a fundamental role in claudin-5–claudin-5 homotypic interactions (34). In contrast, the use of claudin-3 and claudin-4 chimeras has revealed that heterotypic binding is mediated by interactions involving both extracellular loops (6). Finally, the first extracellular loop was shown to be sufficient to promote the formation of claudin-2–claudin-2 homotypic interactions (25). This is in agreement with our results that define the first extracellular loop of claudin-2 as an important determinant in the ability of breast cancer cells to adhere to hepatocytes in vitro and form liver metastases in vivo. We further demonstrate that a portion of claudin-2 is localized at the cell membrane and would be available to participate in claudin-2–claudin-2 homotypic interactions between breast cancer cells and hepatocytes.
Our studies raise the possibility of targeting claudin-2 during liver metastasis and define a region within claudin-2 to which therapeutic agents should be directed. Indeed, there has been a recent surge in interest surrounding claudins and the metastatic process (8). Claudin-3 and claudin-4 are overexpressed in a range of human epithelial cell-derived tumors, including breast, prostate, ovarian, and pancreatic cancers (4). Interestingly, these tight-junctional proteins are bound by the Clostridium perfringens enterotoxin (CPE), which interacts with the second extracellular loop of claudin-3 (9). Several studies have used the C-terminal fragment of CPE, fused with various cytotoxic drugs, to specifically kill tumor cells expressing claudin-3 and claudin-4 (16, 49, 50, 52). This approach has also been successfully employed to target chemotherapy-resistant ovarian carcinomas or to reduce the formation of B16 melanoma cell-derived lung metastases (4, 41). Based on these observations and our findings, we speculate that blocking peptides or neutralizing antibodies raised to the first extracellular loop of claudin-2 may be employed to limit the dissemination of cancer cells to the liver. Such therapeutic agents may find clinical utility not only in breast cancer but also in other liver-metastatic cancers, such as colorectal cancer.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the Goodman Cancer Research Centre and the Centre for Bone and Periodontal Research histology core facilities (McGill University) for routine histological services. We are grateful to James M. Anderson for kindly providing us with the claudin-4 and derived chimeric constructs, J. J. Lebrun for the HaCaT cell line, and J. Massagué for the HEK-293 and Mv1Lu cell lines. We thank members of the Siegel laboratory and Josie Ursini-Siegel for thoughtful discussions and critical reading of the manuscript.
This work was supported by a program project grant from the Terry Fox Foundation (grant number 17003 to P.M.S.). E.A. and M.C. received funding assistance from the Canadian Breast Cancer Foundation, Ontario Chapter, and a donation from the Tina and Her Angels of Hope Fund. S.T. acknowledges support from the McGill University Department of Medicine, F.D. was supported by a fellowship from the Défi Corporatif Canderel, and P.M.S. is a research scholar (junior II) of the Fonds de Recherche en Santé du Québec.
Footnotes
Published ahead of print 29 May 2012
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1. Aung PP, et al. 2006. Differential expression of claudin-2 in normal human tissues and gastrointestinal carcinomas. Virchows Arch. 448:428–434 [DOI] [PubMed] [Google Scholar]
- 2. Chao Y, Wu Q, Shepard C, Wells A. 2012. Hepatocyte induced re-expression of E-cadherin in breast and prostate cancer cells increases chemoresistance. Clin. Exp. Metastasis 29:39–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chao YL, Shepard CR, Wells A. 2010. Breast carcinoma cells re-express E-cadherin during mesenchymal to epithelial reverting transition. Mol. Cancer 9:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cocco E, et al. 2010. Clostridium perfringens enterotoxin carboxy-terminal fragment is a novel tumor-homing peptide for human ovarian cancer. BMC Cancer 10:349 doi:10.1186/1471-2407-10-349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Colegio OR, Van Itallie C, Rahner C, Anderson JM. 2003. Claudin extracellular domains determine paracellular charge selectivity and resistance but not tight junction fibril architecture. Am. J. Physiol. Cell Physiol. 284:C1346–C1354 [DOI] [PubMed] [Google Scholar]
