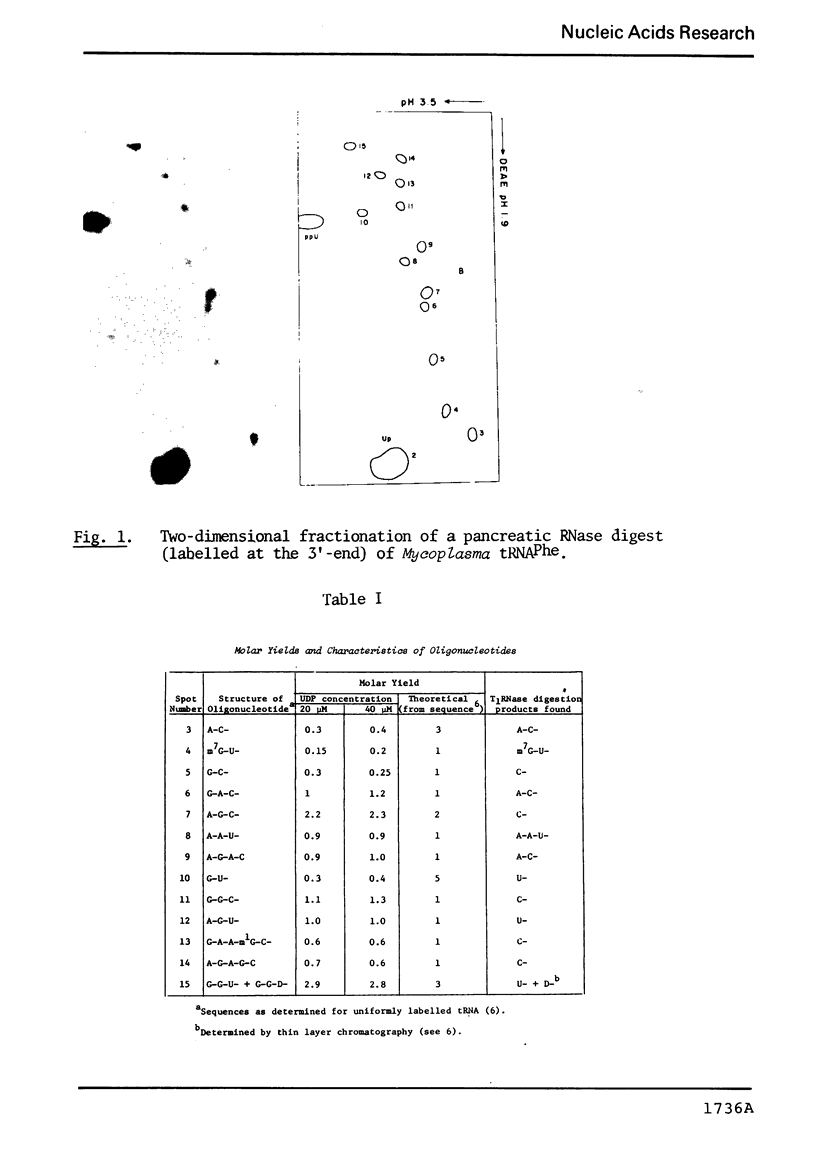
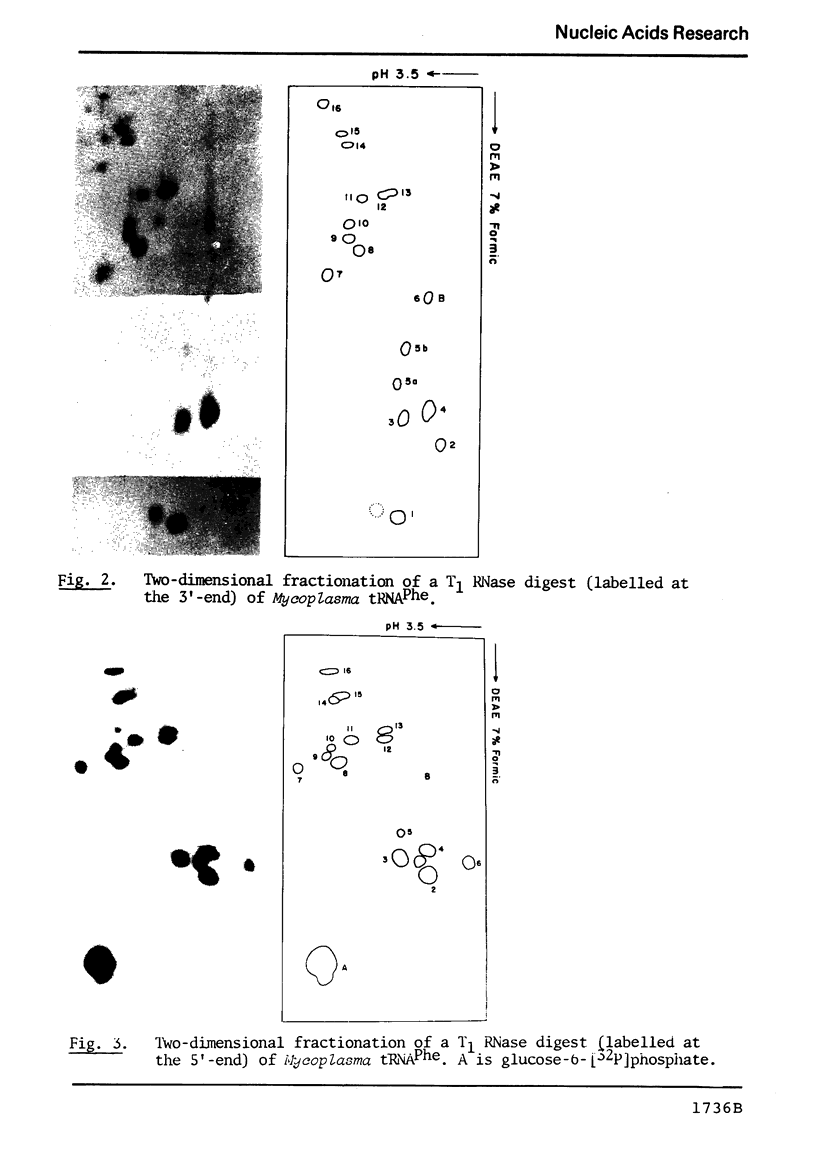
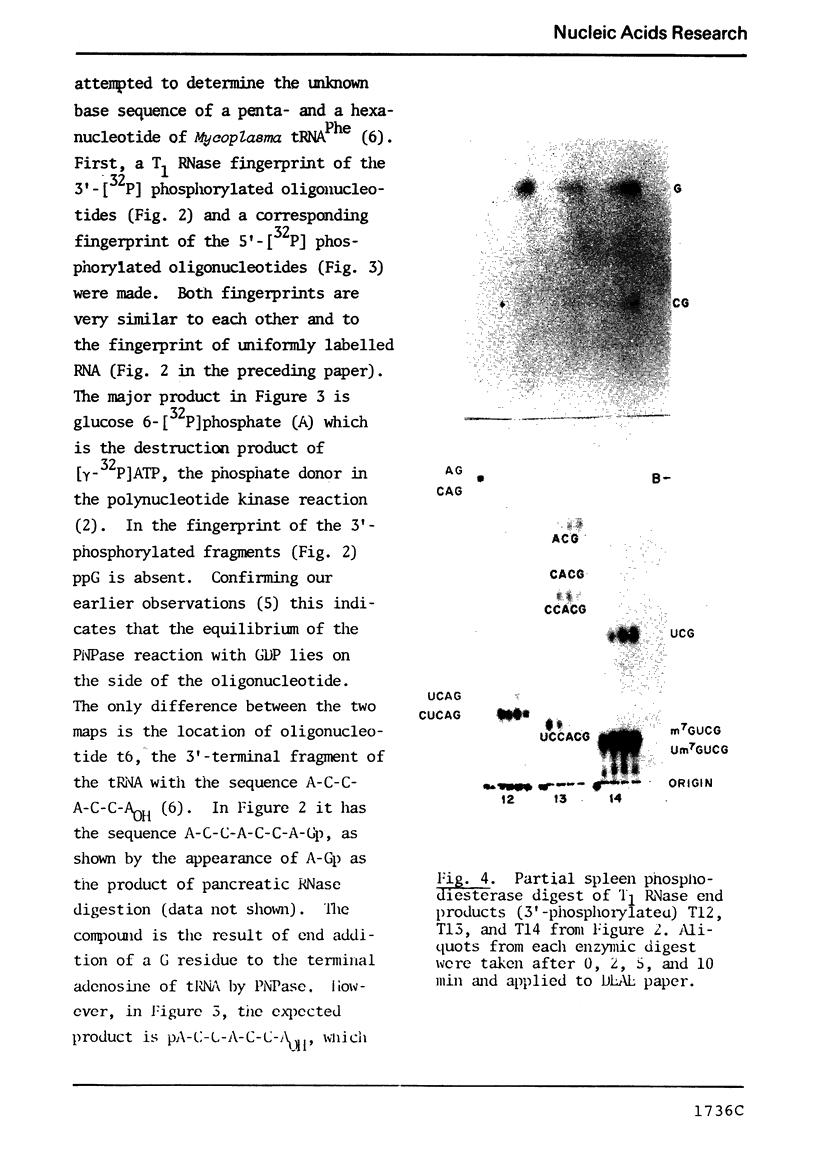
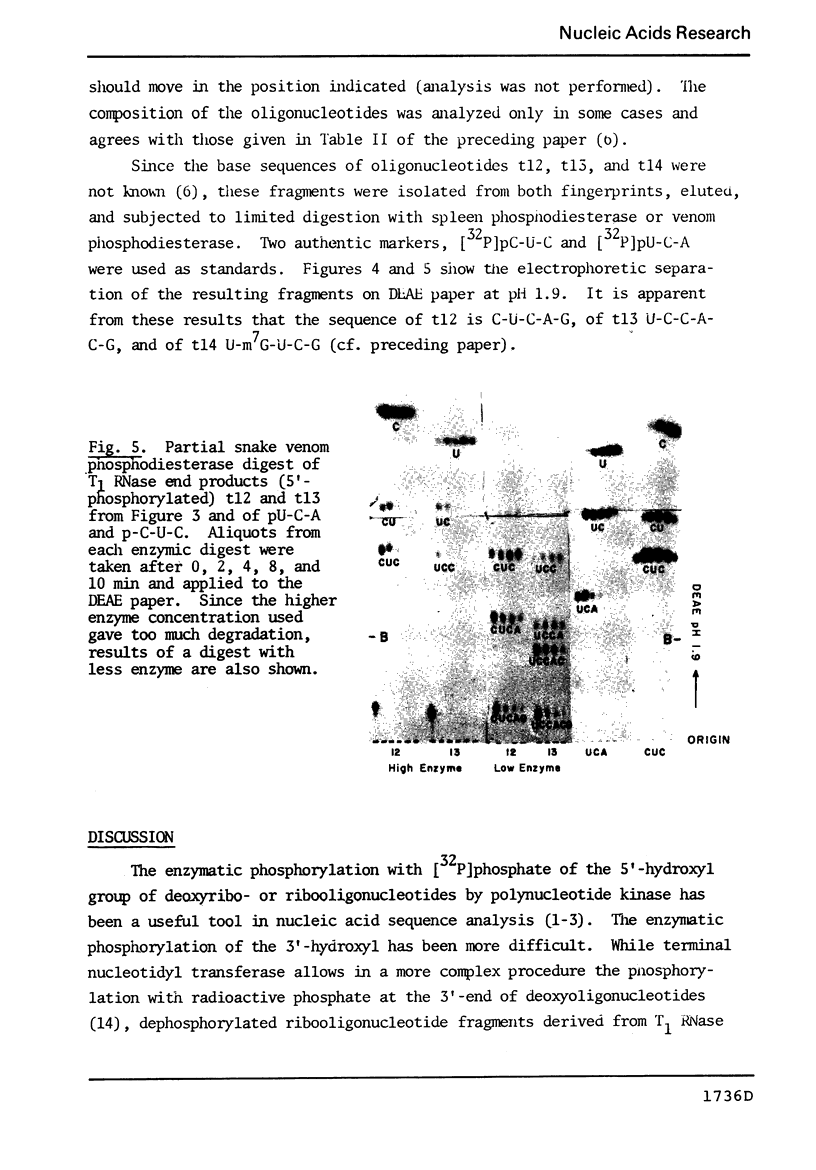
Abstract
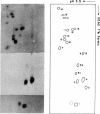
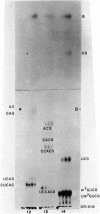
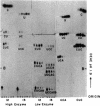
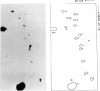
The known methods of enzymatic phosphorylation with [32P]phosphate of the 3′- or 5′-hydroxyl group of an oligonucleotide have been applied to oligonucleotides derived from Mycoplasma tRNAPhe. The fingerprints obtained by both methods are very similar to each other and to that of uniformly labelled tRNA. The sequence of some oligonucleotides was determined by partial digestion of the 3′-phosphorylated fragment with spleen phosphodiesterase and of the corresponding 5′-phosphorylated fragment with venom phosphodiesterase.