Abstract
Uropathogenic Escherichia coli (UPEC) strains are a leading cause of infections in humans, but the mechanisms governing host colonization by this bacterium remain poorly understood. Previous studies have identified numerous gene clusters encoding proteins involved in sugar transport, in pathogen-specific islands. We investigated the role in fitness and virulence of the vpe operon encoding an EII complex of the phosphotransferase (PTS) system, which is found more frequently in human strains from infected urine and blood (45%) than in E. coli isolated from healthy humans (15%). We studied the role of this locus in vivo, using the UPEC E. coli strain AL511, mutants, and complemented derivatives in two experimental mouse models of infection. Mutant strains displayed attenuated virulence in a mouse model of sepsis. A role in kidney colonization was also demonstrated by coinfection experiments in a mouse model of pyelonephritis. Electron microscopy examinations showed that the vpeBC mutant produced much smaller amounts of a capsule-like surface material than the wild type, particularly when growing in human urine. Complementation of the vpeBC mutation led to an increase in the amount of exopolysaccharide, resistance to serum killing, and virulence. It was therefore clear that the loss of vpe genes was responsible for all the observed phenotypes. We also demonstrated the involvement of the vpe locus in gut colonization in the streptomycin-treated mouse model of intestinal colonization. These findings confirm that carbohydrate transport and metabolism underlie the ability of UPEC strains to colonize the host intestine and to infect various host sites.
INTRODUCTION
Escherichia coli is a normal resident bacterium of the intestines of healthy humans. However, in certain circumstances, it may also cause serious disease in humans. Uropathogenic E. coli (UPEC) strains are facultative pathogens that are present as commensal organisms in the normal flora of some healthy people. They are responsible for 80 to 90% of urinary tract infections (UTI) in humans (3, 17, 53). Many UTI are asymptomatic, but some UPEC strains cause significant clinical symptoms, ranging from pain in uncomplicated cases of cystitis to sepsis in cases of pyelonephritis. Antibiotic treatment may be difficult if the strains concerned are multiresistant (27). Despite tremendous advances in our understanding of the genetic bases of pathogenicity and of the evolutionary diversity of UPEC strains over the last 10 years (28), the mechanisms by which UPEC strains colonize the human intestine, which serves as their reservoir, and then travel to and persist in the urinary tract (UT) remain poorly understood. These bacteria frequently express adhesin/invasin and toxin genes and are equipped with iron acquisition systems and mechanisms for evading the immune response, through the production of extracellular polysaccharides, for example. However, no virulence factor or set of factors has yet been identified as essential for gut colonization and infection of the bladder or kidney. This implies that a number of alternative factors may mediate each step in the infection process and that each strain may have a unique combination of such factors.
It also remains unclear how UPEC strains adapt their metabolism to derive carbon and energy from the environment, allowing them to grow, to survive, and to colonize their hosts at both intestinal and extraintestinal sites. UTI generally develop following contamination of the UT by the intestinal or vaginal microflora (46). The UT constitutes a unique environment, in which UPEC strains grow better than other bacteria. Several studies have shown that the ability of UPEC strains to synthesize or to acquire amino acids (arginine) and nucleosides (guanine) that are limiting in urine plays an essential role in maximizing virulence within the UT (43, 44). The impact of metabolic functions on urovirulence first became clear when effective iron acquisition systems were shown to be required for urovirulence (21, 45), and association was demonstrated between d-serine metabolism and the intracellular accumulation and expression of virulence genes during ascending UTI in a mouse model (20). Comparative and functional analyses have shown that the metabolic flexibility of E. coli reflects genetic diversity and the dynamic organization of the genome and have highlighted the key role of carbohydrate metabolism in the adaptation of pathogenic strains to different ecological niches (30, 51). Many genomic islands specific to UPEC strains harbor genes encoding proteins involved in the transport and use of carbohydrates such as sucrose (50), cellobiose (33), and deoxyribose (6). The catabolism of deoxyribose is catalyzed by the products of the deoK operon, which has been transferred from Salmonella enterica to E. coli (6). Using a mouse model of intestinal colonization, we recently demonstrated the involvement of this operon in gut colonization by pathogenic E. coli strains, including a UPEC strain (32). The deoK operon is frequently associated with strains isolated from infected urine and blood, in which it is always located in large specific islands carrying genes contributing to the intrinsic virulence and/or adaptive properties of the strain. The characterization of PAI-I from the sepsis strain AL862, which carries the deoK operon, identified and localized three genes encoding hypothetical proteins displaying 30, 27, and 35% identity to SgaT, -B, and -A, respectively, the components of the tripartite L-ascorbate-specific permease of E. coli (29) (GenBank accession number GQ497943). Based on this sequence similarity and the location of the genes in a PAI, the locus was named vpe for virulence-associated phosphotransferase (PTS) in E. coli. This locus contains the vpeA, vpeB, and vpeC genes, which encode the EIIA, EIIB, and EIIC constituents, respectively, of a putative carbohydrate-specific permease of the SgaTBA family (23, 54).
The aim of this work was to explore the role of carbohydrate metabolism in urovirulence by investigating the vpe locus. We first documented the association of this locus with strains from urine and blood. The UPEC AL511 strain is used as a model organism for studies in our laboratory (10, 36). A clear link between expression of the vpe locus and the intrinsic virulence of AL511 was demonstrated in a standardized mouse model of lethal infection. Competition experiments on human urine and in the mouse model of ascending pyelonephritis provided information about the role of vpe genes in the development of UTI. Finally, a role for vpe genes in intestine colonization was also suggested in the streptomycin mouse model of gut colonization.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
We studied 235 well-characterized clinical isolates from our laboratory collection (32), including 88 isolates obtained from the urine or blood of patients, 20 enteroaggregative E. coli (EAEC) strains, and 127 E. coli isolates obtained from the stool samples of healthy subjects. Isolate AL511 was used as the prototype for characterization of the genes of interest (Table 1). Strain AL511, from phylogenetic group A, adheres to mouse renal epithelial collecting ducts both in vitro and in vivo. This interaction stimulates an innate immune response (10), and the uptake of AL511 bacteria into these cells is dependent on the flagella (36).
Table 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Reference or source |
|---|---|---|
| E. coli strains | ||
| AL511 | Urine isolate (O9:H12:F11) from a patient with acute pyelonephritis; Apr Cmr Strr Spr Tcr | 2, 18 |
| AL511 vpeBC | vpeBC::Km, resistance cassette from MC4100ybeW::GB | This study |
| AL511 vpeBC (Km-FRT) | vpeBC::Km-FRT, resistance cassette from pKD4 | This study |
| AL511 vpeBC (FRT) | vpeBC::FRT, removal of the Km-FRT cassette | This study |
| AL511 vpeR | vpeR::Km, resistance cassette from MC4100ybeW::GB | This study |
| AL862 | Human blood isolate from a patient with both UTI and bacteremia | 29 |
| MG1655 | Nonpathogenic K-12 reference strain | 7 |
| Plasmids | ||
| pKOBEGA | Derivative of pKOBEG, Apr | 11 |
| pKOBAPRA | Unpublished derivative of pKOBEG, Aprar | 11 |
| pCP20.Zeo | Flipase (Flp) expression plasmid for removal of the Km-FRT cassette; Zeor | This study |
| pZEZeoGFP | Zeor derivative of pZE21GFP (13) | 32 |
| pZEZeovpeBC | vpeB-C genes from AL511 inserted in place of the GFP gene in pZEZeoGFP | This study |
Apr, ampicillin resistance; Aprar, apramycin resistance; Cmr, chloramphenicol resistance; Kmr, kanamycin resistance; Spr, spectinomycin resistance; Strr, streptomycin resistance; Tcr, tetracycline resistance; Zeor, zeocin resistance.
Bacterial strains were routinely cultured in Luria-Bertani (LB) broth, with shaking (140 rpm), or on LB agar plates at 37°C. In various experiments, the strains were cultured in human urine or serum. Urine composition is highly variable. We therefore carried out assays with independent urine samples (including samples collected first thing in the morning) from several healthy volunteers (at least five) who had not been treated with antibiotics in the preceding 2 months. Each urine sample was filter sterilized (passed through a filter with 0.22-μm pores). The samples were pooled, and aliquots were frozen at −80°C before use. Three independent urine pools were constituted. The sera from healthy subjects were provided by the ICAReB platform (Investigation Clinique et Accès aux ressources Biologiques, Institut Pasteur, Paris, France). Pooled serum samples from at least two donors were either used fresh or stored in aliquots at −20°C until required. Antibiotics were used, as required, at the following concentrations: kanamycin, 100 mg/liter; Zeocin, 60 mg/liter; apramycin, 30 mg/liter; and carbenicillin, 100 mg/liter.
Carbohydrate fermentation.
We routinely assessed the ability of the various strains to metabolize carbohydrates by culturing the bacteria overnight on LB plates. For some experiments, bacteria were grown overnight on K5 minimal medium (16) plates supplemented with 50% human serum or urine. API 50 CH carbohydrate fermentation strips (bioMérieux, France) were used in accordance with the manufacturer's instructions. Bacterial growth was also assessed on K5 plates containing 0.1% to 0.4% concentrations of the carbohydrate of interest as the sole source of carbon. The plates were incubated at 37°C for 24 to 48 h.
Construction of deletion mutants.
We generated mutants of AL511 by the allelic exchange recombination method, using derivatives of the thermosensitive plasmid pKOBEG, which carries lambda red recombination genes (Table 1). Deletion strains were obtained by a one-step (14) or a three-step (11) method, with primers targeting the 5′ and 3′ ends of the target genes and kanamycin resistance cassettes derived from either pKD4 (14) or MG4100ybe::GB (40) as templates. All allelic exchanges were verified by PCR with primers flanking the gene of interest. We checked that allelic exchanges resulted in mutants with no growth defect phenotype, as assessed in independent cultures in LB broth, urine, and heated serum-containing media.
For complementation assays, the vpeBC sequence was amplified from AL511 genomic DNA with the Expand high-fidelity PCR system kit (Roche Applied Science), with the vpeBC-KpnI and vpeBC-HindIII primers. The PCR product was inserted into pZEZeoGFP cut with the appropriate restriction enzymes and the resulting recombinant plasmid was introduced, by electroporation, into vpeBC mutant strains. All the primers used are listed in Table S1 in the supplemental material.
In vitro growth curves.
We checked that the independent growth of the mutant was similar to that of the wild-type strain, using colonies grown from a freezer stock to initiate overnight cultures in 5 ml of appropriate medium. The following morning, the culture was diluted 1:100 in fresh medium and incubated at 37°C, with shaking at 140 rpm. Strain growth was monitored at hourly intervals until stationary phase was reached. Growth was monitored by both determining the optical density at 600 nm (OD600) and counting the CFU.
