Abstract
The pathogenesis of the diarrheal disease caused by Vibrio parahaemolyticus, a leading cause of seafood-associated enteritis worldwide, is dependent upon a type III secretion system, T3SS2. This apparatus enables the pathogen to inject bacterial proteins (effectors) into the cytosol of host cells and thereby modulate host processes. T3SS effector proteins transit into the host cell via a membrane pore (translocon) typically formed by 3 bacterial proteins. We have identified the third translocon protein for T3SS2: VopW, which was previously classified as an effector protein for a homologous T3SS in V. cholerae. VopW is a hydrophilic translocon protein; like other such proteins, it is not inserted into the host cell membrane but is required for insertion of the two hydrophobic translocators, VopB2 and VopD2, that constitute the membrane channel. VopW is not required for secretion of T3SS2 effectors into the bacterial culture medium; however, it is essential for transfer of these proteins into the host cell cytoplasm. Consequently, deletion of vopW abrogates the virulence of V. parahaemolyticus in several animal models of diarrheal disease. Unlike previously described hydrophilic translocators, VopW is itself translocated into the host cell cytoplasm, raising the possibility that it functions as both a translocator and an effector.
INTRODUCTION
Vibrio parahaemolyticus is a Gram-negative marine bacterium that is a leading cause of diarrhea linked to consumption of raw or undercooked seafood (30). Typically, infection of the gastrointestinal tract with V. parahaemolyticus manifests as acute, self-limited watery diarrhea, which is often accompanied by abdominal pain and vomiting. Additionally, intestinal biopsy samples from patients have revealed disruption of the intestinal epithelium and the presence of an acute inflammatory response (27). Using animal models of V. parahaemolyticus infection, we and others have demonstrated that a type III secretion system (T3SS) encoded on chromosome II of pathogenic isolates of V. parahaemolyticus (T3SS2) plays a critical role in inducing these signs of disease (11, 25, 28). Export of several bacterial proteins encoded within this gene cluster, e.g., VopT, VopL, VopC, VopV, and VPA1380, has been found to be dependent upon T3SS2 (12, 17, 19). However, the precise roles for at least some of these proteins, as well as the roles for numerous other proteins encoded within the T3SS2 gene cluster, have yet to be identified. A second secretion system (T3SS1), which unlike T3SS2 is found in all V. parahaemolyticus strains, including environmental/nonpathogenic isolates, appears to play a relatively minor role in intestinal disease (11, 28).
T3SSs are associated with virulence in numerous bacterial pathogens (3). These complex multiprotein apparati enable bacteria to transfer proteins—termed effectors—from the bacterial cytoplasm into the host cell cytoplasm, where they have been found to modulate a wide variety of processes, including apoptosis, membrane ruffling, actin nucleation and polymerization, microtubule polymerization, ubiquitination, and transcription (4). The protein components of the transfer apparatus, or needle complex, are relatively well conserved; however, there is extensive heterogeneity among the effectors that are delivered by different pathogens. Furthermore, effectors do not necessarily share distinguishing features, although at least a subset displays homology to eukaryotic proteins. Effectors are often encoded within or adjacent to the gene clusters that encode the structural components of the T3SS; thus, nonconserved open reading frames (ORFs) of unknown function carried in the neighborhood of T3SS genes are often good candidates for potential effectors.
Protein transfer by T3SS is typically a regulated and hierarchical process (7, 22). Expression of T3SS genes and/or release of effectors can be triggered by contact with a host cell; they can also be induced by environmental conditions, even in the absence of a eukaryotic cell, in which case proteins are secreted into the culture medium rather than translocated. Injection of proteins into a host cell is dependent upon translocator proteins, which are released in a T3SS-dependent manner and form a pore (also known as a translocon) in the host cell membrane. In general, each T3SS has at least three translocator proteins that are encoded within an operon, often accompanied by at least one cytoplasmic chaperone (21, 23). For systems with just three translocators, two of these proteins are hydrophobic and can be assembled into a heteromultimeric pore that spans the host cell membrane. The third translocator protein is hydrophilic and is not a structural component of the pore; instead, it is thought to aid in pore assembly and/or insertion, perhaps via chaperoning the other components as they are released from the secretion apparatus. The hydrophilic translocator is also likely to serve as a bridge between the primary needle subunit and the transmembrane pore (14, 21). None of the translocon proteins are required for secretion of T3SS proteins into the bacterial culture medium; however, in the absence of the hydrophilic component, regulation of secretion is often perturbed (18). Translocator proteins can also be secreted into the bacterial supernatant. At least one hydrophilic translocator protein (LcrV) also has immunomodulatory effects, mediated by its signaling through a TLR2/TLR6/CD14 complex (5).
Previous studies have identified the hydrophobic constituents of the V. parahaemolyticus T3SS2 translocon, VopB2 and VopD2 (VPA1362 and VPA1361, respectively) (15, 20). VopB2 shows some homology (22 and 27% identity) to translocon proteins (AopB and EspD, respectively) from two distinct families of T3SS, while VopD2 lacks homology to any characterized translocon components, suggesting that the V. parahaemolyticus translocon proteins may cluster outside of most T3SS families described to date (15, 23). However, VopD2 and VopB2 homologs with >90% amino acid identity are present within some non-O1/O139 strains of V. cholerae that produce a T3SS (15). Neither the V. parahaemolyticus nor the V. cholerae gene clusters encode obvious homologs to hydrophilic translocon proteins, and their known translocon genes are flanked on the chromosome by genes annotated as chaperones.
