Abstract
Cholera is classically considered a noninflammatory diarrheal disease, in comparison to invasive enteric organisms, although there is a low-level proinflammatory response during early infection with Vibrio cholerae and a strong proinflammatory reaction to live attenuated vaccine strains. Using an adult mouse intestinal infection model, this study examines the contribution of neutrophils to host defense to infection. Nontoxigenic El Tor O1 V. cholerae infection is characterized by the upregulation of interleukin-6 (IL-6), IL-10, and macrophage inflammatory protein 2 alpha in the intestine, indicating an acute innate immune response. Depletion of neutrophils from mice with anti-Ly6G IA8 monoclonal antibody led to decreased survival of mice. The role of neutrophils in protection of the host is to limit the infection to the intestine and control bacterial spread to extraintestinal organs. In the absence of neutrophils, the infection spread to the spleen and led to increased systemic levels of IL-1β and tumor necrosis factor alpha, suggesting the decreased survival in neutropenic mice is due to systemic shock. Neutrophils were found not to contribute to either clearance of colonizing bacteria or to alter the local immune response. However, when genes for secreted accessory toxins were deleted, the colonizing bacteria were cleared from the intestine, and this clearance is dependent upon neutrophils. Thus, the requirement for accessory toxins in virulence is negated in neutropenic mice, which is consistent with a role of accessory toxins in the evasion of innate immune cells in the intestine. Overall, these data support that neutrophils impact disease progression and suggest that neutrophil effectiveness can be manipulated through the deletion of accessory toxins.
INTRODUCTION
Global pandemics of the diarrheal disease cholera have occurred throughout human history. The disease is caused by ingestion of the Gram-negative bacterium Vibrio cholerae in contaminated water. Cholera is typified by symptoms of severe, watery diarrhea that leads to life-threatening dehydration and loss of electrolytes (48). There have been seven cholera pandemics in recorded history. The first six were caused by V. cholerae O1 of the classical biotype. The seventh and currently ongoing pandemic began in 1961, when V. cholerae O1 strains of the El Tor biotype began to predominate and spread throughout the world (22). Recent whole-genome analysis of single-nucleotide polymorphisms revealed that the seventh pandemic consists of three distinct waves of transmission from the pandemic center in South Asia. Strain diversification has resulted from continuous local evolution punctuated by sporadic long-range transmission to countries where this pathogen is not endemic, such as in the case of Haiti in 2010 (34). It is estimated that 1.4 billion people worldwide are at risk for cholera, with approximately 2.8 million cholera cases occurring in areas of endemicity each year (1).
The innate immune response to V. cholerae infection is poorly understood. Recent studies in humans of the immune response to natural V. cholerae El Tor O1 infection reveal that persons who naturally acquire cholera transition through an early proinflammatory phase characterized by an increase in markers of inflammation as demonstrated by gut biopsy, stool sample, and peripheral blood analysis. Patients show increased levels of C-reactive protein, total white blood cells, neutrophils, and bactericidal proteins lactoferrin (Lf) and myeloperoxidase (MPO) (12, 43, 44). This proinflammatory phase persists during acute, symptomatic illness and is then followed by a noninflammatory convalescent phase, during which expression of inflammatory markers are suppressed (44).
It is possible that the phases of the immune response to infection are partially driven by the immunomodulatory activity of the primary virulence factor cholera toxin (CT). Studies in vitro have demonstrated that CT suppresses induction of proinflammatory and regulatory cytokines tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), and IL-12 by lipopolysaccharide (LPS)-stimulated macrophages (6, 10). CT is also known to be a potent adjuvant, enhancing antigen presentation by antigen-presenting cells and intestinal epithelial cells (3, 4, 8). Administration of CT as an immunoadjuvant has demonstrated its ability to direct CD4+ T cells to a Th2 and/or Th17 lineage, while suppressing Th1 differentiation (23, 24, 31). Given the myriad immunomodulatory effects of CT, it would be unsurprising if nontoxigenic V. cholerae strains elicit immune responses distinct from that of toxigenic strains.
Studies in humans have suggested that diarrhea is more inflammatory in nature when caused by vaccine strains deleted of genes for CT (26). These studies correlate with clinical observations in which naturally occurring “nontoxigenic” V. cholerae O1 strains that have not acquired the CTXΦ prophage induce diarrhea that is more inflammatory (5, 33). In an infant rabbit model of infection, this diarrhea has been specifically linked to expression of flagellin that may be functioning by increasing inflammatory signaling through TLR5 (20, 46, 47). Studies using lung infection in mice have also linked proinflammatory signaling from CT-negative strains to LPS-dependent signaling through TLR4 (18), whereas lipoprotein has been shown in vitro to stimulate inflammation via TLR1/TLR2 (16, 25). Thus, the prevailing models suggest that bacterial factors induce a proinflammatory response that then causes significant diarrhea, resulting in either serious clinical symptoms during natural infection with nontoxigenic strains or reactogenic symptoms in vaccine studies.
Use of mouse models has been a major asset to understanding innate immunity in other bacterial pathogens; however, the emphasis of cholera research on human, rabbit, and infant mouse studies has prevented detailed analysis of how protection against cholera develops, since reagents are predominantly developed for studies in adult mice. The use of ketamine as an anesthetic has been shown to promote small intestine infections, allowing for development of adult mouse models of infection for the purpose of studying immune responses (40). Applications of these models have focused on understanding the role of toxins other than CT during infection (39, 41) and analysis of vaccine efficacy (38).
Adult mice have also been critical in demonstrating the roles of secreted “accessory toxins” of V. cholerae, including hemolysin, multifunctional autoprocessing repeats-in-toxin (MARTX), and hemagglutinin (HA)/protease. Hemolysin is a pore-forming cytotoxin that induces vacuolation and cell lysis and is linked to induction of autophagy (7, 11, 17). MARTX is a multifunctional toxin that depolymerizes and cross-links actin, thereby rounding cells and disabling phagocytosis (14, 28). HA/protease is a zinc metalloprotease that can degrade various host proteins, including mucin, thus acting primarily as a means for bacteria to detach from epithelial surfaces and promote further spread (36). Recent studies in adult mice demonstrate that the accessory toxins contribute to prolonged colonization of the small intestine (40).
For V. cholerae small intestine infection, it is proposed that this animal model mimics well the proinflammatory phase of infection with a transition after 24 h to an asymptomatic carriage state rather than to disease (41). Notably, a recent study in human patients in an area of endemicity indicates that only 57% of exposures to V. cholerae El Tor O1 result in clinical symptoms (19). The high incidence of asymptomatic infections can likely be explained by a number of factors, including preexisting immunity, coinfections with other pathogenic organisms, blood type, nutrition status, etc., all of which may affect whether the proinflammatory phase of infection is successful in containing the infection as an asymptomatic colonization or clearing the bacteria entirely (35). Thus, studying this phase of infection in mice will provide insight into host protection against natural infection.
Neutrophils are considered a first-line cellular defense against bacterial infection (21). However, histological studies demonstrated a low abundance of neutrophils in a patient pool from Vellore, India, with moderate-to-severe cholera disease, suggesting that they may not have a significant impact on V. cholerae infection (32). In contrast, other reports do indicate a neutrophil response during the acute phase of V. cholerae infection (12, 43, 44). In the present study, we use mice depleted of neutrophils to determine whether and how neutrophils contribute to cholera disease during the proinflammatory phase of infection. We found that neutrophils are unable to effectively clear bacteria from the small intestine, except in a strain lacking secreted bacterial accessory toxins. However, circulating neutrophils are critical to blocking systemic inflammation and extraintestinal infections that are typical of nontoxigenic V. cholerae infection in humans.