For cocultures in LB broth and urine, overnight precultures of wild-type and mutant strains in the medium of interest were mixed 1:1 (vol/vol). Aliquots of these mixtures were diluted and plated on LB plates and on LB plates supplemented with kanamycin to determine the input, in CFU/ml, of the two strains. The numbers of mutants were determined directly from the kanamycin-containing plates. Counts for the parental strain were obtained by subtracting the number of CFU on kanamycin-containing selective plates from that on LB plates without antibiotic. We used a toothpick to transfer 100 colonies from plates without antibiotic to plates with or without kanamycin to check these counts. The fraction of colonies susceptible to kanamycin was determined. These two methods gave highly consistent estimates of the number of parental strain colonies. The mixtures diluted 1:100 in fresh medium (5 and 2 ml of LB medium and urine, respectively) were incubated at 37°C with shaking. They were passaged (dilution, 1:500) every 8 and 16 h for 2 weeks. At the end of each 24-h period, we determined the number of viable CFU of parental and mutant strains by plating on LB and antibiotic-containing LB plates. The detection limit for plate counts was 102 CFU/ml. Each coculture experiment was carried out at least twice. At the end of the experiment, we checked the identity of each strain by PCR analysis and determination of an antibiogram.
RNA extraction and cDNA synthesis.
Bacterial cultures were stopped during the exponential (OD600 of 0.6 or 0.8 for LB broth and 0.3 for urine) or stationary (after 9 or 24 h) phase. The cells were harvested by centrifugation and immediately frozen at −80°C. Total RNA was extracted with TRIzol reagent according to the manufacturer's instructions (Invitrogen). Dried RNA pellets were resuspended in diethyl pyrocarbonate (DEPC)-treated water. RNA extraction was followed by DNase I treatment (Ambion) to eliminate contaminating genomic DNA. RNA quality was determined with RNANano chips and a Bioanalyzer (Agilent Technologies).
Total RNA was used as a template for cDNA synthesis with the AMV reverse transcriptase (Roche). Reaction mixtures (20 μl) containing 200 ng of random hexamers (Roche), 0.5 mM deoxynucleotide triphosphate, and 4 μg of total RNA were heated at 65°C for 5 min to denature the nucleic acid and placed on ice. Reverse transcription was carried out with AMV reverse transcriptase (25 U) and the 1× buffer supplied in the kit, according to the manufacturer's protocol.
Quantitative reverse transcription-PCR (qRT-PCR).
Specific primer pairs were designed with BEACON designer software, version 4.02 (Premier Biosoft International, Palo Alto, CA) (see Table S1 in the supplemental material). PCR amplification was performed in a reaction volume of 25 μl containing 5 μl of a 1:100 dilution of cDNA, 1 μl of gene-specific primers (10 μM), and 12.5 μl of iQ SYBR green Supermix (Bio-Rad), according to the manufacturer's instructions. Control reactions with no reverse transcriptase were also performed. Amplification, detection, and analysis were performed with the MyiQ single-color real-time PCR detection system (Bio-Rad). Each assay was performed in triplicate and repeated with at least two independent RNA samples. All data were normalized with respect to an internal standard (16S rRNA). The fold change ratio between growth conditions for each gene was calculated by the cycle threshold (ΔΔCT) method (31).
Experimental mouse models.
All animal experiments were performed according to protocols approved by the institutional animal care and use committee. Groups of at least five mice per coinoculation were used to determine defects in the fitness and virulence of mutants. The coinfection was repeated whenever a defect was apparent. At the end of the experiments, the identity of the bacteria recovered from the biological samples was checked by bacteriological and genetic approaches. The data were plotted and analyzed with Prism 5.0a (GraphPad software). P values below 0.05 were considered significant.
Lethality model.
The intrinsic virulence of the E. coli strains was evaluated in a previously described model of systemic infection (35). Briefly, bacterial strains were grown overnight from frozen glycerol stocks in LB broth. The cells were harvested by centrifugation, washed, and suspended in Ringer solution to obtain inocula of about 2 × 108 CFU in 200 μl, which were injected into the peritoneal cavities of 8-week-old female Swiss mice (Janvier Laboratories, France). The mice were observed 6 h after inoculation to detect whether rapid death was linked to endotoxinic shock; no mouse died within the first 6 h. The infected mice were then observed daily for up to 1 week. Death and the time of death were noted for each mouse. Lethality was evaluated on the basis of at least two independent series, with 4 to 10 mice studied for each strain. Each experimental series included the negative-control strain MG1655, which, like a typical commensal E. coli strain, killed no mice during the 7 days of the assay (51).
Urinary tract infection model.
Kidney and bladder colonization by the AL511 strain and its vpe derivatives was assayed in a model of ascending infection, essentially as previously described (10), except that 6- to 8-week old female inbred CBA mice were used within a week of delivery from Charles River Laboratories (France). No bacteria were recovered from the plating of urine from the mice used in the study on Drigalski or Mueller-Hinton agar plates on the day before the experiment. Before infection, the bladders of the mice were voided by gentle compression of the abdomen, and the sterility of the urine was again tested. The mice were anesthetized by intraperitoneal injection of a mixture of ketamine and xylazine and infected with 50 μl of a bacterial suspension. The inoculum was prepared from E. coli strains grown overnight from frozen stocks in static LB broth; it contained approximately 108 bacteria suspended in phosphate-buffered saline (PBS). For competitive infection experiments, the parental and mutant strains were grown independently and then mixed in a 1:1 ratio to obtain inocula. Bacteria were delivered to the bladder via the transurethral route through a polyethylene catheter (Insyte Autoguard soft catheter, 0.7-mm external diameter; Vygon, France). We monitored the colonization dynamics of strains by sequential urine cultures during the first 2 days. Four days after infection, mice were killed humanely by CO2 asphyxiation, and the bladders and kidneys were excised, weighed, and homogenized in PBS supplemented with 0.025% Triton X-100. Bacterial counts were determined by plating serial dilutions on LB agar plates with and without kanamycin, to differentiate between the two strains in coinfections. Raw data were adjusted for the number of CFU per gram of organ. We excluded animals with sterile kidney cultures from strain comparisons. The minimum thresholds of detection for infection were 30 CFU/g for the kidney and 300 CFU/g for the bladder. The degree of attenuation of the mutant was estimated by determining the competitive index (CI). The CI was calculated as the ratio of mutant to wild-type CFU recovered from the organs on day 4 divided by the initial ratio of mutant to wild-type CFU. The results are expressed as log CI in the figures. A log CI of zero indicates that the two strains were recovered in the same ratio as that in which they were injected; negative values indicate that the parental strain outcompeted the mutant strain, and positive values indicate that the mutant outcompeted the parental strain. Mutants were considered attenuated if the log CI was below −0.3 (26).
Intestinal colonization model.
The intestine-colonizing abilities of the AL511 strain and its vpe derivative were compared in a previously described competition-based model (32) in streptomycin-treated BALB/c female mice (7 weeks old; Janvier Laboratories). After 18 to 20 h of food and water starvation, the mice were fed 200 μl of a sucrose (20%)-bicarbonate (2.6%) solution supplemented with 105 to 106 CFU of bacteria grown overnight in LB broth with shaking. The mice were then returned to their normal diet, including streptomycin (5 g/liter). After the bacterial suspension had been ingested, mice were housed individually in cages without bedding that were cleaned daily, and the food and streptomycin-treated water supply was reestablished. Fresh fecal samples collected after 24 h and at the times indicated in the figures were weighed, homogenized, and diluted in 1× PBS. Bacterial counts were determined by plating serial dilutions of homogenates on LB agar plates. Raw data were adjusted according to the number of CFU per gram of feces. The threshold for the detection of infection was 6 × 102 CFU/g of feces. For competitive infection experiments, the parental and mutant strains were grown independently and then mixed in a 1:1 ratio to obtain inocula. Bacteria were counted by plating biological samples on LB agar plates with and without kanamycin to differentiate between the two strains. The degree of attenuation of the mutant was estimated by determining log CI.
Exopolysaccharide staining and electron microscopy.
We visualized polysaccharide material at the surface of the bacteria by staining with cationized ferritin (39). Bacteria were grown overnight in 2 ml of LB broth or urine at 37°C with shaking, collected by centrifugation, and washed in 2 ml of 1× PBS. They were then fixed and stained with polycationic ferritin (Sigma) as previously described (4). Thin sections of samples embedded in epoxy resin were stained with 2% uranyl acetate and lead citrate. They were observed in a JEOL JEM 1010 transmission electron microscope operating at 80 kV, and images were recorded with a Keen View camera (Eloise Ltd.).
Serum bactericidal assay.
We evaluated changes in cell viability following the exposure of E. coli strains to human serum. Bacteria from an overnight culture in LB broth (containing antibiotics when required) were washed twice in 1× PBS. For the assay, we mixed 100 μl of 1× PBS containing approximately 106 bacteria with 100 μl of 40% serum. We withdrew 5-μl samples at 30 min, 1 h, 2 h, 3 h, and 4 h of incubation at 37°C for the counting of viable bacteria. All assays were carried out in triplicate at least twice. When required, the complement was inactivated by incubating the serum for 30 min at 56°C before mixing it with bacteria.
Nucleotide sequence accession numbers.
The GenBank accession numbers for the sequence of the 9,092-bp chromosomal region from AL511 carrying the deoK and vpe loci and the 59,151-bp region of PAI-IAL862 reported here are FJ999998 and GQ497943, respectively. The PAI-IAL862 sequence was deduced from the sequence of three cosmid clones (pILL1255, pILL1269, and pILL1270) as described in a previous study (29).
RESULTS
The vpe locus is associated with pathogenic E. coli strains.
BLAST analyses showed that the vpe locus from PAI-IAL862 was strongly conserved in the genomes of four pathogenic E. coli isolates (UPEC isolate CFT073, EAEC isolates 55989 and O42, and Shiga toxin-producing isolate 4797/97). The vpe cluster was also identified in the genome of one commensal isolate (SE15). Comparative genomic analysis revealed that the region of PAI-IAL862 conserved in these five genomes spanned 3,318 bp, with 100% coverage and 99% identity. In addition to vpeA, -B, and -C, it includes a divergent transcriptional unit, vpeR, encoding a putative 343-amino-acid protein (Fig. 1). BLAST analysis also showed the sequence of VpeR to be very similar to those of transcriptional regulators of the LacI family (33% identity to GntR), with a predicted N-terminal helix-turn-helix motif coinciding with a DNA-binding domain at residues 16 to 67, a putative dimerization interface at residues 72 to 313, and a ligand-binding domain at residues 72 to 342. The vpe and deoK operons were found to be linked, in a conserved 9-kb region, in all genomes studied other than that of the 4797/97 isolate. The two loci are separated by about 700 bp in length, the features of this region being reminiscent of an insertion sequence.
