Abstract
Recent studies suggest shared pathogenic pathways during malaria and allergy. Indeed, IgE, histamine, and the parasite-derived Plasmodium falciparum histamine-releasing factor translationally controlled tumor protein (PfTCTP) can be found at high levels in serum from patients experiencing malaria, but their relationship with basophil activation remains unknown. We recruited P. falciparum-infected patients in Senegal with mild malaria (MM; n = 19) or severe malaria (SM; n = 9) symptoms and healthy controls (HC; n = 38). Levels of serum IgE, PfTCTP, and IgG antibodies against PfTCTP were determined by enzyme-linked immunosorbent assays (ELISA). Basophil reactivities to IgE-dependent and -independent stimulations were measured ex vivo using fresh blood by looking at the expression level of the basophil activation marker CD203c with flow cytometry. Unstimulated basophils from MM had significantly lower levels of CD203c expression compared to those from HC and SM. After normalization on this baseline level, basophils from SM showed an enhanced reactivity to calcimycin (A23187) and hemozoin. Although SM reached higher median levels of activation after anti-IgE stimulation, great interindividual differences did not allow the results to reach statistical significance. When primed with recombinant TCTP before anti-IgE, qualitative differences in terms of a better ability to control excessive activation could be described for SM. IgE levels were very high in malaria patients, but concentrations in MM and SM were similar and were not associated with basophil responses, which demonstrates that the presence of IgE alone cannot explain the various basophil reactivities. Indeed, PfTCTP could be detected in 32% of patients, with higher concentrations for SM. These PfTCTP-positive patients displayed significantly higher basophil reactivities to any stimulus. Moreover, the absence of anti-PfTCTP IgG was associated with higher responses in SM but not MM. Our results show an association between basophil reactivity and malaria severity and suggest a pathogenic role for plasmodial PfTCTP in the induction of this allergy-like mechanism.
INTRODUCTION
Severe forms of Plasmodium falciparum malaria are still responsible for 1 million deaths each year, mainly in African countries (43). Acquisition of a clinical immunity is the result of a tightly regulated balance between pro- and anti-inflammatory signals. As 20% of patients admitted to intensive care units still succumb to malaria despite administration of effective antiplasmodial drugs, understanding the immunopathogenic mechanisms associated with severe malaria cases (SM) is of major importance to identify new therapeutic targets aiming at reducing disease mortality.
Recent findings have raised the hypothesis that clinical susceptibility to malaria may be related to allergy-type responses. In a family-based genetic study, Sakuntabhai et al. identified a significant linkage between the number of malaria attacks and loci previously related to allergic susceptibility (36). In mouse models of malaria infection, histamine seems to be crucial, as mice deficient for the histamine-producing enzyme, or wild-type mice treated with antihistaminic compounds, failed to develop cerebral symptoms (1). These findings are in accordance with the high level of circulating histamine reported in patients with severe malaria (10, 39) and the marked elevation of Plasmodium-specific IgE during malaria attacks. However, association of these IgE responses with protection (2, 19) or pathogenicity (7, 27, 30, 38) remains controversial. Antimalarial IgE is able to induce ex vivo an FcεRI-dependent release of interleukin-4 (IL-4) from basophils of healthy volunteers (26) and an FcεRII-dependent release of tumor necrosis factor alpha (TNF-α) by mononuclear cells (28, 29), but our knowledge of IgE functionality is still very limited, and the reactivity of peripheral blood basophils from humans experiencing malaria has never been studied. Moreover, the discovery of a plasmodial homolog of the human histamine-releasing factor translationally controlled tumor protein (hTCTP), Plasmodium falciparum TCTP (PfTCTP), which is secreted in vivo and enhances basophil responses to IgE-dependent challenge in vitro (23), reinforces the need to explore these pathways, as it could represent a significant player in the induction of allergic-type responses during malaria.
In this work, we used for the first time with malaria patients a flow cytometric technique that allows the reliable detection and quantitation of basophil activation in blood samples. We compared basophil reactivities to IgE-dependent and IgE-independent stimulations in three groups of individuals with different clinical presentations, looked for the relationship between basophil responses, IgE levels, and PfTCTP, and discuss our results with regard to malaria severity.
MATERIALS AND METHODS
Patients.
Patients were recruited in Dakar (Senegal) during the rainy season. All patients or their relatives signed an informed consent form before participation, and this protocol was approved by the Senegalese Ethics Committee.
Severe malaria cases (SM) were recruited at the intensive care unit of the “Service des Maladies Infectieuses” (CHNU de Fann). Initially, we decided to enroll each patient attending with an axillary temperature > 37.5°C, a positive blood smear for Plasmodium falciparum, and at least one clinical sign of severity as defined by WHO criteria (impaired consciousness, prostration, respiratory distress, organ failure, etc.). Due to the similarity of symptoms to those of arboviral fevers, all plasma samples (and cerebrospinal fluid samples when available) from SM were screened for IgM against the six most prevalent arboviruses in Senegal (dengue virus, yellow fever virus, Rift Valley fever virus, Crimean-Congo hemorrhagic fever virus, West Nile virus, and Chikungunya virus). Mild malaria cases (MM) were recruited consecutively among patients attending the St Martin dispensary in Dakar with fever (axillary temperature > 37.5°C) and a rapid diagnostic test (RDT) positive for P. falciparum, without signs of severity. RDT results were secondarily confirmed by microscopic examination of blood smears.
Healthy controls (HC) were recruited at the Laboratoire d'Analyses Médicales, Institut Pasteur de Dakar, from healthy subjects attending the laboratory for routine biological examination. None of them displayed fever or other signs of infection or disease. Absence of Plasmodium in their blood was confirmed by quantitative PCR (qPCR) detection (40). No subjects were under medical treatment.
Antigen preparation and reagents.
Goat polyclonal anti-human IgE and calcimycin (calcium ionophore A23187) were purchased from Sigma. Hemozoin (HZ) was prepared from supernatants of P. falciparum (strain 3D7) in vitro culture at 1% parasitemia. Supernatants were pooled and centrifuged twice at 200 × g for 5 min to remove erythrocyte debris and merozoites. Pellet was submitted to 3 cycles of washing in sterile water (centrifugation at 700 × g for 10 min) before storage at −20°C. Before use, HZ was diluted at 1:10 in phosphate-buffered saline (PBS). A single preparation was used for all experiments.