Here, we report that we have identified an additional translocator protein from V. parahaemolyticus T3SS2. Atypically, this protein, VPA1345 (also known as VopW), is not encoded in an operon with the other translocon proteins. VopW, like known translocon proteins, is secreted into culture medium in a T3SS2-dependent manner. Strains that lack vopW cannot transfer known T3SS2 effectors to host cells, nor do they permeabilize or introduce other translocon proteins into host cell membranes. Furthermore, vopW mutants are avirulent in 2 animal models of V. parahaemolyticus infection. These findings, in conjunction with the size and predicted secondary structure of VopW, suggest that it functions as a hydrophilic translocon component for V. parahaemolyticus, which may guide assembly of VopD2 and VopB2 into a membrane pore. Unexpectedly, however, unlike most such proteins it also appears to be translocated into the cytoplasm of host cells, raising the possibility that it may serve as a T3SS effector as well as a translocator.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
All strains are derivatives of RIMD2210633 (20). POR-2, POR-2 vcrD2, vscN1, and vscN1 vscN2 strains have been previously described (17, 25). Additional strains were created by allele exchange, using derivatives of the suicide vector pDM4 as previously described (33). Strains were cultured in LB medium at 37°C and supplemented to induce protein expression as indicated. The plasmids pVopW-FLAG and pVopW-CyaA were constructed using the vectors pACYC184 and pMMB207, respectively.
Analysis of secreted proteins.
Secreted proteins were prepared as described previously (15). Briefly, bacterial strains were grown for 5 h in LB medium supplemented with 0.5% NaCl and 0.04% bile (Sigma). Secreted proteins were harvested by precipitation with cold trichloroacetic acid to a final concentration of 10% (vol/vol) on ice for 60 min, followed by centrifugation at 48,000 × g for 60 min. The pellets were rinsed in cold 100% acetone and solubilized in Laemmli buffer.
Immunoblot analysis.
Samples for Western blot analysis were separated by SDS-PAGE (12% polyacrylamide). The transferred membrane was probed with polyclonal antisera against VopB2, VopD2, VopV, VopC, VopW, RNA polymerase (RNAP), tubulin, calnexin, CyaA, or FLAG (10, 12, 15, 16) and with horseradish peroxidase-conjugated goat anti-rabbit antibody (Zymed). The blots were developed by using the ECL Western blotting kit (Amersham).
Protein translocation analyses.
Adenylate cyclase reporter gene assays were performed as previously described (12, 15, 17). Caco-2 cells (∼10-day cultures) were infected with the indicated strains of V. parahaemolyticus harboring expression constructs for CyaA fusion at a multiplicity of infection (MOI) of 10 for 1 h. After infection, the cyclic AMP (cAMP) levels in infected cells were determined using the cAMP Biotrak enzyme immunoassay (EIA) kit (Amersham Biosciences) according to the manufacturer's instructions.
Fractionation of infected host cells was performed as described previously (17). Briefly, Caco-2 cells were infected with V. parahaemolyticus for 1.5 h at an MOI of 100. Infected cells were washed with ice-cold phosphate-buffered saline (PBS) and then suspended in homogenization buffer (3 mM imidazole, 250 mM sucrose, 0.5 mM EDTA, pH 7.4) supplemented with protease inhibitor cocktail (Sigma) and mechanically disrupted by passage through a 22-gauge needle. The homogenate was centrifuged at 3,000 × g for 15 min to pellet unbroken cells, bacteria, nuclei, and cytoskeletal components. The supernatant was subjected to ultracentrifugation at 41,000 × g for 20 min to separate host cell membrane (pellet) from cytoplasm (supernatant). Fractions were analyzed by immunoblotting as described above.
Type III secretion system-dependent pore formation assay.
The T3SS-dependent permeability assay was performed as described previously (15). Briefly, Caco-2 cells were infected with V. parahaemolyticus for 1.5 h at an MOI of 100, followed by exposure to 25 mg/ml ethidium bromide and 5 mg/ml acridine orange.
Rabbit ileal loop infection.
Rabbit ileal loop tests were performed as previously described (15). The bacteria tested (109 CFU) were suspended in 1 ml LB broth with 0.5% NaCl and injected into the ligated ileal loops of rabbits, and then fluid accumulation in each loop was measured 18 h after challenge. The fluid accumulation is expressed as the amount of accumulated fluid (in milliliters) per length (in centimeters) of ligated rabbit small intestine.
Infection of infant rabbits.
