Abstract
The transcription factor HNF4α (hepatocyte nuclear factor-4α) is required for increased β-cell proliferation during metabolic stress in vivo. We hypothesized that HNF4α could induce proliferation of human β-cells. We employed adenoviral-mediated overexpression of an isoform of HNF4α (HNF4α8) alone, or in combination with cyclin-dependent kinase (Cdk)6 and Cyclin D3, in human islets. Heightened HNF4α8 expression led to a 300-fold increase in the number of β-cells in early S-phase. When we overexpressed HNF4α8 together with Cdk6 and Cyclin D3, β-cell cycle entry was increased even further. However, the punctate manner of bromodeoxyuridine incorporation into HNF4αHigh β-cells indicated an uncoupling of the mechanisms that control the concise timing and execution of each cell cycle phase. Indeed, in HNF4α8-induced bromodeoxyuridine+,punctate β-cells we observed signs of dysregulated DNA synthesis, cell cycle arrest, and activation of a double stranded DNA damage-associated cell cycle checkpoint mechanism, leading to the initiation of loss of β-cell lineage fidelity. However, a substantial proportion of β-cells stimulated to enter the cell cycle by Cdk6 and Cyclin D3 alone also exhibited a DNA damage response. HNF4α8 is a mitogenic signal in the human β-cell but is not sufficient for completion of the cell cycle. The DNA damage response is a barrier to efficient β-cell proliferation in vitro, and we suggest its evaluation in all attempts to stimulate β-cell replication as an approach to diabetes treatment.
Since the first successful transplantation of cadaveric islets into brittle type 1 diabetic patients using a glucocorticoid-free immunosuppressive regiment resulted in temporary insulin independence (1), there has been much interest in the basic mechanisms and factors required to obtain fully mature β-cells in vitro, because the demand for transplants far exceeds islets supply. Approaches toward this therapeutic goal include the replication of preexisting β-cells (2). To date, an efficient protocol to drive efficient nononcogenic proliferation of human β-cells in vitro remains elusive.
Because nearly all adult human β-cells reside in G0-phase (3), the promotion of β-cell proliferation needs to accomplish 1) cell cycle entry and licensing of replication origins (G1-phase); 2) initiation of DNA replication at licensed origins resulting in genome duplication (S-phase); and 3) progression through the remaining cell cycle phases (G2- and M-phase). Thus far, only overexpression of the winged helix transcription factor FoxM1 (4), or combinations of a G1/S-phase specific cyclin-dependent kinase (Cdk) with its D-type cyclin partner have shown progression through multiple phases of the cell cycle in adult human β-cells, with Cdk6 overexpression translating to continued β-cell replication in the face of hyperglycemia in vivo (5). In addition, even if a factor is sufficient to induce progression through one complete cell cycle, protection of the newly formed β-cells from apoptosis must also be achieved (6).
One potential sufficiency factor is hepatocyte nuclear factor (HNF) 4α (mutated in MODY1), because its transcriptional activity is required for the physiological increase of β-cell replication during murine pregnancy, and glucose stimulated insulin secretion of the β-cell in nonstressful metabolic conditions in mice (7, 8). Both its membership in the nuclear hormone receptor family and the dissimilar expression pattern of specific isoforms in various tissues suggest HNF4α as a potential drug target (9, 10). In the current study, we overexpress an isoform of HNF4α physiologically expressed in the pancreas (HNF4α8) specifically in human β-cells and demonstrate that HNF4α8-overexpression alone and in combination with other mitogenic factors is sufficient for cell cycle entry. We further demonstrate that the previously unacknowledged analysis of the DNA damage response is critical for the evaluation of mitogenic signals in human β-cells.
Results
HNF4αHigh β-cells incorporate bromodeoxyuridine (BrdU) in a punctate, not diffuse, manner
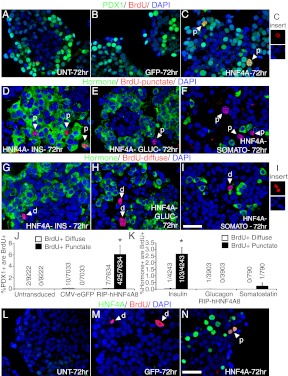
Immunofluorescent analysis of adult human cadaveric islets was performed to confirm expression of HNF4α in human β-cells. HNF4α was detected in β-cells, albeit at low and variable levels between individual β-cells (Supplemental Fig. 1A published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). We next investigated whether enhanced transcriptional activity of HNF4α is sufficient to drive human β-cell replication, normally very low as measured upon receipt of the islets (0.05% ± 0.04 of Pdx1+ cells were Ki67+) (Supplemental Fig. 1, B–D). We used a non-replication-competent adenovirus containing the rat insulin promoter to overexpress HNF4α8 specifically in human β-cells (designated as HNF4αHigh relative to endogenous expression; Supplemental Fig. 1, E and F). Subsequent figures portraying HNF4α-expression in isolated human islets depict only HNF4αHigh levels. Approximately 35% of human β-cells expressed high HNF4α protein levels (Supplemental Fig. 1, G–K), a transduction efficiency similar to that of AdCMV (cytomegalovirus)-eGFP (enhanced green fluorescent protein) (Supplemental Fig. 1, L and M). To determine whether HNF4α8 overexpression led to increased β-cell proliferation, we added BrdU continuously for 72 h after adenoviral transduction. No appreciable BrdU incorporation of any kind occurred into the Pdx1+ population in both untransduced and AdCMV-eGFP transduced islets (Fig. 1, A, B, and J). However, overexpression of HNF4α8 caused a dramatic increase in BrdU incorporation within the β-cell population, because 6.2% ± 1.6 of Pdx1+ cells colocalized with BrdU 72 h after transduction (Fig. 1, C and J). Surprisingly, closer examination shows that all of BrdU incorporation into Pdx1+, insulin+ cells occurred in distinct punctate domains with reduced 4′,6-diamidino-2-phenylindole (DAPI) signal [Fig. 1C (inset), and panels D–F and J and K]. In contrast, diffuse BrdU incorporation throughout the nucleus was readily detectable in all experimental conditions but only present in nonendocrine islet cells (or defined here as negative for insulin, glucagon, somatostatin expression) [Fig. 1, G–I, I (inset), and panels J and K]. Furthermore, all BrdU+,punctate, but not BrdU+,diffuse, cells were part of the HNF4αHigh population (Fig. 1, L–O). Of the entire HNF4αHigh population 12 ± 3.0% incorporated BrdU in a punctate manner, and this was similar in islets isolated from type 2 diabetic donors (9.3 ± 2.9%) (Fig. 1P). Thus, a detectable fraction of β-cells overexpressing HNF4α8 entered S-phase, similar to what had been reported for single factor overexpression of Cyclin D1, Cyclin D2, Cdk6, and cMyc, and low-dose combinatorial overexpression of Cdk6/Cyclin D1 (5, 11–13).
Fig. 1.