MATERIALS AND METHODS
Depletion of neutrophils.
The 1A8 monoclonal antibody (MAb; BioXCell, West Lebanon, NH) is an anti-Ly6-G MAb that specifically depletes neutrophils, preserving GR-1+ blood monocytes (9). At 16 to 24 h prior to inoculation, C57BL/6 mice were injected intraperitoneally (i.p.) with 50 μg of 1A8 in phosphate-buffered saline (PBS). Control mice were mock injected with PBS or 50 μg of rat IgG (Sigma-Aldrich, St. Louis, MO), as noted. Preliminary studies found that this dose of antibody treatment was sufficient to deplete neutrophils from 5.4% ± 0.5% to 1% ± 1% of total peripheral blood leukocytes by 24 h (P = 0.004) and that this depletion persisted until 72 h. Neutropenia was confirmed in each experiment by peripheral blood smear at the time of inoculation.
Bacterial strains and growth media.
All strains have previously been described and were derived from V. cholerae El Tor O1 Inaba strain P27459, a clinical isolate from Bangladesh (13). Strain P4 has a kanamycin cassette inserted as a replacement for the ctxA and ctxB genes of P27459. Strain KFV101 is further modified with unmarked deletions in the rtxA, hlyA, and hapA genes (13). Overnight V. cholerae cultures were grown from single colonies in Luria-Bertani (LB) medium with 100 μg of streptomycin/ml overnight, shaking, at 30°C. Overnight cultures were then subcultured 1:1,000 in LB medium with streptomycin, shaking, at 37°C until mid-log phase (A600 ≈ 0.5). Bacterial pellets were then washed with PBS and diluted in PBS to the appropriate CFU/ml, using A600 to estimate culture density. The actual administered dose was confirmed by plating of dilutions of the inocula on LB agar.
Mouse inoculations.
Five- to six-week-old female C57BL/6 mice (Harlan, Indianapolis, IN) were housed in the Northwestern University barrier facility. Mice were given food and sterile water ad libitum until use, except for mice used for histopathology, where food was removed 12 h prior to infection to clear the gut lumen of its contents. Mouse inoculations were performed according to Northwestern University IACUC-approved protocols and as previously described (40). Briefly, the mice were anesthetized with 60 to 70 mg of ketamine/kg and 12 to 14 mg of xylazine/kg by i.p. injection. A total of 50 μl of 8.5% (wt/vol) NaHCO3 was administered intragastrically (i.g.) with a 22-gauge feeding needle, followed immediately by 50 μl of bacterial suspension in PBS. The mice were weighed before inoculation and daily thereafter as an indicator of health. For survival studies, mice were monitored and weighed daily for 7 days postinoculation (p.i.) Mice that become severely moribund, as evidenced by scruffy fur, extreme lethargy, and severe weight loss, were euthanized and counted as nonsurvivors.
Quantification of intestinal colonization and dissemination.
At appropriate time points p.i., mice were euthanized by cervical dislocation under anesthesia. The small intestines, livers, and/or spleens were removed, measured, weighed, and then homogenized in PBS. The homogenate was serially diluted in PBS and plated on LB agar plates containing 100 μg of streptomycin/ml in order to quantify the CFU recovered. Mice were considered noncolonized if fewer than 1 colony in 50 μl of undiluted homogenate was recovered (100 CFU in the small intestine); this is the detection limit of the experiment.
Gene expression and histopathology.
For histopathological analysis, sections of distal small intestine representing 1 cm of the ileocecal junction were dissected and fixed in 10% buffered formalin, embedded in paraffin, sliced and affixed to glass slides, and stained by hematoxylin and eosin (H&E) at the Mouse Histology and Phenotyping Laboratory of the Robert H. Lurie Comprehensive Cancer Center, Northwestern University, Chicago, IL. Analysis was performed by a pathologist blinded to the experimental sample set (G. K. Haines, Yale University, New Haven, CT). For gene expression studies, the intestinal tissue was preserved in RNALater (Invitrogen, Grand Island, NY) and stored at −20°C. These samples were then homogenized and lysed, and RNA was extracted using Clontech Nucleospin RNA II kit, according to the manufacturer's protocol (Bethlehem, PA). RNA quality extracted by this method was assessed (Bio-Rad Experion automated electrophoresis system; Bio-Rad, Hercules, CA) and reverse transcribed to cDNA (qScript cDNA synthesis kit; Quanta Biosciences, Gaithersburg, MA). cDNA was analyzed by quantitative real-time PCR (qRT-PCR) in triplicate. Target genes were identified by using validated TaqMan primers (Applied Biosystems, Carlsbad, CA), and a Bio-Rad IQ5 real-time detection system (Bio-Rad) was performed according to the following program: 2 min at 50°C, 10 min at 95°C, followed by 40 cycles of 15 s at 95°C and 1 min at 60°C. The data were analyzed using the 2−ΔΔCT method, using GAPDH (glyceraldehyde-3-phosphate dehydrogenase) as a control housekeeping gene (27).
Cytokine enzyme-linked immunosorbent assays (ELISAs).
For serum samples, blood was collected from mice by cardiac puncture and allowed to clot overnight at 4°C. Serum was collected by centrifugation at 3,000 rpm for 15 min at 4°C and stored at −20°C. For intestinal samples, 1 ml of whole intestinal homogenate (prepared as described above) was centrifuged at 13,000 rpm for 5 min at 4°C, and the supernatant was stored at −80°C until ready for use. All samples were analyzed by quantitative sandwich enzyme colorimetric immunoassays according to the manufacturer's instructions (R&D Systems, Minneapolis, MN), and the plates were read on a SpectraMax M5 plate reader (Molecular Devices, Sunnyvale, CA).
Statistical analysis.
All statistical analyses were performed using GraphPad Prism 4 for Macintosh software (GraphPad Software, Inc., San Diego, CA). For morbidity assays, data were pooled from multiple experiments and analyzed by using the Fisher exact test for comparison of surviving versus nonsurviving animals. Colonization and dissemination data were pooled from multiple experiments and analyzed by two-tailed Mann Whitney t test to compare medians. Cytokine ELISA data were analyzed by two-tailed Student t test. P values of <0.05 were considered statistically significant.
RESULTS
Neutropenic mice infected with nontoxigenic V. cholerae have decreased survival rates.