Infant rabbits were infected as described previously (28). Briefly, cimetidine-treated rabbits were orogastrically inoculated with 109 CFU of V. parahaemolyticus, and bacterial colonization (CFU/g intestinal tissue) and fluid accumulation were measured 38 h postinfection. Fluid accumulation ratios for the distal small intestine were determined by isolating an approximately 5-cm length of tissue using silk ligatures. The intestinal section was weighed and then cut every 0.5 cm to release any luminal fluid, and the tissue pieces were reweighed. The fluid accumulation ratio was calculated as the weight of fluid divided by the weight of the drained tissue. The cecal fluid ratio was calculated as the weight of the fluid in the cecum divided by the weight of cecal tissue.
Statistical analyses.
Statistical analyses were performed with PRISM software. Fluid accumulation ratios and bacterial counts (after log transformation) were analyzed using nonparametric one-way analysis of variance (ANOVA) with Bonferroni's multiple-comparison posttest.
RESULTS
Secretion of VopW requires T3SS2.
To screen for V. parahaemolyticus proteins secreted in a T3SS2-dependent manner, we compared supernatants from a T3SS1-deficient derivative (vscN1) of the pathogenic isolate RIMD2210633 and a derivative in which both T3221 and T3SS2 had been inactivated (RIMD2210633 vscN1 vscN2). Secretion of T3SS2 was activated by overexpression of the transcriptional activator VtrB (16). Analysis of supernatants using two-dimensional (2D) gel electrophoresis revealed the presence of an ∼40-kDa protein that was present among proteins released by the vcrN1 mutant but not by the vscN1 vscN2 strain. Mass spectral analyses identified this protein as VPA1345 (data not shown), which is homologous (50% similarity) to a protein (VopW) encoded within the T3SS gene cluster of the O39 V. cholerae strain AM19226. VopW was previously found to be translocated into host cells and to inhibit their growth in the presence of caspofungin, a stressor that perturbs the mitogen-activated protein kinase (MAPK) signaling pathway (1).
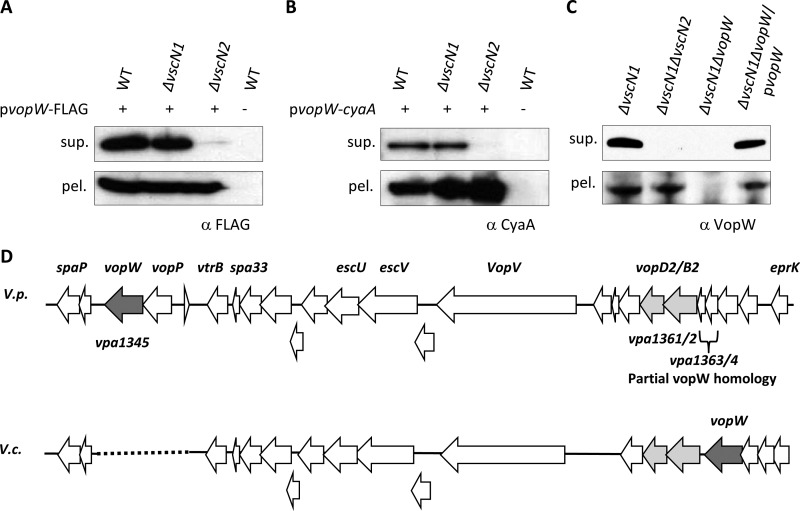
Additional studies, using either tagged, plasmid-encoded VPA1345 (subsequently referred to as VopW) or endogenous VopW, confirmed that secretion of VopW requires functional T3SS2 and demonstrated that secretion is not impaired by inactivation of T3SS1 (Fig. 1A to C). Using immunoblots, no secretion of VopW by a vscN2 (T3SS2) mutant was observed, while secretion by a vscN1 (T3SS1) mutant was equal to that by the wild-type (WT) strain. Furthermore, the abundance of cell-associated (presumably cytoplasmic) VopW fusion proteins was not reduced in the vscN2 mutant; consequently, a defect in expression or protein stability does not account for the absence of VopW secretion by this strain.
Fig 1.
Secretion of VopW (VPA1345), a protein from the T3SS2 gene cluster, requires T3SS2. Tagged VopW (VopW-FLAG [A] and VopW-CyaA [B]) and endogenous VopW (C) in culture supernatants (sup.) and cell pellets (pel.) were detected by immunoblotting. Proteins were isolated from cultures of wild-type (WT), T3SS1-deficient (ΔvscN1), and T3SS2-deficient (ΔvscN2) strains. Antibodies were directed against the protein tags (A and B) or against the native protein (C). (D) Schematic comparison of the VopW genomic neighborhoods in V. parahaemolyticus (V.p.) and V. cholerae AM19226 (V.c.). Selected genes have been labeled, and translocon genes are shaded. The dotted line is inserted to permit alignment of homologous regions in the V. parahaemolyticus and V. cholerae genomes; no sequence is present at the corresponding site in V. cholerae.
V. parahaemolyticus VopW is encoded within the cluster of genes encoding T3SS2 (Fig. 1D). It is encoded between vopP (also known as vopA), which encodes a T3SS2 effector that inhibits MAPK signaling pathways (31), and a hypothetical gene (vpa1344) of unknown function. Although there is significant homology between the V. parahaemolyticus T3SS2 and the T3SS of V. cholerae AM19226 (numerous genes have >80% identity [6]), the V. cholerae VopW is encoded at a different site, upstream of the vopB2 (also known as vspD) homolog. Interestingly, at this site in V. parahaemolyticus are two smaller genes, vpa1363 and vpa1364, each of which displays homology at one end to vpa1345. VopW homologs were not detected in species other than V. parahaemolyticus or V. cholerae.