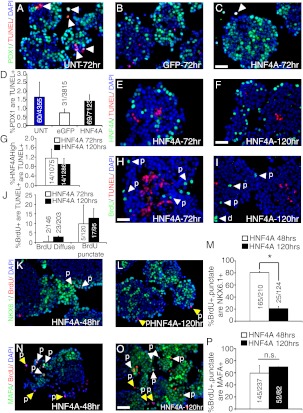
HNF4αHigh β-cells incorporate BrdU in a punctate, not diffuse, manner. Immunofluorescence staining of Pdx1 (green), BrdU (red), and DAPI (blue) in primary human islets (A) not transduced with adenovirus, (B) transduced with AdCMV-eGFP, and (C) transduced with AdRIP-hHNF4α8. C (inset), An example of a nucleus exhibiting punctate BrdU incorporation (red) overlapping with DAPI-dimished regions (blue). Immunofluorescence staining of punctate BrdU incorporation (red) and DAPI (blue) with (D) insulin (green), (E) glucagon (green), and (F) somatostatin (green) cells in primary human islets overexpressing HNF4α8. Similarly, detection of diffuse BrdU incorporation (red) and DAPI (blue) with (G) insulin (green), (H) glucagon (green), and (I) somatostatin (green) cells in AdRIP-hHNF4α8-transduced human islets. I (inset), An example of two DAPI-stained (blue) nuclei showing diffuse BrdU incorporation (red). J, Quantification of the percentage of Pdx1+ cells that are either BrdU+,diffuse or BrdU+,punctate in untransduced, AdCMV-eGFP, and AdRIP-hHNF4α8-transduced islets (HNF4α8, BrdU+,punctate group is statistically significantly higher compared with all other groups; *, P < 0.001 as determined by one-way ANOVA with Tukey post hoc test; n = 3 for each group). K, Quantification of the percentage of insulin+, glucagon+, or somataostatin+ cells that are either BrdU+,diffuse or BrdU+,punctate in AdRIP-hHNF4α8-transduced islets (insulin+, BrdU+,punctate group is statistically significantly higher compared with all other groups; *, P < 0.001 as determined by one-tailed ANOVA with Tukey post hoc test; n = 3–4 for each hormone+ population). Detection of HNF4α (green), BrdU (red), and DAPI (blue) in primary human islets (L) not transduced with adenovirus, (M) transduced with AdCMV-eGFP, and (N) transduced with AdRIP-hHNF4α8. O, Quantification of the percentage of either BrdU+,diffuse or BrdU+,punctate cells that are HNF4αHigh in AdRIP-hHNF4α8-transduced islets. (*, P < 0.001; n = 4 each BrdU incorporation pattern). P, Quantification of the percentage of HNF4αHigh cells are BrdU+,punctate in nondiabetic or type 2 diabetic human islet donations transduced with in AdRIP-hHNF4α8 (n = 3–4 for each diabetic status). All primary human islets were harvested 72 h after transduction. White arrows indicate either diffuse (d) or punctate (p) BrdU incorporation. The scale bar in panels I and N indicates 25 μm. GIUC, glucagon; INS, insulin; SOMATO, somatostatin. n.s., P = 0.28.
HNF4αHigh β-cells do not progress through the cell cycle
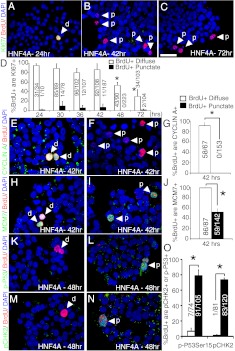
To investigate whether HNF4α8 overexpression is sufficient for cell cycle progression in β-cells, we analyzed Ki67 expression. Surprisingly, 72 h after transduction, HNF4αHigh Pdx1+ cells were not Ki67+ (0.06 ± 0.03% of Pdx1+ cells were Ki67+) similar to the situation in untransduced or AdCMV-eGFP treated controls (Supplemental Fig. 2, A–D). We considered the possibility that HNF4α8-induced proliferation was completed before the 72 h, and collected HNF4α8-overexpressing islets exposed to continuous BrdU at various time points after transduction (24, 30, 36, 42, and 48 h). The BrdU incorporation rate into HNF4αHigh β-cells was negligible until 36 h after transduction and increased until 48 h, with no further increase at 72 h, suggesting that entry into S-phase occurred between 36 and before 48 h (Supplemental Fig. 2, E–H). Strikingly, HNF4αHigh β-cells did not express Ki67 at any time point (Supplemental Fig. 2, I–L). Thus, although a subset of HNF4αHigh β-cells attempt S-phase, the lack of Ki67 expression suggests activation of a cell cycle arrest mechanism.
We hypothesized that the manner of BrdU incorporation can demarcate cell populations with different DNA replication kinetics. Within the HNF4α8-transduced islet, BrdU-incorporation patterns distinguished nonendocrine islet cells (marked by BrdU+,diffuse cells) and HNF4αHigh cells induced to enter S-phase (marked by BrdU+,punctate cells), as mentioned above. Thus, we evaluated both diffuse and punctate BrdU-positive populations with various cell cycle markers to discern differences in cell cycle progression (cell marker validation is shown in Supplemental Fig. 2, M–O). The majority of cells that incorporated BrdU diffusely expressed Ki67 at 24, 30, 36, and 42 h at a high percentage, but not at 48 and 72 h, indicating exit from the cell cycle. In contrast, cells that incorporated BrdU in a punctate manner expressed Ki67 at a very low frequency (Fig. 2, A–D). The rare cells exhibiting both BrdU+,punctate and Ki67 expression did not show characteristically large BrdU domains overlapping with reduced regions of DAPI signal, and their pattern of BrdU incorporation most likely reflected incomplete S-phase progression of nonendocrine islet cells at the time of islet harvest (14, 15). Furthermore, at the 42-h time point, HNF4α8 overexpression never induced the expression of Cyclin A in BrdU+,punctate cells, a marker of S-phase, G2-phase, and early M-phase (16), whereas BrdU+,diffuse cells were Cyclin A positive (Fig. 2, E–G). Because of Ki67 and Cyclin A expression at the time when some HNF4αHigh cells accumulated BrdU, we asked whether the BrdU punctate incorporation pattern was the result of cell cycle entry. We used minichromosome maintenance 7 (Mcm7), a DNA replication licensing factor whose expression is activated in early G1-phase, when Ki67 is not expressed (17), to assess initiation of cell cycle progression. The majority (52 ± 6.3%) of BrdU+,punctate singlets indeed expressed Mcm7 at 42 h after transduction, similar to their BrdU+,diffuse cellular counterparts (Fig. 2, H–J). Together, these data suggested that unlike BrdU+,diffuse cells, which successfully progress through the cell cycle, BrdU+,punctate cells enter the cell cycle, attempt S-phase and arrest.
Fig. 2.
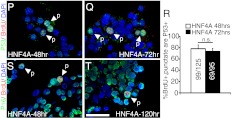
HNF4αHigh β-cells arrest in the cell cycle. Analysis of Ki67 (green), BrdU (red), and DAPI (blue) cells in primary human islets at (A) 24 h, (B) 42 h, and (C) 72 h after transduction with AdRIP-hHNF4α8. D, Quantification of the percentage of BrdU+,Diffuse or BrdU+,Punctate that are Ki67+ at the 24-, 30-, 36-, 42-, 48-, and 72-h time point (72 h, BrdU+,diffuse group is statistically significantly lower then 24, 30, 36, 42 h, BrdU+,diffuse groups, *, P < 0.01; 48 h, BrdU+,diffuse groups are statistically lower then 24-h BrdU+,diffuse group; *, P < 0.01; both determined by one-way ANOVA with Tukey post hoc test; n = 3–5 for each type of BrdU incorporation per time point). E and F, Immunostaining of Cyclin A (green) with BrdU (red) in human islets 42 h after transduction with AdRIP-hHNF4α8. G, Quantification of the percentage of BrdU+,Diffuse or BrdU+,Punctate that are Cyclin A+ (*, P < 0.01; n = 3 per type of BrdU incorporation). H and I, Immunostaining of Mcm7 (green) with BrdU (red) in human islets 42 h after transduction with AdRIP-hHNF4α8. J, Quantification of the percentage of BrdU+,Diffuse or BrdU+,Punctate that are Mcm7+ (*, P < 0.01; n = 3 per type of BrdU incorporation). K and L, Immunostaining of phospho-p53(ser15) (green) and BrdU (red), and (M and N) phospho-Chk2 (green) and BrdU (red) in human islets 48 h after transduction with AdRIP-hHNF4α8. O, Quantification of the percentage of BrdU+,Diffuse or BrdU+,Punctate that are either phospho-p53(Ser15)+ or phospho-Chk2+ (*, P < 0.005; n = 3 per type of BrdU incorporation and protein examined). Immunofluorescence of p53 (green) and BrdU (red) in human islets (P) 48 h and (Q) 72 h after transduction with AdRIP-hHNF4α8. R, Quantification of the percentage of BrdU+,punctate cells that are p53+ at 48-h and 72-h time points (n = 3 per time point). Immunostaining of p16 (green) and BrdU (red) in human islets (S) 48 h and (T) 120 h after transduction with AdRIP-hHNF4α8. White arrows indicate either diffuse (d) or punctate (p) BrdU incorporation. The scale bars in panels C and T indicate 25 μm. n.s., P = 0.30.