Fig 1.
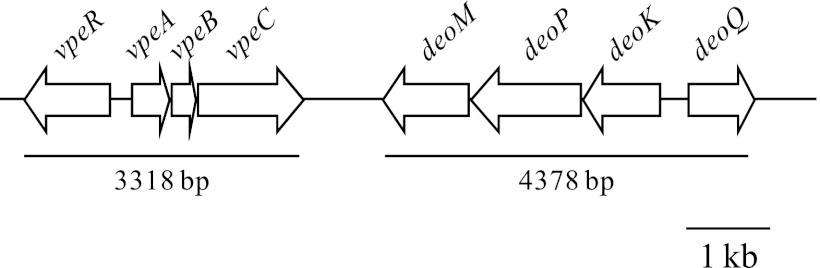
Genomic organization of the vpe gene cluster, which is frequently linked to the deoK gene cluster, in a conserved 9-kb region.
We carried out PCR analyses with primers derived from the PAI-IAL862 sequence to determine whether the vpe locus was present in another of the 233 UPEC, sepsis, EAEC, and commensal strains. Carbohydrate permease specificity is strongly linked to the integral membrane transporter (EIIC) component. We therefore used the vpeC gene as the target for amplification. The AL862 isolate and the E. coli MG1655 strain were used as positive and negative controls, respectively. Our results strongly suggested an association of the vpe locus with strain pathogenicity, despite the presence of the vpeC gene in both pathogenic and commensal strains. This gene was present in 48.15% of pathogenic isolates (52/108) and 15% of commensal strains (19/127); this difference was found to be significant (P < 0.0001) in χ2 analysis. The presence and expression of the deoK operon had previously been investigated in all the strains of the collection (32). For confirmation of the linkage of the two loci observed during genome comparison, we amplified the region between the vpeC and deoM genes by PCR with the vpeC-F and deoM-F primers (see Tables S1 and S2 in the supplemental material and Fig. 1). These loci were found to be linked in 76.1% of the vpe-positive isolates, regardless of the origin of the strain.
We selected E. coli AL511, a pyelonephritic isolate carrying only one copy of the vpe locus (data not shown), for analysis of the expression of this locus and its role in virulence. The nucleotide sequence of the vpe-deoK region from AL511 was 99% identical to those of the AL862 and CFT073 strains over a stretch of 9,092 bp.
The vpe genes and virulence in mice.
We evaluated the link between vpe locus expression and the experimental virulence of AL511 in a standardized mouse lethality assay (35). Reproducible data were obtained for the commensal MG1655 isolate and the parental AL511 strain in 10 independent experiments, indicating that the experimental conditions were well standardized and that the model was not impaired by variability of host susceptibility. MG1655 was innocuous, whereas AL511 killed all of the mice in each experiment (Table 2). The intrinsic virulence of AL511 was strongly attenuated by the vpeBC mutations. The AL511 vpeBC mutant remained lethal but killed only 33% of the mice (mean of nine independent experiments on a total of 74 mice). This model was considered appropriate for tackling this question, because the mean percentage of mice killed remained fairly constant. We investigated four other independent vpeBC mutants (two independent AL511 vpeBC [Km-FRT] and two independent AL511 vpeBC [FRT] clones), each of which killed ≤20% of the mice. We also demonstrated, in this model, that it was possible to restore the virulence of the mutant to wild-type levels by introducing pZEZeovpeBC into AL511 vpeBC. This complementation resulted in a strain that killed more than 90% of the mice (Table 2). Thus, there is clearly a link between expression of the vpe locus from AL511 and lethality in mice.
Table 2.
Virulence of strains evaluated in the mouse lethality model
| Strain(s) | Lethality phenotype |
||||
|---|---|---|---|---|---|
| No. of expts | Total no. of mice | Total no. of mice killeda | Mean % of mice killed/expt | Pb | |
| MG1655 | 10 | 46 | 0 | 0 | |
| AL511 | 10 | 57 | 57 | 100 | |
| vpeBC mutants of AL511 | |||||
| AL511 vpeBC | 9 | 74 | 24 | 33 ± 8.3 | <0.0001 |
| AL511 vpeBC (Km-FRT) | 20 | <0.0001 | |||
| clone 1 | 1 | 10 | 2 | ||
| clone 2 | 1 | 5 | 1 | ||
| AL511 vpeBC (FRT) | 15 | <0.0001 | |||
| clone 1 | 1 | 10 | 1 | ||
| clone 2 | 1 | 5 | 1 | ||
| Total | 13 | 104 | 29 | 28 | |
| Complementation experiments | |||||
| AL511 + pZEZeoGFPc | 2 | 20 | 20 | 100 | |
| AL511 vpeBC + pZEZeoGFPc | 3 | 25 | 6 | 23.3 ± 15.3 | <0.0001 |
| AL511 vpeBC + pZEZeovpeBC | 3 | 25 | 24 | 96.7 ± 5.8 | 1 |
Mice die 24 to 48 h postinfection. No deaths occur after this period.
The significance of differences in the lethality phenotype between mutants and the wild type was estimated by Fisher's exact test; P values of <0.05 were considered significant.
The presence of pZEZeoGFP has no significant effect on the lethality phenotype of AL511 or AL511 vpeBC.
Analysis of vpe gene expression in human urine.
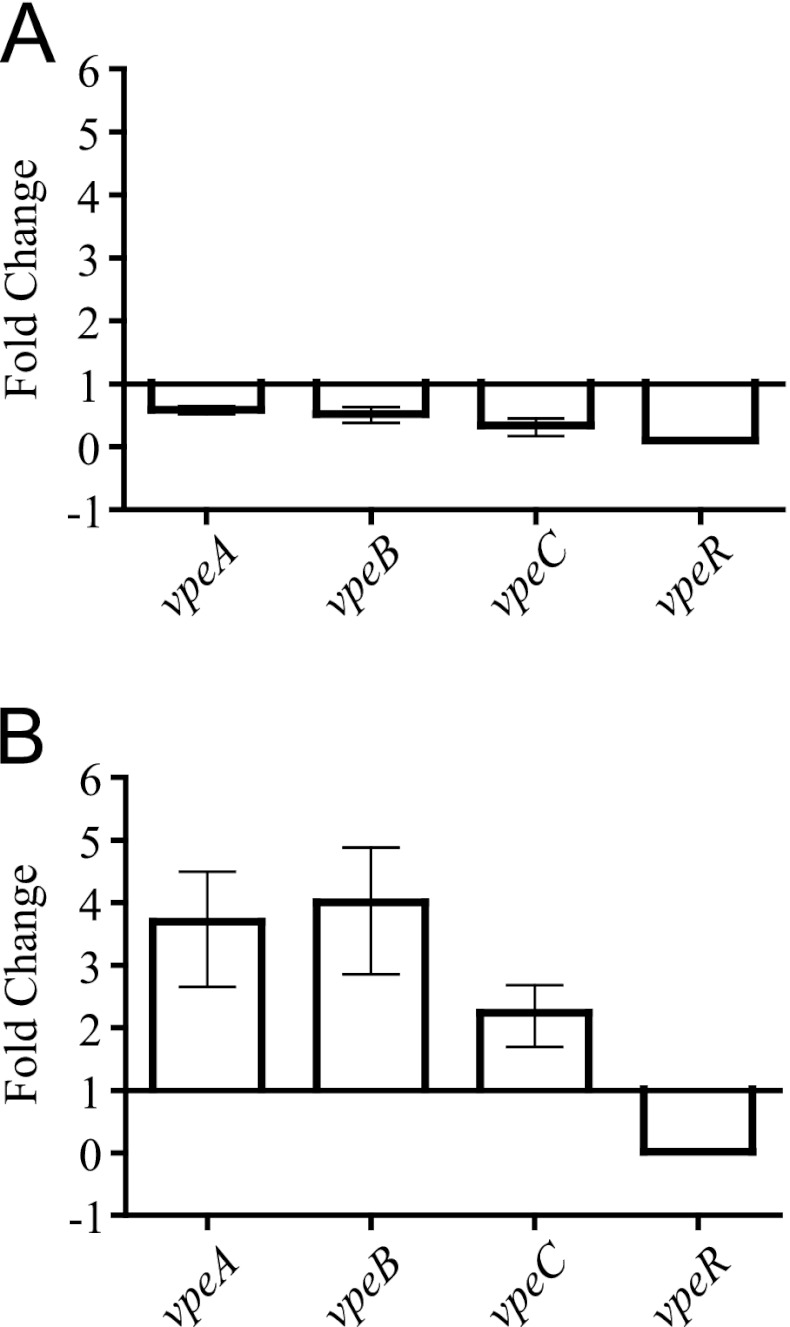
The AL511 strain was cultured in human urine, and the levels of transcripts for the vpeA, -B, -C, and -R regions were evaluated by qRT-PCR experiments. Transcript levels for the vpeA, -B, and -C genes halved in stationary phase, with no significant difference in transcription level observed between the three genes, strongly suggesting that these genes, which are separated by only a few nucleotides (15 and 12, respectively), were cotranscribed (Fig. 2A). The structure of the transcriptional unit was confirmed by RT-PCR on the same cDNA sample with the vpeA-F and vpeC-R primers, which amplified a 1,210-bp fragment, consistent with the production of a single transcript corresponding to all three genes (data not shown). A stronger effect of the change of growth phase was observed for the expression of vpeR, which decreased by a factor of about 10 in the stationary phase (Fig. 2A). We carried out qRT-PCR assays on total RNA extracted from cultures in LB broth to control for the specificity of the changes in vpe gene transcription in response to urine. We observed no significant effect of growth phase in LB broth on mRNA levels (data not shown). Due to differences in the rates of growth of the bacterium in urine and LB broth, significant results were obtained only for comparisons of gene expression during the stationary phase. The induction rate for vpeABC expression was about three times higher in urine than in LB broth during the stationary growth phase, and that for vpeR in urine was 1/10 that in LB broth (Fig. 2B).
Fig 2.