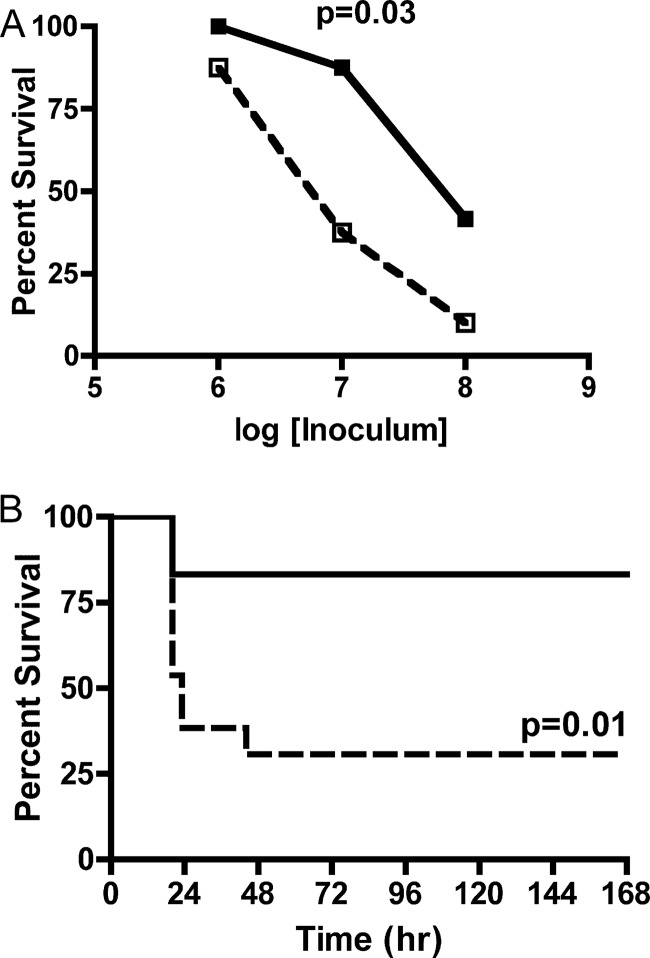
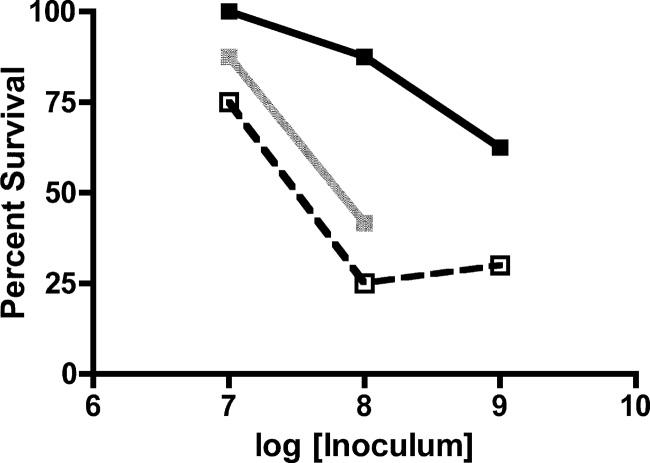
Previous studies have indicated no significant role for CT in establishment of small intestine colonization or virulence in the adult mouse infection model, making this model an appropriate choice for study of nontoxigenic infection (41). To determine the importance of neutrophils in controlling infection with nontoxigenic V. cholerae, C57BL/6 mice were depleted of neutrophils by prior treatment with anti-Ly6-G MAb 1A8 or mock treated. Mice were inoculated i.g. with a range of doses of V. cholerae El Tor O1 strain P4, derived from the clinical isolate P27459 via genetic replacement of the ctxAB genes with a kanamycin resistance cassette (15). Neutrophil-depleted mice succumbed in greater numbers to all three doses tested (Fig. 1A), with a significant survival difference at 107 CFU as determined by chi-square (P = 0.036) and log-rank sum (P = 0.01) analyses. At this dose, 83% (10/12) of neutrophil-replete mice survived inoculation compared to 33% (4/12) of neutrophil-depleted mice, with a majority of nonsurviving mice in both groups recorded within the first 24 h after infection (Fig. 1B). Survival at a dose of 106 CFU was similar in both neutropenic and neutrophil-replete mice, with 100% (4/4) and 89% (8/9) of mice surviving infection, respectively (P = 1.0). Survival at a dose of 108 CFU was 39% (5/13) in control mice and only 11% (1/9) in neutrophil-depleted mice (P = 0.33). Thus, depletion of neutrophils was detrimental to the host at increasing bacterial load, indicating that neutrophils protect against infection with nontoxigenic V. cholerae El Tor O1 at moderate doses. However, at the highest inocula, this protective effect of neutrophils is likely overwhelmed by the bacterial burden.
Fig 1.
Survival after infection with nontoxigenic V. cholerae. Mock-PBS-treated or neutrophil-depleted C57BL/6 mice were inoculated with a range of doses of nontoxigenic V. cholerae El Tor O1 strain P4, and survival was monitored over the course of 7 days. (A) Total survival of replete (solid line) or neutropenic (dotted line) mice inoculated with (1.4 to 2.1) × 106 CFU, (1.3 to 2.0) × 107 CFU, or (2.4 to 3.7) × 108 CFU. (B) Survival curve of replete (solid line) and neutropenic (dotted line) mice inoculated with (1.3 to 2.0) × 107 CFU plotted over time. The data are pooled from two experiments and analyzed by using the Fisher exact test (A) or log-rank test (B).
Neutropenic mice are colonized equally well as neutrophil-replete mice.
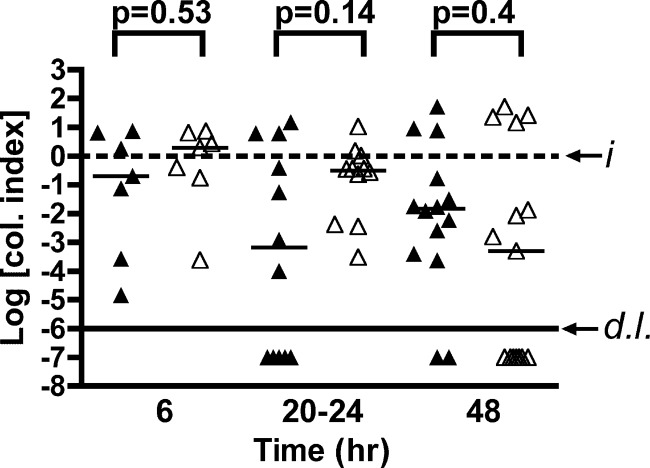
Previous studies established that strain P4 colonizes the distal small intestine quickly and replicates for up to 12 h p.i. After 12 h, the bacteria are partially cleared, but a stable population colonizes the small intestine for at least 72 h (41). To determine whether neutrophils are important for impairment of either the establishment or persistence of infection, neutrophil-replete and neutropenic mice were inoculated with a sublethal dose of strain P4, and small intestine colonization was assessed after 6, 20 to 24, or 48 h. Recovered CFU demonstrate no significant difference (Fig. 2) between neutrophil-replete and neutrophil-depleted mice in median intestinal colonization index either early in infection or in subsequent days (P = 0.53, P = 0.27, and P = 0.4, respectively). In contrast to the hypothesis that neutrophils are important for clearing the colonizing bacteria, these data indicate no role for neutrophils in controlling bacterial load in the small intestine at any point during the first 48 h of infection with nontoxigenic strain P4.
Fig 2.
Small intestine colonization. Mock-PBS-treated (▲) or neutrophil-depleted (△) C57BL/6 mice were inoculated with a sublethal dose of (0.57 to 2.8) × 106 CFU of nontoxigenic V. cholerae El Tor O1 strain P4. At 6, 20 to 24, or 48 h p.i., the small intestines were collected, homogenized in PBS, and plated for CFU counting. The data for individual mice are pooled from two to three experiments per endpoint and normalized by dividing the recovered CFU by the input CFU; thus, data are plotted as the log colonization index (median shown by black line) with the dashed line at 0 representing recovered CFU that is identical to the input (i). Values below the solid line (d.l.) indicate mice colonized below the detection limit of 100 CFU in the small intestine. Indicated P values were obtained by using a two-tailed Mann-Whitney nonparametric t test comparing medians.