To assess whether HNF4αHigh β-cells activate cell cycle checkpoints, we used phospho-Chk2 (Thr68) and phospho-p53(Ser15) staining, and the expression of tumor suppressors p16INK4a and p53, known to contribute to cell cycle arrest in β-cells (18, 19). Expression of both checkpoint markers phospho-Chk2(Thr68) and phospho-p53(Ser15) was present in BrdU+,punctate cells (73.4% ± 2.2 and 78.7% ± 7.5, respectively), but not in BrdU+,diffuse cells 48 h after transduction (Fig. 2, K–O). p53 expression was present on the protein level in AdRIP-HNF4α8 treated islets as indicated by Western blot analysis (Supplemental Fig. 2P). When quantified by immunostaining, p53 was easily detectable in BrdU+,punctate cells at 48 and 72 h after transduction, with 78.2% ± 6.6 and 73.3% ± 5.1 staining positive, respectively (Fig. 2, P-R). We also found p16INK4a expression in BrdU+,punctate cells at 48 and 120 h after transduction (Fig. 2, S and T). Thus, although HNF4α8 was sufficient to stimulate a percentage of transduced β-cells to enter the cell cycle, it also activated a checkpoint response. The activation of tumor suppressor genes indicated a failure to complete the cell cycle by the HNF4αHigh, BrdU+,punctate population.
Overexpression of HNF4α8 leads to activation of the DNA damage response associated with replication stress
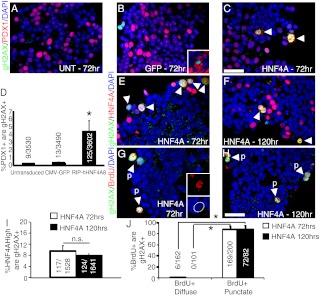
The punctate manner of BrdU incorporation into HNF4α8-overexpressing β-cells is reminiscent of the patterns of DNA replication seen during S-phase in mammalian nuclei (15, 20). Although BrdU+,punctate cells are able to license their DNA for replication, as demonstrated by increased Mcm7 expression, the appearance of large punctate domains points to misregulation of the licensing system, allowing cells to inappropriately relicense their already replicated DNA (14). Phosphorylation of Chk2 on threonine 68 and p53 on serine 15 are downstream substrates of Ataxia telangiectasia mutated, a key mediator of the DNA damage response, and these modifications ultimately lead to the stabilization and subsequent accumulation of p53 protein (21, 22). We hypothesized that overexpression of HNF4α8 caused repeated licensing of DNA replication from single origins (or rereplication), resulting in enlarged punctate foci, double-stranded DNA breaks (defined here as replication stress), and induction of the DNA damage response. Therefore, we assessed the effects of HNF4α8 overexpression on phosphorylation of histone H2AX (γH2AX), an indicator of DNA double-stranded breaks (23). Indeed, overexpression of HNF4α8 caused a dramatic increase in the number of γH2AX+ Pdx1+ cells, with 4.4% ± 1.4 of Pdx1+ cells colocalizing with γH2AX 72 h after transduction, whereas less than 0.5% of Pdx1+ cells stained for γH2AX in untransduced or CMV-eGFP-treated controls (Fig. 3, A–D). In AdCMV-eGFP transduced islets, γH2AX colocalized with cells positive for green fluorescent protein (GFP), reflecting rare toxicity of this adenovirus to islet cells (Fig. 3B, inset). The accumulation of γH2AX+ in Pdx1+ cells mirrored the accrual of BrdU+,punctate in Pdx1+ cells, demonstrating that the DNA damage response was restricted to this cell population (Fig. 3D). Indeed, γH2AX expression was present in 9.50% ± 1.97 HNF4αHigh cells and 88.1% ± 5.6 of BrdU+,punctate cells at 72 h after transduction and was sustained for up to 120 h (Fig. 3, E–J). No significant number of BrdU+,diffuse cells expressed γH2AX during either time point (Fig. 3J). Many HNF4αHigh BrdU+,punctate cells showed enlarged, dysmorphic nuclei not present in control islets (Fig. 3G, inset), suggesting that HNF4α8-overexpression in β-cells stimulated double-stranded DNA damage that was not repaired over time, leading to a sustained DNA damage response.
Fig. 3.
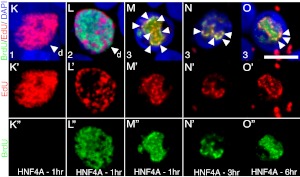
Overexpression of HNF4α8 leads to activation of the DNA damage response associated with replication stress. Immunodetection of γH2AX (green), Pdx1 (red), and DAPI (blue) in untransduced (panel A), GFP- (panel B), and HNF4α8-overexpressing (panel C) primary human islets at 72 h after transduction. The inset in panel B shows GFP (red), γH2AX (green), and DAPI (blue) colocalization. D, Quantification of the percentage of Pdx1+ cells that are γH2AX+ in all experimental conditions (AdRIP-hHNF4α8 group is statistically significantly higher then untransduced and AdCMV-eGFP groups; *, P < 0.03 as determined by one-way ANOVA with Tukey post hoc test; n = 3 for each group). Immunolocalization of γH2AX (green), and both (panels E and F) HNF4α (red) and (panels G and H) BrdU (red) in HNF4α8-tranduced primary human islets at (panels E and G) 72 h and (panels F and H) 120 h after transduction. G (inset), Example of a BrdU+,punctate cell with enlarged nucleus at 72-h time point. I, Quantification of the percentage of HNF4αHigh cells that are γH2AX+ at 72 and 120 h after AdRIP-hHNF4α8 transduction (n = 3–4 per time point). J, Quantification of the percentage of either BrdU+,diffuse or BrdU+,punctate cells that are γH2AX+ at 72 h and 120 h (n = 3; *, P < 0.001 vs. BrdU+,Diffuse condition for each time point). Simultaneous immunodetection of EdU (red) and BrdU (green) in HNF4α8-transduced islets of (K) single thymidine analog-labeled diffuse nucleus with 1-h nonlabeling time, (L) dual labeled noncolocalizing diffuse nucleus with 1-h labeling time, dual-labeled colocalizing punctate nucleus with (M) 1 h, (N) 3 h, and (O) 6 h nonlabeling times. Individual red channels (K′–O′), and green channels (K″–O″) are shown. The white arrows indicate dual-labeled replication foci. The scale bars in panels C and H indicate 25 μm and in panel O indicate 5 μm, respectively. n.s., P = 0.29.
To assess whether DNA damage was indeed associated with rereplication, we pulse labeled HNF4α8 transduced islets between 36 and 48 h, the established time of thymidine incorporation, sequentially with BrdU and EdU (5-ethynyl-2′-deoxyuridine) (Supplemental Fig. 3A). We confirmed absence of cross-reactivity between the two thymidine analogs by labeling HNF4α8-transduced islets with either BrdU-only or EdU-only, and using detection methods for both thymidine analogs simultaneously (Supplemental Fig. 3, B–C”). To demonstrate occurrence of rereplication, we varied the nonlabeling times between pulses (1, 3, and 6 h). Rereplication is defined here as the incorporation of both BrdU and EdU into the same locus. Several patterns of thymidine analog incorporation were observed. In HNF4α8-transduced islets with a 1-h nonlabeling interval, thymidine-analog+ cells incorporated a single analog in a diffuse manner (possibility no. 1), undergoing S-phase during the first or second labeling period (Fig. 3, K–K”). Thymidine-analog+ cells also incorporated both analogs in mutually exclusive areas in a diffuse manner (possibility no. 2), representing a cell undergoing proper S-phase during both labeling periods (Fig. 3, L–L”). The percentages of cells with diffuse thymidine analog incorporation integrating only one analog increased with increased nonlabeling interval, as expected (Supplemental Fig. 3D). Strikingly, in the thymidine-analog+,punctate domains, we saw overlapping incorporation of both EdU and BrdU into at least one focus per nucleus, regardless of the length of the nonlabeling interval (possibility no. 3), demonstrating rereplication (Fig. 3, M–O”). 87.9% ± 7.5, 89.8% ± 6.9, and 83.3% ± 8.4 of all thymidine-analog+,punctate cells showed rereplication with a 1-, 3-, and 6-h pulse, respectively (Supplemental Fig. 3E). These data suggest that replication stress prompted an uncoupling of the mechanisms that controls the replication timing program during S-phase, leading to both DNA damage and subsequent activation of the checkpoint response exhibited in HNF4αHigh BrdU+,punctate β-cells.