A P. falciparum total antigen extract was prepared from synchronized cultures (strains 3D7 and W2). Infected erythrocytes were concentrated using a VarioMACS column (Miltenyi Biotech) at a final parasitemia level of 90%. They were pooled, washed with PBS, and lysed with 3 cycles of freeze-thaw. Debris was removed by two centrifugation cycles at 200 × g for 5 min, and extracts were diluted in sterile PBS to the final concentration of 5 mg/ml. Aliquots were stored at −20°C until use.
Production and purification of recombinant TCTPs (rTCTPs) and anti-PfTCTP monoclonal antibodies.
The pGEX-2T vector carrying the human TCTP coding sequence was obtained from MacDonald (24). We constructed the PfTCTP expression plasmid ourselves by inserting the PfTCTP coding sequence (NCBI accession number XM_001351631.1) into a pET16b (Novagen) vector carrying a His10 tag and transfected it into Escherichia coli BL21. After expression in IPTG (isopropyl-β-d-thiogalactopyranoside) medium, recombinant proteins were purified on glutathione-Sepharose (GST) or Talon metal affinity columns for GST-hTCTP or PfTCTP-His10, respectively. Endotoxins were removed by washing the columns with 0.1% cold Triton X-114 and then by loading recombinant proteins on a polymyxin-agarose column (Detoxi-Gel; Pierce). Final endotoxin levels were lower than 5 U/mg (Limulus amebocyte lysate assay; Lonza).
Production of monoclonal antibodies against PfTCTP was performed at Institut Pasteur de Paris and followed ethical laws. Briefly, mice were immunized with 10 μg of recombinant protein in complete Freund adjuvant, followed by 3 further injections in incomplete adjuvant at 15-day intervals. The polyclonal response was checked by enzyme-linked immunosorbent assay (ELISA), and mice showing the best response were chosen for fusion. Splenocytes were harvested, isolated, and fused with myeloma cells (5/1) by the use of polyethylene glycol and standard protocols (Sigma). Fused cells were then diluted in selective medium (azaserine-hypoxanthine) and distributed in 24-well plates at 105 cells per well. After 10 days of culture, antibody-secreting cells were screened by ELISA. Positive hybridomas were cloned by limit dilution, and the supernatants were tested by ELISA after 12 to 15 days. For antibody production, positive clones were injected into mice pretreated with pristane. Each mouse received 5 million cells, and ascites were collected after 15 days. Monoclonal antibodies against PfTCTP were counterselected against hTCTP to avoid cross-reactivity. Two clones (H13-53 and J15-65) were selected.
Serum IgE and tryptase measurements.
Total IgE and tryptase concentrations were measured using an ImmunoCAP immunoassay (Phadia, Sweden). The plasmodium-specific IgE concentration was determined by ELISA. Briefly, 96-well plates (Nunc Maxisorp) were coated overnight at 4°C with 50 μl of P. falciparum antigens (10 μg/ml) diluted in PBS and then blocked for 1 h at 37°C with PBS-Tween (PBT) (0.05%) plus 5% bovine serum albumin (BSA). Serum samples were diluted 1:5 in PBT–BSA (0.5%) (PBTB) in duplicate on the coated plates and incubated overnight at room temperature. A mouse IgG anti-human IgE (diluted 1:500 in PBTB) was further incubated for 3 h at 37°C followed by incubation with a horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (diluted 1:500 in PBTB for 1 h at 37°C) and revealed with a standard TMB (3,3′,5,5′-tetramethylbenzidine) procedure. On each plate, a mixture representing a standard curve was coated using human myeloma IgEs. The concentration of P. falciparum-specific IgE in serum samples was calculated with GraphPad Prism 5.0 using a 4th-order polynomial equation interpolation of the absorbance values. The threshold of detection (5 ng/ml) was calculated as the mean absorbance of a pool of negative serum from European donors who had never experienced malaria (+ 3 standard deviations [SD]).
PfTCTP and anti-PfTCTP IgG measurement.
Plates were coated overnight at 4°C with 50 μl of H13-53 (10 μg/ml) and then blocked with PBTB (1%) for 2 h at 37°C. Serum samples (dilution, 1:20 in PBTB) were added for 1 h 30 min followed by addition of biotinylated J15-65 (2 μg/ml) for an additional hour and HRP-streptavidin (1:2,000 in PBTB) for 1 h. The reaction was revealed as described above. A standard curve was established on each plate by diluting known concentrations of rPfTCTP in naïve serum from European healthy donors. The threshold for positivity calculated as described above was 50 ng/ml.
For anti-PfTCTP detection, plates were coated overnight at 4°C with 50 μl of rPfTCTP (1 μg/ml). After 2 h of saturation with PBTB (1%) at 37°C, sera (diluted 1:1,000 in PBTB) were incubated for 1 h and then rabbit anti-human HRP-IgG (diluted 1:5,000 in PBTB) was added for 1 h and revealed as described above. The threshold of positivity was calculated as described for IgE, and concentrations were expressed as the ratio between the mean optical density (OD) of the positive sample and the mean OD of the negative serum samples.
Basophil activation tests.
Basophil activation was measured following manufacturer's recommendations, using a flow cytometry method which relies on the expression of the activation marker CD203c (Allergenicity kit; Beckman Coulter). A Flow2CAST kit from Bühlmann (activation marker CD63) was also used in preliminary experiments performed on European donors, following the manufacturer's recommendations. All samples were kept at room temperature, and much attention was paid to avoidance of shaking during transportation between sites. Samples were processed within the 4 h following blood collection. Whole-blood samples were incubated for 15 min with 20 μl of rTCTP or PBS before stimulation with 20 μl of A23187, hemozoin, or anti-IgE (aIgE) for 15 min. Each concentration of these 20 μl of stimuli was adapted in order to reach the desired final concentrations in each well. Data acquisition was done for 500 basophils. Samples with less than 300 recorded basophils were not retained for analysis. Basophils were identified on dot plots as lowSSC/CD3−/CRTH2+/CD203c+ cells (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). Data were analyzed using FCS Express version 3 (DeNovo Software). For each set of stimulation conditions, the CD203c geometric mean fluorescence intensity (MFI) was registered. The CD203c ΔMFI (MFI of stimulated sample minus MFI of unstimulated sample) was then calculated and retained for analysis of basophil reactivity.
Statistical analysis.
Medians of comparisons between two or multiple groups of unpaired data were determined using a nonparametric Mann-Whitney test or a Kruskal-Wallis unpaired rank test, respectively. Fisher's exact test was used to compare frequencies, the Student t test for comparisons of means, and the Spearman rank test for correlations. Statistics and graphics were performed with GraphPad Prism version 5.0.