Effect of growth phase and urine on the transcription of the vpe genes from AL511. Transcription of the vpeA, -B, -C, and -R genes was analyzed by qRT-PCR after growth in either LB broth or urine. (A) A growth phase-dependent effect of urine on gene expression was demonstrated. Fold change indicates the ratio of growth in stationary phase to that in exponential phase. (B) The induction of transcription by urine is illustrated for bacteria in the stationary growth phase. Fold change refers to the ratio of growth in urine to that in LB broth.
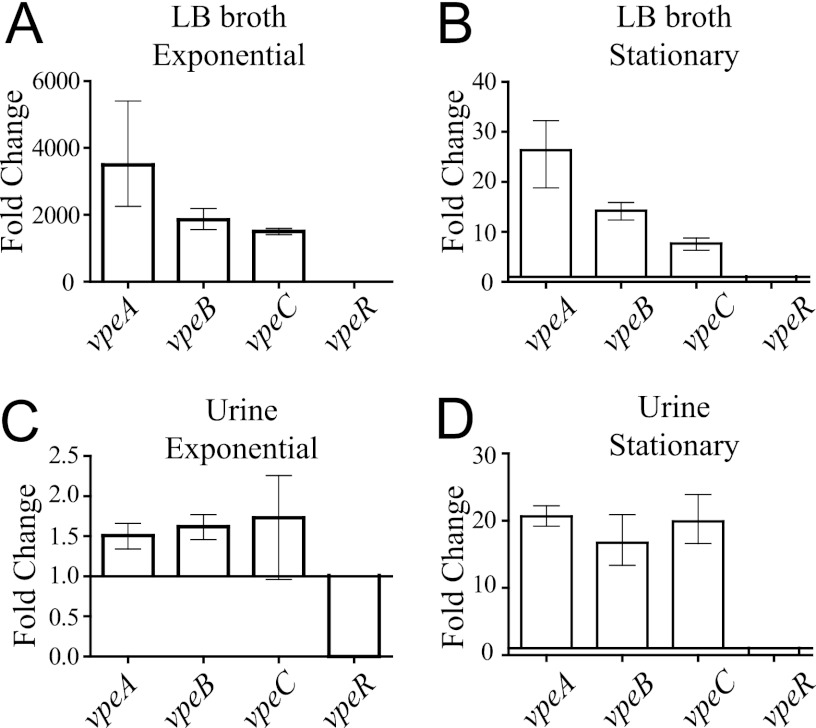
We investigated the potential regulatory role of VpeR by comparing levels of expression of the vpeA, -B, and -C genes in the parental isolate and the AL511 vpeR derivative. As expected, no vpeR transcription was detected in the mutant, whether it was grown in urine or LB broth (Fig. 3). In LB broth cultures, inactivation of the vpeR gene induced a large increase in expression of the vpeA, -B, and -C genes, regardless of the growth phase, providing clear evidence of an inhibitory effect of VpeR on the expression of the vpeABC operon (Fig. 3A and B). In contrast, in urine, the induction of vpeABC operon expression in the vpeR mutant depended on growth phase, with little or no effect (an increase of about 1.5 times) during the exponential growth phase and an effect similar to that observed in LB broth in stationary phase (induction by a factor of more than 15) (Fig. 3C and D). These data confirm that VpeR belongs to the GntR superfamily of transcriptional regulators, which typically respond to metabolite effector molecules. Putative effectors present in human urine seem to induce the repression of vpeR transcription. These data also indicate that the regulation of vpeABC operon expression is not purely dependent on VpeR. Instead, it is complex and controlled at multiple levels, as suggested by the presence of binding sites for the CRP and Mlc transcriptional regulators in the promoter region of vpeABC genes (data not shown).
Fig 3.
Regulatory effect of vpeR on transcription of the vpeABC operon. Relative expression of the genes in the AL511 vpeR and AL511 strains was analyzed by qRT-PCR after growth in either LB broth or urine. Downregulation was observed for cells grown in LB broth (A and B) and in urine (C and D), in the exponential growth and stationary phases.
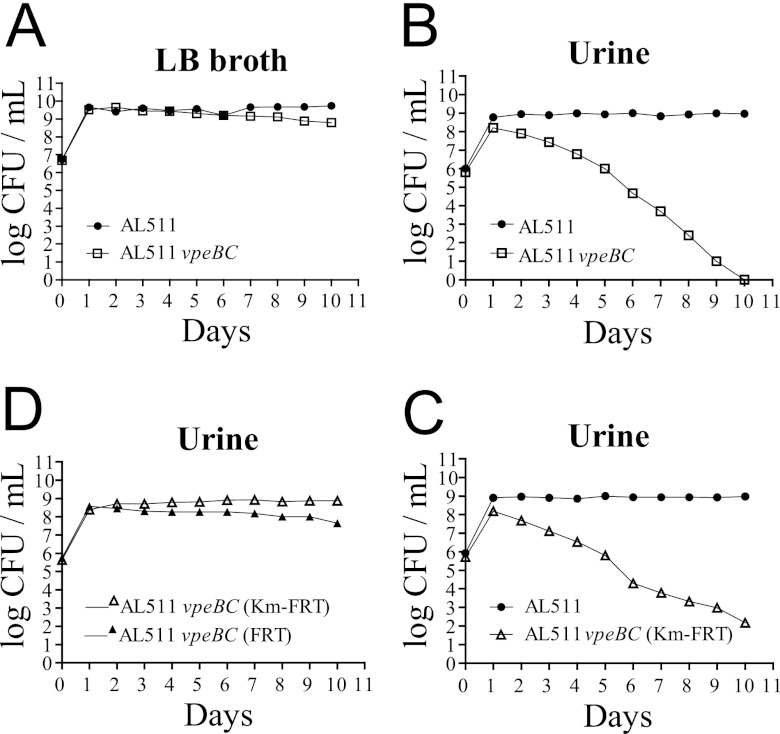
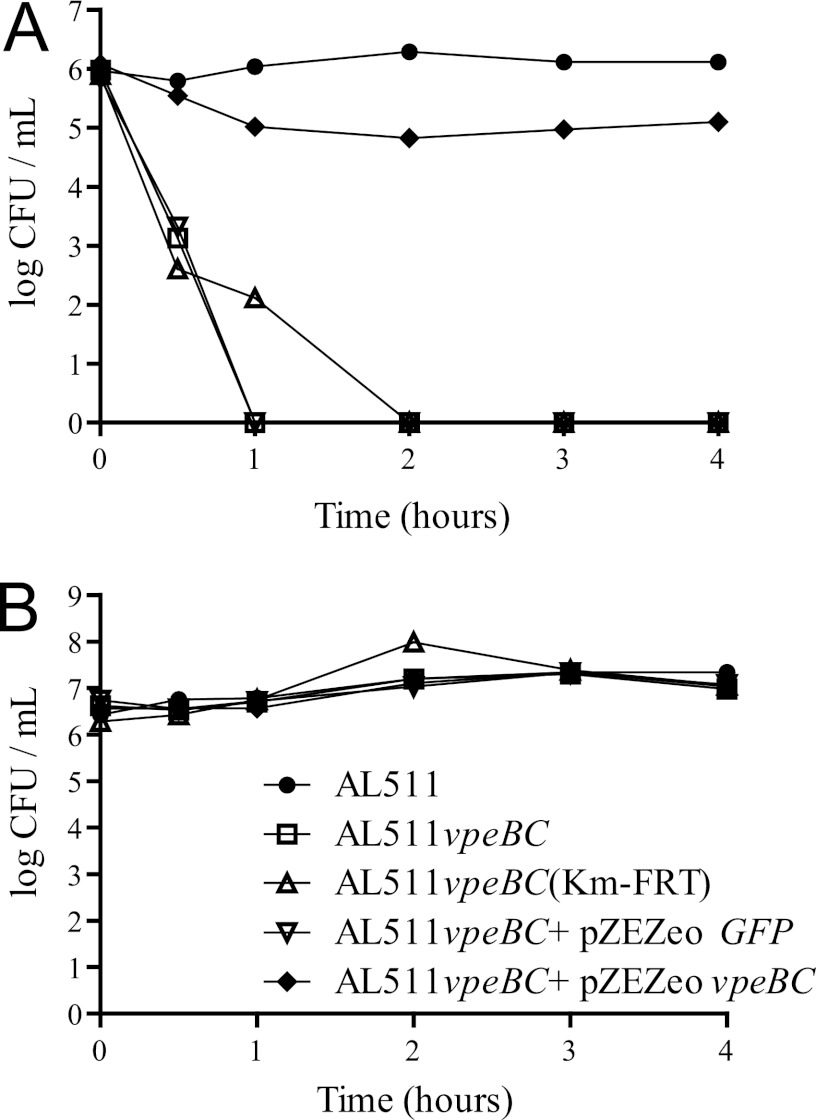
Our data suggest that the vpeABC operon encodes an EII permease specific for a substrate present in human urine. The use of this substrate as an additional carbon source might increase the fitness of the bacterium in urine. We tested this hypothesis by carrying out coculture experiments with the AL511 strain and isogenic vpeBC mutants. The growth rates of the two strains did not differ significantly in LB broth cocultures (Fig. 4A), but the AL511 vpeBC mutant was less competitive than the parental strain in urine cocultures. The parental strain reached a density of 109 CFU/ml after 24 h, this density then being maintained through the 10 days of coculture, whereas the density of the vpeBC mutant did not exceed 108 CFU/ml after 24 h and subsequently decreased until the end of the experiment. The mutant strain was eliminated from the culture after 8 to 10 days (Fig. 4B). Similar results were obtained for coculture experiments with a mutant constructed with a different kanamycin cassette (AL511 vpeBC [Km-FRT]) (Fig. 4C). Similar results were also obtained in experiments with three independent urine pools, indicating that the substrate specifically transported by the EII permease is a constituent of human urine in general.
Fig 4.
Survival of independent AL511 vpeBC mutants in coculture experiments. The parental AL511 strain and the AL511 vpeBC mutant were cocultured in both LB broth (A) and human urine (B) for 2 weeks. The mutant was outcompeted by the wild-type strain only in urine. Similar results were obtained when AL511 was cocultured with an independent AL511 vpeBC derivative (vpeBC [Km-FRT]) in urine (C). No competition was observed in urine cocultures of two independent AL511 derivatives (vpeBC [Km-FRT] and vpeBC [FRT]) (D). Similar results were obtained in at least two independent experiments.