Partial loss of lineage fidelity, independent of apoptosis, in β-cells overexpressing HNF4α8
Once expressed, p53 functions as an integrator of diverse stress signals into different cellular outcomes, including cell cycle arrest, senescence, DNA repair, and apoptosis (24, 25). Sustained mitogenic signaling in the β-cell can cause apoptosis (26). We used the TUNEL assay to determine whether HNF4αHigh BrdU+,punctate β-cells succumb to cell death. Pdx1+ cells rarely exhibited TUNEL staining in untransduced, eGFP-overexpresssing, and even HNF4α8-overexpressing islets 72 h after transduction (Fig. 4, A–D). The percentage of HNF4αHigh cells that were TUNEL+ was only 1.2% ± 0.4 at 72 h and 1.1% ± 0.1 at 120 h after transduction (Fig. 4, E–G). Whereas there was a very low level of BrdU+,diffuse cells that were TUNEL+, the percentage of BrdU+,punctate cells that were TUNEL+ was only 9.8% ± 5.0, and 12.7% ± 9.9 at 72 and 120 h after transduction, respectively (Fig. 4, H–J), demonstrating that apoptosis was not the predominant fate of HNF4αHigh BrdU+,punctate β-cells.
Fig. 4.
Partial loss of lineage fidelity is the predominant fate of β-cells overexpressing HNF4α8. Immunofluorescence analysis of Pdx1 (green), TUNEL (red), and DAPI (blue) in (A) untransduced, (B) AdCMV-eGFP-, and (C) AdRIP-hHNF4α8-transduced islets at 72-h time point. D, Quantification of the percentage of Pdx1+ cells that are TUNEL+ in each experimental condition 72 h after transduction (n = 3–4 for each group). Immunostaining for HNF4α (green), TUNEL (red), and DAPI (blue) in AdRIP-hHNF4α8-transduced islets at (E) 72-h, and (F) 120-h time points. G, Quantification of the percentage of HNF4High cells that are Tunel+ at 72 and 120 h after transduction (n = 3 per time point). Analysis of TUNEL (red) positive cells that are BrdU+ (green) at (H) 72 h and (I) 120 h after transduction with AdRIP-hHNF4α8. J, Quantification of the percentage of either BrdU+,diffuse or BrdU+,punctate cells that are TUNEL+ at 72 h and 120 h (n = 3–5 per time point). Immunodetection of Nkx6.1 (green), BrdU (red), and DAPI (blue) in HNF4α8 transduced islets (K) 48 h and (L) 120 h after transduction. M, Quantification of the percentage of BrdU+,punctate cells that are Nkx6.1+ at 48 and 120 h (*, P < 0.001; n = 3 per time point). Immunodetection of MafA (green), BrdU (red), and DAPI (blue) in HNF4α8-transduced islets (N) 48 h and (O) 120 h after transduction. P, Quantification of the percentage of BrdU+,punctate cells that are MafA+ at 48 and 120 h (n = 2–3 per time point). The yellow arrows indicate BrdU+,punctate, Nkx6.1−, or MafA− cells, and white arrows highlight their colocalization. The scale bars in panels C, F, H, and O indicate 25 μm. n.s., P = 0.28.
The inability of the β-cell to repair DNA damage over time may lead to cellular senescence and loss of gene expression required for maintaining the β-cell terminal differentiated state (19, 27, 28). Indeed, we detected some BrdU+,punctate cells positive for senescence associated β-galactosidase activity 72 h after transduction (Supplemental Fig. 4, A and B). To determine whether a sustained DNA damage response was accompanied by loss of differentiation, we analyzed the expression of two transcription factors required for β-cell maturation, Nkx6.1 and MafA, by immunofluorescence (29–31). Remarkably, there was a time-dependent decrease of Nkx6.1 expression in BrdU+,punctate β-cells, with only 20% of the cells retaining Nkx6.1 5 d after viral transduction (Fig. 4, K–M). Although we did not record a similar temporal decrease in MafA production, there were a significant number of BrdU+,punctate β-cells without detectable MafA expression at both 72-h and 120-h time points (Fig. 4, N–P).
To assess the effect of HNF4α-overexpression on β-cell function, we performed static glucose-stimulated insulin assays. The absolute amount of insulin released upon glucose stimulus by AdRIP-HNF4α8 treated islets was not statistically different from untransduced, AdCMV-eGFP treated islets and untransduced islets at receipt of donation (Supplemental Fig. 4C). Also, there were no statistical differences in insulin content in any of the aforementioned groups 72 h after transduction (Supplemental Fig. 4D), suggesting no loss of β-cell function or dedifferentiation in β-cells overexpressing HNF4α8. However, it was not possible to assess the function of the small HNF4αHigh BrdU+,punctate β-cell population separate from the remainder of the islet; thus, loss of function in these cells remains a likely possibility as, in fact, indicated by the overall deterioration of β-cell lineage marker expression described above.
HNF4α8 synergizes with known factors sufficient for promoting cell cycle entry in the human β-cell
Although HNF4α8 was not sufficient to promote full β-cell replication alone, we hypothesized that overexpression of HNF4α8 might augment human β-cell proliferation stimulated by Cdk6 and Cyclin D3 (5). The overexpression of Cdk6 and Cyclin D3 caused a significant increase in the number of Pdx1+ cells that also expressed Ki67 (3.1% ± 0.8 of Pdx1+ were Ki67+,total) (Fig. 5A). Strikingly, when we applied HNF4α8, Cdk6, and Cyclin D3 adenoviruses together, we saw a further increase in the number of Pdx1+ cells that were in the cell cycle 72 h after transduction (8.0% ± 2.2 of Pdx1+ were Ki67+,total) (Fig. 5, B and C, left graph). This finding suggested that HNF4α8 overexpression in combination with known cell cycle regulators is a mitogenic signal sufficient to increase the number of human β-cells initiating cell cycle progression.
Fig. 5.
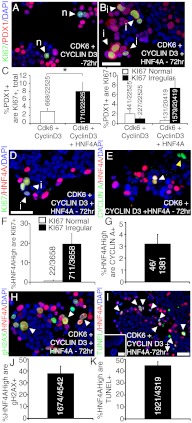
HNF4α8 synergizes with factors known to be sufficient for promoting cell cycle entry in human β-cells. Immunofluorescence detection of Ki67 (green), Pdx1 (red), and DAPI (blue) in (A) Cyclin D3 and Cdk6-, and (B) HNF4α, Cyclin D3, Cdk6-transduced primary human islets at 72 h with (C, left graph) quantification of the percentage of Pdx1+ cells that are Ki67+,total (*, P < 0.04; n = 6 per group). Image (A) is shown as an example of normal Ki67 staining pattern in Cyclin D3/Cdk6 transduction cocktail. C (right graph), Quantification of the percentage of Pdx1+ cells that are either Ki67+,normal or Ki67+,irregular in Cdk6/CyclinD3 and Cdk6/Cyclin D3/HNF4α8 conditions (n = 6 per group). Immunofluorescent detection of HNF4α (red), DAPI (blue), and (D) Ki67, (E) Cyclin A in HNF4α8, Cyclin D3, Cdk6-transduced primary human islets at 72 h with quantification of the percentage of HNF4αHigh cells that are (F) either Ki67+,normal or Ki67+,irregular, and (G) CyclinA+ (n = 3 per group). Immunostaining of HNF4α (red), DAPI (blue), and (H) γH2AX (green), (I) TUNEL (red) in HNF4α8, Cyclin D3, Cdk6-transduced islets at 72 h with quantification of the percentage of HNF4αHigh cells that are (J) γH2AX+ and (K) TUNEL+ (n = 3 per group). The inset in panel I shows representative DAPI staining (blue) of HNF4AHigh TUNEL+ cells. The scale bars in panels B, D, and I indicate 25 μm and in inset of I indicates 5 μm. n, Normal; I, irregular.