RESULTS
Control of rPfTCTP biological activity.
Basophil activation tests were first conducted on whole-blood samples from four European, nonallergic blood donors by using in parallel the activation markers CD63 or CD203c, and their validity was assessed for our study. We also used hTCTP as a positive control, which gave the same responses as PfTCTP (not shown). We found a good correlation between the results determined with the two markers (r > 0.77; P < 0.01), with a slightly higher sensitivity for CD203c, which is why we chose this marker for malaria samples. Whatever the marker, large interindividual variations in terms of the magnitude of the response and sensitivity to anti-IgE (aIgE) were observed, but bell-shaped dose-response curves were a common feature (Fig. 1). Stimulation with PfTCTP or hTCTP alone never induced basophil activation, which confirmed the goodness of the results with respect to removal of endotoxins from our recombinant preparations. As expected, PfTCTP priming enhanced aIgE responses by 10% to 75%. It also decreased the concentration of aIgE needed to obtain the maximal response (optimal concentration) up to two times (1 to 0.5 μg/ml with the highest concentration of PfTCTP), thus shifting the curve to the left (Fig. 1). As a result, for an equivalent aIgE concentration (1 μg/ml), enhancing the PfTCTP concentration could result in a lower response. This earlier engagement of the supraoptimal phase characterized by a decreasing response to higher stimulation served as a conceptual frame to interpret subsequent results from malaria patients.
Fig 1.
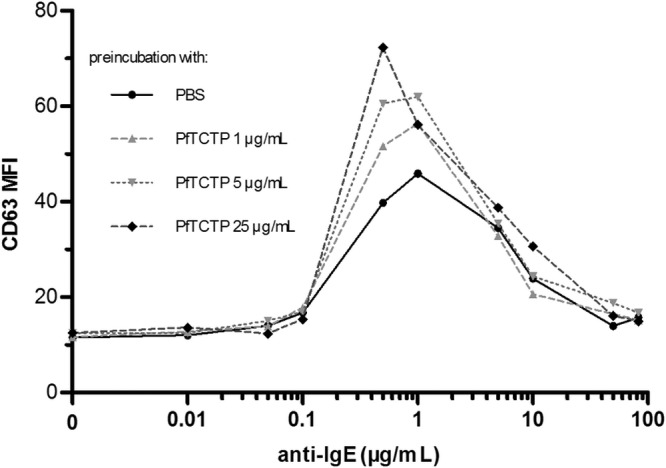
Effect of PfTCTP priming on basophil response to anti-IgE challenge in a representative individual. Basophils were preincubated with 3 concentrations of PfTCTP before challenge with 9 anti-IgE (aIgE) concentrations. In this representative experiment performed on a European nonallergic donor, basophil activation was evaluated by measuring the level of CD63 expression. Preincubation with PfTCTP enhanced aIgE responses and decreased the optimal aIgE concentration up to 2 times. Experiments using CD63 or CD203C as an activation marker were performed in parallel, and the results were well correlated (Spearman's correlation coefficient r > 0.77; P < 0.01). This figure shows only the results determined with CD63, as it was the more representative of the two activation markers.
Characteristics of patients, serology, and basophils.
Thirty-four healthy controls (HC), 19 mild malaria cases (MM), and 9 severe malaria cases (SM) were included in our study. Although enrollment criteria were capacious, all patients with SM displayed an impaired consciousness. They were seronegative for arbovirus. The data for mean ages, temperatures, and parasitemias were similar in MM and SM, but the two groups differed with respect to the delay between the beginning of symptoms and consultation (5.3 days versus 2.2 days for SM and MM, respectively; P = 0.01) (Table 1).
Table 1.
Characteristics of patients included in the studya
| Parameter | Value(s) for group |
P value |
|||
|---|---|---|---|---|---|
| Healthy controlsb | Mild malariac | Severe malariad | All groups | MM vs SM | |
| Patients | |||||
| Age in yr (mean) | 36.0 (19.0) | 25.6 (20.9) | 28.2 (12.1) | 0.16 | 0.69 |
| No. of males/no. of females | 17/17 | 13/6 | 6/3 | 0.36 | 0.27 |
| Mean temp (°C) | <37.5 | 38.9 (0.8) | 39.1 (1.2) | <0.001 | 0.76 |
| % parasitemia (mean) | 0.0 | <0.01 | <0.01 | 0.82 | |
| Mean duration of illness (days) | 2.2 (1.1) | 5.3 (3.0) | 0.01 | ||
| Serologye | |||||
| IgEtot (ng/ml) | 216 (41–2,020) | 777 (19–4,500) | 1,586 (107–2,371) | 0.01 | 0.64 |
| IgEspe (ng/ml) | 7.5 (0–27.5) | 33.5 (6–92.8) | 26.2 (19.3–32.2) | <0.001 | 0.18 |
| IgEspe/IgEtot (%) | 5.2 (0–29.9) | 2.9 (0.7–84.3) | 1.8 (1.0–24.4) | 0.57 | 0.33 |
| Tryptase (ng/ml) | 4.34 (1.15–10) | 3.02 (1.34–8.82) | 2.89 (1–3.99) | 0.02 | 0.48 |
| Basophilse | |||||
| % of all leukocytes | 0.54 (0.22–1.62) | 0.29 (0.02–0.59) | 0.11 (0.03–0.37) | <0.001 | 0.14 |
| CD203c MFI | 81.4 (33.8–134.2) | 43.2 (21.2–132.2) | 89.3 (36.0–200.0) | 0.02 | 0.02 |
ANOVA (patients) or Kruskal-Wallis tests (serology and basophils) were used for comparisons between the three groups. MM and SM data were compared using Student t tests (patients) or Mann-Whitney U tests (serology and basophils).
n = 34, 23, and 19 for patient data, serology data, and basophil data, respectively.
n = 19, 19, and 14 for patient data, serology data, and basophil data, respectively.
n = 9, 9, and 8 for patient data, serology data, and basophil data, respectively.
Data represent median values (minimum-maximum).
Serologic studies could be performed on 51 subjects, and interpretable results for basophil analysis were obtained for 41 subjects. MM and SM patients presented a significantly higher level of total and malaria-specific IgE than HC but without differences between the two groups (Table 1). The proportions of anti-plasmodial IgE and total IgE were similar in the three groups, with a positive correlation between total and specific IgE (r = 0.40; P < 0.01). Considering all groups together, total IgE levels increased with age (r = 0.32; P = 0.02) whereas specific IgE levels decreased (r = −0.31; P = 0.02), resulting in a decreasing ratio of specific/total IgE with age (r = −0.35; P = 0.02). Tryptase levels decreased significantly from HC to SM, although MM and SM data did not display significant differences (Table 1).