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Kimball M. E., Soll D. The phenylalanine tRNA from Mycoplasma sp. (Kid): a tRNA lacking hypermodified nucleosides functional in protein synthesis. Nucleic Acids Res. 1974 Dec;1(12):1713–1720. [PMC free article] [PubMed] [Google Scholar]
- Kimball M. E., Szeto K. S., Soll D. The nucleotide sequence of phenylalanine tRNA from Mycoplasma sp. (Kid). Nucleic Acids Res. 1974 Dec;1(12):1721–1732. [PMC free article] [PubMed] [Google Scholar]
- Kössel H., Roychoudhury R. Synthetic polynucleotides. Ther terminal addition of riboadenylic acid to deoxyoligonucleotides by terminal deoxynucleotidyl transferase as a tool for the specific labelling of deoxyoligonucleotides at the 3'-ends. Eur J Biochem. 1971 Sep 24;22(2):271–276. doi: 10.1111/j.1432-1033.1971.tb01541.x. [DOI] [PubMed] [Google Scholar]
- Lohrmann R., Söll D., Hayatsu H., Ohtsuka E., Khorana H. G. Studies on polynucleotides. LI. Syntheses of the 64 possible ribotrinucleotides derived from the four major ribomononucleotides. J Am Chem Soc. 1966 Feb 20;88(4):819–829. doi: 10.1021/ja00956a039. [DOI] [PubMed] [Google Scholar]