VopW is translocated into host cell cytoplasm.
The VopW fusion proteins were also used to explore whether VopW is translocated into host cells. Since T3SS1 induces cytotoxicity in cultured cells (11), all translocation assays were performed in a vscN1 strain background. Initially, VopW-Cya was expressed in the vscN1 mutant and in strains also lacking vscN2, vopD2, or vopB2, and these strains were used to infect Caco-2 cells. Adenylate cyclase activity in host cells, which reflects effector fusion translocation, was then monitored using an enzyme-linked immunosorbent assay (ELISA) to detect cAMP. After a 1-h infection, the cAMP level in the cells infected with vscN1 bacteria was markedly higher than in cells infected with any of the double mutants (Fig. 2A). As T3SS2 is inactive in all the double mutants, these data strongly suggest that VopW is translocated into host cells in a T3SS2-dependent manner. V. cholerae VopW has also been found to be translocated, albeit at extremely low levels, and has consequently previously been described as an effector protein (1).
Fig 2.
Translocation of VopW to Caco-2 cells requires a functional T3SS2. (A) cAMP accumulation, which reflects translocation of CyaA fusion protein, was measured in Caco-2 cells that had been infected with V. parahaemolyticus expressing VopW-CyaA. All mutants lacked functional T3SS1 (ΔvscN1); T3SS2 is also inactivated in each of the double mutants. Error bars reflect standard deviations for experiments performed at least in triplicate. (B) Caco-2 cells were infected with T3SS1 (ΔvscN1)- and T3221/T3SS2 (ΔvscN1 ΔvscN2)-deficient V. parahaemolyticus expressing VopW-FLAG, and VopW translocation was assayed in membrane and cytosolic fractions from the infected cells. Translocated proteins (VopW-FLAG and VopD2) and markers for host cytosol (tubulin) and membranes (calnexin) were detected via immunoblotting with anti-FLAG or antisera generated against the native proteins. RNAP (bacterial) served as a marker for bacterial contamination of samples.
Subsequently, fractionation studies were performed on Caco-2 cells infected with various V. parahaemolyticus mutants expressing VopW-FLAG, in order to further characterize the fate of VopW within host cells. As in the previous analyses, translocation of VopW appeared to be dependent upon T3SS2, as it was abrogated by deletion of vscN2 (Fig. 2B). Notably, translocated VopW was detectable only within the cytoplasm of infected Caco-2 cells; no VopW was observed within the membrane fraction. In contrast, the hydrophobic translocon protein VopD2 was present in both the cytoplasmic and the membrane fractions.
VopW is essential for T3SS2-mediated pore formation.
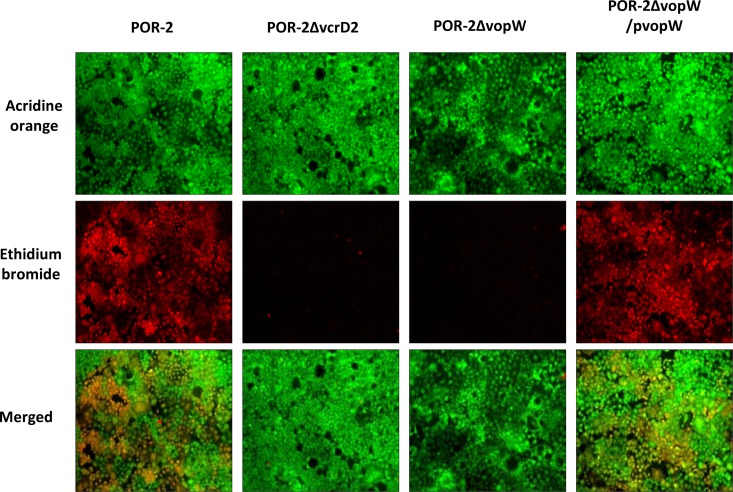
Although translocation of VopW is consistent with a potential role as an effector, we also investigated whether VopW might function as a translocon component. VopW is similar in size to most hydrophilic translocator proteins (383 amino acids [aa] versus ∼330 aa for PcrV, Lcr, IpaD, and SipD), and like these proteins it is predicted to be rich in alpha helices and lacks putative transmembrane domains. Furthermore, the relative chromosomal locations of VopW and VopB2/VspD in V. cholerae (although not in V. parahaemolyticus) match those of known translocator proteins. Consequently, we tested whether deletion of vopW altered the capacity of V. parahaemolyticus to permeabilize host cells. Assembly of a T3SS translocon pore across host cell membranes often increases their permeability to small molecules, and such an effect has previously been observed for the T3SS2 translocons VopB2 and VopD2 (15). Caco-2 cells were incubated with various strains of the bacteria and then treated with the fluorescent dyes acridine orange, which can penetrate intact cells, and ethidium bromide, which is taken up only by cells with perforated membranes. Notably, the efficient uptake of ethidium bromide that occurred following infection of Caco-2 cells with POR-2 cells (RIMD2210633 tdhAS vcrD1; used so that cytotoxicity from hemolysins and T3SS1 did not mask T3SS2-dependent pore formation) was completely absent in cells infected with either the known T3SS2-deficient mutant POR-3 (POR-2 vcrD2) or with POR-2 vopW (Fig. 3). Permeabilization capacity was restored to the vopW mutant by introduction of plasmid-encoded VopW. Collectively, these data suggest that VopW is essential for T3SS2-mediated pore formation in host cell membranes, consistent with a role as a translocon protein.