A significant decrease from the HC to the MM and SM groups in the number of circulating basophils was also observed (P < 0.001 for all). However, SM patients displayed significantly higher CD203c MFI than MM patients (P = 0.02), representing higher activation of circulating basophils. Duration of illness, age, parasite count, fever, and IgE levels had no effect on basophil count or basal CD203c expression (P > 0.35 for all).
Basophil reactivities to IgE-independent and -dependent stimulations.
To assess basophil responses to IgE-independent stimuli, we first used calcimycin (calcium ionophore; A23187). SM were highly reactive to this stimulus (ΔMFI = 30), whereas HC and MM were poorly sensitive (ΔMFI = 5; P = 0.01). Basophil responses to the plasmodial antigen hemozoin (HZ) were then assessed and shown to be higher in SM basophils (ΔMFI = 86) than in MM basophils (ΔMFI = 17; P = 0.01) (Fig. 2).
Fig 2.
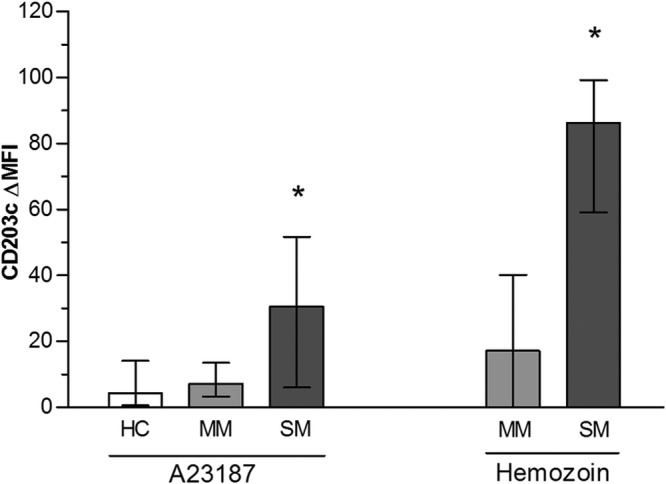
Basophil reactivity to IgE-independent stimulation. Blood samples from healthy controls (HC), mild malaria cases (MM), and severe malaria cases (SM) were stimulated for 15 min with A23187 calcium ionophore (1 μg/ml) or hemozoin (HZ). Histograms represent median values calculated as [MFI (stimulated) − MFI (unstimulated)], and error bars are for interquartile ranges. For technical reasons, reactivity to HZ could not be tested in HC. *, significant difference between SM and other groups (P = 0.01; Mann-Whitney test).
For all groups, basophil responses to IgE-dependent stimulation significantly increased with 1 and 10 μg/ml of aIgE. A higher amplitude of median response to aIgE was observed in SM than in HC or MM (ΔMFI = 171, 105, or 98, respectively) (Fig. 3A); however, this difference did not reach statistical significance due to great variability of individual responses (Fig. 3B). We classified these individual responses to aIgE into two categories according to the shapes of dose-response curves.
Fig 3.
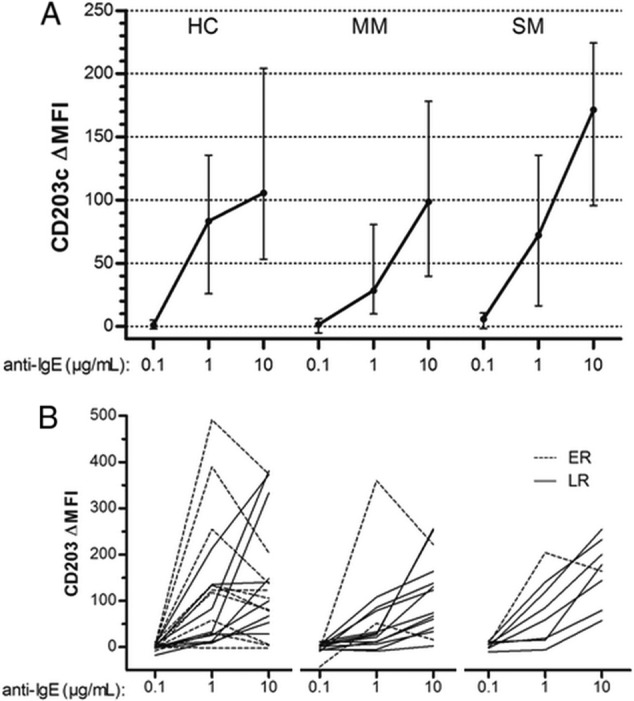
Basophil reactivity to anti-IgE challenge. Blood samples from healthy controls (HC), mild malaria cases (MM), or severe malaria cases (SM) were stimulated for 15 min with anti-IgE at 0.1, 1, and 10 μg/ml. (A) Median levels of CD203c ΔMFI with interquartile ranges (error bars). (B) Individual variations of CD203c ΔMFI. Continuous lines represent late responders (LR; see text), and dashed lines are for early responders (ER; see text).
Early responders (ER) were defined as basophils that reached the supraoptimal phase when stimulated with more than 1 μg/ml of aIgE while late responders (LR) were still in the suboptimal phase, with an increasing response. ER were more frequently found in healthy than in infected individuals (6/19 HC, 2/14 MM, and 1/8 SM) and displayed a higher reactivity to aIgE stimulation at 1 μg/ml (3.1-fold increase of MFI versus 1.7-fold increase for LR; P < 0.01) (Fig. 3B). No significant differences between ER and LR in IgE levels, age, duration of illness, fever, parasite count, or basophil percentage were found, whatever the clinical group (P > 0.35 for all).
PfTCTP and anti-PfTCTP antibodies are associated with basophil reactivity in vivo.