Neutropenic and neutrophil-replete mice have equivalent levels of inflammatory markers in the intestine.
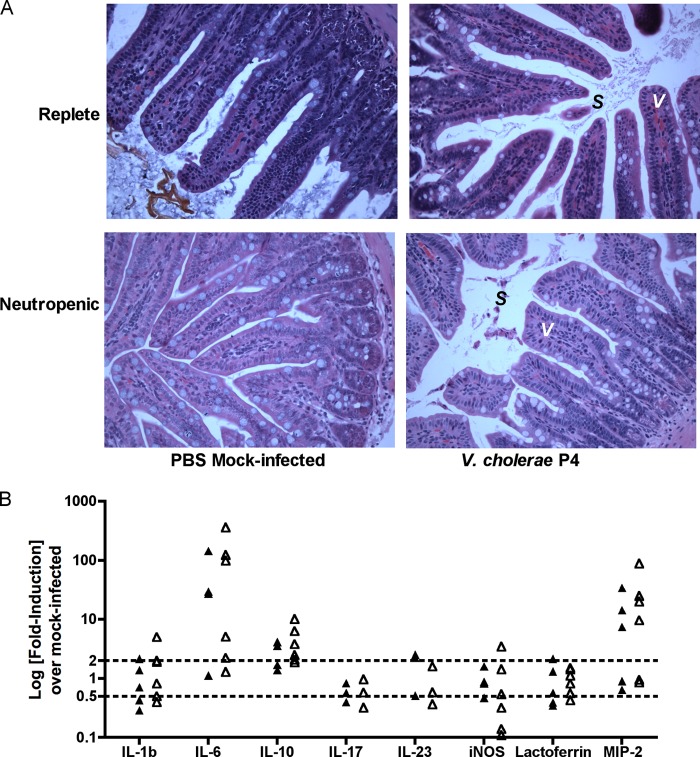
It was considered that decreased survival of neutropenic mice could relate to a heightened inflammatory response during the acute phase of infection. Histological analysis of the distal small intestine at 6 h p.i. with 108 CFU of strain P4 indicates similar levels of very mild inflammation in both replete and neutropenic animals, with no overt damage to the epithelium and minor accumulations of sloughed cells in the intestinal lumen (Fig. 3A). Previous studies have implicated secreted accessory toxins in the accumulation of these as-yet-unidentified sloughed cells (29, 39). Importantly, these sloughed cells were observed in neutropenic mice, indicating that the cells are not neutrophils.
Fig 3.
Intestinal histology and inflammatory gene expression. (A) Mock-PBS-treated or neutrophil-depleted C57BL/6 mice were inoculated with 1.5 × 108 CFU of nontoxigenic V. cholerae El Tor O1 strain P4 or mock inoculated with PBS. At 6 h p.i., the distal 1 cm of the small intestine at the ileocecal junction was collected, fixed, embedded in paraffin, and stained with H&E. Representative ×40 images are shown, with epithelial villi (V) and sloughed cells (S) noted as marked. (B) Mock-IgG-treated or neutrophil-depleted C57BL/6 mice were inoculated with (1.5 to 2.5) × 108 CFU of nontoxigenic V. cholerae El Tor O1 strain P4 or mock-inoculated with PBS. At 6 h p.i., the distal 1 cm of the small intestine at the ileocecal junction was collected, and RNA was extracted, converted to cDNA, and used for qRT-PCR analysis of the indicated genes, compared to PBS-mock-inoculated controls, and normalized to housekeeping gene GAPDH. Dotted lines indicate 2-fold up-or-down regulation compared to PBS control mice.
Gene expression profiles of inflammatory and regulatory cytokines and chemokines in the distal small intestine were also assessed (Fig. 3B); mice infected with V. cholerae strain P4 were characterized by upregulated expression of IL-6 (1- to 143-fold) and macrophage inflammatory protein 2 alpha (MIP-2) (1- to 34-fold), and a modest increase in IL-10 expression (1- to 4-fold), compared to uninfected controls. However, there was no significant difference in gene expression between neutropenic and replete animals. Other markers of inflammation and innate signaling were not significantly upregulated compared to uninfected controls, including IL-1β, IL-17, IL-23, Lf, and iNOS, indicating a low-level inflammatory response. Altogether, these data support increased expression of regulatory cytokines during acute V. cholerae infection but a limited role for neutrophils in orchestrating early innate immune signals in the intestine.
Neutrophils contribute to host defense by limiting bacterial dissemination and systemic inflammation.
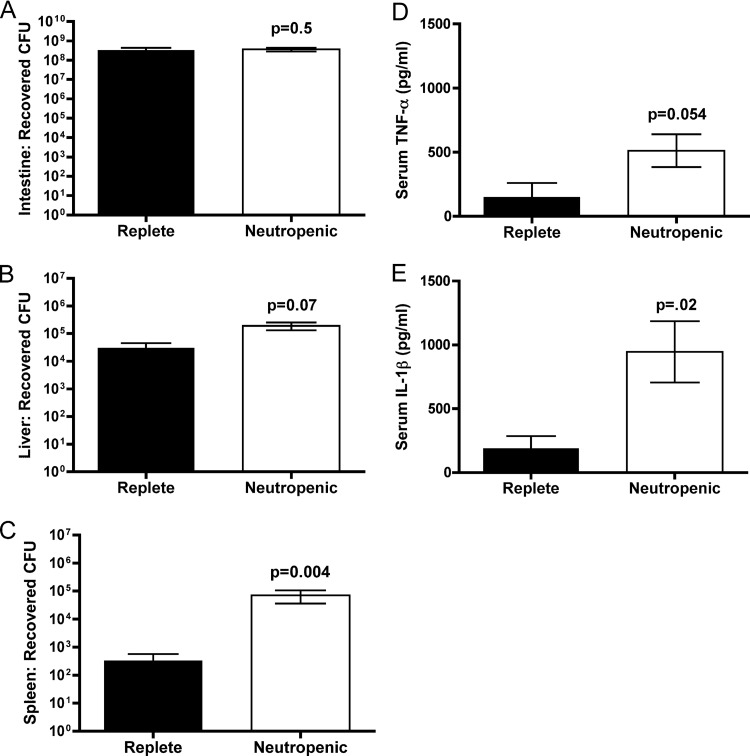
Nontoxigenic cholera is linked clinically not only to inflammatory diarrhea but also to extraintestinal infections, including septicemia (37, 45). Previous studies established that V. cholerae strain P4 can disseminate from either a lung infection (13) or gut infection (39) in mice. To determine whether dissemination is normally controlled by neutrophils, neutropenic mice were infected with a high dose (108 CFU) of V. cholerae strain P4 and assessed for dissemination to extraintestinal organs. Neutropenic mice have significantly higher levels of bacterial dissemination at 6 h compared to control mice. These mice demonstrated no difference in small intestine colonization at 6 h p.i. (Fig. 4A), again pointing to a mechanism other than high intestinal bacterial load for the previously observed rapid animal death in the neutropenic mice. However, CFU enumerated from the spleens indicate a mean splenic colonization 2.5 logs higher in neutropenic animals compared to replete animals (Fig. 4C), many of which have no disseminated bacteria present in the spleen (P = 0.004). Neutropenic mice show a trend of liver dissemination 1 log higher than control animals (P = 0.07) (Fig. 4B). In addition, although showing a limited local response, these mice do mount a robust systemic inflammatory response to infection (Fig. 4D and E). The levels of the proinflammatory cytokines IL-1β and TNF-α in serum are significantly higher in neutropenic mice (P = 0.023 and P = 0.054, respectively). Thus, neutrophils are critical to control of circulating bacteria that have escaped the local infection in the small intestine and progression of disease to septicemia.