As two independent mutants displayed the same growth defect in urine cocultures, we were able to exclude the hypothesis that unknown mutations were responsible for the phenotype. We demonstrated that the vpeBC gene deletions, rather than expression of the kanamycin cassettes, were responsible for the lower fitness of the mutants in cocultures in urine, by introducing pZEZeovpeBC into the AL511 vpeBC mutant to complement the growth defect and restore fitness. qRT-PCR analysis of transcript levels in the resulting strain confirmed that expression of the vpeC gene in urine resulted in levels of vpeC expression about 10 times higher than those of the parental AL511 strain, regardless of the growth phase (data not shown). Despite this expression, we were unable to demonstrate complementation of the deficiency of the vpeBC mutant in cocultures in urine. This suggests that overexpression of the vpeBC genes inhibits the complementation process in urine. We therefore set up mixed cultures of two mutant strains simultaneously (AL511 vpeBC [Km-FRT] and AL511 vpeBC [FRT]). We found that the two strains displayed similar patterns of growth and maintenance over the 10 days of the experiment (Fig. 4D). These experiments confirmed that the kanamycin cassettes used had no significant effect on the results obtained.
Various assays were carried out to identify substrates transported and phosphorylated by the Vpe permease. We compared the abilities of the parental and mutant strains to metabolize carbohydrates present on API 50 CH strips, but no metabolic change attributable to the mutation could be identified. There are several possible reasons for this: the carbohydrate of interest was not tested, it was not present in large enough amounts to induce expression of the vpe genes in the parental strain, or it has an affinity for AL511 transporters other than Vpe. In addition, no change in cell physiology associated with the loss of vpe gene function was observed in analyses of the various strains on phenotypic microarrays (Biolog plates), in which we investigated carbon sources together with the metabolism of nitrogen, phosphorus, and sulfur.
The vpe genes and colonization of the mouse urinary tract.
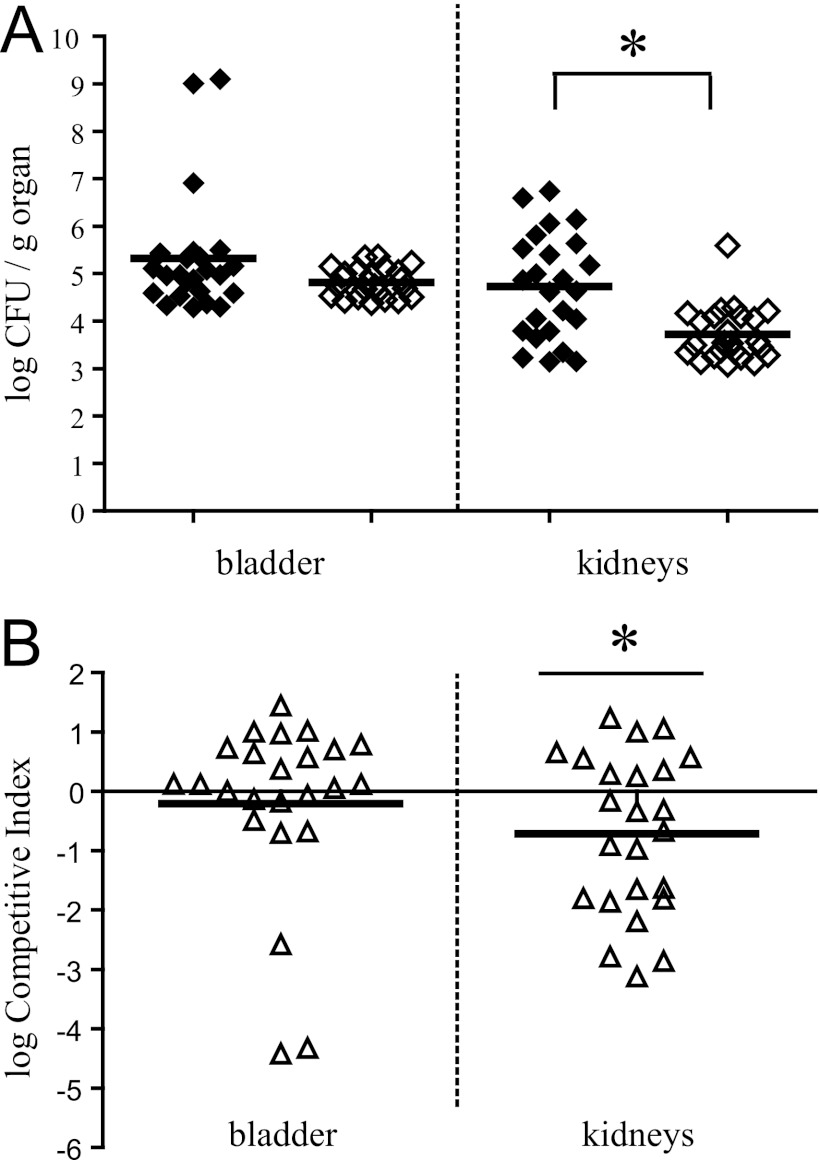
We investigated the association between the growth advantage in urine conferred by expression of the vpeABC operon and urovirulence by comparing the survival of vpeBC mutants with that of the wild type in a mouse model of UTI. Preliminary experiments were performed with the parental AL511 strain to optimize the protocol and achieve efficient colonization of the bladder and kidneys. With an inoculum of 108 CFU per mouse, significant organ colonization (>104 CFU/g) was observed 4 days after the challenge in animals for which urine samples tested positive for the presence of E. coli 2 days after inoculation (>104 CFU/ml). Five of six mice with AL511-infected urine displayed colonization of both the bladder (1.7 × 104 to 2.6 × 108 CFU/g of organ) and the two kidneys (6 × 104 to 9.1 × 106/g of organ). One mouse with no detectable bladder infection had bacteria in only one kidney (6 × 104 CFU/g of organ). Variability in tissue titers between mice, as observed in our experiments, has been reported in several other studies (19, 22) and may be accounted for by various factors, such as differences in the administration of the inoculum, the degree of vesicoureteral reflux, and the susceptibility of individual animals, even for mice of the same genetic background. In analyses of the survival of the AL511 vpeBC mutant in the UT, bladder and/or kidney colonization was also observed, and survival in the UT did not differ significantly from that of the wild type. Only two of six mice in which the UT was colonized by the mutant displayed bladder colonization, with 107 CFU/g of organ. However, five of these six mice displayed colonization of one or both kidneys, with 104 to 106 CFU/g of organ (data not shown). We corrected for the variations inherent to the model by carrying out experiments involving mixed infections for estimation of the relative persistence of the strains in the UT for each mouse (Fig. 5). The wild type colonized the bladder and kidneys to an extent similar to that observed in single infections (from 104 to 109 CFU/g of bladder tissue and 104 to 5 × 106 CFU/g of kidney tissue). AL511 vpeBC survived almost as well as the wild type in the bladder (P = 0.212), but its survival was markedly lower in the kidneys (P = 0.0014) (Fig. 5A). The mutant was outcompeted by the wild type in the kidneys of 58% of the mice, with a mean log CI value of −0.7 (P = 0.038), indicating strong attenuation of the strain. No trend toward a disadvantage of the mutant in the bladder was detected in CI analysis (mean log CI of −0.2, P = 0.483) (Fig. 5B). No complementation of the poor competitiveness of the mutant was obtained in urine cocultures. We therefore did not carry out in vivo complementation experiments. However, we carried out two series of coinfections (with five mice in each case), which provided evidence for a specific role of the vpeBC deletion in the poor survival of the mutant in the kidneys. Like the AL511 vpeBC mutant, the AL511 vpeBC (Km-FRT) strain colonized the bladder, but its survival in the kidneys was lower than that of the wild type. Indeed, four mice had approximately 104 CFU of each strain/g of bladder tissue, and two mice had only the wild type in the kidneys (104 CFU/g of organ). Assuming that no cost was associated with the introduction of the kanamycin cassette in place of the vpeBC genes in the mutant, we expected AL511 vpeBC (Km-FRT) and its kanamycin-sensitive derivative (AL511 vpeBC [FRT]) to have similar colonizing abilities. Mice were infected with the two strains simultaneously, and the two strains were recovered at similar levels from both the bladder and kidneys: four mice had 103 to 104 CFU of each strain/g of bladder tissue, and three mice had 103 to 105 CFU of each strain/g of kidney tissue (data not shown).
Fig 5.
Competitive colonization of the urinary tract (UT) by AL511 and AL511 vpeBC. Two independent colonization experiments were performed on a total of 24 mice displaying UT colonization in the bladder 4 days after the simultaneous administration of the two strains (1:1 ratio). (A) The results are reported as log CFU/g of organ for AL511 (◆) and AL511 vpeBC (♢). The horizontal bars represent the mean values. The asterisks indicate significant differences, with P values of <0.05 in a paired one-tailed t test. (B) The results are reported as log CI values. Each point (△) corresponds to a single mouse, and the horizontal bars represent the mean values. The asterisk indicates mean values of less than −0.3, indicating significant attenuation of the mutant.
Exopolysaccharide production in urine and vpe genes.
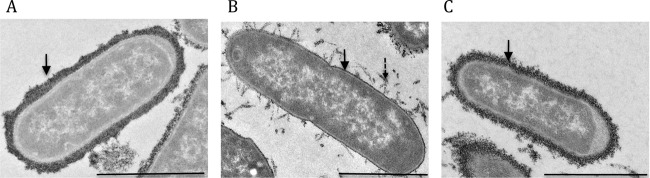
E. coli strains produce many cell surface polysaccharides with biological functions. We investigated whether differences in exopolysaccharide production reflected differences in the virulence of the AL511 and AL511 vpeBC strains by culturing bacteria overnight in LB broth or human urine and labeling them with cationic ferritin. Under both sets of growing conditions, the vpeBC deletion significantly decreased the amounts of cell surface-associated polysaccharides. These effects were stronger in urine than in LB broth. In urine, substantial amounts of polymer were clearly released from the surface. Complementation of the vpeBC mutation with the pZEZeovpeBC plasmid significantly increased the production of ferritin-labeled material at the cell surface, as observed by electron microscopy (Fig. 6).
Fig 6.
Transmission electron micrographs of exopolysaccharide production by the wild type and vpe mutants of the AL511 strain grown in urine. Surface-expressed polysaccharides are visible as electron-dense material on the surface of bacteria stained with ferritin and examined by transmission electron microscopy. Representative images of wild-type AL511 (A), AL511 vpeBC (B), and complemented AL511 vpeBC (C) are shown. Thick, dark, circumferential staining (solid arrow) was observed at the surface of most (89%) AL511 cells. In contrast, only a thin layer of irregular staining was identified at the surface of AL511 vpeBC cells. Labeling was discrete, with detached material visible (dashed arrow). The expression of pZEZeovpeBC restored capsule production to wild-type levels, in terms of the number of stained bacteria and the thickness of the ferritin-stained layer. This thickness was estimated by measurements taken at five different points on seven bacteria. The mean scores were 129, 27, and 84 nm for AL511, AL511 vpeBC, and complemented strains, respectively. Bars, 1 μm.