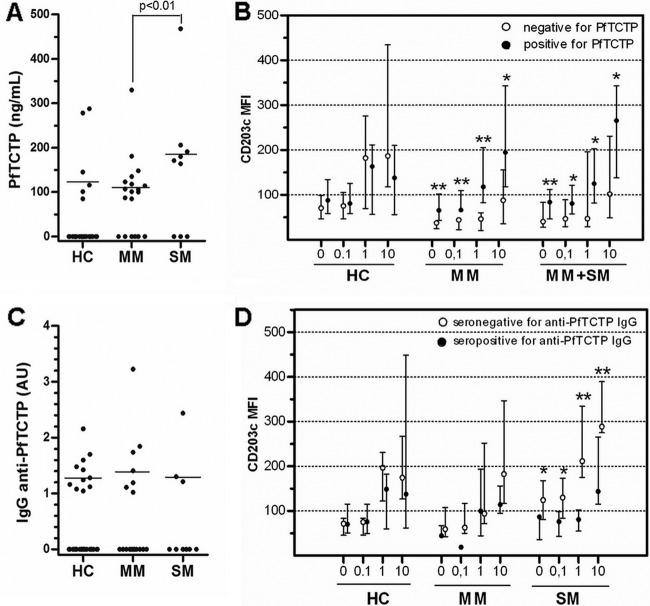
PfTCTP was detected in 32% of infected patients (PfTCTP positive [PfTCTP+], 6/19 MM and 3/9 SM; Fig. 4A), with a higher level in SM than in MM (171 versus 101 ng/ml; P < 0.01). Unexpectedly, 6 out of 33 uninfected HC (18%) were also PfTCTP+. Anti-PfTCTP IgG was detected in 21 out of 59 sera (36%; Fig. 4C), with both similar frequencies and similar median concentrations in each group. Age, fever, duration of illness, parasite count, and IgE levels were not related to the presence or concentration of PfTCTP or anti-PfTCTP IgG.
Fig 4.
Basophil reactivity to anti-IgE according to the presence of PfTCTP or anti-PfTCTP IgG. (A and C) Subjects who had undetectable PfTCTP antibodies (A) or anti-PfTCTP antibodies (C) are plotted on the graph at y = 0. Horizontal lines represent the median levels among seropositive individuals. A statistical comparison (Mann-Whitney test) between MM and SM among these seropositive individuals was made. (B and D) Blood samples were stimulated for 15 min with PBS (0) or anti-IgE at 0.1, 1, and 10 μg/ml. Subjects are separated into two classes according to the presence (full circles) or absence (empty circles) of detectable levels of PfTCTP (B) or anti-PfTCTP IgG (D). **, significant differences in median MFI between seropositive and seronegative groups (P < 0.05; Mann-Whitney test); *, differences with borderline significance (0.055 < P < 0.078; Mann-Whitney test). Comparisons between PfTCTP-seronegative and -seropositive subjects could not be made in SM, because only one subject tested for CD203c expression was seronegative, and then MM and SM data were merged (MM+SM).
PfTCTP+ malaria patients (MM plus SM) exhibited higher basophil activation than PfTCTP− patients (i) before stimulation (CD203c MFI, 84 versus 41; P = 0.04; Fig. 4B), (ii) after stimulation with aIgE (Fig. 4B), and (iii) after stimulation with A23187 (CD203c MFI, 80 versus 43; P = 0.02; data not shown). No difference was found for HC, whatever the stimulus.
Similarly, in SM, being seropositive for anti-PfTCTP IgG was associated with a 1.6-fold reduction of basophil response after stimulation with A23187 (data not shown) and 2.6- and 2.0-fold reductions after stimulation with anti-IgE at 1 and 10 μg/ml (Fig. 4D), respectively (P = 0.02 for all), while this had no impact on the activation level of unstimulated basophils. This relationship between the presence of anti-PfTCTP IgG and basophil activation or reactivity was found in neither HC nor MM (Fig. 4D).
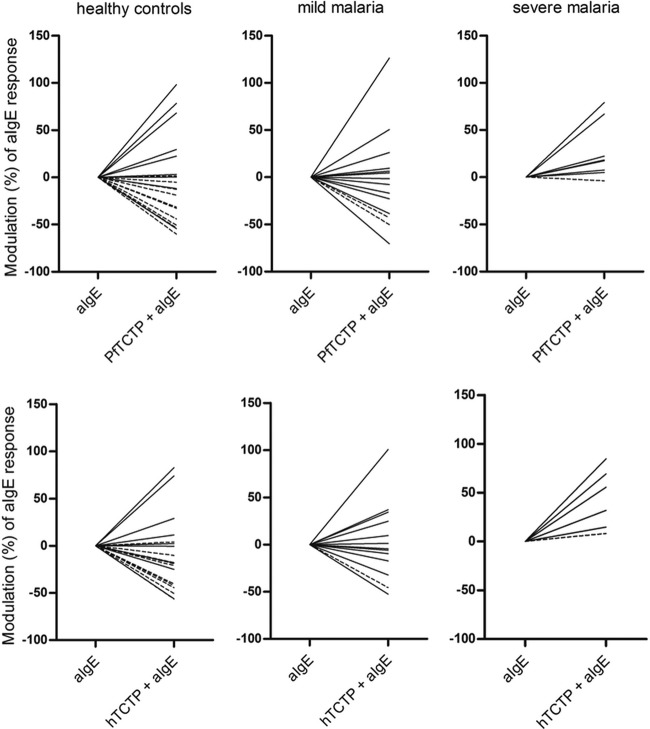
A further priming of cells ex vivo with rTCTP confirmed these differential profiles of basophil reactivities. As for European donors, human or plasmodial rTCTP alone (1 and 10 μg/ml; not shown) had no effect on basophils. rTCTP priming (1 μg/ml) induced either an increase or a decrease in basophil response to aIgE, ranging from −75% to 125% (Fig. 5). In HC and MM subjects, the majority of basophils (all ER plus some LR) stimulated with 1 μg of aIgE/ml after rTCTP priming were engaged in the supraoptimal phase characterized by reduced activation. However, for SM, rTCTP priming constantly enhanced basophil responses to aIgE. This sensitivity to rTCTP priming was not related to age, IgE levels, fever, parasite count, or basal CD203c expression or to the presence of native PfTCTP in blood.
Fig 5.
Impact of TCTP priming ex vivo on reactivity to anti-IgE challenge. After 15 min of preincubation (priming) with PBS or rTCTP (top, hTCTP; bottom, PfTCTP) at 1 μg/ml, blood samples were stimulated with anti-IgE (aIgE) at 1 μg/ml for 15 min. Modulation of the aIgE response due to TCTP priming is represented in percentages of inhibition or enhancement. Dashed and continuous lines represent ER and LR, respectively.