Fig 4.
Dissemination and systemic inflammation. Mock-IgG-treated or neutrophil-depleted C57BL/6 mice were inoculated with (1.4 to 4.6) × 108 CFU of nontoxigenic V. cholerae El Tor O1 strain P4 or mock-inoculated with PBS. At 6 h p.i., the small intestines (A), livers (B), spleens (C), and serum (D and E) were collected. Organs were homogenized and plated for CFU counting. Serum was analyzed for protein concentration by ELISA. The data are pooled from four experiments and analyzed by using a two-tailed Mann-Whitney nonparametric t test comparing medians (A to C) or a two-tailed Student t test (D and E).
Accessory toxins are not required for virulence in neutropenic mice.
Previous studies have demonstrated that the hemolysin and MARTX accessory toxins are essential virulence factors in the adult mouse. It was observed that a ΔhlyA ΔrtxA ΔhapA nontoxigenic V. cholerae El Tor O1 strain was avirulent, with the majority of mice surviving an inoculation of 108 bacteria, a dose that is 100% lethal for parent strain P4 (39). To determine whether accessory toxins are essential for virulence in neutropenic mice, neutrophil-replete and neutrophil-depleted mice were inoculated with a range of doses of ΔhlyA ΔrtxA ΔhapA nontoxigenic V. cholerae El Tor O1 strain KFV101. In control mice inoculated with KFV101, survival is 62.5% even at a dose of 109 CFU (Fig. 5). Thus, as previously reported (39), the accessory toxins play an important role in virulence in immunocompetent mice. However, in mice depleted of neutrophils, the multi-toxin-deficient strain is more virulent, with 75, 50, and 30% surviving inoculation doses of 107, 108, and 109, respectively (compared to replete animals: P = 0.47, P = 0.04, and P = 0.35, respectively). In fact, virulence of strain KFV101 in neutropenic mice is equivalent to that of parent strain P4 in control animals at inoculations of 107 and 108 (P = 1.0 and P = 0.6, respectively). Thus, in neutropenic mice, accessory toxins are no longer required for virulence.
Fig 5.
Survival after infection with multi-toxin-deficient V. cholerae. Mock-PBS-treated or neutrophil-depleted C57BL/6 mice were inoculated with a range of doses of nontoxigenic strain KFV101 (ΔhlyA ΔrtxA ΔhapA), and survival was monitored over the course of 7 days. Total survival of replete (solid black line) or neutropenic (dotted black line) mice inoculated with (2.6 to 3.8) × 107 CFU, (3.1 to 8.4) × 108 CFU, or (3.4 to 5.2) × 109 CFU. Survival is compared to survival of replete mice inoculated with 107 and 108 doses of parent strain P4 (solid gray line), as reported in Fig. 1. The data are pooled from two experiments and analyzed by using the Fisher exact test.
The accessory toxins are required for persistent intestinal colonization in immunocompetent mice but not neutropenic mice.
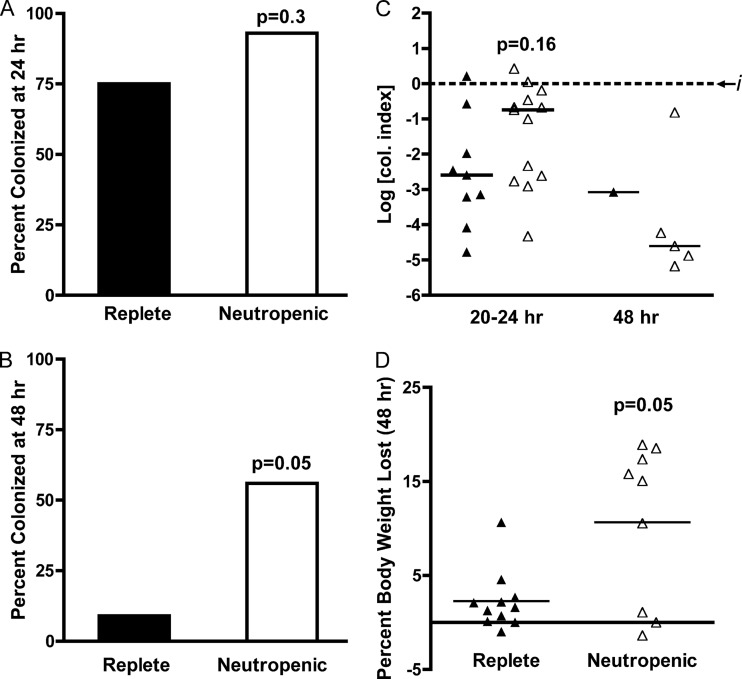
Previous studies have demonstrated that the secreted accessory toxins of V. cholerae El Tor O1 are essential for establishment and maintenance of small intestine colonization in adult mice (41). To determine whether the accessory toxins are essential for prolonged intestinal colonization in neutropenic mice, neutrophil-replete and neutrophil-depleted mice were inoculated with a 107 dose of ΔhlyA ΔrtxA ΔhapA nontoxigenic V. cholerae El Tor O1 strain KFV101. At 20 to 24 h p.i. (Fig. 6A), 9/12 (75%) neutrophil-replete mice remained colonized, while 13/14 (93%) neutropenic mice remained colonized (P = 0.3). Mice that have not cleared V. cholerae by 20 to 24 h (Fig. 6B) were colonized at similar levels (P = 0.16). By 48 h, however, 10/11 (91%) of neutrophil-replete mice had cleared the infection (Fig. 6C), whereas 5/9 (56%) of neutropenic mice remained colonized (P = 0.05). In addition, KFV101-infected replete mice lost significantly less weight than neutropenic mice by 48 h (Fig. 6D), averaging a 2.3% ± 0.95% body weight loss compared to 10.7% ± 2.8% (P = 0.007). Thus, consistent with previous studies, accessory toxins are essential for prolonged colonization to 48 h in the immunocompetent host; however, the need for these toxins is negated in the context of neutropenia.
Fig 6.