However, the majority of Pdx1+ cells exhibited irregular Ki67 staining in islets transduced with Cdk6, Cyclin D3, and HNF4α8 (Fig. 5, B and C right graph), unlike the normal Ki67 staining pattern observed in cycling nonendocrine cells (refer to arrows in Fig. 2, A and D; Supplemental Fig. 2, A and C). We hypothesized that although the triple transgene transduction scheme is sufficient to push more β-cells into the cell cycle, it incites an insult that leads to initiation of cell cycle arrest. Therefore, we assessed the fate of the additional Ki67+ Pdx1+ cells when overexpressing HNF4α8, Cdk6, and Cyclin D3, by analyzing the HNF4αHigh β-cell population for cell cycle progression, DNA damage, and apoptosis 72 h after transduction. Indeed, 20.2 ± 5.8% of HNF4α8 overexpressing cells were Ki67+ and almost always exhibited an irregular Ki67 staining pattern (Fig. 5, D and F). In line with this finding, only 3.2 ± 0.8% of HNF4α8-overexpressing cells were Cyclin A+ (Fig. 5, E and G). Cyclin A+ cells not colocalizing with HNF4αHigh cells could reflect cells transduced with either Cdk6 and/or Cyclin D3 only, because their overexpression is based on a CMV-promoter (Fig. 5E). However, whereas this was an improvement relative to the lack of cell cycle progression seen when overexpressing HNF4α8 alone, the HNF4αHigh cell population in the triple-transduction experiment was frequently positive for γH2AX (37.6% ± 6.4) and TUNEL (44.6% ± 3.4) (Fig. 5, H–K). Additionally, these TUNEL+ cells exhibited nuclear blebbing surrounding a central region of reduced DAPI staining (Fig. 5I, inset). The higher percentage of the HNF4αHigh cells exhibiting γH2AX and TUNEL compared with Ki67 expression suggests that double-stranded DNA damage-induced apoptosis is the predominant fate of the Ki67+ Pdx1+ cell population over time.
Overexpression of Cyclin D3 and Cdk6 also activates the DNA damage response in human β-cells
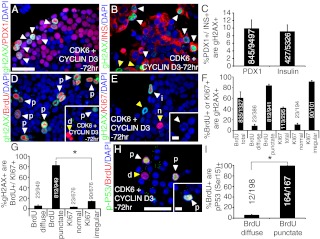
Closer examination of cycling Pdx1+ cells in Cdk6- and Cyclin D3-transduced islets revealed that a significant proportion of these cells also exhibited an irregular Ki67 staining pattern 72 h after transduction (Fig. 5C, right graph). We investigated whether β-cells induced to proliferate by overexpression of Cdk6 and Cyclin D3 alone exhibited expression of γH2AX at the 72-h time point. Indeed, 9.9 ± 2.3% and 9.0 ± 1.8% of Pdx1+ and insulin+ cells, respectively, were positive for γH2AX 72 h after transduction, suggesting that a high percentage of β-cells driven into the cell cycle by Cyclin D3 and Cdk6 are prone to accumulate DNA damage over time (Fig. 6, A–C). To assess whether γH2AX accumulation was specific to cells having entered the cell cycle sometime during the 72-h time period, we evaluated whether BrdU- and Ki67-positive populations exhibited γH2AX expression, and also the percentage of γH2AX+ cells that incorporated BrdU or expressed Ki67. We closely examined the types of possible BrdU and Ki67 staining patterns for each case. Although not all BrdU+ cells were γH2AX+ (Fig. 6D, inset), a significant percentage of cells having progressed through S-phase expressed γH2AX (60.6 ± 11.5% of BrdU+,total were γH2AX+) and exhibited punctate-like BrdU incorporation (83.9 ± 2.4% BrdU+,punctate were γH2AX+) (Fig. 6, D and F). The irregular Ki67 staining pattern was also observed in human islets transduced with Cdk6 and Cyclin D3, and colocalized with γH2AX expression (91.2 ± 2.1% Ki67+,irregular cells were γH2AX+) (Fig. 6, E and F). Only a negligible fraction of either BrdU+,diffuse or Ki67+,normal were γH2AX positive, suggesting that, in line with the overall ability of Cdk6 in combination with a D-cyclin partner to improve human islet function in vivo, a fraction of functional β-cells do arise by Cdk6- and Cyclin D3-stimulated proliferation, although DNA damage analysis at a later time point has not been performed (Fig. 6F) (5). Similarly, the γH2AX-positive population was enriched with cells demonstrating punctate-like BrdU incorporation patterns (82.3 ± 7.5% of γH2AX+ were BrdU+,punctate) similar to what was seen in β-cells when overexpressing HNF4α8 alone, but not diffuse BrdU incorporation (5.23 ± 2.2% of γH2AX+ were BrdU+,diffuse) or a normal Ki67 staining pattern (3.0 ± 0.8% of γH2AX+ were Ki67+,normal) (Fig. 6D, D inset, and panels E and G). Furthermore, the percentage of γH2AX+ cells that incorporated BrdU in a punctate-like manner was much higher than the percentage that expressed Ki67irregular (13.9 ± 1.0% γH2AX+ were Ki67irregular) at 72 h (Fig. 6, E and G). Also, γH2AX+, BrdU+,punctate cells morphologically exhibit nuclear blebbing, indicating apoptosis (Fig. 6E, inset). Based on cell cycle arrest seen in β-cells overexpressing HNF4α8 alone and the fate of HNF4αHigh cells in the triple-transduced condition, this suggests a cell cycle delay in the subpopulation of transduced β-cells accumulating double-stranded DNA damage, ultimately resulting in apoptosis. Indeed, cells incorporating BrdU in a punctate-like manner were positive for the checkpoint marker, phospho-p53 (99.0 ± 0.7% of BrdU+,punctate were phospho-p53(Ser15)+), whereas of cells incorporating BrdU in a diffuse manner only 5.5 ± 1.4% of BrdU+ were phospho-p53(Ser15)+ 72 h after transduction (Fig. 6, H and I). Together, these findings demonstrate that although human β-cells can be stimulated to enter in and progress through the cell cycle by overexpression of Cdk6, Cyclin D3, and further with the addition of HNF4α8, the activation of the DNA damage response highlights the importance of using multiple experimental criteria to adequately assess human β-cell expansion.
Fig. 6.
Overexpression of Cyclin D3 and Cdk6 activates the DNA damage response in human β-cells. Dual immunostaining for γH2AX (green), DAPI (blue), and (A) Pdx1 (red), (B) insulin (red), (D) BrdU (red), and (E) Ki67 (red) in Cyclin D3 and Cdk6-transduced human islets. C, Quantification of the percentage of either Pdx1+, or insulin+ cells that are γH2AX+ in primary human islets transduced with both Cdk6 and Cyclin D3 (n = 3–4 per group). F, Quantification of the percentage of either BrdU+,total, BrdU+,diffuse, BrdU+,punctate, Ki67+,total, Ki67+,normal, or Ki67+,irregular cells that are γH2AX+ in primary human islets transduced with both Cdk6 and Cyclin D3 (n = 3–4 per group). G, Quantification of the percentage of γH2AX+ cells that are either BrdU+,diffuse, BrdU+,punctate, Ki67+,normal, or Ki67+, irregular (n = 3–4 per group; *, P < 0.001 BrdU+,punctate vs. Ki67+,irregular populations). H, Immunostaining for phospho-p53 on serine 15 (green), DAPI (blue), and BrdU (red) in Cyclin D3 and Cdk6-transduced human islets. I, Quantification of the percentage of either BrdU+,diffuse or BrdU+,punctate that are phospho-p53 (serine 15)+ (n = 4; *, P < 0.001). The white arrowheads indicate colocalization, and yellow arrowheads indicate noncolocalization between two markers. All human islets were incubated for 72 h after transduction. The scale bars indicate 25 μm in panels A and H and 5 μm in the insets of panels E and H. p, Punctuate; d, diffuse; n, normal; i, irregular.