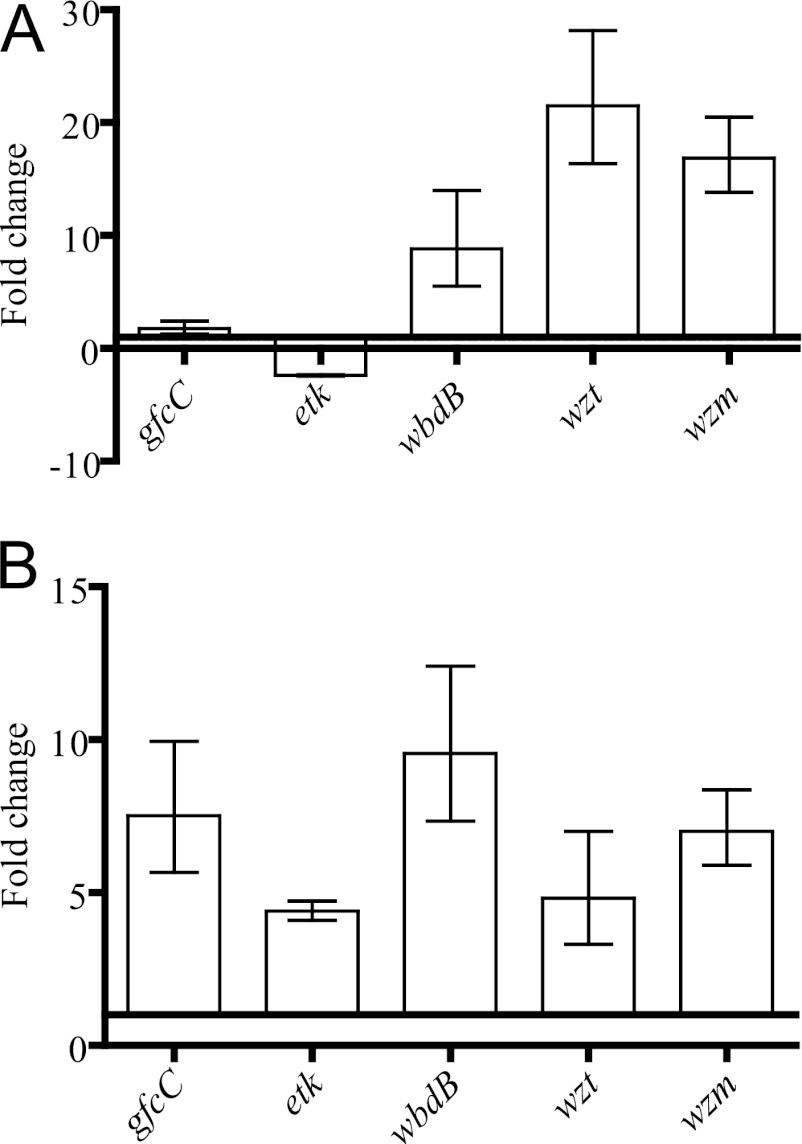
The exopolysaccharides produced by E. coli strains include the O-specific polysaccharide (or O antigen) and the capsular polysaccharide. The AL511 stain has been reported to express the O9 antigen. Four capsule groups (groups 1 to 4) have been defined in E. coli (52). We determined the capsular serotype of AL511 in a PCR-based assay (24, 25, 37, 38). AL511 had typical group 4 capsules (data not shown). This classification was based on the amplification of gfcC and etk, two genes of the gfc operon involved in assembly and export at the cell surface of the capsule (34) (see Table S1 in the supplemental material). We compared the relative expression of gfc genes and O9 antigen (wbdB, wzt, and wzm) genes by qRT-PCR after stationary-phase culture of AL511 in urine and LB broth. The levels of expression of gfcC and etk were similar in the two media, but the rate of induction of wbdB, wzt, and wzm expression was more than eight times higher in urine than in LB broth, suggesting an overproduction of the O9 antigen in urine (Fig. 7A). We then investigated the cross-regulation of vpe genes and the exopolysaccharide genes in assays comparing relative expression in AL511, AL511 vpeBC, and AL511 vpeR in urine. The vpeR mutation had no significant effect on mRNA levels (data not shown), suggesting that VpeR plays no role in exopolysaccharide production. Interestingly, the level of expression of the five genes was significantly modified (P = 0.0056) in the vpeBC mutant. However, this modification was an increase (by a factor of 4 to 9), rather than the expected decrease (Fig. 7B). These data indicate that the expression of vpe genes is linked to that of exopolysaccharide genes in urine and suggest that the carbohydrate transported by the Vpe permease may affect the production of exopolysaccharides by AL511.
Fig 7.
Transcription of genes involved in exopolysaccharide production in AL511. Transcription of genes specific for the assembly and export of group 4 capsule at the cell surface (gfcC and etk) and of genes specific for the ABC-dependent mechanism of O9-antigen biosynthesis (wbdB, wzt, and wzm) was analyzed by qRT-PCR after growth to stationary phase in either LB broth or urine. (A) An effect of urine on the expression of genes involved in O9-antigen synthesis is illustrated. The indicated fold change is the ratio of growth in urine to that in LB broth. (B) The relative expression of the genes in the AL511 vpeBC and AL511 strains was analyzed after growth in urine. The expression of all the genes was induced. The relative expression of the genes in the AL511 vpeR and AL511 strains was set to 1 in urine (data not shown).
Exopolysaccharide production in serum and vpe genes.
Exopolysaccharides contribute to the virulence of many bacterial pathogens, partly by protecting against complement-mediated killing. We therefore compared the survival, in the presence of human serum, of AL511, two independent vpeBC mutants, and a complemented mutant strain (AL511 vpeBC + pZEZeovpeBC) (Fig. 8). No significant difference in survival was observed between the parental and complemented strains. In contrast, 30 min after the start of the incubation period, we recovered significantly fewer CFU of the mutant strains than of the parental or complemented strains. The mutants were no longer viable after 2 h of incubation (Fig. 8A). The inactivation of complement by heating prevented the serum-mediated killing of the vpeBC mutants (Fig. 8B). We therefore suggest that the link between vpe gene expression and the virulence of AL511 may reflect a link between vpe gene expression and the production of exopolysaccharides.
Fig 8.
Kinetics of the killing of AL511 wild type and vpe mutants by human serum. Changes in cell viability were estimated after exposing bacteria to 20% normal (A) or heat-treated (B) human serum. All points are the means of at least three independent determinations in two independent experiments. Similar data were obtained with serum samples from two other healthy people.
The vpe genes and colonization of the mouse intestine.
Pathogenic E. coli infections in humans and animals are transmitted by the orofecal route. Adaptation to the intestinal environment is therefore the first step in infection for UPEC strains. We investigated the possible dependence of intestinal colonization on the expression of vpe genes in the streptomycin-treated mouse model of gut colonization. We fed the AL511 and mutant strains independently to mice, and these strains were found to have colonized the mouse intestine to similar levels (107 to 109 CFU/g of feces) 1 day postinfection. Both strains persisted at this level throughout the 18 days of the assay (data not shown). The relative fitness of the AL511 and vpeBC mutant strains was evaluated in two sets of mixed-infection experiments on a total of 15 mice. Fecal colonization by the parental strain occurred at a level similar to that in single infections, and the bacterium remained present, at a density of 107 to 109 CFU/g of feces, throughout the 18 days. However, the counts of the two parental and mutant strains were similar in feces after 24 h in only 11 of the 15 mice, and in these 11 mice, mutant levels decreased over time (Fig. 9). For both strains, the numbers of CFU per gram of feces differed significantly between 5 days after challenge (P = 0.044) and the end of the experiment (P = 0.032, P = 0.016, P = 0.017, and P = 0.016 on days 8, 12, 15, and 18 postinfection, respectively). Similarly, significant differences in the mean log CI were observed from 8 days after inoculation (−1.08, −1.61, −1.56, and −1.56 on days 8, 12, 15, and 18 postinfection, respectively). Therefore, although the AL511 vpeBC mutant persisted in the intestine when fed to the animals alone, it did not grow well enough to maintain its populations in the intestine when fed to mice simultaneously with the parental strain. We checked that there was no cost associated with the introduction of the cassette replacing the vpeBC genes in AL511 vpeBC by also carrying out a set of mixed-infection experiments with the AL511 vpeBC (Km-FRT) and AL511 vpeBC (FRT) mutant strains. Both these strains increased in number to 107 to 108 CFU/g of feces after infection; colonization was maintained at this level throughout the 12 days of the experiment (data not shown).
Fig 9.
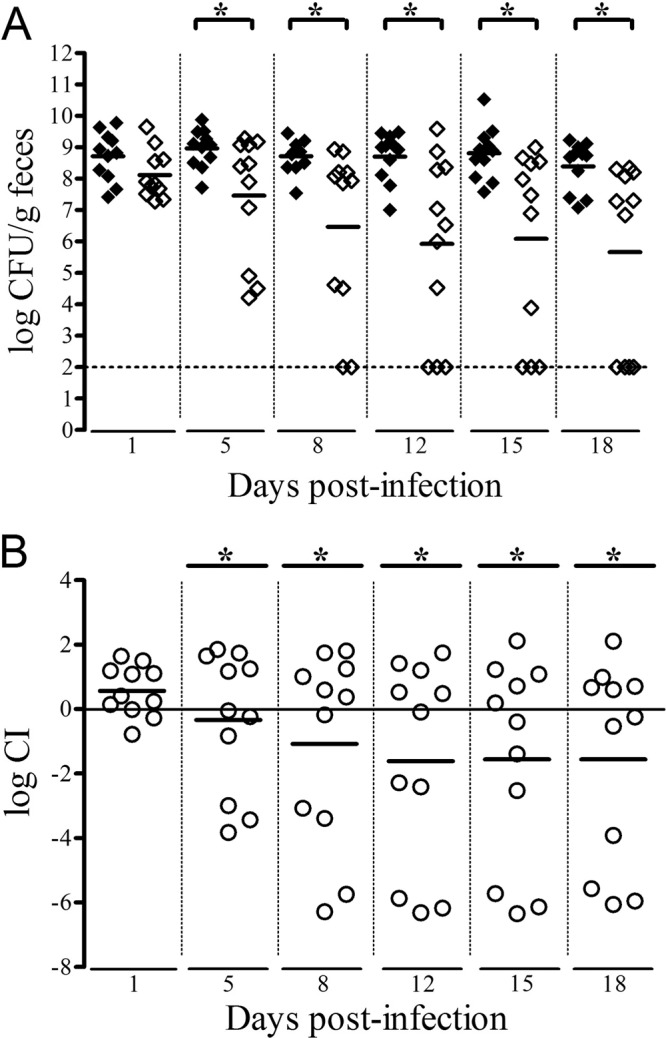
Competitive colonization of the mouse intestine by AL511 and AL511 vpeBC. Two independent colonization experiments were performed. Eleven mice had intestinal colonization 1 day after the simultaneous administration of the two strains (1:1 ratio) by oral force-feeding. At the times indicated, fecal samples were plated on LB agar with and without kanamycin. (A) The results are reported as log CFU/g of feces for AL511 (◆) and AL511 vpeBC (♢). We arbitrarily attributed a value of 102 CFU/g of feces to a strain if no bacteria were recovered on plates. The horizontal bars represent the mean values. The asterisks indicate significant differences, with P values of <0.05 in a paired one-tailed t test. (B) The results of the experiments are reported as log CI values. The CI was calculated as the ratio of colony counts for the mutant to those for the wild type recovered from feces at the various times divided by the initial ratio of mutant to wild-type CFU. Each point (○) corresponds to a single mouse, and the horizontal bars represent the mean values. The asterisks indicate mean values of less than −0.3, reflecting significant attenuation of the mutant.