Prolonged colonization after infection with multi-toxin-deficient V. cholerae. Mock-PBS-treated (filled column or triangles) or neutrophil-depleted (open column or triangles) C57BL/6 mice were inoculated with a dose of (0.6 to 2.7) × 107 CFU of nontoxigenic P4 derivative strain KFV101. At 20 to 24 h or 48 h p.i., small intestines were collected, homogenized in PBS, and plated for CFU counting. The data are pooled from two experiments per endpoint. Mice are reported as either colonized or cleared at 20 to 24 h (A) or 48 h (B), and the data were analyzed by using the Fisher exact test. The CFU in the small intestines of mice remaining colonized (C) are represented as the log colonization index (CFUrecovered/CFUinput) with the median shown (black line) and the dashed line at 0 representing recovered CFU that is identical to the input (i). The percent weight loss of mice at 48 h is indicated (D), with the mean shown (black bar). Indicated P values were obtained by using a two-tailed Mann-Whitney nonparametric t test comparing medians (C) or a two-tailed Student t test (D). Statistical analysis of median colonization index could not be completed for the 48-h time point, since only one neutrophil-replete mouse remained colonized.
DISCUSSION
Cholera is a secretory diarrhea predominantly related to expression of the single virulence factor CT and has classically been regarded as noninflammatory in comparison to dysenteric diarrhea caused by invasive enteropathogens. More recent studies indicate that clinical infection involves a proinflammatory phase and that this phase is amplified in patients and human vaccine trial volunteers infected with nontoxigenic strains of V. cholerae. How this proinflammatory phase of diarrhea affects pathogenesis has not been studied carefully in animal models.
Human volunteers who ingest engineered nontoxigenic V. cholerae vaccines exhibit symptoms of mild diarrhea, abdominal cramping, and some instances of vomiting and fever (26). The bacterial factors causing these adverse reactions are not conclusively known, although several recent studies point to proinflammatory roles for structural components such as flagellins (47), as well as other secreted effectors (29), which likely contribute to vaccine reactogenicity.
Although the absence of flagella reduces the proinflammatory response and thus reduces diarrhea and inflammatory markers in an infant rabbit model, there is no concurrent reduction in colonization, suggesting that the proinflammatory response fails to clear bacterial infection even in the absence of diarrhea, a colonization known as asymptomatic carriage (47). We questioned whether this state was being controlled by the innate immune response even when disease is absent. Since the implications of these studies would be on the ultimate production of safe live attenuated vaccines, we conducted the studies in the context of nontoxigenic infection to model the proinflammatory phase of disease.
The connection of neutrophils to disease is not well understood. Increased numbers of circulating neutrophils, as well as increased concentration of neutrophil products, Lf and MPO, indicate a specific neutrophil response to V. cholerae early during natural human infection (12, 43, 44). Further, biopsies of acutely ill cholera patients demonstrate infiltration of neutrophils into the lamina propria, accompanied by congestion of the vasculature and endothelial damage (32). This is unsurprising, given that neutrophils are known to respond rapidly to mucosal infections by pathogenic bacteria (21).
First, it was necessary to establish whether neutrophils have any role in controlling infection with nontoxigenic V. cholerae. The data based on colonization suggests that there is no role for neutrophils in clearance of bacteria in the gut (Fig. 2). In fact, these data are consistent with rabbit studies wherein both nontoxigenic bacteria that produce flagellin and those deleted of fla genes show equivalent colonization (47). This equivalent colonization is observed despite a reduction of proinflammatory markers in the absence of flagella, which should correlate to decreased recruitment of neutrophils. Further, while infections in a mouse pneumonia model (13), rabbits (47), humans (43), and now adult mice (Fig. 3) show increased expression of regulatory and chemotactic markers IL-6, IL-10, and MIP-2 in the local tissue, there is no difference in these markers when neutrophils are depleted. Altogether, these results show that induction of the immune response by bacterial structural components results in recruitment of neutrophils, which contributes to reactogenic symptoms, including diarrhea in rabbits and humans. The neutrophils and other aspects of the innate immune response fail to clear the bacteria, which continue to colonize throughout the intestinal lumen and crypts (41, 47).
These data support a concept wherein in the absence of CT and at a dose where there is an insufficient immune response to activate proinflammatory diarrhea, as seen in the absence of flagella (47), or low-dose infection with P4 (Fig. 2), the bacteria promote an asymptomatic colonization of the gut. However, at increased inocula, mice that are neutropenic succumb to infection at higher numbers despite equivalent colonization (Fig. 1). This result is consistent with evidence from a pneumonia infection model that neutropenic mice succumb to infection with V. cholerae environmental isolates in far greater numbers than replete mice (30).
To resolve this difference in outcome (i.e., equivalent colonization but disparate survival outcomes), we show that neutrophils are critical to prevent dissemination into extraintestinal organs and an enhanced systemic inflammatory response marked by increased IL-1β and TNF-α in the serum. In fact, the level of these responses may be more severe than measured since it is possible that the cytokine profile was altered during processing, and samples snap-frozen at the time of sample collection would have provided a more accurate picture of inflammatory signaling. The role of neutrophils here is clinically relevant since nontoxigenic strains lacking the CTXΦ prophage are often reported as the agents of local outbreaks of mild-to-moderate diarrheal disease, as well as extraintestinal infections and septicemia, particularly in immunocompromised or liver-damaged individuals. Unlike toxigenic cholera, nontoxigenic V. cholerae induces a more inflammatory diarrhea, characterized by fever, chills, abdominal pain, and bloating (5, 33, 37, 45, 49, 55). Development of these more severe complications of nontoxigenic infection may be occurring in persons with weakened immune systems either from underlying disease or poor nutrition.
That there was no difference in colonization between neutropenic and replete mice indicates that neutrophils are not controlling infection in the gut. This observation may be accounted for by the activities of three “accessory” toxins secreted by El Tor O1 V. cholerae strains: hemolysin, HA/protease, and MARTX. Previous studies have established that nontoxigenic strains defective in the accessory toxins are cleared by 48 h after infection (41). Further, it is known that hemolysin and MARTX have redundant roles in promoting persistent colonization and that HA/protease may alter the efficiency of action of hemolysin and MARTX (41). In addition, it was observed that coinfection with a strain that secretes these toxins can rescue the colonization defect of a multi-toxin-deficient strain (40). These observations argue that accessory toxins, especially hemolysin and MARTX, act not as direct mediators of adherence but by altering the local host environment. Thus, it is hypothesized that they may act, in part, by enabling V. cholerae to survive innate immune clearance.
We found here that a strain that does not secrete the accessory toxins persists in the small intestine in neutropenic mice to 48 h (Fig. 6). We also found that at higher doses, this strain is no longer avirulent in the context of neutropenia (Fig. 5). These observations may indicate that the accessory toxins directly inhibit innate immune clearance by neutrophils in vivo. However, further study is needed to demonstrate how each of these secreted toxins contributes to persistent colonization and whether any individual toxin directly inhibits neutrophils during infection. Previously published in vitro data demonstrate that hemolysin and MARTX have cytopathic effects on innate immune cells, specifically that hemolysin can target neutrophils while MARTX cross-links actin in macrophages (14, 54). These data indicate that the need for accessory toxins to promote persistent colonization and virulence is negated in a neutropenic animal.
Overall, our results indicate that progression of intestinal infection during the proinflammatory phase occurs predominantly without effective clearance of bacteria by neutrophils. This proinflammatory phase is marked by very mild gross pathology, but with marked upregulation of regulatory and proinflammatory cytokines and chemokines, independent of the presence of neutrophils in the gut. Rather, the primary role of neutrophils early during infection is in clearing disseminated bacteria and preventing rapid death by septic shock. These observations of both a local early innate response to infection and systemic proinflammatory response to extraintestinal infection are consistent with previously reported findings in human studies and case reports.