Fig 3.
VopW is essential for T3SS2-mediated pore formation. Caco-2 cells were infected with various strains of V. parahaemolyticus and then treated with the fluorescent dyes acridine orange (which can penetrate all cells) and ethidium bromide (which penetrates only cells with perforated membranes). Cells were visualized under low-power (10×) magnification.
VopW is required for T3SS2-dependent protein translocation but not secretion.
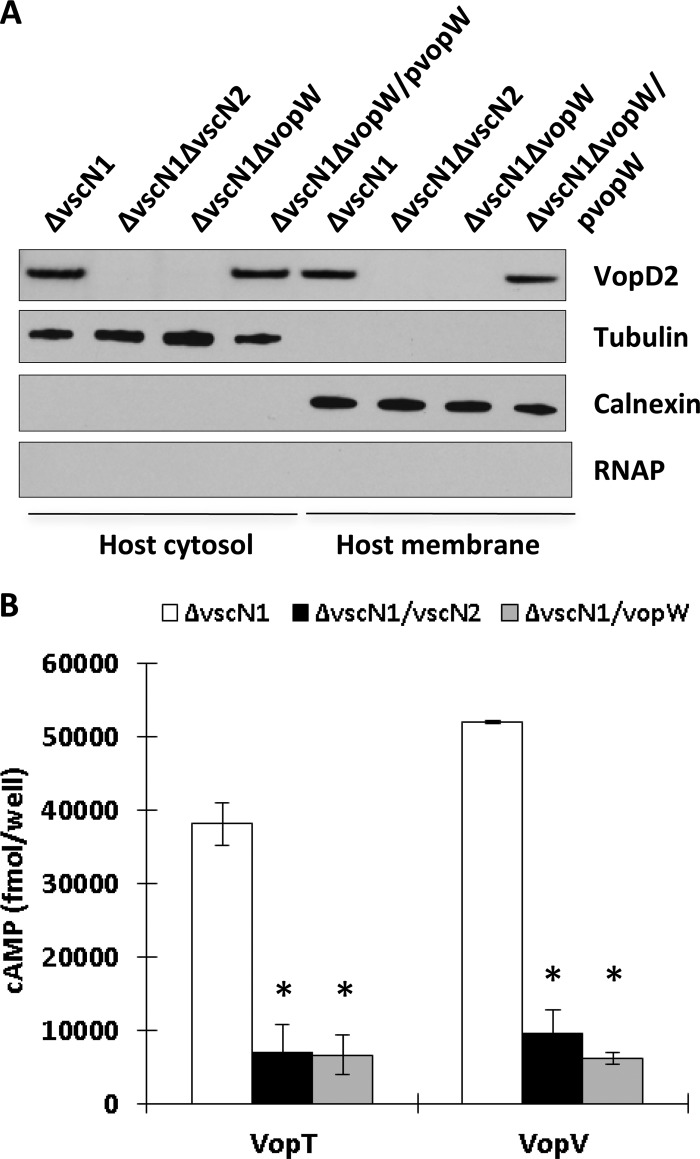
Additional evidence that VopW is a translocon component was obtained by comparing the effects of vopW deletion upon T3SS2-dependent secretion and translocation. In the absence of vopW, the effectors VopC and VopV were secreted into culture supernatants at WT levels (Fig. 4). The translocon proteins VopD2 and VopB2 were also still secreted, although at somewhat reduced levels. Thus, deletion of vopW has markedly different effects upon secretion than does deletion of a structural component, such as vscN2, which completely abolishes T3SS2-mediated secretion (Fig. 4); VopW, unlike VscN2, is clearly not an essential component of the secretory apparatus. However, deletion of vopW had a dramatic effect upon protein translocation. Immunoblot analyses revealed that transfer of VopD2 to Caco-2 cell membranes and cytoplasm was completely blocked by deletion of vopW and that it was restored for the vopW mutant by expression of plasmid-encoded VopW (Fig. 5A). Furthermore, transfer of VopT-CyaA and VopV-CyaA fusion proteins to Caco-2 cells was markedly lower from a vopW vscN1 strain than from a strain containing vopW and comparable to that from a T3SS2-deficient mutant lacking vscN2 and vscN1 (Fig. 5B). This result is consistent with VopW being a translocator that is essential for T3SS2-mediated effector translocation.
Fig 4.