Discussion
In this study we investigated whether an islet-expressed isoform of the transcription factor HNF4α can promote proliferation in primary human β-cells. It has been previously suggested in other cell systems that solely relying on BrdU incorporation into DNA is not sufficient to assess complete cell cycle progression (32). Indeed, through the use of multiple cell cycle markers and their particular staining patterns, in addition to BrdU, we found that overexpression of HNF4α8 was sufficient to initiate only partial cell cycle completion in a subset of transduced human β-cells. The varying response of human β-cells to HNF4α8-overexpression is illustrated in Fig. 7. We provide further evidence that attempts to mimic complete cell cycle transit in human β-cells by induced transcriptional activation uncouples the normally regulated timing at which different chromosomal domains initiate replication during S-phase. Although such an uncoupling event has been achieved before in Xenopus egg extracts by modulating Cdk activity (14), what regulates the replication timing program specifically in pancreatic β-cells is unknown. To ensure that DNA is precisely duplicated during S-phase, the cell must be able to distinguish between replicated and unreplicated DNA. The licensing of origins during G1-phase by the binding to DNA of Mcm2–7 proteins and their subsequent removal after initiation of DNA replication ensures proper initiation of DNA replication only once per locus (33). HNF4αHigh BrdU+,punctate cells were unable to accomplish strict duplication of the genome. Rather, by utilizing two thymidine analogs we detected the occurrence of rereplication as distinct overlapping foci within thymidine+ punctate domains. We suggest that the inappropriate relicensing of already replicated DNA, and subsequent firing of DNA replication more than once per locus during S-phase (represented by the punctate BrdU incorporation pattern) leads to the excision of double stranded DNA fragments, and activation of a DNA damage-associated cell cycle arrest mechanism. Such a DNA damage insult and its effect in halting cell cycle transit are seen in studies of other DNA licensing factors in Xenopus egg extracts. Overexpression of Cdt1 (chromatin licensing and DNA replication factor 1) in the G2-phase of the cell cycle induced rereplication of DNA, activated checkpoint pathways, and blocked further cell cycle progression. Intriguingly, not only was the checkpoint activation a direct result of multiple rounds of DNA rereplication, but it coincided with the appearance of significant double-stranded DNA fragments, consistent with a model of head-to-tail replication fork collision (34). Also, increased expression of Cdt1 and subsequent rereplication occurs in human cancer-derived cell lines upon accumulation of constitutively active mutant form of Cyclin D1 (35). Both activation of the DNA damage checkpoint markers p-Chk2 (Thr68) and p-p53 (Ser15), and expression of γH2AX, occurred in the BrdU+,punctate subpopulation of HNF4αHigh β-cells. Activation of the DNA damage response did not cease over time, suggesting that the DNA damage is too great to repair, preventing any initially cycling HNF4α8-transduced β-cell to successfully complete the cell cycle in a regulated manner.
Fig. 7.
Cell cycle progression of islet cells can be discerned by the manner of thymidine analog incorporation and multiple marker analysis. Schematic illustrating the cell cycle characteristics of cell types present in an HNF4α8-transduced human adult islet in vitro. Left column, Nonendocrine islet cells successfully turn over, distinguished by proper thymidine incorporation (diffuse), and activation of multiple cell cycle proteins. Middle column, Conversely, human adult β-cells are almost all quiescent and do not attempt cell cycle entry. The majority of HNF4α8-overexpressing cells do not show any attempt at cell cycle entry and progression. Right column, However, some of the HNF4αHigh β-cells are able to initiate cell cycle entry but activate cell cycle arrest programs such as the DNA damage response in an attempt to fix an apparent S-phase insult (exemplified by punctate thymidine analog domains). Asterisk, CyclinD3/Cdk6- and CyclinD3/Cdk6/HNF4α8-overexpressing β-cells fall into the latter category as they do show signs of active cell cycling and DNA damage response. However, the successful ability of these cycling β-cells to halt cell cycle progression upon double stranded DNA damage might vary depending on the cocktail of genes being constitutively overexpressed.
Activation of the DNA damage response in human β-cells is not a phenotype specific to HNF4α8 overexpression. Indeed, a significant proportion of β-cells stimulated to progress through the cell cycle by overexpression of Cdk6 and Cyclin D3 alone also exhibited punctate BrdU incorporation, activation of γH2AX expression, an irregular nuclear pattern of Ki67 expression, and checkpoint activation. Although we suggest that a double-stranded DNA damage insult leads to activation of the cell cycle arrest response, identification of the exact DNA damage mechanism requires further investigation (36). We conclude that in comparison with cycling nonendocrine islet cells, human β-cells not only possess innate resistance to entering the cell cycle, but are sensitive to accumulating DNA damage when successfully forced into the cell cycle, as very recently suggested in (37), becoming increasingly predisposed to undesired endpoints such as apoptosis and loss of β-cell differentiation status. Furthermore, genomic instability within β-cells might have clinical relevance in the pathophysiology of type 2 diabetes. A mouse model globally deficient in nonhomologous end-joining and expressing a hypomorphic mutant of p53 becomes defective in apoptosis but not in cell cycle arrest and develops diabetes. In these mice, β-cell mass is progressively depleted due to double stranded DNA damage-induced senescence (19). Strikingly, HNF4αHigh BrdU+,punctate β-cells exhibited activation of p53 expression but did not undergo apoptosis. Rather, over time HNF4α8-overexpressing cells attempting cell cycle progression began to lose their β-cell lineage fidelity, highlighted by the significant loss of β-cell maturation gene expression (Nkx6.1 and MafA), as also seen in the db/db diabetic mouse model (38). Finally, these phenotypes in the β-cell are consistent with the ability of oncogenes to promote senescence by stimulating the DNA damage response through rereplication (39–41), for which pathways encompassing key downstream tumor suppressors p53 and Rb are necessary (42).
The MAPK pathway, dependent on HNF4α during pregnancy in the murine β-cell in vivo (7), can also induce cell cycle arrest and subsequent senescence when deregulated via the increase of both p53 and p21 expression and blocks in hyperphosphorylation of Rb (43). It is therefore tempting to speculate that HNF4α8 overexpression in the β-cell activates this signaling pathway similar to the oncogenes Ras and Mos (44). Indeed, rodent insulinoma cell lines show not only activation of cyclins and cdk, but also increased expression of cell cycle inhibitors (45). Conversely, ablation of Rb and p130 in β-cells does not lead to a net change in β-cell mass despite dramatically increasing β-cell proliferation in vivo (46). Instead, increased β-cell proliferation is associated with increased expression of p-p53 (Ser15) and p21 and matched by β-cell death. We propose that the accumulation of DNA damage resulting from replication stress is a barrier to efficient human β-cell proliferation in vitro. Therefore, as a cautionary note, the criteria for demonstrating sufficiency of a factor to promote β-cell proliferation should be extended to include a detailed analysis of cell cycle entry, the fidelity of duplication of the genome during S-phase, progression through multiple cell cycle phases, and cell cycle exit. Furthermore, activation of the DNA damage response should be assessed in future attempts to faithfully mimic β-cell proliferation in vitro.
Materials and Methods
Adenovirus production
The original cDNA plasmid, pcDNA3.hHNF4α8 (NM_175914.3), was constructed by Dr. Jerome Eeckhoute (47) and is a kind gift of Dr. Frances M. Sladek. An adenovirus containing the islet-expressed human HNF4α8 cDNA and the rat insulin promoter was amplified, purified, and titered.
Culturing, transduction, and harvest of human cadaveric islets
Human islets were supplied by the DERC of the University of Pennsylvania, the National Disease Research Interchange (NDRI) (http://www.ndriresource.org), and the Methodist Hospital Research Institute (http://www.methodisthealth.com/default.cfm). Available information of each human islet donation used and the contribution of each donation to each experiment can be found in Supplemental Table 2. Islets were incubated in CMRL 1066 medium (Mediatech, Manassas, VA) containing 5.5 mm d-glucose, 0.5% human albumin (Talecris Biotherapeutics, Research Triangle Park, NC), 10 U/ml Heparin (Sagent Pharmaceuticals, Schaumberg, IL), 100 μg/ml penicillin/streptomycin, and 2 mm l-glutamine. Islets were transduced with adenoviruses for 24 h followed by washing. Islets were then cultured in the continued presence of the BrdU reagent (Invitrogen, Carlsbad, CA) during the length of incubation after transduction; 5 × 106 infectious particles per islet of AdRIP-HNF4α8, AdCMV-eGFP, and 5 × 105 infectious particles per islet of both AdCMV-Cyclin D3 and AdCMV-Cdk6 were used. Islets were fixed in 4% paraformaldehyde for 1 h at 4 C, paraffin embedded, and sectioned.
mRNA isolation and RT-PCR
Total RNA was isolated, cDNA was synthesized, and quantitative PCR were performed as described previously (48). PCR primer sequences used for E1A are as designed in Ref. 49: HNF4α forward, TGCCTACCTCAAAGCCATCAT and reverse, GCGGTCGTTGATGTAGTCCTC.
Immunofluorescence analysis
Antigen retrieval was performed by pressure cooker heating (Prestige Medical, Northridge, CA) using citrate buffer (pH 6.0). Sections were blocked with CAS-Block (Invitrogen) and incubated with primary antibodies at 4 C overnight (Supplemental Table 1). Secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) were added for 2 h at room temperature, and nuclei were stained with DAPI. High-resolution images were taken on a Yokagawa CSU-10 spinning disk mounted on a Nikon Ti-U inverted microscope, and a Hamamatsu Photonics Orca Charge-Coupled Device Camera (Hamamatsu, Bridgewater, NJ).
Terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling (TUNEL)
Apoptosis was assessed by TUNEL (Roche Applied Science, Minneapolis, MN) (50).
Glucose-stimulated insulin secretion in vitro
Glucose-stimulated insulin secretion was performed under static conditions (51). Insulin release and content was determined using RIA (Millipore Corp., Bedford, MA).
Rereplication assay
BrdU was added to the islet culture, 36 h after adenoviral transduction, for 5.5 h. BrdU was removed by washing, and islets were placed back into fresh medium for 1 h to ensure depletion of BrdU. Then EdU (100 μm; Invitrogen) was added for an additional 5.5 h before harvest. Nonlabeling intervals of 3 h and 6 h were also used. After antigen retrieval, EdU incorporation was detected with the Click-iT EdU Alexa Fluor 555 imaging Kit (Invitrogen).
Statistics
Statistical analysis was done between two data sets using a one-tailed Student's t test, and between three or more data sets using a one-way ANOVA combined with a Tukey post hoc test. Differences were considered significant when P < 0.05. Variation measurements are given as sem unless stated otherwise.
Supplementary Material
Acknowledgments
We thank Mrs. Neena Panackal of the Children's Hospital of Philadelphia Research Institute Pathology Core, and Dr. Gary Swain of the University of Pennsylvania Center for Molecular Studies in Digestive and Liver Diseases for histology assistance, and Dr. Heather Collins and the RIA and Biomarkers Core at the University of Pennsylvania DERC for insulin assays. The HNF4α8 adenovirus was produced by the Penn Vector Core. We acknowledge use of human tissues provided in part by the National Disease Research Interchange (NDRI). The University of Pennsylvania Center for Molecular Studies in Digestive and Liver Diseases is supported through the NIH by P30-050366, the Biomarkers Core at the University of Pennsylvania DERC by P30-DK-19525, the Penn Vector Core by P30-DK-019525 and NDRI by 5 U42 RR006042.
This work was supported by National Institutes of Health (NIH) Pharmacology training grant (T32 GM008076-25) (to S.R.), Juvenile Diabetes Research Foundation (JDRF) Postdoctoral Fellowship Award (to Z.L.), U01 DK 089538 and JDRF grants 1-2008-39/34-2008-7630 (to A.F.S.), and NIH (DK R01-055342) and JDRF grants (17-2011-262) (to K.H.K.).
Author Contributions: S.R. performed experiments and wrote the manuscript. J.Z. performed experiments requested by peer review, L.Z. performed experiments generating the RIP-hHNF4α8 adenovirus. C.L and A.N. provided human islet donations. K.K.T., N.M.F.T., and A.F.S. provided essential reagents. J.A.K provided essential reagents and protocols. K.H.K. edited the manuscript and directed the study. Dr. Kaestner is the guarantor of this work, had full access to all the data, and takes full responsibility for the integrity of data and the accuracy of data analysis.
Disclosure Summary: The authors have nothing to disclose.
NURSA Molecule Pages†:
Nuclear Receptors: HNF4-α.
Annotations provided by Nuclear Receptor Signaling Atlas (NURSA) Bioinformatics Resource. Molecule Pages can be accessed on the NURSA website at www.nursa.org.
- BrdU
- Bromodeoxyuridine
- Cdk
- cyclin-dependent kinase
- CMV
- cytomegalovirus
- DAPI
- 4′,6-diamidino-2-phenylindole
- EdU
- 5-ethynyl-2′-dexyuridine
- eGFP
- enhanced GFP
- GFP
- green fluorescent protein
- HNF4α
- hepatocyte nuclear factor-4α
- TUNEL
- terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling.
References
- 1. Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. 2000. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 343:230–238 [DOI] [PubMed] [Google Scholar]
- 2. Dor Y, Brown J, Martinez OI, Melton DA. 2004. Adult pancreatic β-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429:41–46 [DOI] [PubMed] [Google Scholar]
- 3. Perl S, Kushner JA, Buchholz BA, Meeker AK, Stein GM, Hsieh M, Kirby M, Pechhold S, Liu EH, Harlan DM, Tisdale JF. 2010. Significant human β-cell turnover is limited to the first three decades of life as determined by in vivo thymidine analog incorporation and radiocarbon dating. J Clin Endocrinol Metab 95:E234–E239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Davis DB, Lavine JA, Suhonen JI, Krautkramer KA, Rabaglia ME, Sperger JM, Fernandez LA, Yandell BS, Keller MP, Wang IM, Schadt EE, Attie AD. 2010. FoxM1 is up-regulated by obesity and stimulates β-cell proliferation. Mol Endocrinol 24:1822–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fiaschi-Taesch NM, Salim F, Kleinberger J, Troxell R, Cozar-Castellano I, Selk K, Cherok E, Takane KK, Scott DK, Stewart AF. 2010. Induction of human β-cell proliferation and engraftment using a single G1/S regulatory molecule, cdk6. Diabetes 59:1926–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hohmeier HE, Newgard CB. 2005. Islets for all? Nat Biotechnol 23:1231–1232 [DOI] [PubMed] [Google Scholar]
- 7. Gupta RK, Gao N, Gorski RK, White P, Hardy OT, Rafiq K, Brestelli JE, Chen G, Stoeckert CJ, Jr, Kaestner KH. 2007. Expansion of adult β-cell mass in response to increased metabolic demand is dependent on HNF-4α. Genes Dev 21:756–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gupta RK, Vatamaniuk MZ, Lee CS, Flaschen RC, Fulmer JT, Matschinsky FM, Duncan SA, Kaestner KH. 2005. The MODY1 gene HNF-4α regulates selected genes involved in insulin secretion. J Clin Invest 115:1006–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sladek FM. 2011. What are nuclear receptor ligands? Mol Cell Endocrinol 334:3–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hansen SK, Párrizas M, Jensen ML, Pruhova S, Ek J, Boj SF, Johansen A, Maestro MA, Rivera F, Eiberg H, Andel M, Lebl J, Pedersen O, Ferrer J, Hansen T. 2002. Genetic evidence that HNF-1α-dependent transcriptional control of HNF-4α is essential for human pancreatic β cell function. J Clin Invest 110:827–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fiaschi-Taesch N, Bigatel TA, Sicari B, Takane KK, Salim F, Velazquez-Garcia S, Harb G, Selk K, Cozar-Castellano I, Stewart AF. 2009. Survey of the human pancreatic β-cell G1/S proteome reveals a potential therapeutic role for cdk-6 and cyclin D1 in enhancing human β-cell replication and function in vivo. Diabetes 58:882–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takane KK, Kleinberger JW, Salim FG, Fiaschi-Taesch NM, Stewart AF. 2012. Regulated and reversible induction of adult human β-cell replication. Diabetes 61:418–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Karslioglu E, Kleinberger JW, Salim FG, Cox AE, Takane KK, Scott DK, Stewart AF. 2011. cMyc is a principal upstream driver of β-cell proliferation in rat insulinoma cell lines and is an effective mediator of human β-cell replication. Mol Endocrinol 25:1760–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thomson AM, Gillespie PJ, Blow JJ. 2010. Replication factory activation can be decoupled from the replication timing program by modulating Cdk levels. J Cell Biol 188:209–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dimitrova DS, Gilbert DM. 2000. Temporally coordinated assembly and disassembly of replication factories in the absence of DNA synthesis. Nat Cell Biol 2:686–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pagano M, Pepperkok R, Verde F, Ansorge W, Draetta G. 1992. Cyclin A is required at two points in the human cell cycle. EMBO J 11:961–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Blow JJ, Hodgson B. 2002. Replication licensing–defining the proliferative state? Trends Cell Biol 12:72–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Krishnamurthy J, Ramsey MR, Ligon KL, Torrice C, Koh A, Bonner-Weir S, Sharpless NE. 2006. p16INK4a induces an age-dependent decline in islet regenerative potential. Nature 443:453–457 [DOI] [PubMed] [Google Scholar]