It is notable, however, that systemic V. cholerae infections are not frequently reported in neutropenic patients, but rather in those with liver disease, such as alcoholic cirrhosis (42). It cannot be ruled out that administration of neutrophil-depleting antibody in the present study overwhelmed the reticuloendothelial system, which is responsible for the clearance of apoptotic and activated neutrophils from the circulation (51). It is also well established that Kupffer cells of the liver play an important role in clearing endotoxin and circulating bacteria during sepsis (53). Thus, it is possible that the higher incidence of septicemia in the neutrophil-depleted mice reflects an important role for liver macrophages and not necessarily only a neutrophil effect.
These findings elucidate important roles for host and bacterial factors involved in early innate responses to intestinal V. cholerae infection, and these data may have implications for vaccine development. It has been proposed that optimal next generation live attenuated vaccines would have defined mutations to remove flagellins to prevent reactogenic diarrhea in vaccine recipients (2). However, due to the transcriptional hierarchy of the flagellin system, class II nonmotile mutants such as the ΔflaA mutant have a consequence of increased transcription of numerous adherence and virulence factors, including tcp genes, gbpA, hlx, frhA, and hlyA (52). These factors may be responsible for the residual reactogenicity of Δfla mutants resulting in 12% of animals developing mild to severe diarrhea (47). Therefore, it is possible that a safe vaccine would also require deletion of accessory toxins.
An immunization study in the adult mouse model of infection demonstrated that vaccination with a nontoxigenic strain deleted of the hlyA, hapA, and rtxA genes provided effective protection in mice against subsequent challenge with V. cholerae (39). These data would argue that effective vaccination can be accomplished in the absence of accessory toxins. It is notable that the least reactogenic live attenuated vaccines tested have been Peru-15, which is a nonmotile mutant that also has a defect in production of the MARTX toxin, and strains based on classical strains that are naturally missing accessory toxins (50). In the present study, loss of accessory toxins in the mouse infection model also suggests that they could have a secondary positive role by inducing a more transient colonization, resulting in less transmission back to the environment. This finding warrants future study in humans into the efficacy and safety of vaccine strains lacking the genes for the accessory toxins.
ACKNOWLEDGMENTS
We thank Kristy Wolniak (Northwestern University Department of Pathology) and the Pathology Core Facility of the Robert H. Lurie Cancer Center for leukocyte differentials of peripheral blood smears, the Mouse Histology and Phenotyping Laboratory of the Robert H. Lurie Cancer Center for preparation of histology slides, and G. Kenneth Haines III (Yale University) for analysis of histopathology. We thank Rehman Tungekar, Naglaa El-Abbadi, Jayme Kwak, and Kevin Ziolo for technical assistance.
This study was supported by an Investigators in the Pathogenesis of Infectious Disease award from the Burroughs Wellcome Fund (awarded to K.J.F.S.), by National Institutes of Health grants R01 AI051490 and R21 AI072461 (awarded to K.J.F.S.), and by Ruth L. Kirschstein Research Service Award 1F30 DK084623 (awarded to J.Q.).
Footnotes
Published ahead of print 21 May 2012
REFERENCES
- 1. Ali M.2012. The global burden of cholera. Bull. World Health Organ. 90:209–218A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bishop AL, Camilli A. 2011. Vibrio cholerae: lessons for mucosal vaccine design. Expert Rev. Vaccines 10:79–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bromander A, Holmgren J, Lycke N. 1991. Cholera toxin stimulates IL-1 production and enhances antigen presentation by macrophages in vitro. J. Immunol. 146:2908–2914 [PubMed] [Google Scholar]
- 4. Bromander AK, Kjerrulf M, Holmgren J, Lycke N. 1993. Cholera toxin enhances alloantigen presentation by cultured intestinal epithelial cells. Scand. J. Immunol. 37:452–458 [DOI] [PubMed] [Google Scholar]
- 5. Bubshait SA, Al-Turki K, Qadri MH, Fontaine RE, Cameron D. 2000. Seasonal, nontoxigenic Vibrio cholerae O1 Ogawa infections in the eastern region of Saudi Arabia. Int. J. Infect. Dis. 4:198–202 [DOI] [PubMed] [Google Scholar]
- 6. Burkart V, et al. 2002. Cholera toxin B pretreatment of macrophages and monocytes diminishes their proinflammatory responsiveness to lipopolysaccharide. J. Immunol. 168:1730–1737 [DOI] [PubMed] [Google Scholar]
- 7. Coelho A, Andrade JR, Vicente AC, Dirita VJ. 2000. Cytotoxic cell vacuolating activity from Vibrio cholerae hemolysin. Infect. Immun. 68:1700–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cong Y, Oliver AO, Elson CO. 2001. Effects of cholera toxin on macrophage production of co-stimulatory cytokines. Eur. J. Immunol. 31:64–71 [DOI] [PubMed] [Google Scholar]
- 9. Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. 2008. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J. Leukoc. Biol. 83:64–70 [DOI] [PubMed] [Google Scholar]
- 10. Domingos MO, et al. 2009. Influence of the A and B subunits of cholera toxin (CT) and Escherichia coli toxin (LT) on TNF-alpha release from macrophages. Toxicon 53:570–577 [DOI] [PubMed] [Google Scholar]
- 11. Figueroa-Arredondo P, et al. 2001. Cell vacuolation caused by Vibrio cholerae hemolysin. Infect. Immun. 69:1613–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Flach CF, et al. 2007. Broad upregulation of innate defense factors during acute cholera. Infect. Immun. 75:2343–2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fullner KJ, et al. 2002. The contribution of accessory toxins of Vibrio cholerae O1 El Tor to the proinflammatory response in a murine pulmonary cholera model. J. Exp. Med. 195:1455–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fullner KJ, Mekalanos JJ. 2000. In vivo covalent cross-linking of cellular actin by the Vibrio cholerae RTX toxin. EMBO J. 19:5315–5323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goldberg I, Mekalanos JJ. 1986. Effect of a recA mutation on cholera toxin gene amplification and deletion events. J. Bacteriol. 165:723–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goo SY, Han YS, Kim WH, Lee KH, Park SJ. 2007. Vibrio vulnificus IlpA-induced cytokine production is mediated by Toll-like receptor 2. J. Biol. Chem. 282:27647–27658 [DOI] [PubMed] [Google Scholar]
- 17. Gutierrez MG, et al. 2007. Protective role of autophagy against Vibrio cholerae cytolysin, a pore-forming toxin from V. cholerae. Proc. Natl. Acad. Sci. U. S. A. 104:1829–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Haines GK, III, Sayed BA, Rohrer MS, Olivier V, Satchell KJ. 2005. Role of Toll-like receptor 4 in the proinflammatory response to Vibrio cholerae O1 El Tor strains deficient in production of cholera toxin and accessory toxins. Infect. Immun. 73:6157–6164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harris JB, et al. 2008. Susceptibility to Vibrio cholerae infection in a cohort of household contacts of patients with cholera in Bangladesh. PLoS Negl. Trop. Dis. 2:e221 doi:10.1371/journal.pntd.0000221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harrison LM, et al. 2008. Vibrio cholerae flagellins induce Toll-like receptor 5-mediated interleukin-8 production through mitogen-activated protein kinase and NF-κB activation. Infect. Immun. 76:5524–5534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jones RM, Neish AS. 2011. Recognition of bacterial pathogens and mucosal immunity. Cell Microbiol. 13:670–676 [DOI] [PubMed] [Google Scholar]