VopW is not essential for T3SS2-mediated protein secretion. The abundance of the translocon proteins VopB2 and VopD2 and the T3SS2 effector proteins VopV and VopC in supernatants (sup.) and bacterial pellets (pel.) from cultures of various V. parahaemolyticus strains was assessed by immunoblotting.
Fig 5.
VopW is required for T3SS2-dependent protein translocation. (A) Translocated (VopD2) and host (tubulin, calnexin) proteins in membrane and cytosolic fractions from Caco-2 cells infected with the indicated strains of V. parahaemolyticus were detected via immunoblotting. Probing for bacterial RNAP confirms the absence of bacterial contamination in Caco-2 samples. (B) Translocation of CyaA fusion proteins (VopT-CyaA and VopV-CyaA) was assessed via measurement of cAMP levels in Caco-2 cells that had been infected with various strains of V. parahaemolyticus. The error bars indicate the standard deviations for the results of at least triplicate experiments. Asterisks indicate significant differences (P < 0.01) from the results obtained using the parent strain.
VopW is essential for V. parahaemolyticus pathogenicity.
Previous studies have demonstrated that the pathogenicity of V. parahaemolyticus is dependent upon T3SS2 (11, 12, 28). Inactivation of the secretion apparatus (e.g., via deletion of a structural component) abrogates the bacterium's capacity to colonize the intestines of infant rabbits and all associated pathology, including fluid accumulation, inflammation, and epithelial destruction in rabbit ileal loops. Consistent with these findings, we found that V. parahaemolyticus lacking the translocon protein VopW is also avirulent in these two rabbit-based models of disease. Colonization of the small intestines of infant rabbits by a vopW mutant of RIMD2210633 was at least 3 orders of magnitude lower than that by an isogenic WT strain, and rabbits did not develop diarrhea, intestinal inflammation, or other signs of disease (Fig. 6B and C and 7; also data not shown). In all assays using infant rabbits, the attenuation of virulence due to deletion of vopW was indistinguishable from the attenuation that results from the absence of the T3SS2 structural component VscN2. Similarly, injection of POR-2 vopW into rabbit ileal loops did not induce enterotoxicity; fluid accumulation in loops infected with the vopW mutant was significantly lower than in loops injected with POR-2 and was equivalent to that seen in uninfected loops or in loops infected with a vscN2 mutant (Fig. 6A).
Fig 6.
VopW is required for T3SS2-dependent fluid accumulation in adult and infant rabbit tissues infected with V. parahaemolyticus. The enterotoxigenicity is indicated by the fluid accumulation ratios, which reflect the amount of fluid released per unit of tissue following infection with the indicated strains. Fluid accumulation was measured in ligated ileal loops from adult rabbits (A) and in the distal small intestines (B) and ceca (C) of infected infant rabbits. For panel A, the error bars indicate the standard deviations for the results of at least triplicate experiments. NI, not infected. For panels B and C, error bars indicate the standard deviation in results from two litters of rabbits. Twelve animals were infected with the WT bacterial strain, and 14 animals were infected with each mutant strain. Variance was analyzed using nonparametric one-way ANOVA with Bonferroni's multiple-comparison posttest. Asterisks indicate significant differences from the results obtained using the parent strain (P < 0.01).
Fig 7.
VopW is required for colonization of the infant rabbit small intestine by V. parahaemolyticus. Tissue samples were isolated from the proximal small intestine (A), middle small intestine (B), and distal small intestine (C) ∼2 days postinfection, and bacterial colonization was calculated as log CFU/gram intestinal tissue. Two independent experiments were performed for each strain. Twelve animals were infected with the WT bacterial strain, and 14 animals were infected with each mutant strain. Variance was analyzed using nonparametric one-way ANOVA with Bonferroni's multiple-comparison posttest. The statistical significance of variance is indicated on the figure.
DISCUSSION
We have identified a component of V. parahaemolyticus T3SS2 that is dispensable for secretion of translocon and effector proteins into culture medium but essential for translocation of these proteins into host cells. A variety of results suggest that this protein, VopW (also known as VPA1345), is a hydrophilic translocon protein, functionally homologous (although lacking in sequence similarity) to proteins such as LcrV, PcrV, IpaD, and SipD. In the absence of vopW, the hydrophobic translocon proteins of T3SS2 do not form pores in host cell membranes and are not inserted there, and T3SS2 effectors are not translocated. However, vopW is not required for secretion of the T3SS2 translocon proteins VopB2 and VopD2, and deletion of vopW does not impair secretion of T3SS2 effector proteins. Furthermore, VopW can itself be secreted into the bacterial supernatant in a T3SS2-dependent fashion, as has been observed for other hydrophilic translocators. Thus, VopW, which to date has been identified only in the T3SS of pathogenic V. parahaemolyticus and some atypical pathogenic V. cholerae strains, is likely the founding member of a new subtype within the family of hydrophilic translocator proteins.