DISCUSSION
Basophils have been poorly studied in the context of malaria, and, to our knowledge, this report is the first to describe basophil activation in P. falciparum-infected individuals. We provide evidence that patients with severe malaria display enhanced basophil reactivities, likely associated with an inability to control excessive activation, and that the plasmodial cytokine-like PfTCTP might play an important role in the modulation of these responses. During this work, we took advantage of a flow cytometry strategy that is widely used in allergy studies to analyze basophils separately from other leukocytes in whole blood and to assess their reactivity to allergens (4, 35). In these studies, it is widely accepted that expression of the basophil activation marker CD203c is well correlated with histamine release, which is no longer analyzed due to its great instability during storage. This strategy has begun to be more widely used in studies of other pathologies, such as helminth infections (11) or chronic idiopathic urticaria (22), although their clinical presentation and pathogenesis differ from those of malaria.
We observed a decrease in the number of peripheral blood basophils that was related to the severity of symptoms. Such a decrease may be due to the recruitment and accumulation of these cells in tissues. Indeed, IL-3-activated endothelial cells can selectively promote the in vitro interaction and adhesion of basophils (21), which can then cross the endothelium (17). This mechanism is relevant in the inflammatory context of malaria, where sequestration of infected erythrocytes to microvascular sites is associated with local activation. Basophils may thus accumulate to these sites of inflammation. Another study in a mouse model has reported a significant accumulation of basophils in the spleen during malaria (31), and this could account for a reduction of their numbers in peripheral blood. Altogether, these hypotheses of local arrest on the endothelium or recruitment in a secondary lymphoid organ imply that a modification of the basophil activation status occurs during malaria.
Our findings confirm this change. In addition to basophils from mild malaria cases (MM) displaying a lower activation status than healthy controls (HC) and severe malaria cases (SM), basophils from SM were found to be significantly more reactive to calcimycin and hemozoin than basophils from MM or HC. Activation of basophils by hemozoin, an inorganic byproduct of hemoglobin digestion released during erythrocyte rupture, has not previously been described. Hemozoin can induce IgG- and IgM-specific immune responses (3) and, following phagocytosis, an activation of dendritic cells (DC) via Toll-like receptor 9 (TLR9) (8, 9) and an activation of monocytes (33), both of which result in the production of inflammatory mediators such as TNF-α. Basophils might be sensitive to TNF-α, but it is unlikely that this indirect activation occurred within our short stimulation times. This issue requires further exploration.
IgE-dependent basophil activation relies on cross-linking of the high-affinity IgE receptor. It is a highly regulated process with a bell-shaped dose-response curve, with supraoptimal stimulations leading to reduced activation, as displayed in our preliminary experiment performed on European donors. This supra-activation—related to desensitization (25)—is associated with endogenous inhibitory mechanisms that overtake the activating signals above a threshold of stimulation, among which the SHIP protein plays a central role (13). Similar intracellular pathways determine basophil releasability, i.e., intensity of the response to a given stimulus (15, 16, 41), providing an intimate link between the amount of released mediators and the cell's ability to control its response to an excessive stimulation.
Although basophils from SM could reach higher median levels of activation than those from MM following anti-IgE stimulation, the great interindividual differences prevented the results from reaching statistical significance. Great variation in individual basophil responses is a common feature. However, this might represent a limitation of the study due to a lack of enrollment of well-defined patients in Dakar, in a context of low and seasonal transmission of malaria. Ex vivo priming of basophils with recombinant TCTP prior to anti-IgE stimulation shed light on another difference between MM and SM basophils. It is striking that the majority of MM basophils displayed a reduced response to the same anti-IgE concentration when primed with TCTP, as if MM basophils had been stimulated with a 10-times-higher concentration of anti-IgE, whereas SM basophils displayed a continuous increase in activation. This suggests that, during severe malaria, the endogenous inhibitory mechanisms leading the supra-activation phase are dampened. As IgE levels were high but similar between MM and SM, with no correlation to basophil responses, it is therefore difficult to assign a direct role of IgE concentration to account for this difference.
High serum concentrations of PfTCTP were reported in malaria patients (23), with the same priming function as seen with its human counterpart. Therefore, it has been hypothesized that PfTCTP could prime basophils in vivo. Our recombinant preparation confirmed this priming effect and the relevance of flow cytometry to explore it. In accordance with the results reported by MacDonald et al. (23), we found that not all infected subjects had a detectable level of PfTCTP and that concentrations were not linked to parasitemia. However, the low levels of parasitemia reported here may underestimate the real parasite biomass, because of likely parasite sequestrations. These low levels of parasitemia (related to a low transmission level in Senegal) may also help to explain the lower PfTCTP concentrations we found compared to Malawian patients. Unexpectedly, we also found detectable levels of PfTCTP in some HC individuals. The monoclonal antibodies used in our assay were selected on the basis of the absence of cross-reactivity with human TCTP, but we cannot exclude the possibility that a cross-reaction with TCTP from other prevalent organisms occurred as schistosomiasis (14, 34). Moreover, such a result could be due to a past asymptomatic Plasmodium infection, as PfTCTP is still detectable up to 14 days after parasite clearance (personal observations).
Indeed, our report is the first to note an association of PfTCTP with enhanced basophil reactivities in vivo. We hypothesize that, in addition to the generalized inflammatory context present during SM, the continuous exposure of basophils to PfTCTP has been responsible for an attenuation of the inhibitory mechanisms which control releasability and desensitization, leading to an increase in basophil responsiveness in the same way as occurs with IL-3 (6, 20, 42). Consistently, the presence of anti-PfTCTP antibodies in serum is negatively correlated with these reactivities in SM. These antibodies could prevent the TCTP dimerization necessary to its function (18), thus avoiding further basophil activation during SM, while in MM, this protective effect would be dispensable due to the cell's intrinsic ability to limit its ongoing responses.
Overall, what would be the significance of these results? Ex vivo, activated basophils release histamine and cytokines in amounts that positively correlate with the level of CD203c expression (22). In addition to various immunomodulatory functions, histamine enhances endothelial permeability and has been associated with many pathophysiological features of cerebral malaria in a mouse model (1). Moreover, in vitro, histamine-activated endothelial cells almost instantly acquire a proadhesive state that allows the sequestration of Plasmodium-infected erythrocytes via the release of Von Willebrand strings and platelet tethering (5). Basophils are also the most important producers of IL-4 in the body; thus, they might play a crucial role in the initiation of Th2 responses (12), facilitating IgE class switching and Th1/Th2 imbalance (26). Whatever the consequences of basophil activation, these two potentially important mechanisms could play a pathogenic role throughout the disease and support the notion that basophil responsiveness has to be tightly regulated.