DISCUSSION
Studies are increasingly demonstrating a link between the transport and metabolism of various carbohydrates and virulence in enterobacteria (30). However, in most cases, the precise role of such metabolic traits in the expression and regulation of virulence remains to be determined. They may play a fundamental role in facilitating the adaptation of the bacteria to their environment, by enabling the bacteria to use the carbohydrates present in the host for growth. This may promote host colonization and increase the pathogenic potential of the bacteria. It has also been suggested that the enzymes involved in carbohydrate transport may control gene expression in response to nutrient availability (12). Extraintestinal pathogenic E. coli (ExPEC) strains, including UPEC, are unique among pathogenic E. coli strains in being able to adapt their metabolism first to the intestine, their reservoir, in which they must outcompete members of the normal microflora for nutrients, and then to the various normally sterile sites at which they need to survive and grow. Many operons involved in carbohydrate metabolism have been identified in the genomes of ExPEC strains, but only the fos, frz, and deoK operons have been reported to play a role in gut colonization (30, 41, 48). We investigated the role of the PTS system in the development of E. coli-associated infections outside the gastrointestinal tract.
The PTS system is a sensing and transport system present in many bacteria that allows substrates to cross the inner membrane. It uses phosphoenolpyruvate (PEP) as a source of energy. It consists of two general cytoplasmic components, both of which are energy-coupling proteins required for the transfer of phosphate from PEP to any of a number of membrane-associated sugar permease complexes, also referred to as enzymes II (EII), each specific for a substrate (15). Comparative genomics studies of E. coli strains led to identification of the vpe locus, which encodes the three components of an EII complex (VpeA, VpeB, and VpeC) and a putative transcriptional regulator (VpeR). These genes are present in large strain-specific islands that frequently harbor other genes related to carbohydrate transport and metabolism. In most cases, the vpe and deoK loci are strongly linked (>75% association). We previously showed that the deoK operon encodes proteins involved in deoxyribose utilization and was acquired by horizontal transfer from Salmonella enterica (6, 32). The high level of similarity between the vpe locus and a chromosomal sequence in Yersinia (75% identical to the sequence of Y. enterocolitica strain 8081) and its G+C content of 46.8% suggested that E. coli acquired this sequence from Yersinia. The 9-kb region carrying these two loci seems to have resulted from a stepwise acquisition of DNA sequences from foreign sources.
Previous studies have reported UPEC strains to have some virulence characteristics in common with diarrheagenic E. coli—particularly those associated with the EAEC pathotype—suggesting that some EAEC strains may be potential uropathogens or that some UPEC strains might infect the gastrointestinal tract (1). The occurrence of the deoK-vpe region not only in strains isolated from infected urine and blood but also in EAEC isolates provides support for this hypothesis and raises questions about the role of this region in intestinal and extraintestinal infections. The genetic linkage of the deoK and vpe clusters suggests possible complementary functions. The recent demonstration that deoxyribose catabolism is involved in colonization of the intestine by pathogenic E. coli is consistent with a role for the vpe operon in host colonization. Carbohydrate metabolism has been shown to be important for colonization of the mammalian intestine by E. coli (9). We therefore investigated the role of the VpeABC EII permease in the provision of an additional source of nutrients. We found that it increased bacterial fitness during in vivo assays of competition in colonization of the mouse gut. Interestingly, similar results were obtained for the UPEC AL511 and EAEC 55989 (5) strains (data not shown).
We demonstrated that expression of the vpe cluster also plays a role in the virulence of AL511, increasing resistance to killing by complement and favoring the development of systemic infection. We suggest that the vpe locus responds to the availability of nutrients present in human urine, and we have identified the UT as a site at which the vpe-specific PTS system may also be involved in the expression of bacterial virulence. As the induction of expression in urine increased bacterial fitness during coculture experiments, we initially investigated the involvement of the PTS system in fitness in vivo and UT colonization. We developed an ascending UTI model of infection in mice, in which we showed that the vpe locus was involved in kidney colonization.
Exopolysaccharides (lipopolysaccharides [LPS], O antigens, and capsular polysaccharides) play a crucial role in bacterial survival in hostile environments. Many studies have been carried out, but conflicting conclusions have been drawn regarding the role of exopolysaccharides in urovirulence, based on the quantification of UT colonization and mortality due to urosepsis in various mouse models (42, 47). The K2 capsule of the CFT073 strain was recently shown to play a role in such infections. It was shown to be important for kidney colonization in a mouse model of UTI and to provide protection against complement-mediated killing (8). The AL511 strain expresses the O9 antigen and is the first UPEC isolate reported to carry the gfc operon required for the production of a group 4 capsule (BLAST analysis on 3 January 2012). We demonstrated cross-regulation of the expression of vpe genes and production of exopolysaccharides in biological assays on strains cultured in human fluids (urine and serum). A cross-regulation of vpe and exopolysaccharide gene expression was also detected in qRT-PCR assays on RNA samples extracted from overnight cultures in urine. In summary, an absence of vpeBC gene expression in the mutant was found to be correlated with a significantly higher level of expression of both group 4 capsule and O9-antigen genes and with the excretion of polysaccharide chains retaining only a limited association with the cell surface, suggesting potential defects in the final stages of assembly or polymer export when too many of these polysaccharides are produced. No such modifications were detected in the vpeR mutant, suggesting that the substrate of the Vpe permease may play a role in exopolysaccharide expression and production. Further investigations of the interaction of the phosphorylated carbohydrate with polysaccharide gene expression are required and will be facilitated by determination of the complete genome sequence of the AL511 strain. In diarrhea-associated E. coli strains, group 4 capsule polysaccharides are produced and assembled from the same O-antigen repeat unit as used to produce LPS (34). We need to determine whether a similar process occurs in AL511.
UPEC isolates classically belong to phylogenetic group B2 and have group 2 and 3 capsules. AL511 is, thus, an atypical UPEC strain, because it belongs to phylogenetic group A and has a group 4 capsule. Preliminary studies determined the potential urovirulence of this strain (10, 36). We provide here the first evidence of a role for O9 antigen and/or the group 4 capsule in urovirulence and serum resistance and suggest that the production of these exopolysaccharides may be under metabolic control. A previous analysis of the transcriptome of a well-characterized group B2 UPEC strain, CFT073, provided evidence for upregulation of the vpe locus in vitro, during growth in human urine, and in vivo, during UTI (49). We now need to determine whether the group 2 and 3 capsules produced by classical group B2 UPEC isolates are also under the control of the PTS system, to improve our understanding of the development of urinary tract infections. Interestingly, characterization of the PAI-V from the UPEC strain 536 revealed that the K15 (group 2/3) capsule locus involved in the urovirulence of this strain was genetically linked to a region of about 10 kb in length carrying the deoK operon and an EII complex-encoding locus (47).
Pathogens require nutrients provided by the host to grow and cause disease. A comprehensive understanding of pathogen nutrition and metabolism may therefore provide a rational basis for the development of new pathogen identification tools and therapeutic strategies. It is becoming increasingly apparent that there is a link between the transport and metabolism of carbohydrates and virulence in enterobacteria. We identified a new metabolic trait influencing the expression of full virulence in a uropathogenic E. coli strain. However, as in most previous studies, the carbohydrate metabolized has yet to be identified, perhaps because it is not commercially available or is transported by another system that complements the vpeBC mutation. The recent massive development of equipment, software, and powerful analytical methods should facilitate more global studies of the link between metabolism and virulence. In particular, additional studies on the metabolism of carbohydrates present in newly developed foods and dietary supplements are required, because the catabolism of such molecules by pathogenic bacteria might have major implications for human health.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants PTR-165 from the Institut Pasteur and ANR-06-PATHO-002-03 from the Agence Nationale de la Recherche “ERA-NET PathoGenoMics.” V.M.-J. held a fellowship from the Ministère de l'Enseignement Supérieur et de la Recherche. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We thank I. Lequeutre and L. Ma (Institut Pasteur) for expert technical assistance. We thank A. M. Gilles (Institut Pasteur) and Y. Guérardel (Université des Sciences et Technologies de Lille, France) for identifying the carbohydrate transported by the product of vpe genes and for helpful discussions. We also thank P. Trieu-Cuot for critical reading of the manuscript.