- Randerath K., Randerath E., Chia L. S., Gupta R. C., Sivarajan M. Sequence analysis of nonradioactive RNA fragments by periodate-phosphatase digestion and chemical tritium labeling: characterization of large oligonucleotides and oligonucleotides containing modified nucleosides. Nucleic Acids Res. 1974 Sep;1(9):1121–1141. doi: 10.1093/nar/1.9.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINGER M. F., HEPPEL L. A., HILMOE R. J. Oligonucleotides as primers for polynucleotide phosphorylase. J Biol Chem. 1960 Mar;235:738–750. [PubMed] [Google Scholar]
- Sanger F., Brownlee G. G., Barrell B. G. A two-dimensional fractionation procedure for radioactive nucleotides. J Mol Biol. 1965 Sep;13(2):373–398. doi: 10.1016/s0022-2836(65)80104-8. [DOI] [PubMed] [Google Scholar]
- Simsek M., RajBhandary U. L., Boisnard M., Petrissant G. Nucleotide sequence of rabbit liver and sheep mammary gland cytoplasmic initiatory transfer RNAs. Nature. 1974 Feb 22;247(5442):518–520. doi: 10.1038/247518a0. [DOI] [PubMed] [Google Scholar]
- Simsek M., Ziegenmeyer J., Heckman J., Rajbhandary U. L. Absence of the sequence G-T-psi-C-G(A)- in several eukaryotic cytoplasmic initiator transfer RNAs. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1041–1045. doi: 10.1073/pnas.70.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto K. S., Söll D. Fingerprinting nonradioactive ribonucleic acid with the aid of polynucleotide phosphorylase. Nucleic Acids Res. 1974 Jan;1(1):171–181. doi: 10.1093/nar/1.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Székely M., Sanger F. Use of polynucleotide kinase in fingerprinting non-radioactive nucleic acids. J Mol Biol. 1969 Aug 14;43(3):607–617. doi: 10.1016/0022-2836(69)90362-3. [DOI] [PubMed] [Google Scholar]