- 6. Daugherty BL, Ward C, Smith T, Ritzenthaler JD, Koval M. 2007. Regulation of heterotypic claudin compatibility. J. Biol. Chem. 282:30005–30013 [DOI] [PubMed] [Google Scholar]
- 7. Dhawan P, et al. 2011. Claudin-2 expression increases tumorigenicity of colon cancer cells: role of epidermal growth factor receptor activation. Oncogene 30:3234–3247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Escudero-Esparza A, Jiang WG, Martin TA. 2011. The claudin family and its role in cancer and metastasis. Front. Biosci. 16:1069–1083 [DOI] [PubMed] [Google Scholar]
- 9. Fujita K, et al. 2000. Clostridium perfringens enterotoxin binds to the second extracellular loop of claudin-3, a tight junction integral membrane protein. FEBS Lett. 476:258–261 [DOI] [PubMed] [Google Scholar]
- 10. Furuse M, Furuse K, Sasaki H, Tsukita S. 2001. Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J. Cell Biol. 153:263–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Furuse M, Sasaki H, Tsukita S. 1999. Manner of interaction of heterogeneous claudin species within and between tight junction strands. J. Cell Biol. 147:891–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hess KR, et al. 2006. Metastatic patterns in adenocarcinoma. Cancer 106:1624–1633 [DOI] [PubMed] [Google Scholar]
- 13. Hugo H, et al. 2007. Epithelial-mesenchymal and mesenchymal-epithelial transitions in carcinoma progression. J. Cell. Physiol. 213:374–383 [DOI] [PubMed] [Google Scholar]
- 14. Joyce JA, Pollard JW. 2009. Microenvironmental regulation of metastasis. Nat. Rev. Cancer 9:239–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jung H, Jun KH, Jung JH, Chin HM, Park WB. 2011. The expression of claudin-1, claudin-2, claudin-3, and claudin-4 in gastric cancer tissue. J. Surg. Res. 167:e185–e191 [DOI] [PubMed] [Google Scholar]
- 16. Kakutani H, et al. 2010. Claudin-4-targeting of diphtheria toxin fragment A using a C-terminal fragment of Clostridium perfringens enterotoxin. Eur. J. Pharm. Biopharm. 75:213–217 [DOI] [PubMed] [Google Scholar]
- 17. Kemperman H, Driessens M, La Riviere G, Meijne AM, Roos E. 1994. The role of integrins and integrin activation in liver metastasis. Invasion Metastasis 14:98–108 [PubMed] [Google Scholar]
- 18. Kemperman H, Wijnands Y, de Rijk D, Roos E. 1993. The integrin alpha 6 beta 4 on TA3/Ha mammary carcinoma cells is involved in adhesion to hepatocytes. Cancer Res. 53:3611–3617 [PubMed] [Google Scholar]
- 19. Kemperman H, Wijnands Y, Meijne AM, Roos E. 1994. TA3/St, but not TA3/Ha, mammary carcinoma cell adhesion to hepatocytes is mediated by alpha 5 beta 1 interacting with surface-associated fibronectin. Cell Adhes. Commun. 2:45–58 [DOI] [PubMed] [Google Scholar]
- 20. Kim TH, et al. 2008. Down-regulation of claudin-2 in breast carcinomas is associated with advanced disease. Histopathology 53:48–55 [DOI] [PubMed] [Google Scholar]
- 21. Kinugasa T, et al. 2007. Selective up-regulation of claudin-1 and claudin-2 in colorectal cancer. Anticancer Res. 27:3729–3734 [PubMed] [Google Scholar]
- 22. Kojima T, Sawada N. 2011. Expression and function of claudins in hepatocytes. Methods Mol. Biol. 762:233–244 [DOI] [PubMed] [Google Scholar]
- 23. Lacerte A, et al. 2008. Transforming growth factor-beta inhibits telomerase through SMAD3 and E2F transcription factors. Cell. Signal. 20:50–59 [DOI] [PubMed] [Google Scholar]
- 24. Langley RR, Fidler IJ. 2011. The seed and soil hypothesis revisited—the role of tumor-stroma interactions in metastasis to different organs. Int. J. Cancer 128:2527–2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lim TS, Vedula SR, Hunziker W, Lim CT. 2008. Kinetics of adhesion mediated by extracellular loops of claudin-2 as revealed by single-molecule force spectroscopy. J. Mol. Biol. 381:681–691 [DOI] [PubMed] [Google Scholar]
- 26. Lopez-Rovira T, Chalaux E, Massague J, Rosa JL, Ventura F. 2002. Direct binding of Smad1 and Smad4 to two distinct motifs mediates bone morphogenetic protein-specific transcriptional activation of Id1 gene. J. Biol. Chem. 277:3176–3185 [DOI] [PubMed] [Google Scholar]
- 27. Marceau N, Gilbert S, Loranger A. 2004. Uncovering the roles of intermediate filaments in apoptosis. Methods Cell Biol. 78:95–129 [DOI] [PubMed] [Google Scholar]
- 28. Mathew J, Galarneau L, Loranger A, Gilbert S, Marceau N. 2008. Keratin-protein kinase C interaction in reactive oxygen species-induced hepatic cell death through mitochondrial signaling. Free Radic. Biol. Med. 45:413–424 [DOI] [PubMed] [Google Scholar]