- 22. Kaper JB, Morris JG, Jr, Levine MM. 1995. Cholera. Clin. Microbiol. Rev. 8:48–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. la Sala A, et al. 2009. Cholera toxin inhibits IL-12 production and CD8alpha+ dendritic cell differentiation by cAMP-mediated inhibition of IRF8 function. J. Exp. Med. 206:1227–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee JB, Jang JE, Song MK, Chang J. 2009. Intranasal delivery of cholera toxin induces Th17-dominated T-cell response to bystander antigens. PLoS One 4:e5190 doi:10.1371/journal.pone.0005190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee NY, Lee HY, Lee KH, Han SH, Park SJ. 2011. Vibrio vulnificus IlpA induces MAPK-mediated cytokine production via TLR1/2 activation in THP-1 cells, a human monocytic cell line. Mol. Immunol. 49:143–154 [DOI] [PubMed] [Google Scholar]
- 26. Levine MM, et al. 1988. Volunteer studies of deletion mutants of Vibrio cholerae O1 prepared by recombinant techniques. Infect. Immun. 56:161–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 28. Ma AT, McAuley S, Pukatzki S, Mekalanos JJ. 2009. Translocation of a Vibrio cholerae type VI secretion effector requires bacterial endocytosis by host cells. Cell Host Microbe 5:234–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ma AT, Mekalanos JJ. 2010. In vivo actin cross-linking induced by Vibrio cholerae type VI secretion system is associated with intestinal inflammation. Proc. Natl. Acad. Sci. U. S. A. 107:4365–4370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Makri S, Purdy AE, Bartlett D, Fierer J. 2007. Pathogenicity of environmental isolates of Vibrio cholerae in mice. Microbes Infect. 9:1351–1358 [DOI] [PubMed] [Google Scholar]
- 31. Marinaro M, et al. 1995. Mucosal adjuvant effect of cholera toxin in mice results from induction of T helper 2 (Th2) cells and IL-4. J. Immunol. 155:4621–4629 [PubMed] [Google Scholar]
- 32. Mathan MM, Chandy G, Mathan VI. 1995. Ultrastructural changes in the upper small intestinal mucosa in patients with cholera. Gastroenterology 109:422–430 [DOI] [PubMed] [Google Scholar]
- 33. Morris JG, Jr, et al. 1984. Isolation of nontoxigenic Vibrio cholerae O group 1 from a patient with severe gastrointestinal disease. J. Clin. Microbiol. 19:296–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mutreja A, et al. 2011. Evidence for several waves of global transmission in the seventh cholera pandemic. Nature 477:462–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nelson EJ, Harris JB, Morris JG, Jr, Calderwood SB, Camilli A. 2009. Cholera transmission: the host, pathogen and bacteriophage dynamic. Nat. Rev. Microbiol. 7:693–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nielsen AT, et al. 2006. RpoS controls the Vibrio cholerae mucosal escape response. PLoS Pathog. 2:e109 doi:10.1371/journal.ppat.0020109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ninin E, et al. 2000. Nontoxigenic Vibrio cholerae O1 bacteremia: case report and review. Eur. J. Clin. Microbiol. Infect. Dis. 19:489–491 [DOI] [PubMed] [Google Scholar]
- 38. Nygren E, Li BL, Holmgren J, Attridge SR. 2009. Establishment of an adult mouse model for direct evaluation of the efficacy of vaccines against Vibrio cholerae. Infect. Immun. 77:3475–3484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Olivier V, Haines GK, III, Tan Y, Satchell KJ. 2007. Hemolysin and the multifunctional autoprocessing RTX toxin are virulence factors during intestinal infection of mice with Vibrio cholerae El Tor O1 strains. Infect. Immun. 75:5035–5042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Olivier V, Queen J, Satchell KJ. 2009. Successful small intestine colonization of adult mice by Vibrio cholerae requires ketamine anesthesia and accessory toxins. PLoS One 4:e7352 doi:10.1371/journal.pone.0007352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Olivier V, Salzman NH, Satchell KJ. 2007. Prolonged colonization of mice by Vibrio cholerae El Tor O1 depends on accessory toxins. Infect. Immun. 75:5043–5051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Patel NM, et al. 2009. Vibrio cholerae non-O1 infection in cirrhotics: case report and literature review. Transpl. Infect. Dis. 11:54–56 [DOI] [PubMed] [Google Scholar]
- 43. Qadri F, et al. 2004. Acute dehydrating disease caused by Vibrio cholerae serogroups O1 and O139 induce increases in innate cells and inflammatory mediators at the mucosal surface of the gut. Gut 53:62–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Qadri F, et al. 2002. Increased levels of inflammatory mediators in children and adults infected with Vibrio cholerae O1 and O139. Clin. Diagn. Lab. Immunol. 9:221–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Restrepo D, Huprikar SS, VanHorn K, Bottone EJ. 2006. O1 and non-O1 Vibrio cholerae bacteremia produced by hemolytic strains. Diagn. Microbiol. Infect. Dis. 54:145–148 [DOI] [PubMed] [Google Scholar]
- 46. Rolfs A, et al. 2008. Production and sequence validation of a complete full-length ORF collection for the pathogenic bacterium Vibrio cholerae. Proc. Natl. Acad. Sci. U. S. A. 105:4364–4369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rui H, et al. 2010. Reactogenicity of live-attenuated Vibrio cholerae vaccines is dependent on flagellins. Proc. Natl. Acad. Sci. U. S. A. 107:4359–4364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sack DA, Sack RB, Nair GB, Siddique AK. 2004. Cholera. Lancet 363:223–233 [DOI] [PubMed] [Google Scholar]
- 49. Saha PK, et al. 1996. Nontoxigenic Vibrio cholerae O1 serotype Inaba biotype El Tor associated with a cluster of cases of cholera in southern India. J. Clin. Microbiol. 34:1114–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Satchell KJ. 2003. Activation and suppression of the proinflammatory immune response by Vibrio cholerae toxins. Microbes Infect. 5:1241–1247 [DOI] [PubMed] [Google Scholar]
- 51. Shi J, Gilbert GE, Kokubo Y, Ohashi T. 2001. Role of the liver in regulating numbers of circulating neutrophils. Blood 98:1226–1230 [DOI] [PubMed] [Google Scholar]
- 52. Syed KA, et al. 2009. The Vibrio cholerae flagellar regulatory hierarchy controls expression of virulence factors. J. Bacteriol. 191:6555–6570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Szabo G, Romics L, Jr, Frendl G. 2002. Liver in sepsis and systemic inflammatory response syndrome. Clin. Liver Dis. 6:1045–1066 [DOI] [PubMed] [Google Scholar]
- 54. Valeva A, et al. 2008. Proinflammatory feedback activation cycle evoked by attack of Vibrio cholerae cytolysin on human neutrophil granulocytes. Med. Microbiol. Immunol. 197:285–293 [DOI] [PubMed] [Google Scholar]
- 55. Vogt AP, et al. 2010. Acute cholecystitis caused by nontoxigenic Vibrio cholerae O1 Inaba. J. Clin. Microbiol. 48:1002–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]