Footnotes
Published ahead of print 21 May 2012
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1. Abe CM, et al. 2008. Uropathogenic Escherichia coli (UPEC) strains may carry virulence properties of diarrhoeagenic E. coli. FEMS Immunol. Med. Microbiol. 52:397–406 [DOI] [PubMed] [Google Scholar]
- 2. Archambaud M, Courcoux P, Labigne-Roussel A. 1988. Detection by molecular hybridization of pap, afa, and sfa adherence systems in Escherichia coli strains associated with urinary and enteral infections. Ann. Inst. Pasteur Microbiol. 139:575–588 [DOI] [PubMed] [Google Scholar]
- 3. Bagshaw SM, Laupland KB. 2006. Epidemiology of intensive care unit-acquired urinary tract infections. Curr. Opin. Infect. Dis. 19:67–71 [DOI] [PubMed] [Google Scholar]
- 4. Bahrani-Mougeot FK, et al. 2002. Type 1 fimbriae and extracellular polysaccharides are preeminent uropathogenic Escherichia coli virulence determinants in the murine urinary tract. Mol. Microbiol. 45:1079–1093 [DOI] [PubMed] [Google Scholar]
- 5. Bernier C, Gounon P, Le Bouguénec C. 2002. Identification of an aggregative adhesion fimbria (AAF) type III-encoding operon in enteroaggregative Escherichia coli as a sensitive probe for detecting the AAF-encoding operon family. Infect. Immun. 70:4302–4311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bernier-Fébreau C, et al. 2004. Use of deoxyribose by intestinal and extraintestinal pathogenic Escherichia coli strains: a metabolic adaptation involved in competitiveness. Infect. Immun. 72:6151–6156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blattner FR, et al. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453–1462 [DOI] [PubMed] [Google Scholar]
- 8. Buckles EL, et al. 2009. Role of the K2 capsule in Escherichia coli urinary tract infection and serum resistance. J. Infect. Dis. 199:1689–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chang DE, et al. 2004. Carbon nutrition of Escherichia coli in the mouse intestine. Proc. Natl. Acad. Sci. U. S. A. 101:7427–7432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chassin C, et al. 2006. Renal collecting duct epithelial cells react to pyelonephritis-associated Escherichia coli by activating distinct TLR4-dependent and -independent inflammatory pathways. J. Immunol. 177:4773–4784 [DOI] [PubMed] [Google Scholar]
- 11. Chaveroche MK, Ghigo JM, d'Enfert C. 2000. A rapid method for efficient gene replacement in the filamentous fungus Aspergillus nidulans. Nucleic Acids Res. 28:E97 doi:10.1093/nar/28.22.e97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Commichau FM, Stulke J. 2008. Trigger enzymes: bifunctional proteins active in metabolism and in controlling gene expression. Mol. Microbiol. 67:692–702 [DOI] [PubMed] [Google Scholar]
- 13. Da Re S, Ghigo JM. 2006. A CsgD-independent pathway for cellulose production and biofilm formation in Escherichia coli. J. Bacteriol. 188:3073–3087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Deutscher J, Francke C, Postma PW. 2006. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol. Mol. Biol. Rev. 70:939–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Epstein W, Kim BS. 1971. Potassium transport loci in Escherichia coli K-12. J. Bacteriol. 108:639–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Foxman B, Brown P. 2003. Epidemiology of urinary tract infections: transmission and risk factors, incidence, and costs. Infect. Dis. Clin. North Am. 17:227–241 [DOI] [PubMed] [Google Scholar]
- 18. Girardeau JP, Lalioui L, Said AM, De Champs C, Le Bouguénec C. 2003. Extended virulence genotype of pathogenic Escherichia coli isolates carrying the afa-8 operon: evidence of similarities between isolates from humans and animals with extraintestinal infections. J. Clin. Microbiol. 41:218–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hagberg L, et al. 1983. Ascending, unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coli of human origin. Infect. Immun. 40:273–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Haugen BJ, et al. 2007. In vivo gene expression analysis identifies genes required for enhanced colonization of the mouse urinary tract by uropathogenic Escherichia coli strain CFT073 dsdA. Infect. Immun. 75:278–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Henderson JP, et al. 2009. Quantitative metabolomics reveals an epigenetic blueprint for iron acquisition in uropathogenic Escherichia coli. PLoS Pathog. 5:e1000305 doi:10.1371/journal.ppat.1000305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hung CS, Dodson KW, Hultgren SJ. 2009. A murine model of urinary tract infection. Nat. Protoc. 4:1230–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hvorup R, Chang AB, Saier MH., Jr 2003. Bioinformatic analyses of the bacterial L-ascorbate phosphotransferase system permease family. J. Mol. Microbiol. Biotechnol. 6:191–205 [DOI] [PubMed] [Google Scholar]
- 24. Johnson JR, O'Bryan TT. 2004. Detection of the Escherichia coli group 2 polysaccharide capsule synthesis gene kpsM by a rapid and specific PCR-based assay. J. Clin. Microbiol. 42:1773–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Johnson JR, Stell AL. 2000. Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J. Infect. Dis. 181:261–272 [DOI] [PubMed] [Google Scholar]
- 26. Kelly M, et al. 2006. Essential role of the type III secretion system effector NleB in colonization of mice by Citrobacter rodentium. Infect. Immun. 74:2328–2337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Khouri N, et al. 2006. An unusual case of dramatic acute bilateral pyelonephritis with systemic bacterial dissemination caused by uropathogenic Escherichia coli. Nephrol. Dial. Transplant. 21:1423–1426 [DOI] [PubMed] [Google Scholar]
- 28. Köhler CD, Dobrindt U. 2011. What defines extraintestinal pathogenic Escherichia coli? Int. J. Med. Microbiol. 301:642–647 [DOI] [PubMed] [Google Scholar]
- 29. Lalioui L, Le Bouguénec C. 2001. afa-8 gene cluster is carried by a pathogenicity island inserted into the tRNA(Phe) of human and bovine pathogenic Escherichia coli isolates. Infect. Immun. 69:937–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Le Bouguénec C, Schouler C. 2011. Sugar metabolism, an additional virulence factor in enterobacteria. Int. J. Med. Microbiol. 301:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 32. Martinez-Jéhanne V, et al. 2009. Role of deoxyribose catabolism in colonization of the murine intestine by pathogenic Escherichia coli strains. Infect. Immun. 77:1442–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Neelakanta G, Sankar TS, Schnetz K. 2009. Characterization of a beta-glucoside operon (bgc) prevalent in septicemic and uropathogenic Escherichia coli strains. Appl. Environ. Microbiol. 75:2284–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Peleg A, et al. 2005. Identification of an Escherichia coli operon required for formation of the O-antigen capsule. J. Bacteriol. 187:5259–5266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Picard B, et al. 1999. The link between phylogeny and virulence in Escherichia coli extraintestinal infection. Infect. Immun. 67:546–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pichon C, et al. 2009. Uropathogenic Escherichia coli AL511 requires flagellum to enter renal collecting duct cells. Cell. Microbiol. 11:616–628 [DOI] [PubMed] [Google Scholar]
- 37. Power ML, Littlefield-Wyer J, Gordon DM, Veal DA, Slade MB. 2005. Phenotypic and genotypic characterization of encapsulated Escherichia coli isolated from blooms in two Australian lakes. Environ. Microbiol. 7:631–640 [DOI] [PubMed] [Google Scholar]
- 38. Rahn A, Drummelsmith J, Whitfield C. 1999. Conserved organization in the cps gene clusters for expression of Escherichia coli group 1 K antigens: relationship to the colanic acid biosynthesis locus and the cps genes from Klebsiella pneumoniae. J. Bacteriol. 181:2307–2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Reid AN, Whitfield C. 2005. Functional analysis of conserved gene products involved in assembly of Escherichia coli capsules and exopolysaccharides: evidence for molecular recognition between Wza and Wzc for colanic acid biosynthesis. J. Bacteriol. 187:5470–5481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rossi MS, Paquelin A, Ghigo JM, Wandersman C. 2003. Haemophore-mediated signal transduction across the bacterial cell envelope in Serratia marcescens: the inducer and the transported substrate are different molecules. Mol. Microbiol. 48:1467–1480 [DOI] [PubMed] [Google Scholar]
- 41. Rouquet G, et al. 2009. A metabolic operon in extraintestinal pathogenic Escherichia coli promotes fitness under stressful conditions and invasion of eukaryotic cells. J. Bacteriol. 191:4427–4440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Russo T, Brown JJ, Jodush ST, Johnson JR. 1996. The O4 specific antigen moiety of lipopolysaccharide but not the K54 group 2 capsule is important for urovirulence of an extraintestinal isolate of Escherichia coli. Infect. Immun. 64:2343–2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Russo TA, Carlino UB, Mong A, Jodush ST. 1999. Identification of genes in an extraintestinal isolate of Escherichia coli with increased expression after exposure to human urine. Infect. Immun. 67:5306–5314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Russo TA, Jodush ST, Brown JJ, Johnson JR. 1996. Identification of two previously unrecognized genes (guaA and argC) important for uropathogenesis. Mol. Microbiol. 22:217–229 [DOI] [PubMed] [Google Scholar]
- 45. Russo TA, et al. 2002. IroN functions as a siderophore receptor and is a urovirulence factor in an extraintestinal pathogenic isolate of Escherichia coli. Infect. Immun. 70:7156–7160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Russo TA, Stapleton A, Wenderoth S, Hooton TM, Stamm WE. 1995. Chromosomal restriction fragment length polymorphism analysis of Escherichia coli strains causing recurrent urinary tract infections in young women. J. Infect. Dis. 172:440–445 [DOI] [PubMed] [Google Scholar]
- 47. Schneider G, et al. 2004. The pathogenicity island-associated K15 capsule determinant exhibits a novel genetic structure and correlates with virulence in uropathogenic Escherichia coli strain 536. Infect. Immun. 72:5993–6001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schouler C, Taki A, Chouikha I, Moulin-Schouleur M, Gilot P. 2009. A genomic island of an extraintestinal pathogenic Escherichia coli strain enables the metabolism of fructooligosaccharides, which improves intestinal colonization. J. Bacteriol. 191:388–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Snyder JA, et al. 2004. Transcriptome of uropathogenic Escherichia coli during urinary tract infection. Infect. Immun. 72:6373–6381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sorsa LJ, Feldmann F, Hildinger K, Dufke S, Schubert S. 2007. Characterization of four novel genomic regions of uropathogenic Escherichia coli highly associated with the extraintestinal virulent phenotype: a jigsaw puzzle of genetic modules. Int. J. Med. Microbiol. 297:83–95 [DOI] [PubMed] [Google Scholar]
- 51. Touchon M, et al. 2009. Organised genome dynamics in the Escherichia coli species results in highly diverse adaptive paths. PLoS Genet. 5:e1000344 doi:10.1371/journal.pgen.1000344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Whitfield C. 2006. Biosynthesis and assembly of capsular polysaccharides in Escherichia coli. Annu. Rev. Biochem. 75:39–68 [DOI] [PubMed] [Google Scholar]
- 53. Zhang L, Foxman B. 2003. Molecular epidemiology of Escherichia coli mediated urinary tract infections. Front. Biosci. 8:e235–e244 [DOI] [PubMed] [Google Scholar]
- 54. Zhang Z, Aboulwafa M, Smith MH, Saier MH., Jr 2003. The ascorbate transporter of Escherichia coli. J. Bacteriol. 185:2243–2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.