- 29. Mego M, Mani SA, Cristofanilli M. 2010. Molecular mechanisms of metastasis in breast cancer—clinical applications. Nat. Rev. Clin. Oncol. 7:693–701 [DOI] [PubMed] [Google Scholar]
- 30. Mina LA, Sledge GW., Jr 2011. Rethinking the metastatic cascade as a therapeutic target. Nat. Rev. Clin. Oncol. 8:325–332 [DOI] [PubMed] [Google Scholar]
- 31. Mook OR, et al. 2008. Interactions between colon cancer cells and hepatocytes in rats in relation to metastasis. J. Cell. Mol. Med. 12:2052–2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mook OR, et al. 2003. Visualization of early events in tumor formation of eGFP-transfected rat colon cancer cells in liver. Hepatology 38:295–304 [DOI] [PubMed] [Google Scholar]
- 33. Patonai A, et al. 2011. Claudins and tricellulin in fibrolamellar hepatocellular carcinoma. Virchows Arch. 458:679–688 [DOI] [PubMed] [Google Scholar]
- 34. Piontek J, et al. 2008. Formation of tight junction: determinants of homophilic interaction between classic claudins. FASEB J. 22:146–158 [DOI] [PubMed] [Google Scholar]
- 35. Polyak K, Weinberg RA. 2009. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat. Rev. Cancer 9:265–273 [DOI] [PubMed] [Google Scholar]
- 36. Roos E, Dingemans KP, Van de Pavert IV, Van den Bergh-Weerman MA. 1978. Mammary-carcinoma cells in mouse liver: infiltration of liver tissue and interaction with Kupffer cells. Br. J. Cancer 38:88–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Roos E, Van de Pavert IV, Middelkoop OP. 1981. Infiltration of tumour cells into cultures of isolated hepatocytes. J. Cell Sci. 47:385–397 [DOI] [PubMed] [Google Scholar]
- 38. Rose AA, et al. 2010. ADAM10 releases a soluble form of the GPNMB/Osteoactivin extracellular domain with angiogenic properties. PLoS One 5:e12093 doi:10.1371/journal.pone.0012093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rose AA, et al. 2007. Osteoactivin promotes breast cancer metastasis to bone. Mol. Cancer Res. 5:1001–1014 [DOI] [PubMed] [Google Scholar]
- 40. Rosenthal R, et al. 2010. Claudin-2, a component of the tight junction, forms a paracellular water channel. J. Cell Sci. 123:1913–1921 [DOI] [PubMed] [Google Scholar]
- 41. Saeki R, et al. 2010. A claudin-targeting molecule as an inhibitor of tumor metastasis. J. Pharmacol. Exp. Ther. 334:576–582 [DOI] [PubMed] [Google Scholar]
- 42. Sasaki H, et al. 2003. Dynamic behavior of paired claudin strands within apposing plasma membranes. Proc. Natl. Acad. Sci. U. S. A. 100:3971–3976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Soini Y. 2004. Claudins 2, 3, 4, and 5 in Paget's disease and breast carcinoma. Hum. Pathol. 35:1531–1536 [DOI] [PubMed] [Google Scholar]
- 44. Szasz AM, et al. 2011. Prognostic significance of claudin expression changes in breast cancer with regional lymph node metastasis. Clin. Exp. Metastasis 28:55–63 [DOI] [PubMed] [Google Scholar]
- 45. Tabariès S, et al. 2011. Claudin-2 is selectively enriched in and promotes the formation of breast cancer liver metastases through engagement of integrin complexes. Oncogene 30:1318–1328 [DOI] [PubMed] [Google Scholar]
- 46. Thiery JP, Acloque H, Huang RY, Nieto MA. 2009. Epithelial-mesenchymal transitions in development and disease. Cell 139:871–890 [DOI] [PubMed] [Google Scholar]
- 47. Turksen K, Troy TC. 2004. Barriers built on claudins. J. Cell Sci. 117:2435–2447 [DOI] [PubMed] [Google Scholar]
- 48. Van Itallie CM, Anderson JM. 2006. Claudins and epithelial paracellular transport. Annu. Rev. Physiol. 68:403–429 [DOI] [PubMed] [Google Scholar]
- 49. Yamaguchi H, et al. 2011. Effects of Clostridium perfringens enterotoxin via claudin-4 on normal human pancreatic duct epithelial cells and cancer cells. Cell. Mol. Biol. Lett 16:385–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yao Q, et al. 2010. Turn a diarrhoea toxin into a receptor-mediated therapy for a plethora of CLDN-4-overexpressing cancers. Biochem. Biophys. Res. Commun. 398:413–419 [DOI] [PubMed] [Google Scholar]
- 51. Yates CC, Shepard CR, Stolz DB, Wells A. 2007. Co-culturing human prostate carcinoma cells with hepatocytes leads to increased expression of E-cadherin. Br. J. Cancer 96:1246–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yuan X, et al. 2009. Recombinant CPE fused to tumor necrosis factor targets human ovarian cancer cells expressing the claudin-3 and claudin-4 receptors. Mol. Cancer Ther. 8:1906–1915 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.