The chromosomal location of vopW in V. parahaemolyticus is unusual, in that it is not directly adjacent to genes encoding other translocon components. In contrast, V. cholerae vopW is encoded directly upstream of translocon homologues. Furthermore, in V. parahaemolyticus, genes that exhibit similarity to vopW (vpa1364 and vpa1363) are found in this position, raising the possibility that vopW, vopD2, and VopB2 were once adjacent and were separated by chromosomal rearrangement. vpa1363 has been annotated in the JCVI CMR database as encoding a putative chaperone, an annotation consistent with its small size and its chromosomal position, but no biological role for this gene has been proven. In some databases (e.g., SEED), Vpa1345 is also described as a chaperone; however, our observations that it is secreted and translocated, as well as its size (384 aa versus ∼160 aa for most translocon chaperones [26]), argue strongly against this possibility.
Structural analyses of T3SSs in other organisms have revealed that hydrophilic translocators often localize to the tip of the needle complex (8, 14, 24). From this position they can potentially anchor hydrophobic translocon proteins to the needle prior to their insertion in the host membrane, although such interactions between translocon components have not been detectable in all systems. Additionally, hydrophilic translocators may serve as a physical link between the translocon pore and the T3SS needle, thereby enabling uninterrupted migration of effector proteins from the bacterium to the host. Interestingly, there is significant variability in the needle-linked structures formed by hydrophilic translocon proteins. Electron microscopy analyses suggest that several proteins (e.g., LcrV, IpaD) (8, 24) assemble into relatively small oligomeric complexes, while others form extensive polymers (e.g., EspA [29]). To date, VopW has not been visualized as a component of the T3S apparatus; consequently, the nature of any structure formed by it remains to be determined.
The most unusual feature of VopW, which to our knowledge distinguishes it from all other hydrophilic translocator proteins, is that it is translocated into host cells. Hydrophobic translocon proteins are by definition mobilized to host cell membranes, and several have also been localized to the host cytoplasm as well; from both positions, they can act as T3SS effectors (2, 13, 15, 32). However, we are not aware of other hydrophilic translocator proteins that are transferred into host cells in a T3SS-dependent manner. LcrV has been detected within host cell cytoplasm; however, its entry into host cells is independent of its cognate T3SS and instead appears to be mediated by a distinct, as-yet-uncharacterized pathway (9). Since deletion of vopW disrupts transfer of all T3SS2 effectors from V. parahaemolyticus into host cells, the specific effects of VopW translocation cannot be observed in the assays we have performed to date. However, in future studies it should be possible to investigate the effects of VopW on host cell signaling or other processes via expressing it in host cells. Preliminary studies suggest that VopW is distributed diffusely throughout host cells (data not shown); however, no activity for VopW within host cells has yet been defined. Future studies should clarify whether VopW plays a dual role in V. parahaemolyticus pathogenicity, both as an essential mediator of T3SS2-dependent protein translocation and as an effector of this secretion system.
ACKNOWLEDGMENTS
This work was supported by NIH-AI-R37-42347 and HHMI. T. Kodama was supported by a grant-in-aid from the Ministry of Health, Labor and Welfare of Japan and a Grant-in-Aid for Young Scientists (B) (23790474) from The Ministry of Education, Culture, Sports, Science and Technology (MEXT).
The authors declare that they have no conflict of interest regarding this work.
Footnotes
Published ahead of print 14 May 2012
REFERENCES
- 1. Alam A, Miller KA, Chaand M, Butler JS, Dziejman M. 2011. Identification of Vibrio cholerae type III secretion system effector proteins. Infect. Immun. 79:1728–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chang J, Chen J, Zhou D. 2005. Delineation and characterization of the actin nucleation and effector translocation activities of Salmonella SipC. Mol. Microbiol. 55:1379–1389 [DOI] [PubMed] [Google Scholar]
- 3. Coburn B, Sekirov I, Finlay BB. 2007. Type III secretion systems and disease. Clin. Microbiol. Rev. 20:535–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dean P. 2011. Functional domains and motifs of bacterial type III effector proteins and their roles in infection. FEMS Microbiol. Rev. 35:1100–1125 [DOI] [PubMed] [Google Scholar]
- 5. Depaolo RW, et al. 2008. Toll-like receptor 6 drives differentiation of tolerogenic dendritic cells and contributes to LcrV-mediated plague pathogenesis. Cell Host Microbe 4:350–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dziejman M, et al. 2005. Genomic characterization of non-O1, non-O139 Vibrio cholerae reveals genes for a type III secretion system. Proc. Natl. Acad. Sci. U. S. A. 102:3465–3470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Enninga J, Mounier J, Sansonetti P, Nhieu GTV. 2005. Secretion of type III effectors into host cells in real time. Nat. Methods 2:959–965 [DOI] [PubMed] [Google Scholar]