- 19. Tavana O, Puebla-Osorio N, Sang M, Zhu C. 2010. Absence of p53-dependent apoptosis combined with nonhomologous end-joining deficiency leads to a severe diabetic phenotype in mice. Diabetes 59:135–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sengupta S, Linke SP, Pedeux R, Yang Q, Farnsworth J, Garfield SH, Valerie K, Shay JW, Ellis NA, Wasylyk B, Harris CC. 2003. BLM helicase-dependent transport of p53 to sites of stalled DNA replication forks modulates homologous recombination. EMBO J 22:1210–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sancar A, Lindsey-Boltz LA, Unsal-Kaçmaz K, Linn S. 2004. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem 73:39–85 [DOI] [PubMed] [Google Scholar]
- 22. Kastan MB, Bartek J. 2004. Cell-cycle checkpoints and cancer. Nature 432:316–323 [DOI] [PubMed] [Google Scholar]
- 23. Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. 1998. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem 273:5858–5868 [DOI] [PubMed] [Google Scholar]
- 24. Murray-Zmijewski F, Slee EA, Lu X. 2008. A complex barcode underlies the heterogeneous response of p53 to stress. Nat Rev Mol Cell Biol 9:702–712 [DOI] [PubMed] [Google Scholar]
- 25. Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. 2010. The essence of senescence. Genes Dev 24:2463–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Laybutt DR, Weir GC, Kaneto H, Lebet J, Palmiter RD, Sharma A, Bonner-Weir S. 2002. Overexpression of c-Myc in β-cells of transgenic mice causes proliferation and apoptosis, downregulation of insulin gene expression, and diabetes. Diabetes 51:1793–1804 [DOI] [PubMed] [Google Scholar]
- 27. Halvorsen TL, Beattie GM, Lopez AD, Hayek A, Levine F. 2000. Accelerated telomere shortening and senescence in human pancreatic islet cells stimulated to divide in vitro. J Endocrinol 166:103–109 [DOI] [PubMed] [Google Scholar]
- 28. Russ HA, Bar Y, Ravassard P, Efrat S. 2008. In vitro proliferation of cells derived from adult human β-cells revealed by cell-lineage tracing. Diabetes 57:1575–1583 [DOI] [PubMed] [Google Scholar]
- 29. Nelson SB, Schaffer AE, Sander M. 2007. The transcription factors Nkx6.1 and Nkx6.2 possess equivalent activities in promoting β-cell fate specification in Pdx1+ pancreatic progenitor cells. Development 134:2491–2500 [DOI] [PubMed] [Google Scholar]
- 30. Zhang C, Moriguchi T, Kajihara M, Esaki R, Harada A, Shimohata H, Oishi H, Hamada M, Morito N, Hasegawa K, Kudo T, Engel JD, Yamamoto M, Takahashi S. 2005. MafA is a key regulator of glucose-stimulated insulin secretion. Mol Cell Biol 25:4969–4976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Matsuoka TA, Kaneto H, Stein R, Miyatsuka T, Kawamori D, Henderson E, Kojima I, Matsuhisa M, Hori M, Yamasaki Y. 2007. MafA regulates expression of genes important to islet β-cell function. Mol Endocrinol 21:2764–2774 [DOI] [PubMed] [Google Scholar]
- 32. Pang L, Reddy PV, McAuliffe CI, Colvin G, Quesenberry PJ. 2003. Studies on BrdU labeling of hematopoietic cells: stem cells and cell lines. J Cell Physiol 197:251–260 [DOI] [PubMed] [Google Scholar]
- 33. Blow JJ, Dutta A. 2005. Preventing re-replication of chromosomal DNA. Nat Rev Mol Cell Biol 6:476–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Davidson IF, Li A, Blow JJ. 2006. Deregulated replication licensing causes DNA fragmentation consistent with head-to-tail fork collision. Mol Cell 24:433–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Aggarwal P, Lessie MD, Lin DI, Pontano L, Gladden AB, Nuskey B, Goradia A, Wasik MA, Klein-Szanto AJ, Rustgi AK, Bassing CH, Diehl JA. 2007. Nuclear accumulation of cyclin D1 during S phase inhibits Cul4-dependent Cdt1 proteolysis and triggers p53-dependent DNA rereplication. Genes Dev 21:2908–2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sage J, Straight AF. 2010. RB's original CIN? Genes Dev 24:1329–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee SH, Hao E, Levine F, Itkin-Ansari P. 2011. Id3 upregulates BrdU incorporation associated with a DNA damage response, not replication, in human pancreatic β-cells. Islets 3:358–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Matsuoka TA, Kaneto H, Miyatsuka T, Yamamoto T, Yamamoto K, Kato K, Shimomura I, Stein R, Matsuhisa M. 2010. Regulation of MafA expression in pancreatic β-cells in db/db mice with diabetes. Diabetes 59:1709–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D, Issaeva N, Vassiliou LV, Kolettas E, Niforou K, Zoumpourlis VC, Takaoka M, Nakagawa H, Tort F, Fugger K, Johansson F, Sehested M, Andersen CL, Dyrskjot L, Ørntoft T, Lukas J, Kittas C, Helleday T, Halazonetis TD, Bartek J, Gorgoulis VG. 2006. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature 444:633–637 [DOI] [PubMed] [Google Scholar]
- 40. Di Micco R, Fumagalli M, Cicalese A, Piccinin S, Gasparini P, Luise C, Schurra C, Garre' M, Nuciforo PG, Bensimon A, Maestro R, Pelicci PG, d'Adda di Fagagna F. 2006. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature 444:638–642 [DOI] [PubMed] [Google Scholar]
- 41. Bartkova J, Horejsi Z, Koed K, Krämer A, Tort F, Zieger K, Guldberg P, Sehested M, Nesland JM, Lukas C, Ørntoft T, Lukas J, Bartek J. 2005. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 434:864–870 [DOI] [PubMed] [Google Scholar]
- 42. Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. 1997. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88:593–602 [DOI] [PubMed] [Google Scholar]
- 43. Lin AW, Barradas M, Stone JC, van Aelst L, Serrano M, Lowe SW. 1998. Premature senescence involving p53 and p16 is activated in response to constitutive MEK/MAPK mitogenic signaling. Genes Dev 12:3008–3019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shibuya EK, Morris J, Rapp UR, Ruderman JV. 1996. Activation of the Xenopus oocyte mitogen-activated protein kinase pathway by Mos is independent of Raf. Cell Growth Differ 7:235–241 [PubMed] [Google Scholar]
- 45. Cozar-Castellano I, Harb G, Selk K, Takane K, Vasavada R, Sicari B, Law B, Zhang P, Scott DK, Fiaschi-Taesch N, Stewart AF. 2008. Lessons from the first comprehensive molecular characterization of cell cycle control in rodent insulinoma cell lines. Diabetes 57:3056–3068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Harb G, Vasavada RC, Cobrinik D, Stewart AF. 2009. The retinoblastoma protein and its homolog p130 regulate the G1/S transition in pancreatic β-cells. Diabetes 58:1852–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chartier FL, Bossu JP, Laudet V, Fruchart JC, Laine B. 1994. Cloning and sequencing of cDNAs encoding the human hepatocyte nuclear factor 4 indicate the presence of two isoforms in human liver. Gene 147:269–272 [DOI] [PubMed] [Google Scholar]
- 48. Rieck S, White P, Schug J, Fox AJ, Smirnova O, Gao N, Gupta RK, Wang ZV, Scherer PE, Keller MP, Attie AD, Kaestner KH. 2009. The transcriptional response of the islet to pregnancy in mice. Mol Endocrinol 23:1702–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lavine JA, Raess PW, Davis DB, Rabaglia ME, Presley BK, Keller MP, Beinfeld MC, Kopin AS, Newgard CB, Attie AD. 2010. Contamination with E1A-positive wild-type adenovirus accounts for species-specific stimulation of islet cell proliferation by CCK: a cautionary note. Mol Endocrinol 24:464–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Soleimanpour SA, Crutchlow MF, Ferrari AM, Raum JC, Groff DN, Rankin MM, Liu C, De León DD, Naji A, Kushner JA, Stoffers DA. 3010. Calcineurin signaling regulates human islet β-cell survival. J Biol Chem 285:40050–40059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hardy OT, Hohmeier HE, Becker TC, Manduchi E, Doliba NM, Gupta RK, White P, Stoeckert CJ, Jr, Matschinsky FM, Newgard CB, Kaestner KH. 2007. Functional genomics of the β-cell: short-chain 3-hydroxyacyl-coenzyme A dehydrogenase regulates insulin secretion independent of K+ currents. Mol Endocrinol 21:765–773 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.