Unfortunately, measuring the levels of circulating histamine was not feasible in the context of our study. Blood samples were collected in health structures, and biological analysis was performed at a distant site within the 4 h following blood collection. Histamine has a very short half-life and great instability ex vivo. To be relevant, histamine measurements have to be performed on blood samples processed in the hour following collection, which was hardly feasible. In clinical studies of immediate allergic reactions, histamine measurement is progressively replaced by measurement of the level of tryptase, which is more stable and correlates well with the level of histamine released by mast cells during anaphylactic shock (37). We found that tryptase levels paralleled the decrease of the basophil percentage among leukocytes associated with disease severity (data not shown) and did not help to resolve the issue of histamine release by basophils during malaria.
In conclusion, although patients with mild malaria and patients with severe malaria harbored similarly high levels of serum IgE, their intrinsic basophil reactivities clearly differed. Our findings could help to improve understanding of the conflicting results from reports of the association between IgE levels and malaria severity. As for allergies, they suggest that activation of basophils, enhanced by the presence of plasmodial PfTCTP, might play an important role in malaria pathogenesis, in relation with the genetic or immunological background of each individual. Therefore, as seen with allergy, not all patients should have the same probability of developing SM. This consideration could help to reconcile our results with those obtained in a murine model whose allergic susceptibility remains to be determined (32). Further studies are required to identify the determinants of basophil sensitization, such as mosquito bites (D. Aldebert, unpublished data), allergen sensitization, or helminth coinfection, in areas of malaria endemicity, but this new field of investigation has already provided additional keys for a better understanding of malaria immunopathogenesis.
ACKNOWLEDGMENTS
We are grateful to Aissatou Toure for providing access to her laboratory and to Amadou Alpha Sall for viral assays. We are also grateful to the medical staff who helped in the recruitment of patients, in particular, Stella Ndene, Benoit Garin, Marie-Madeleine Ndour, Rose Ndour, and Viviane Dos Santos. Many thanks to all patients and their companions for their participation in the study. We thank Susan MacDonald for the gift of the plasmid producing human TCTP. Thanks to Beckman and Buhlmann for providing some basophil activation kits. Many thanks to Eric Baret for his technical help in antigen preparation, and thanks to Véronique Durand and Caroline Maier from Becton, Dickinson, for their support regarding flow cytometry.
This study received financial contribution from PEA 06-CO 2008 and APRI for biological studies in Marseille, from the Institut Pasteur Grands Projets Horizontaux Anopheles for the clinical studies, and from Genopole Pasteur (PF5), which supported cloning and expression of recombinant proteins and monoclonal antibodies. S.P. was supported by a grant from Lavoisier Program and a grant from Fondation des Treilles.
Footnotes
Published ahead of print 2 July 2012
REFERENCES
- 1. Beghdadi W, et al. 2008. Inhibition of histamine-mediated signaling confers significant protection against severe malaria in mouse models of disease. J. Exp. Med. 205:395–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bereczky S, et al. 2004. Elevated anti-malarial IgE in asymptomatic individuals is associated with reduced risk for subsequent clinical malaria. Int. J. Parasitol. 34:935–942 [DOI] [PubMed] [Google Scholar]
- 3. Biswas S, Karmarkar MG, Sharma YD. 2001. Antibodies detected against Plasmodium falciparum haemozoin with inhibitory properties to cytokine production. FEMS Microbiol. Lett. 194:175–179 [DOI] [PubMed] [Google Scholar]
- 4. Boumiza R, Debard AL, Monneret G. 2005. The basophil activation test by flow cytometry: recent developments in clinical studies, standardization and emerging perspectives. Clin. Mol. Allergy 3:9 doi:10.1186/1476-7961-3-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bridges DJ, et al. 2010. Rapid activation of endothelial cells enables Plasmodium falciparum adhesion to platelet-decorated von Willebrand factor strings. Blood 115:1472–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brunner T, Heusser CH, Dahinden CA. 1993. Human peripheral blood basophils primed by interleukin 3 (IL-3) produce IL-4 in response to immunoglobulin E receptor stimulation. J. Exp. Med. 177:605–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Calissano C, et al. 2003. IgE antibodies to Plasmodium falciparum and severity of malaria in children of one ethnic group living in Burkina Faso. Am. J. Trop. Med. Hyg. 69:31–35 [PubMed] [Google Scholar]
- 8. Coban C, et al. 2010. Immunogenicity of whole-parasite vaccines against Plasmodium falciparum involves malarial hemozoin and host TLR9. Cell Host Microbe 7:50–61 [DOI] [PubMed] [Google Scholar]
- 9. Coban C, et al. 2005. Toll-like receptor 9 mediates innate immune activation by the malaria pigment hemozoin. J. Exp. Med. 201:19–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Enwonwu CO, Afolabi BM, Salako LO, Idigbe EO, Bashirelah N. 2000. Increased plasma levels of histidine and histamine in falciparum malaria: relevance to severity of infection. J. Neural Transm. 107:1273–1287 [DOI] [PubMed] [Google Scholar]
- 11. Falcone FH, et al. 2009. Antigen-driven basophil activation is indicative of early Necator americanus infection in IgE-seronegative patients. J. Allergy Clin. Immunol. 124:1343–1350 [DOI] [PubMed] [Google Scholar]
- 12. Falcone FH, Zillikens D, Gibbs BF. 2006. The 21st century renaissance of the basophil? Current insights into its role in allergic responses and innate immunity. Exp. Dermatol. 15:855–864 [DOI] [PubMed] [Google Scholar]
- 13. Gibbs BF, Rathling A, Zillikens D, Huber M, Haas H. 2006. Initial Fc epsilon RI-mediated signal strength plays a key role in regulating basophil signaling and deactivation. J. Allergy Clin. Immunol. 118:1060–1067 [DOI] [PubMed] [Google Scholar]
- 14. Gnanasekar M, et al. 2002. Molecular characterization of a calcium binding translationally controlled tumor protein homologue from the filarial parasites Brugia malayi and Wuchereria bancrofti. Mol. Biochem. Parasitol. 121:107–118 [DOI] [PubMed] [Google Scholar]