- 8. Espina M, et al. 2006. IpaD localizes to the tip of the type III secretion system needle of Shigella flexneri. Infect. Immun. 74:4391–4400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fields KA, Straley SC. 1999. LcrV of Yersinia pestis enters infected eukaryotic cells by a virulence plasmid-independent mechanism. Infect. Immun. 67:4801–4813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gotoh K, et al. 2010. Bile acid-induced virulence gene expression of Vibrio parahaemolyticus reveals a novel therapeutic potential for bile acid sequestrants. PLoS One 5:e13365 doi:10.1371/journal.pone.0013365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hiyoshi H, Kodama T, Iida T, Honda T. 2010. Contribution of Vibrio parahaemolyticus virulence factors to cytotoxicity, enterotoxicity, and lethality in mice. Infect. Immun. 78:1772–1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hiyoshi H, et al. 2011. VopV, an F-actin-binding type III secretion effector, is required for Vibrio parahaemolyticus-induced enterotoxicity. Cell Host Microbe 10:401–409 [DOI] [PubMed] [Google Scholar]
- 13. Iizumi Y, et al. 2007. The enteropathogenic E. coli effector EspB facilitates microvillus effacing and antiphagocytosis by inhibiting myosin function. Cell Host Microbe 2:383–392 [DOI] [PubMed] [Google Scholar]
- 14. Knutton S, et al. 1998. A novel EspA-associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. EMBO J. 17:2166–2176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kodama T, et al. 2008. Identification of two translocon proteins of Vibrio parahaemolyticus type III secretion system 2. Infect. Immun. 76:4282–4289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kodama T, et al. 2010. Two regulators of Vibrio parahaemolyticus play important roles in enterotoxicity by controlling the expression of genes in the Vp-PAI region. PLoS One 5:e8678 doi:10.1371/journal.pone.0008678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kodama T, et al. 2007. Identification and characterization of VopT, a novel ADP-ribosyltransferase effector protein secreted via the Vibrio parahaemolyticus type III secretion system 2. Cell. Microbiol. 9:2598–2609 [DOI] [PubMed] [Google Scholar]
- 18. Lee P-C, Stopford CM, Svenson AG, Rietsch A. 2010. Control of effector export by the Pseudomonas aeruginosa type III secretion proteins PcrG and PcrV. Mol. Microbiol. 75:924–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liverman ADB, et al. 2007. Arp2/3-independent assembly of actin by Vibrio type III effector VopL. Proc. Natl. Acad. Sci. U. S. A. 104:17117–17122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Makino K, et al. 2003. Genome sequence of Vibrio parahaemolyticus: a pathogenic mechanism distinct from that of V. cholerae. Lancet 361:743–749 [DOI] [PubMed] [Google Scholar]
- 21. Matteï P-J, et al. 2011. Membrane targeting and pore formation by the type III secretion system translocon. FEBS J. 278:414–426 [DOI] [PubMed] [Google Scholar]
- 22. Mills E, Baruch K, Charpentier X, Kobi S, Rosenshine I. 2008. Real-time analysis of effector translocation by the type III secretion system of enteropathogenic Escherichia coli. Cell Host Microbe 3:104–113 [DOI] [PubMed] [Google Scholar]
- 23. Mueller CA, Broz P, Cornelis GR. 2008. The type III secretion system tip complex and translocon. Mol. Microbiol. 68:1085–1095 [DOI] [PubMed] [Google Scholar]
- 24. Mueller CA, et al. 2005. The V-antigen of Yersinia forms a distinct structure at the tip of injectisome needles. Science 310:674–676 [DOI] [PubMed] [Google Scholar]
- 25. Park K-S, et al. 2004. Functional characterization of two type III secretion systems of Vibrio parahaemolyticus. Infect. Immun. 72:6659–6665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Parsot C, Hamiaux C, Page A-L. 2003. The various and varying roles of specific chaperones in type III secretion systems. Curr. Opin. Microbiol. 6:7–14 [DOI] [PubMed] [Google Scholar]
- 27. Qadri F, et al. 2003. Adaptive and inflammatory immune responses in patients infected with strains of Vibrio parahaemolyticus. J. Infect. Dis. 187:1085–1096 [DOI] [PubMed] [Google Scholar]
- 28. Ritchie JM, et al. Inflammation and disintegration of intestinal villi in an experimental model for Vibrio parahaemolyticus-induced diarrhea. PLoS Pathog. 8:e1002593 doi:10.1371/journal.ppat.1002593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sekiya K, et al. 2001. Supermolecular structure of the enteropathogenic Escherichia coli type III secretion system and its direct interaction with the EspA-sheath-like structure. Proc. Natl. Acad. Sci. U. S. A. 98:11638–11643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Su Y-C, Liu C. 2007. Vibrio parahaemolyticus: a concern of seafood safety. Food Microbiol. 24:549–558 [DOI] [PubMed] [Google Scholar]
- 31. Trosky JE, et al. 2004. Inhibition of MAPK signaling pathways by VopA from Vibrio parahaemolyticus. J. Biol. Chem. 279:51953–51957 [DOI] [PubMed] [Google Scholar]
- 32. Wolff C, Nisan I, Hanski E, Frankel G, Rosenshine I. 1998. Protein translocation into host epithelial cells by infecting enteropathogenic Escherichia coli. Mol. Microbiol. 28:143–155 [DOI] [PubMed] [Google Scholar]
- 33. Zhou X, Shah DH, Konkel ME, Call DR. 2008. Type III secretion system 1 genes in Vibrio parahaemolyticus are positively regulated by ExsA and negatively regulated by ExsD. Mol. Microbiol. 69:747–764 [DOI] [PMC free article] [PubMed] [Google Scholar]