- 15. Huber M, et al. 1998. The src homology 2-containing inositol phosphatase (SHIP) is the gatekeeper of mast cell degranulation. Proc. Natl. Acad. Sci. U. S. A. 95:11330–11335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huber M, et al. 1998. Targeted disruption of SHIP leads to Steel factor-induced degranulation of mast cells. EMBO J. 17:7311–7319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Iikura M, et al. 2004. Transendothelial migration of human basophils. J. Immunol. 173:5189–5195 [DOI] [PubMed] [Google Scholar]
- 18. Kashiwakura J, et al. 2012. Histamine-releasing factor has a proinflammatory role in mouse models of asthma and allergy. J. Clin. Invest. 122:218–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Khusmith S, Panitchakorn J, Krudsood S, Wilairatana P, Looareesuwan S. 2001. IgE elevation and anti-plasmodium falciparum IgE antibodies: association of high level with malaria resistance. Southeast Asian J. Trop. Med. Public Health 32:696–701 [PubMed] [Google Scholar]
- 20. Kurimoto Y, De Weck AL, Dahinden CA. 1991. The effect of interleukin 3 upon IgE-dependent and IgE-independent basophil degranulation and leukotriene generation. Eur. J. Immunol. 21:361–368 [DOI] [PubMed] [Google Scholar]
- 21. Lim LH, et al. 2006. Stimulation of human endothelium with IL-3 induces selective basophil accumulation in vitro. J. Immunol. 176:5346–5353 [DOI] [PubMed] [Google Scholar]
- 22. Lourenço FD, et al. 2008. Activated status of basophils in chronic urticaria leads to interleukin-3 hyper-responsiveness and enhancement of histamine release induced by anti-IgE stimulus. Br. J. Dermatol. 158:979–986 [DOI] [PubMed] [Google Scholar]
- 23. MacDonald SM, et al. 2001. Immune mimicry in malaria: Plasmodium falciparum secretes a functional histamine-releasing factor homolog in vitro and in vivo. Proc. Natl. Acad. Sci. U. S. A. 98:10829–10832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. MacDonald SM, Rafnar T, Langdon J, Lichtenstein LM. 1995. Molecular identification of an IgE-dependent histamine-releasing factor. Science 269:688–690 [DOI] [PubMed] [Google Scholar]
- 25. MacGlashan D, Jr, Lavens-Phillips S, Katsushi M. 1998. IgE-mediated desensitization in human basophils and mast cells. Front. Biosci. 3:d746–d756 [DOI] [PubMed] [Google Scholar]
- 26. Nyakeriga MA, et al. 2003. Immunoglobulin E (IgE) containing complexes induce IL-4 production in human basophils: effect on Th1-Th2 balance in malaria. Acta Trop. 86:55–62 [DOI] [PubMed] [Google Scholar]
- 27. Perlmann H, et al. 1994. IgE elevation and IgE anti-malarial antibodies in Plasmodium falciparum malaria: association of high IgE levels with cerebral malaria. Clin. Exp. Immunol. 97:284–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Perlmann P, Perlmann H, ElGhazali G, Blomberg MT. 1999. IgE and tumor necrosis factor in malaria infection. Immunol. Lett. 65:29–33 [DOI] [PubMed] [Google Scholar]
- 29. Perlmann P, et al. 1997. Immunoglobulin E, a pathogenic factor in Plasmodium falciparum malaria. Infect. Immun. 65:116–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Perlmann P, et al. 2000. Contrasting functions of IgG and IgE antimalarial antibodies in uncomplicated and severe Plasmodium falciparum malaria. Am. J. Trop. Med. Hyg. 62:373–377 [DOI] [PubMed] [Google Scholar]
- 31. Poorafshar M, Helmby H, Troye-Blomberg M, Hellman L. 2000. MMCP-8, the first lineage-specific differentiation marker for mouse basophils. Elevated numbers of potent IL-4-producing and MMCP-8-positive cells in spleens of malaria-infected mice. Eur. J. Immunol. 30:2660–2668 [DOI] [PubMed] [Google Scholar]
- 32. Porcherie A, et al. 2011. Critical role of the neutrophil-associated high-affinity receptor for IgE in the pathogenesis of experimental cerebral malaria. J. Exp. Med. 208:2225–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Prato M, Gallo V, Giribaldi G, Aldieri E, Arese P. 2010. Role of the NF-kappaB transcription pathway in the haemozoin- and 15-HETE-mediated activation of matrix metalloproteinase-9 in human adherent monocytes. Cell. Microbiol. 12:1780–1791 [DOI] [PubMed] [Google Scholar]
- 34. Rao KV, Chen L, Gnanasekar M, Ramaswamy K. 2002. Cloning and characterization of a calcium-binding, histamine-releasing protein from Schistosoma mansoni. J. Biol. Chem. 277:31207–31213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sainte-Laudy J, Sabbah A, Vallon C, Guerin JC. 1998. Analysis of anti-IgE and allergen induced human basophil activation by flow cytometry. Comparison with histamine release. Inflamm. Res. 47:401–408 [DOI] [PubMed] [Google Scholar]
- 36. Sakuntabhai A, et al. 2008. Genetic determination and linkage mapping of Plasmodium falciparum malaria related traits in Senegal. PLoS One 3:e2000 doi:10.1371/journal.pone.0002000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schwartz LB, Yunginger JW, Miller J, Bokhari R, Dull D. 1989. Time course of appearance and disappearance of human mast cell tryptase in the circulation after anaphylaxis. J. Clin. Invest. 83:1551–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Seka-Seka J, Brouh Y, Yapo-Crezoit AC, Atseye NH. 2004. The role of serum immunoglobulin E in the pathogenesis of Plasmodium falciparum malaria in Ivorian children. Scand. J. Immunol. 59:228–230 [DOI] [PubMed] [Google Scholar]
- 39. Srichaikul T, Archararit N, Siriasawakul T, Viriyapanich T. 1976. Histamine changes in Plasmodium falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 70:36–38 [DOI] [PubMed] [Google Scholar]
- 40. Vo TK, et al. 2007. Evaluation of a real-time PCR assay for malaria diagnosis in patients from Vietnam and in returned travellers. Trans. R. Soc. Trop. Med. Hyg. 101:422–428 [DOI] [PubMed] [Google Scholar]
- 41. Vonakis BM, Gibbons S, Jr, Sora R, Langdon JM, MacDonald SM. 2001. Src homology 2 domain-containing inositol 5′ phosphatase is negatively associated with histamine release to human recombinant histamine-releasing factor in human basophils. J. Allergy Clin. Immunol. 108:822–831 [DOI] [PubMed] [Google Scholar]
- 42. Vonakis BM, et al. 2008. Distinct characteristics of signal transduction events by histamine-releasing factor/translationally controlled tumor protein (HRF/TCTP)-induced priming and activation of human basophils. Blood 111:1789–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. WHO 2009. World malaria report. World Health Organization, Geneva, Switzerland [Google Scholar]